Abstract
Background
Postmenopausal women typically experience accelerated muscle loss which has a negative effect on strength. The maximum daily recommended dosage of ibuprofen (1,200 mg) following resistance exercise has been shown to increase muscle hypertrophy and strength in older adults. This study aimed to determine the effects of low-dose ibuprofen (400 mg) immediately following resistance exercise sessions on muscle mass and strength in postmenopausal women.
Methods
Participants were randomized to ingest ibuprofen (IBU: n = 15, 57.8 ± 5.1 years, 75.9 ± 9.0 kg, 165.9 ± 6.2 cm, BMI = 28 ± 4 kg/m2) or placebo (PLA: n = 13, 56.5 ± 4.4 years, 73.0 ± 10.4 kg, 163.1 ± 5.9 cm, BMI = 26 ± 9 kg/m2) immediately following resistance exercise (11 whole-body exercises), which was performed 3 days/week, on nonconsecutive days, for 9 weeks. Prior to and following training, measures were taken for lean tissue mass (dual-energy X-ray absorptiometry), muscle size of the elbow and knee flexors and extensors and ankle dorsiflexors and plantar flexors (ultrasound), and strength (one-repetition maximum leg press and chest press).
Results
Over the 9 weeks of training, there were significant changes (p < 0.05) in lean tissue mass (IBU, −1.1 ± 1.0 kg; PLA, −0.7 ± 1.4 kg), muscle size of the knee extensors (IBU, 0.3 ± 0.6 cm; PLA, 0.2 ± 0.7 cm), ankle dorsiflexors (IBU, 0.5 ± 0.8 cm; PLA, 0.1 ± 0.5 cm), and ankle plantar flexors (IBU, 0.3 ± 0.9 cm; PLA, 0.5 ± 0.9 cm), leg press strength (IBU, 20.6 ± 18.0 kg; PLA, 20.0 ± 20.0 kg), and chest press strength (IBU, 5.1 ± 9.5 kg; PLA, 8.1 ± 7.6 kg), with no differences between groups.
Conclusion
Low-dose ibuprofen following resistance exercise has no greater effect on muscle mass or strength over exercise alone in postmenopausal women.
Keywords: Aging, Sarcopenia, Muscle protein, Dosage, Frequency
Introduction
The loss of muscle mass with aging (i.e., sarcopenia) has a negative effect on strength [1] and the ability to perform tasks of daily living [2]. Females typically experience an accelerated decline in muscle mass (0.6 %/year) after menopause [3], possibly because of an imbalance between muscle protein synthesis and protein catabolism [4]. Resistance exercise increases the rates of muscle protein synthesis [5], which may lead to significant muscle accretion and strength over time in older adults [6]. In addition to resistance exercise, nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen may also benefit aging muscle. Ibuprofen inhibits cyclooxygenase (COX-1, COX-2) activity, which decreases the synthesis of prostaglandin E2 (PGE2), a biological regulator of protein catabolism [7]. In a recent study by Trappe et al. [8], healthy older adults (nine males, four females; 64 years) who ingested the maximum daily recommended dosage of ibuprofen (1,200 mg) during structured resistance exercise for 12 weeks experienced a significant increase in quadriceps muscle volume and muscle strength over a placebo group who performed the same training program. The authors speculate that ibuprofen inhibited COX and PGE2 activity and decreased muscle protein catabolism more so than protein synthesis following exercise, which resulted in net muscle protein retention and muscle accretion over time. Previous work by the same researchers showed that 1,200 mg of ibuprofen following an acute bout of resistance exercise inhibited the fractional synthetic rate of muscle proteins [9] and prostaglandin F2α, a regulator of muscle protein synthesis [10]. Based on these findings, higher-dose ibuprofen (i.e., 1