Abstract
Objective
To examine the clinical factors associated with increased opioid dose among mechanically ventilated children in the Pediatric Intensive Care Unit (PICU).
Design
Prospective, observational study with 100% accrual of eligible patients.
Setting
Seven PICUs from tertiary-care children’s hospitals in the Collaborative Pediatric Critical Care Research Network.
Patients
419 children treated with morphine or fentanyl infusions.
Interventions
None
Measurements and Main Results
Data on opioid use, concomitant therapy, demographic and explanatory variables were collected. Significant variability occurred in clinical practices, with up to 100-fold differences in baseline opioid doses, average daily or total doses, or peak infusion rates. Opioid exposure for 7 or 14 days required doubling of the daily opioid dose in 16% patients (95%CI: 12–19%) and 20% patients (95%CI: 16–24%) respectively. Among patients receiving opioids for longer than 3 days (n=225), this occurred in 28% (95%CI 22–33%) and 35% (95%CI 29–41%) by 7 or 14 days respectively. Doubling of the opioid dose was more likely to occur following opioid infusions for 7 days or longer (OR 7.9, 95%CI 4.3–14.3; p<0.001) or co-therapy with midazolam (OR 5.6, 95%CI 2.4–12.9; p<0.001), and it was less likely to occur if morphine was used as the primary opioid (vs. fentanyl) (OR 0.48, 95%CI 0.25–0.92; p=0.03), for patients receiving higher initial doses (OR 0.96, 95%CI 0.95–0.98; p<0.001), or if patients had prior PICU admissions (OR 0.37, 95%CI 0.15–0.89, p=0.03).
Conclusions
Mechanically ventilated children require increasing opioid doses, often associated with prolonged opioid exposure or the need for additional sedation. Efforts to reduce prolonged opioid exposure and clinical practice variation may prevent the complications of opioid therapy.
Keywords: intensive care, pain, agitation, anxiolysis, clinical practices, variability, child, infant
Children requiring mechanical ventilation and invasive monitoring routinely receive opioids for analgesia/sedation in the Pediatric Intensive Care Unit (PICU) (1, 2). Use of opioid analgesics and other sedatives helps to reduce pain, anxiety, or agitation, facilitate mechanical ventilation, prevent physiological stress responses, and avoid secondary complications (3–5). Opioid therapy may lead to opioid-induced hyperalgesia or opioid tolerance, dependence, and withdrawal (6). These effects occur more commonly in children than in adults, because of developmental changes in metabolism, excretion, drug efficacy, receptor subtypes, signal transduction, receptor induction, or cellular regulatory pathways (6–11).
Surveys of analgesia/sedation practices in PICUs demonstrated wide variability in clinical practices (1, 12). The use of several drug classes, multiple agents, large variations in the doses, frequency, and routes of administration, off-label use of analgesic drugs, or untested drug combinations occurs routinely, often driven by individual preferences or local culture (1, 6, 13). Consensus guidelines for sedation, analgesia, or neuromuscular blockade in PICU patients have highlighted the paucity of high-quality evidence and called for more randomized trials in this area (14, 15).
Given this variability, it is difficult to define best practices, develop guidelines, or launch scientific efforts to investigate the key hypotheses in this area. Randomized trials in this population await descriptive observational studies to determine associations between clinical practices and patient outcomes, needed for generating hypotheses that can be tested formally. The Collaborative Pediatric Critical Care Research Network (CPCCRN) designed this prospective, observational study to characterize the exposure to opioid analgesia among mechanically ventilated children in the PICU, in order to prepare for a randomized trial comparing alternative analgesic strategies.
Methods
Study Design
The MOTIF (Measuring Opioid Tolerance Induced by Fentanyl (or morphine)) Study was conducted in the seven PICUs participating in CPCCRN. Institutional Review Boards (IRBs) of all clinical centers and the Data Coordinating Center (DCC) approved the protocol. No interventions were performed and a waiver of informed consent was obtained in order to allow for the accrual of all eligible patients.
Participants
Patients were eligible for enrollment if they were: 1) over 37 weeks post-conceptual age and less than 18 years; 2) receiving ventilatory assistance via endotracheal tube or tracheostomy; and 3) receiving morphine or fentanyl infusions for analgesia. Patients were excluded if they: 1) had a history of drug abuse or alcohol dependence, 2) if the patient was less than 3 months old and the patient’s mother had a history of drug abuse during pregnancy, 3) were likely to be placed on extra-corporeal support soon after admission, 4) were receiving continuous drug infusions for epidural analgesia, nerve blocks, or plexus blocks, 5) were receiving opioid therapy via patient-controlled analgesia (PCA) pumps, 6) were receiving chronic opioid therapy before PICU admission, 7) were previously enrolled in this study, or 8) if parents were considering withdrawal of care or do-not-resuscitate orders.
Outcomes
Our primary outcome was defined as the need for doubling the initial opioid doses in order to achieve the same pharmacological effects as those seen at initiation of therapy. Patients met the primary outcome if the total daily dose required to maintain adequate analgesia was doubled as compared to opioid doses required in the first 24 hours of therapy. Any subjects who died, were discharged from the PICU, or exited the study prior to Day 14 (e.g., for initiation of ECMO) were included in the denominator for the primary endpoint. Secondary outcomes included the average daily opioid dose (μg/kg/day, in fentanyl equivalent doses, as defined below), peak opioid infusion rate (μg/kg/hour), total cumulative opioid dose (μg/kg) and the duration of opioid exposure (hours).
Data collection
We also collected data on demographic and clinical variables, history of prior opioid exposure and the use of sedation with benzodiazepines, to identify potential risk factors or mitigating factors for increased opioid dosage in children. Daily doses of opioids and benzodiazepines were calculated using the total infusion-based dosing as well as the pro re nata (PRN) or bolus doses required each day. Data were collected daily by research coordinators at the CPCCRN sites and entered into an electronic data system. Data collection began with initiation of opioid therapy in the PICU (Day 1) and continued until Day 14 unless one of the study exit criteria were met. Study exit criteria were defined as death, PICU discharge, discontinuation of opioid therapy, or initiation of ECMO. Since some agents are more lipophilic than others, we calculated the BMI (body mass index) and evaluated its impact using the BMI percentile for age. Morphine doses were converted to fentanyl equivalents using well-known opioid potency ratios (1:80) (6, 16), whereas midazolam and lorazepam doses were combined assuming a potency ratio of 1:1 (17, 18).
Statistical analyses
Demographic and clinical characteristics were summarized for all subjects and by site, using counts and percentages for categorical data, and the median and interquartile range (IQR: 25th and 75th percentiles) for continuous data. Differences across sites were evaluated using Chi-square or Fisher’s exact test for categorical variables and Kruskal-Wallis test for continuous variables.
Our primary outcome (defined as doubling of the daily opioid dose) after 7 and 14 days of opioid therapy was described using proportions and 95% confidence intervals (95%CI). Univariable associations of demographic and clinical factors with increased opioid use by 14 days were evaluated using the Chi-square test for categorical variables and the Wilcoxon rank-sum test for continuous variables. Multivariable logistic regression analyses were performed for all patients and separately for postoperative and non-postoperative patients. All models included a priori factors, such as age, sex, baseline opioid dose, and primary opioid used (morphine vs. fentanyl). Additional factors were selected for inclusion in the final model based on backward variable selection at a significance level of 0.05. Adjusted odds ratios and corresponding 95%CI were reported. A Kaplan-Meier “freedom from event” curve was generated for the outcome of time to doubling of the initial opioid dose. Patients not achieving this outcome were censored at the time of study exit.
Analyses were performed in SAS v9.2 (SAS Institute Inc., North Carolina), using a significance level of 0.05 for all analyses. In analyses examining the associations between patient characteristics and opioid use, or for comparisons between clinical sites we made no adjustment for multiple comparisons because these investigations are descriptive and hypothesis-generating in nature.
Results
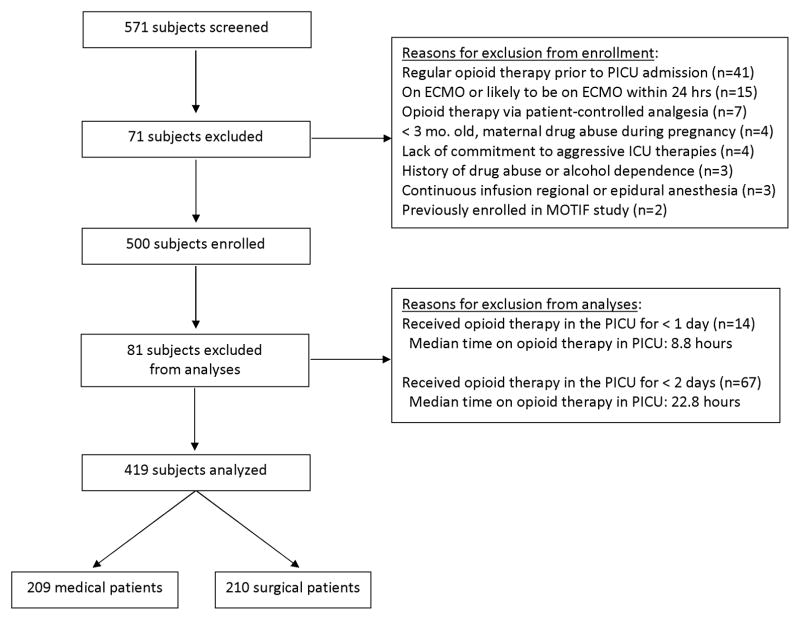
Total 571 subjects were screened, 500 were enrolled, and 419 subjects were eligible for study analyses (Figure 1). Baseline characteristics of study subjects (N=419) are listed in Table 1. Patients had a median age of 16 months (IQR 4, 80), a Pediatric Risk of Mortality (PRISM III) score of 6 (IQR 3, 11), height of 75 cm (IQR 58, 112), weight of 10.0 kg (IQR 5.5, 21.3), and BMI of 16.6 (IQR 14.7, 18.9). Factors possibly associated with previous opioid exposure included a history of NICU admission (n=96, 24%), PICU admission (n=81, 20%) or surgical operation (n=161, 39%) prior to this PICU admission.
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) Diagram showing subjects screened, enrolled, and included in this analysis.
Table 1.
Patient Characteristics
| Variable | n (%) Yes |
|---|---|
| Female | 177 (42) |
| Male | 242 (58) |
| Age groups: | |
| Newborn (< 1 mo) | 42 (10) |
| Infant (1 mo – < 1 yr) | 148 (35) |
| Pre-school (1 yr – < 5 yrs) | 109 (26) |
| Pre-adolescent (6 yrs – < 10 yrs) | 44 (11) |
| Adolescent (10 yrs–< 18 yrs) | 76 (18) |
| Ethnicity: | |
| Hispanic or Latino | 78 (19) |
| Not Hispanic or Latino | 259 (62) |
| Unknown | 82 (20) |
| Race: | |
| Black or African American | 95 (23) |
| White | 236 (56) |
| Other/Unknown | 88 (21) |
| Primary Diagnosis: | |
| Cardiac | 131 (31) |
| Neurological | 14 (3) |
| Respiratory | 131 (31) |
| Shock | 32 (8) |
| Surgical/Trauma | 54 (13) |
| Transplant | 21 (5) |
| Other | 36 (9) |
| Post Operative | 210 (50) |
| Types of Surgery (N = 210): | |
| Cardiac surgery | 97 (46) |
| Neurosurgery | 13 (6) |
| Orthopedic surgery | 6 (3) |
| Transplant | 21 (10) |
| Trauma | 7 (3) |
| Other | 66 (31) |
| Opioid therapy initiated with: | |
| Fentanyl | 267 (64) |
| Morphine | 152 (36) |
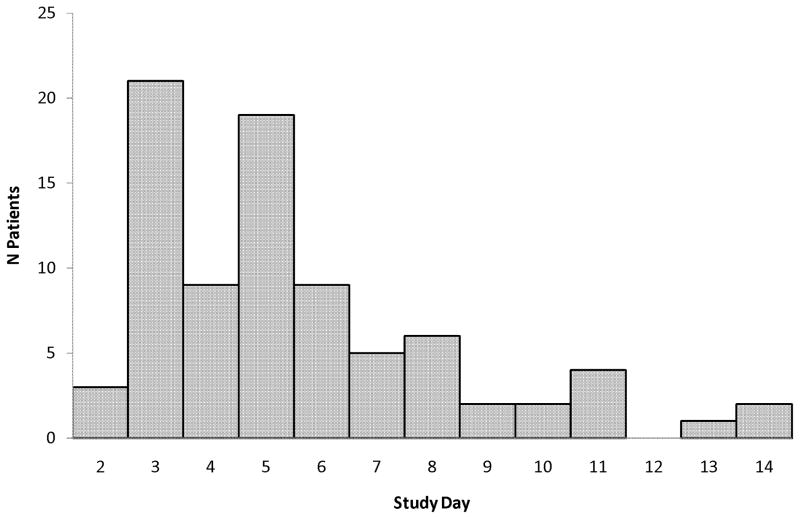
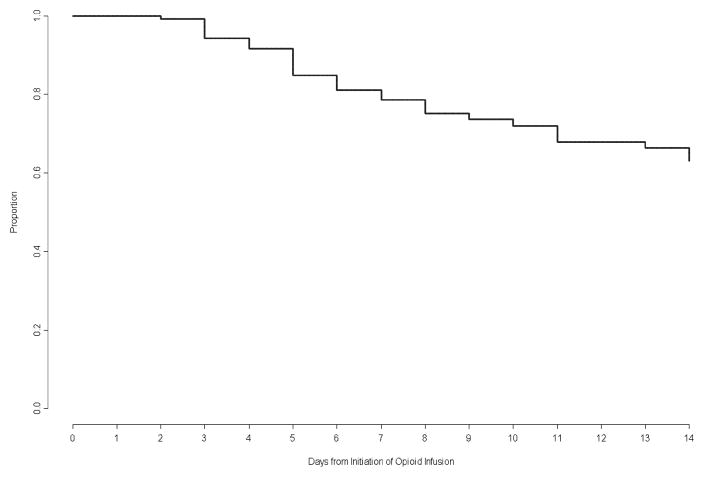
Therapeutic variables related to opioid and benzodiazepine use are listed in Table 2 and adjuvant therapies are listed in Table 3. Striking variability occurred in the opioids administered to individual patients, with more than 100-fold differences in the initial opioid doses (0–24 hrs), average daily or total cumulative doses, and peak infusion rates. Doubling of the opioid dose from the initial baseline occurred in 16% patients by Day 7 (95%CI 12–19%) and 20% patients by Day 14 (95%CI 16–24%). Days on which individual patients achieved the primary outcome are presented in Figure 2 and cumulative estimates are presented in Figure 3. Among patients receiving opioids for at least 96 hours (n=225), doubling of the opioid dose occurred in 28% by Day 7 (95%CI 22–33%) and 35% by Day 14 (95%CI 29–41%). Reasons for study exit prior to Day 14 included discontinuation of opioid therapy (73%), PICU discharge (8%), initiation of ECMO (2%), or death (2%). Opioid doses had doubled among 43% (95%CI 30–55%) of the 61 patients still receiving opioids on Day 14.
Table 2.
Opioid and Benzodiazepine Usein All Patients
| Opioids, N=419 | Minimum, Maximum | Median (Interquartile Range) |
|---|---|---|
| Baseline opioid dose (μg/kg/day) | 1.2, 161.5 | 34.9 (20.9, 60.2) |
| Average daily dose (μg/kg/day) | 1.1, 185.5 | 34.0 (15.4, 53.8) |
| Total cumulative dose (μg/kg) | 3.4, 2431 | 179.7 (72.5, 436.1) |
| Peak infusion rate (μg/kg/hr) | 0.1, 16.0 | 2.5 (1.0, 4.0) |
| Duration of opioid infusion (hrs) | 5.3, 336.0 | 104.1 (57.7, 213.0) |
| Duration on study (hrs) | 33.0, 336.0 | 133.2 (74.5, 233.5) |
| Interval between unit admission and opioid infusion (hrs) | 0, 865.7 | 3.3 (1.1, 8.8) |
|
|
||
| Rate | 95% Confidence Interval | |
| Doubling of opioid dose at 7 days | 0.16 (66/419) | 0.12, 0.19 |
| Doubling of opioid dose at 14 days | 0.20 (83/419) | 0.16, 0.24 |
|
| ||
| Benzodiazepines, N=387a | Minimum, Maximum | Median (Interquartile Range) |
|
| ||
| Days receiving benzodiazepines | 1, 14 | 5 (3, 9) |
| Average daily dose (mg/kg) | 0.02, 23.4 | 1.1 (0.2, 2.9) |
| Total dose (mg/kg) | 0.02, 227 | 4.1 (0.7, 18.1) |
IQR = interquartile range (25th, 75th percentile). Morphine doses were converted to fentanyl equivalents using well-definedopioid potency ratios (1:80).
387/419 (92%) of patients received midazolam or lorazepam during the study period.
Average daily dose is basedonly on the days when any benzodiazepines were received.
Table 3.
Adjuvant Sedatives and Analgesics Administered
| Sedatives | n (%) | Analgesics | n (%) |
|---|---|---|---|
| Ketamine | 73 (17) | Acetaminophen | 317 (76) |
| Propofol | 60 (14) | Dexmedetomidine | 90 (21) |
| Chloral hydrate | 62 (15) | Methadone | 73 (17) |
| Pentobarbital | 26 (6) | Ibuprofen | 51 (12) |
| Phenobarbital | 24 (6) | Oxycodone | 32 (8) |
| Clonazepam | 9 (2) | Hydromorphone | 30 (7) |
| Diphenhydramine | 9 (2) | Ketorolac | 25 (6) |
| Diazepam | 5 (1) | Acetylsalicylic acid | 25 (6) |
| Hydroxyzine | 5 (1) | Clonidine | 8 (2) |
| Isoflurane | 4 (1) | Meperidine | 7 (2) |
| Sevoflurane | 3 (1) | Nalbuphine | 3 (1) |
| Haloperidol | 3 (1) | Hydrocodone | 2 (0) |
| Promethazine | 3 (1) | Remifentanil | 1 (0) |
| Thiopental | 2 (0) | Codeine | 1 (0) |
| Etomidate | 2 (0) | ||
| Droperidol | 1 (0) |
Figure 2.
Number of patients who required doubling of their opioid doses after initiation of opioid therapy.
Figure 3.
Kaplan-Meier “freedom from event” curve for patients meeting the criterion for the primary outcome (time to doubling of the initial opioid dose). Patients not achieving this outcome were censored at the time of study exit.
Clinical and demographic factors included in univariable and multivariable analyses are listed in Table 4. Age (p=0.50), sex (p=0.82), race (p=0.08), ethnicity (p=0.13), BMI (p=0.33), severity of illness (PRISM III scores, p=0.47), previous NICU admission (p=0.54) or previous surgical operations (p=0.81) were not associated with this outcome. Doubling of the initial opioid dose occurred less frequently in patients admitted just after surgery (p<0.001) and those with previous PICU admission(s) (p=0.01). Subjects receiving lower opioid doses at baseline (p=0.003) and those requiring surgery while on the study (p=0.001) had greater likelihood of doubling their daily opioid doses. The median baseline (0–24 hr) fentanyl doses were 29 μg/kg/day (IQR 20–46) vs. 41 μg/kg/day (IQR 21–66) respectively for those who did or did not require doubling of their initial opioid dose. Children who required doubling of the opioid dose were more likely to receive additional opioids (other than morphine or fentanyl; 49% vs. 27%, p<0.001) and benzodiazepines (99% vs. 91%, p=0.02), require multiple adjuvant therapies (median 5 (IQR 3–7) vs. 3 (IQR 2–4), p<0.001) and to receive methadone therapy (39% vs. 12%, p<0.001).
Table 4.
Factors associated with Doubling of the DailyOpioidDose
| Variable | n (%) | p |
|---|---|---|
| Sex: | 0.82 | |
| Female | 36 (20) | |
| Male | 47 (19) | |
| Age group: | 0.50 | |
| Newborn (< 1 mo) | 8 (19) | |
| Infant (1 mo – < 1 yr) | 23 (16) | |
| Pre-school (1 yr – < 5 yrs) | 24 (22) | |
| Pre-adolescent (6 yrs – < 10 yrs) | 9 (20) | |
| Adolescent (10 yrs – < 18 yrs) | 19 (25) | |
| Postoperative Status: | < 0.001 | |
| Postoperative | 26 (12) | |
| Not postoperative | 57 (27) | |
| Surgery Required While On Study: | 0.001 | |
| Yes | 31 (31) | |
| No | 52 (16) | |
| NICU Admission(s) in Previous Year: | 0.54 | |
| Yes | 17 (18) | |
| No | 63 (21) | |
| PICU Admission(s) in Previous Year: | 0.01 | |
| Yes | 8 (10) | |
| No | 73 (23) | |
| Surgical Operation(s) in Previous Year: | 0.81 | |
| Yes | 31 (19) | |
| No | 52 (20) | |
| Primary Opioid Used: | 0.30 | |
| Fentanyl | 47 (18) | |
| Morphine | 36 (22) |
Multivariable logistic regression analyses are presented in Table 5. Factors associated with our primary outcome included opioid infusions for 7 days or longer (OR=7.9) and concomitant infusions of midazolam (OR=5.6). This risk was mitigated in patients receiving primarily morphine vs. fentanyl (OR=0.48), those given higher initial opioid doses (OR=0.96), or those with prior PICU admissions (OR=0.37). Logistic regression analyses limited to postoperative patients identified similar factors, but females were more likely than males (OR=2.8) to require doubling of their initial opioid dose, as were those receiving co-therapy with midazolam (OR=6.3) or lorazepam (OR=5.2). Using morphine primarily appeared to reduce the risk of doubling opioid doses in the surgical patients (OR=0.27), but not in the medical or non-postoperative patients (OR=0.60).
Table 5.
Factors associated with Doubling of the Daily Opioid Dose
| Variables | All Patients (N=419) Adjusted OR (95% Confidence Intervals) |
P-values | Postoperative (N=210) Adjusted OR (95% Confidence Intervals) |
P-values | Medical (N = 209) Adjusted OR (95% Confidence Intervals) |
P-values |
|---|---|---|---|---|---|---|
| Age groups: | 0.50 | 0.53 | 0.90 | |||
| Infant (< 1 yr) | 0.81 (0.37, 1.79) | 0.47 (0.12, 1.79) | 0.95 (0.37, 2.50) | |||
| Child (1 yr – < 10 yrs) | 1.21 (0.55, 2.65) | 0.69 (0.17, 2.81) | 1.15 (0.45, 2.95) | |||
| Adolescent (10 yrs–< 18 yrs) | Reference | Reference | Reference | |||
| Female (vs. male) | 1.10 (0.61, 1.96) | 0.75 | 2.79 (0.99, 7.87) | 0.052 | 0.68 (0.33, 1.40) | 0.29 |
| Baseline opioid dose (1 unit mcg/kg) | 0.96 (0.95, 0.98) | <0.001 | 0.96 (0.94, 0.98) | 0.001 | 0.97 (0.95, 0.99) | 0.004 |
| Primary opioid morphine (vs. fentanyl) | 0.48 (0.25, 0.92) | 0.03 | 0.27 (0.08, 0.88) | 0.03 | 0.60 (0.27, 1.33) | 0.21 |
| Midazolam used | 5.57 (2.41, 12.9) | <0.001 | 6.31 (1.73, 23.0) | 0.005 | 6.91 (2.21, 21.7) | <0.001 |
| Lorazepam used | N.S. | -- | 5.21 (1.49, 18.2) | 0.01 | N.S. | -- |
| Opioid infusion ≥ 7 days | 7.85 (4.32, 14.3) | <0.001 | 5.86 (2.10, 16.3) | <0.001 | 7.06 (3.33, 15.0) | <0.001 |
| History of previous PICU admission | 0.37 (0.15, 0.89) | 0.03 | N.S. | -- | N.S. | -- |
Note that age group, sex, baseline opioid dose, and primary opioid used were included a priori in all models. Other variables were selected based on backward variable selection with a significance level of 0.05. The variable selection procedure was performed separatelyfor each of the three analyses above. The adolescent age group was used as a reference group because it is closest to adult patients. OR = odds ratio, NS = not selected in the logistic model because p>0.05.
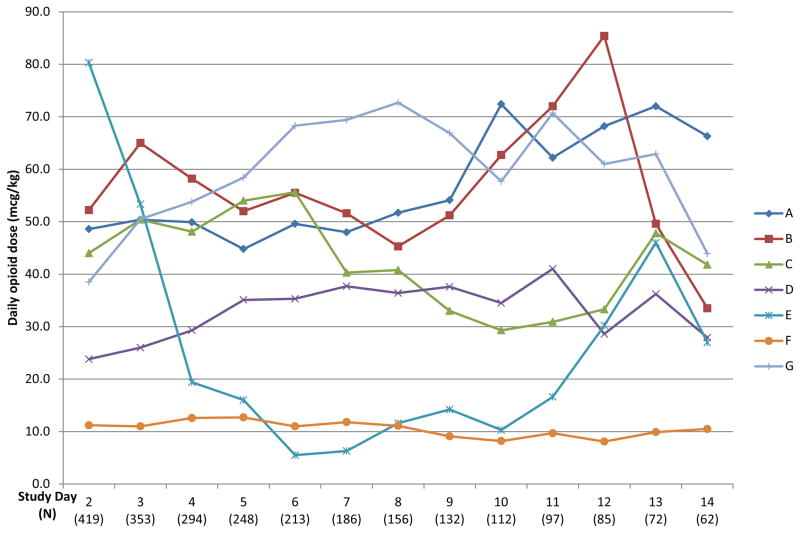
Opioid and benzodiazepine usage by clinical site is shown in Table 6. Significant differences occurred in the baseline opioid doses used, the average daily doses, the cumulative total doses, peak opioid infusion rates, and duration of opioid infusions between the 7 clinical sites (p<0.001). Four sites used fentanyl as the primary opioid, two sites preferred morphine, whereas one site used both drugs equally. Variations in median daily opioid doses for all clinical sites are displayed in Figure 4. Site E enrolled a relatively high percentage of cardiac surgical patients and used high initial opioid doses (fentanyl at 3–5 mcg/kg/hr). No patients at this site required doubling of the opioid dose during this study. Site F was the only institution that followed an explicit sedation protocol at the time of this study. Minimal variation occurred in their median daily doses and very few patients at this site (3%) required doubling of their initial opioid dose. Benzodiazepines were used for sedation for most patients at each clinical site (83–98%), although the drugs used, the average daily benzodiazepine doses, and the total cumulative doses differed significantly between the clinical sites (p<0.001).
Table 6.
Variability of Opioid and Benzodiazepine Use by Clinical Site, N=419
| Variable | A | B | C | D | E | F | G | |
|---|---|---|---|---|---|---|---|---|
| Opioids | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | p |
|
| ||||||||
| Baseline opioid dose (μg/kg) | 48 (29, 58) | 54 (35, 87) | 41 (29, 59) | 24 (15, 30) | 77 (57, 97) | 14 (9, 19) | 37 (28, 70) | < .001 |
| Average daily dose (μg/kg) | 42 (30, 54) | 49 (32, 76) | 37 (27, 65) | 26 (15, 41) | 44 (20, 68) | 9 (6, 13) | 46 (14, 70) | < .001 |
| Total cumulative dose (μg/kg) | 281 (124, 531) | 320 (129, 682) | 227 (102, 458) | 169 (64, 463) | 202 (102, 394) | 39 (18, 100) | 291 (71, 634) | < .001 |
| Peak infusion rate (μg/kg/hr) | 3.0 (2.0, 4.0) | 4.0 (3.0, 6.0) | 2.5 (2.0, 4.8) | 1.5 (1.0, 2.5) | 4.0 (3.0, 5.0) | 0.4 (0.3, 0.6) | 3.0 (2.0, 5.0) | < .001 |
| Duration of opioid infusion (hrs) | 132 (77, 240) | 134 (69, 254) | 115 (65, 198) | 161 (80, 287) | 60 (34, 99) | 75 (40, 158) | 100 (45, 204) | < .001 |
| Duration on study (hrs) | 169 (82, 275) | 148 (75, 260) | 136 (83, 218) | 178 (81, 298) | 123 (77, 174) | 83 (53, 167) | 136 (60, 233) | .009 |
|
| ||||||||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||
|
| ||||||||
| Primary Opioid Used | ||||||||
| Fentanyl | 57 (95) | 54 (98) | 42 (52) | 24 (30) | 46 (88) | 5 (8) | 30 (100) | < .001 |
| Morphine | 3 (5) | 1 (2) | 39 (48) | 55 (70) | 6 (12) | 57 (92) | 0 (0) | |
| Primary outcome at 7 days | 12 (20) | 6 (11) | 20 (25) | 18 (23) | 0 (0) | 2 (3) | 8 (27) | <.001 |
| Primary outcome at 14 days | 19 (32) | 10 (18) | 22 (27) | 21 (27) | 0 (0) | 2 (3) | 9 (30) | < .001 |
|
| ||||||||
| Benzodiazepines | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
|
| ||||||||
| Any benzodiazepine used | 56 (93) | 49 (89) | 67 (83) | 77 (97) | 51 (98) | 58 (94) | 29 (97) | .01 |
| Midazolam used | 36 (60) | 21 (38) | 66 (81) | 76 (96) | 51 (98) | 18 (29) | 26 (87) | < .001 |
| Lorazepam used | 47 (78) | 44 (80) | 24 (30) | 34 (43) | 11 (21) | 55 (89) | 20 (67) | < .001 |
|
| ||||||||
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | ||
|
| ||||||||
| Days receiving benzodiazepines | 4 (3, 8) | 4 (3, 7) | 6 (3, 10) | 8 (4, 13) | 5 (4, 8) | 3 (2,6) | 5 (3, 10) | < .001 |
| Average daily dose (mg/kg)* | 0.2 (0.1, 0.3) | 0.2 (0.1, 0.4) | 2.4 (1.1, 4.1) | 2.1 (1.4, 3.6) | 2.9 (1.7, 4.2) | 0.2 (0.1, 0.4) | 1.5 (0.8, 3.2) | < .001 |
| Total cumulative dose (mg/kg) | 0.6 (0.3, 2.5) | 0.9 (0.4, 2.5) | 11.9 (3.0, 28.7) | 15.7 (6.0, 31.0) | 13.7 (5.4, 29.6) | 0.6 (0.3, 1.9) | 5.4 (2.6, 20.2) | < .001 |
IQR = interquartile range (25th, 75th percentile); Primary Outcome was defined as doubling of the initially effectiveopioid dose (0–24 hours of therapy). All totals included infusion and bolus doses; morphine doses were converted to fentanyl equivalents using well-defined opioid potency ratios (1:80).
Average daily benzodiazepine dose is based only on the days when any benzodiazepines were received.
Figure 4.
Patterns of opioid dosing at the participating sites. Each line represents a single clinical site, showing the median daily opioid dose used at that site each day during the study. Morphine doses were converted to fentanyl equivalents using well-defined opioid potency ratios (1:80).
Discussion
In this study, we found that 16% of all studied patients required doubling of their opioid dose by 7 days, and 20% by 14 days. This outcome was more likely among patients exposed to opioid infusions for 7 days or longer, those receiving midazolam infusions, and among non-postoperative patients. Among postoperative patients, doubling of the opioid dose occurred more frequently in patients receiving opioid infusions for 7 days or longer, and those receiving fentanyl (vs. morphine), midazolam, or lorazepam infusions. We noted a striking variability in the opioid and benzodiazepine use between CPCCRN sites. Finally, we enrolled 500 subjects who met the inclusion and exclusion criteria, but 81 (16%) of these patients required opioid management for less than 24 hours. In the design of future randomized trials, this loss of evaluable subjects must be taken into account when planning accrual and power calculations.
Prolonged opioid exposure may lead to opioid tolerance and withdrawal in children requiring intensive care (19–22); therefore we wanted to identify the patients at risk for these complications. While tolerance may occur in any patient, those exposed to opioids for longer than 3 days and those who require a doubling of their initially effective opioid doses are likely to be at highest risk for these complications (6). This doubling occurred in up to 20% of all ventilated patients receiving opioids for 24 hours, 35% of those receiving opioids for 96 hours or longer, and 43% of those receiving opioids for 14 days or more. These data are consistent with previously reported rates of opioid tolerance in 36–57% of PICU patients (19, 23–28) and were further corroborated in a systematic review (29).
Critically ill children require frequent adjustments of their analgesia/sedation, in response to rapidly changing clinical parameters, worsening illness, necessary invasive procedures, or surgical operations and other indications, but also because of poorly defined goals of sedation, subjective assessments of pain or discomfort, varying expectations of different nurses or physicians, and deeply entrenched practice patterns. These challenges can be addressed in formal analgesia/anxiolysis titration protocols designed for both rapid and effective pain and anxiety relief, but also proactive weaning of these drugs if the patient remains comfortable (30). Research in this area should receive high priority, because iatrogenic injury, with major complications (31, 32) and prolonged hospital stays (27, 33) may occur among children with prolonged opioid exposure (23–25, 34, 35).
In mechanically ventilated children, the effectiveness of opioid analgesia may vary because of worsening pain, or opioid-induced hyperalgesia, or opioid tolerance (6). Currently, there are no clinical, physiological, or biochemical markers to identify patients developing opioid tolerance. In animal models, opioid tolerance is assessed by a need for increased doses and the signs of opioid withdrawal precipitated by giving opioid antagonists (36, 37). This approach is not clinically feasible; therefore, future research must focus on a search for reliable biomarkers that can allow us to investigate the real-time relationships between opioid analgesia, hyperalgesia, tolerance, and withdrawal. We explored a pragmatic definition (doubling of the total initial opioid dose, assuming that effective analgesia was achieved with the initial and subsequent opioid doses) to identify a high-risk group for developing opioid tolerance.
We found that prolonged opioid exposure was associated with doubling of the opioid dose, also noted previously (19, 38). Patients receiving lower initial doses were more likely to develop opioid tolerance compared to those receiving high doses. Inadequate analgesia associated with low initial dosing leads to ongoing pain, hyperalgesia and windup (39), which require much higher opioid doses to finally regain pain control. Benzodiazepines are often added for sedation, but their impact on opioid effectiveness remains unclear. In young animals, midazolam may increase analgesic requirements because it potentiates nociceptive behaviors, sensitizes cutaneous reflexes, and appears to have no sedative effects (40). Other animal studies suggest that midazolam promotes acute opioid tolerance (41). There are no data to suggest similar effects in humans, but our findings support the need to perform similar studies in children.
Univariable analyses showed no differences between morphine and fentanyl, but the logistic regression model showed that primarily using morphine reduced the need for escalating opioid doses, particularly among postoperative patients. This is consistent with the mechanisms of opioid tolerance (6, 20), was noted previously in neonates requiring ECMO (33) but has not been reported in PICU patients. Females receiving postoperative analgesia appeared to have greater odds for doubling of their opioid dose than males, but this effect was barely significant. Gender differences in postoperative pain, opioid analgesia and tolerance are well-known from animal and adult human studies (42–44), but have not been reported in children. Further studies are needed to explore this association.
We confirmed a significant degree of variability in the clinical practices used for opioid analgesia/sedation. Guidelines to reduce practice variability are associated with improved outcomes (45–47). The American College of Critical Care Medicine developed guidelines for analgesia/sedation in critically ill adults in 1995, updated in 2002 (48, 49), but similar guidelines are not available for children. Use of a sedation protocol at one site was associated with reduced variation in median daily opioid doses (Figure 4) and fewer patients at this site required doubling of their initial opioid doses. Practice variation in the use of opioid analgesia may lead to a higher incidence of complications such as oversedation, respiratory depression, hypotension, and opioid withdrawal. Conducting a prospective observational study to define clinical outcomes and identify the populations at risk for complications is important to prepare for clinical trials of opioid analgesia in children (50).
This observational study has several limitations. To achieve 100% accrual of eligible patients, we did not assess the confounding effects of adjuvant drugs on opioid analgesia in this study. Some drugs alter the mechanisms of opioid analgesia and can delay tolerance (e.g. ketamine, clonidine, methadone), although most drug interactions are not well-characterized in children. We did find that children developing tolerance required multiple adjuvant therapies (median 5 vs. 3, p<0.001), perhaps identifying a “difficult to sedate” phenotype among these patients. Another limitation is that we truncated the data collection at 14 days of opioid therapy, thus missing the primary outcome in some patients. However, we assumed that patients likely to require a doubling of their opioid dose would have met this primary outcome by 14 days (27). Another limitation is that these data were obtained from PICUs at tertiary-care children’s hospitals, but the vast majority of PICUs are not located at academic medical centers. We feel that the latter two limitations may underestimate the true incidence of opioid tolerance, which may be greater than 20% in non-academic centers or if data collection was extended beyond 14 days.
Despite these limitations, the present study is timely and relevant (6, 14), as it brings attention to clinical practices associated with unacceptably high risks of iatrogenic injury (1, 2, 5, 6, 13, 19, 23, 29, 31–33), prolonged ICU stays (27, 33) and increased healthcare costs (51–53). Despite the paucity of scientific evidence related to specific drugs or approaches (2, 6, 12–14), developing consensus guidelines for analgesia/sedation in infants and children and defining their daily therapeutic goals based on objective clinical criteria, may help to focus the expectations of bedside clinicians, bring greater consistency to their practice patterns, and thereby prevent some of the complications associated with opioid therapy.
Supplementary Material
Acknowledgments
FUNDING
This work was supported, in part, by cooperative agreements (U10HD500009, U10HD050096, U10HD049981, U10HD049945, U10HD049983, U10HD050012 and U01HD049934) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Department of Health and Human Services.
CPCCRN members participating in this study included: Arkansas Children’s Hospital, Little Rock, AR: KJS Anand, MBBS, D.Phil., Parthak Prodhan, MD, Ronald C. Sanders, Jr., MD, Glenda Hefley, MNSc, RN, Patti Korehbandi, RN; University of Utah (Data Coordinating Center), Salt Lake City, UT: J. Michael Dean, MD, MBA, Jeri Burr, MS, RN-BC, CCRC, Amy Clark, MS, Richard Holubkov, PhD, Stephanie Bisping, BSN, RN, CCRP, Teresa Liu, MPH, Rene Enriquez, BS, Jeff Yearley, BA; Children’s National Medical Center, Washington DC: John Berger, MD, Angela Wratney, MD, Jean Reardon, MA, BSN, RN; Children’s Hospital of Pittsburgh, Pittsburgh, PA: Joseph Carcillo, MD, Michael Bell, MD, Alan Abraham, BA, CCRC, Amanda Geyser, RN, Jennifer Jones, RN, and Luther Springs; Children’s Hospital of Michigan, Detroit, MI: Kathleen L. Meert, MD, Sabrina Heidemann, MD, Ann Pawluszka, BSN, RN; Seattle Children’s Hospital, Seattle, WA: Jerry Zimmerman, MD, PhD, David Jardine, MD, Ruth Barker, RRT; Children’s Hospital Los Angeles, Los Angeles, CA: Christopher J.L. Newth, MB, ChB, J. Francisco Fajardo, CLS (ASCP), RN, MD; Mattel Children’s Hospital at University of California Los Angeles, Los Angeles, CA: Rick Harrison, MD; University of Virginia Children’s Hospital, Charlottesville VA: Douglas F. Willson, MD; National Institute of Child Health and Human Development, Bethesda, MD: Carol Nicholson, MD, Tammara Jenkins, MSN, RN.
Footnotes
Conflict of Interest Declaration: Authors have no conflicts of interest related to the data or concepts presented in this article.
References
- 1.Jenkins IA, Playfor SD, Bevan C, et al. Current United Kingdom sedation practice in pediatric intensive care. Paediatr Anaesth. 2007;17(7):675–683. doi: 10.1111/j.1460-9592.2006.02180.x. [DOI] [PubMed] [Google Scholar]
- 2.Tobias JD. Sedation and analgesia in the pediatric intensive care unit. Pediatr Ann. 2005;34(8):636–645. doi: 10.3928/0090-4481-20050801-12. [DOI] [PubMed] [Google Scholar]
- 3.Anand KJS, Aranda JV, Berde CB, et al. Summary proceedings from the neonatal pain-control group. Pediatrics. 2006;117(3 Pt 2):S9–S22. doi: 10.1542/peds.2005-0620C. [DOI] [PubMed] [Google Scholar]
- 4.Anand KJS. Relationships between stress responses and clinical outcome in newborns, infants, and children. Critical Care Medicine. 1993;21(9 Suppl):S358–359. doi: 10.1097/00003246-199309001-00035. [DOI] [PubMed] [Google Scholar]
- 5.Simons SH, Anand KJS. Pain control: opioid dosing, population kinetics and side-effects. Semin Fetal Neonatal Med. 2006;11(4):260–267. doi: 10.1016/j.siny.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Anand KJS, Willson DF, Berger J, et al. Tolerance and withdrawal from prolonged opioid use in critically ill children. Pediatrics. 2010;125(5):e1208–1225. doi: 10.1542/peds.2009-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu JG, Anand KJS. Protein kinases modulate the cellular adaptations associated with opioid tolerance and dependence. Brain Res Brain Res Rev. 2001;38(1–2):1–19. doi: 10.1016/s0165-0173(01)00057-1. [DOI] [PubMed] [Google Scholar]
- 8.Baba H, Doubell TP, Moore KA, et al. Silent NMDA receptor-mediated synapses are developmentally regulated in the dorsal horn of the rat spinal cord. Journal of Neurophysiology. 2000;83(2):955–962. doi: 10.1152/jn.2000.83.2.955. [DOI] [PubMed] [Google Scholar]
- 9.Chahal H, D’Souza SW, Barson AJ, et al. Modulation by magnesium of N-methyl-D-aspartate receptors in developing human brain. Archives of Disease in Childhood Fetal & Neonatal Edition. 1998;78(2):F116–120. doi: 10.1136/fn.78.2.f116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebert B, Thorkildsen C, Andersen S, et al. Opioid analgesics as noncompetitive N-methyl-D-aspartate (NMDA) antagonists. Biochemical Pharmacology. 1998;56(5):553–559. doi: 10.1016/s0006-2952(98)00088-4. [DOI] [PubMed] [Google Scholar]
- 11.Rahman W, Dashwood MR, Fitzgerald M, et al. Postnatal development of multiple opioid receptors in the spinal cord and development of spinal morphine analgesia. Brain Research Developmental Brain Research. 1998;108(1–2):239–254. doi: 10.1016/s0165-3806(98)00054-6. [DOI] [PubMed] [Google Scholar]
- 12.Mehta S, Burry L, Fischer S, et al. Canadian survey of the use of sedatives, analgesics, and neuromuscular blocking agents in critically ill patients. Crit Care Med. 2006;34(2):374–380. doi: 10.1097/01.ccm.0000196830.61965.f1. [DOI] [PubMed] [Google Scholar]
- 13.Matthews AJ. An audit of sedation, analgesia and muscle relaxation in pediatric intensive care in the United Kingdom. Paediatric Anaesthesia. 1993;3:107–115. [Google Scholar]
- 14.Playfor S, Jenkins I, Boyles C, et al. Consensus guidelines on sedation and analgesia in critically ill children. Intensive Care Med. 2006;32(8):1125–1136. doi: 10.1007/s00134-006-0190-x. [DOI] [PubMed] [Google Scholar]
- 15.Playfor S, Jenkins I, Boyles C, et al. Consensus guidelines for sustained neuromuscular blockade in critically ill children. Paediatr Anaesth. 2007;17(9):881–887. doi: 10.1111/j.1460-9592.2007.02313.x. [DOI] [PubMed] [Google Scholar]
- 16.Berde CB, Sethna NF. Analgesics for the treatment of pain in children. New England Journal of Medicine. 2002;347(14):1094–1103. doi: 10.1056/NEJMra012626. [DOI] [PubMed] [Google Scholar]
- 17.Jacqz-Aigrain E, Burtin P. Clinical pharmacokinetics of sedatives in neonates. Clinical Pharmacokinetics. 1996;31(6):423–443. doi: 10.2165/00003088-199631060-00003. [DOI] [PubMed] [Google Scholar]
- 18.Blumer JL. Clinical pharmacology of midazolam in infants and children. Clinical Pharmacokinetics. 1998;35(1):37–47. doi: 10.2165/00003088-199835010-00003. [DOI] [PubMed] [Google Scholar]
- 19.Katz R, Kelly HW, Hsi A. Prospective study on the occurrence of withdrawal in critically ill children who receive fentanyl by continuous infusion. Critical Care Medicine. 1994;22(5):763–767. doi: 10.1097/00003246-199405000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Suresh S, Anand KJS. Opioid tolerance in neonates: a state-of-the-art review. Paediatric Anaesthesia. 2001;11(5):511–521. doi: 10.1046/j.1460-9592.2001.00764.x. [DOI] [PubMed] [Google Scholar]
- 21.Anand KJS, Ingraham J. Tolerance, dependence, and strategies for compassionate withdrawal of analgesics and anxiolytics in the pediatric ICU. Crit Care Nurse. 1996;16(6):87–93. [PubMed] [Google Scholar]
- 22.Krantz MJ, Mehler PS. Treating opioid dependence. Growing implications for primary care. Archives of Internal Medicine. 2004;164:277–288. doi: 10.1001/archinte.164.3.277. [DOI] [PubMed] [Google Scholar]
- 23.Fonsmark L, Rasmussen YH, Carl P. Occurrence of withdrawal in critically ill sedated children. Crit Care Med. 1999;27(1):196–199. doi: 10.1097/00003246-199901000-00052. [DOI] [PubMed] [Google Scholar]
- 24.Tobias JD, Schleien CL, Haun SE. Methadone as treatment for iatrogenic narcotic dependency in pediatric intensive care unit patients. Critical Care Medicine. 1990;18(11):1292–1293. doi: 10.1097/00003246-199011000-00024. [DOI] [PubMed] [Google Scholar]
- 25.Tobias JD. Subcutaneous administration of fentanyl and midazolam to prevent withdrawal after prolonged sedation in children [see comments] Critical Care Medicine. 1999;27(10):2262–2265. doi: 10.1097/00003246-199910000-00033. [DOI] [PubMed] [Google Scholar]
- 26.Malviya S, Pandit UA, Merkel S, et al. A comparison of continuous epidural infusion and intermittent intravenous bolus doses of morphine in children undergoing selective dorsal rhizotomy. Regional Anesthesia & Pain Medicine. 1999;24(5):438–443. doi: 10.1016/s1098-7339(99)90011-1. [DOI] [PubMed] [Google Scholar]
- 27.Robertson RC, Darsey E, Fortenberry JD, et al. Evaluation of an opiate-weaning protocol using methadone in pediatric intensive care unit patients. Pediatric Critical Care Medicine. 2000;1(2):119–123. doi: 10.1097/00130478-200010000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Siddappa R, Fletcher JE, Heard AM, et al. Methadone dosage for prevention of opioid withdrawal in children. Paediatr Anaesth. 2003;13(9):805–810. doi: 10.1046/j.1460-9592.2003.01153.x. [DOI] [PubMed] [Google Scholar]
- 29.Birchley G. Opioid and benzodiazepine withdrawal syndromes in the paediatric intensive care unit: a review of recent literature. Nurs Crit Care. 2009;14(1):26–37. doi: 10.1111/j.1478-5153.2008.00311.x. [DOI] [PubMed] [Google Scholar]
- 30.Deeter KH, King MA, Ridling D, et al. Successful implementation of a pediatric sedation protocol for mechanically ventilated patients. Crit Care Med. 2011;39(4):683–688. doi: 10.1097/CCM.0b013e318206cebf. [DOI] [PubMed] [Google Scholar]
- 31.Bergman I, Steeves M, Burckart G, et al. Reversible neurologic abnormalities associated with prolonged intravenous midazolam and fentanyl administration. J Pediatr. 1991;119(12):644–649. doi: 10.1016/s0022-3476(05)82420-5. [DOI] [PubMed] [Google Scholar]
- 32.Lane JC, Tennison MB, Lawless ST, et al. Movement disorder after withdrawal of fentanyl infusion. J Pediatr. 1991;119:649–651. doi: 10.1016/s0022-3476(05)82421-7. [DOI] [PubMed] [Google Scholar]
- 33.Franck LS, Vilardi J, Durand D, et al. Opioid withdrawal in neonates after continuous infusions of morphine or fentanyl during extracorporeal membrane oxygenation. American Journal of Critical Care. 1998;7(5):364–369. [PubMed] [Google Scholar]
- 34.Tobias JD, Deshpande JK, Gregory DF. Outpatient therapy of iatrogenic drug dependency following prolonged sedation in the pediatric intensive care unit. Intensive Care Medicine. 1994;20(7):504–507. doi: 10.1007/BF01711905. [DOI] [PubMed] [Google Scholar]
- 35.Tobias JD, Berkenbosch JW. Tolerance during sedation in a pediatric ICU patient: effects on the BIS monitor. J Clin Anesth. 2001;13(2):122–124. doi: 10.1016/s0952-8180(00)00232-4. [DOI] [PubMed] [Google Scholar]
- 36.McPhie AA, Barr GA. Regional Fos expression induced by morphine withdrawal in the 7-day-old rat. Dev Psychobiol. 2009;51(7):544–552. doi: 10.1002/dev.20392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stoller DC, Smith FL. Buprenorphine blocks withdrawal in morphine-dependent rat pups. Paediatr Anaesth. 2004;14(8):642–649. doi: 10.1111/j.1460-9592.2004.01264.x. [DOI] [PubMed] [Google Scholar]
- 38.Arnold JH, Truog RD, Orav EJ, et al. Tolerance and dependence in neonates sedated with fentanyl during extracorporeal membrane oxygenation. Anesthesiology. 1990;73(6):1136–1140. doi: 10.1097/00000542-199012000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288(5472):1765–1768. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 40.Koch SC, Fitzgerald M, Hathway GJ. Midazolam potentiates nociceptive behavior, sensitizes cutaneous reflexes, and is devoid of sedative action in neonatal rats. Anesthesiology. 2008;108(1):122–129. doi: 10.1097/01.anes.0000296079.45446.15. [DOI] [PubMed] [Google Scholar]
- 41.Schwieger IM, Hall RI, Szlam F, et al. Anesthetic interactions of midazolam and fentanyl: is there acute tolerance to the opioid? Anesthesiology. 1989;70(4):667–671. doi: 10.1097/00000542-198904000-00021. [DOI] [PubMed] [Google Scholar]
- 42.Loyd DR, Morgan MM, Murphy AZ. Sexually dimorphic activation of the periaqueductal gray-rostral ventromedial medullary circuit during the development of tolerance to morphine in the rat. Eur J Neurosci. 2008;27(6):1517–1524. doi: 10.1111/j.1460-9568.2008.06100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dahan A, Kest B, Waxman AR, et al. Sex-specific responses to opiates: animal and human studies. Anesth Analg. 2008;107(1):83–95. doi: 10.1213/ane.0b013e31816a66a4. [DOI] [PubMed] [Google Scholar]
- 44.Wiesenfeld-Hallin Z. Sex differences in pain perception. Gend Med. 2005;2(3):137–145. doi: 10.1016/s1550-8579(05)80042-7. [DOI] [PubMed] [Google Scholar]
- 45.Trafton JA, Humphreys K, Harris AH, et al. Consistent adherence to guidelines improves opioid dependent patients’ first year outcomes. J Behav Health Serv Res. 2007;34(3):260–271. doi: 10.1007/s11414-007-9074-2. [DOI] [PubMed] [Google Scholar]
- 46.Iliadis EA, Klein LW, Vandenberg BJ, et al. Clinical practice guidelines in unstable angina improve clinical outcomes by assuring early intensive medical treatment. J Am Coll Cardiol. 1999;34(6):1689–1695. doi: 10.1016/s0735-1097(99)00405-2. [DOI] [PubMed] [Google Scholar]
- 47.Simpson RJ, Jr, Sueta CA, Boccuzzi SJ, et al. Performance assessment model for guideline-recommended pharmacotherapy in the secondary prevention of coronary artery disease and treatment of left ventricular dysfunction. Am J Cardiol. 1997;80(8B):53H–56H. doi: 10.1016/s0002-9149(97)00821-7. [DOI] [PubMed] [Google Scholar]
- 48.Shapiro BA, Warren J, Egol AB, et al. Practice parameters for intravenous analgesia and sedation for adult patients in thee intensive care unit: An executive summary. Critical Care Medicine. 1995;23:1596–1600. doi: 10.1097/00003246-199509000-00021. [DOI] [PubMed] [Google Scholar]
- 49.Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Critical Care Medicine. 2002;30:119–141. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 50.Curley MAQ. ClinicalTrials.gov. 2009. Sedation Management in Pediatric Patients With Acute Respiratory Failure (The RESTORE Study) NCT00814099. [Google Scholar]
- 51.Horn SD. Quality, clinical practice improvement, and the episode of care. Manag Care Q. 2001;9(3):10–24. [PubMed] [Google Scholar]
- 52.Horn SD. Overcoming obstacles to effective treatment: use of clinical practice improvement methodology. J Clin Psychiatry. 1997;58 (Suppl 1):15–19. [PubMed] [Google Scholar]
- 53.Horn SD. Data-driven clinical outcomes improvement to reduce health care cost. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.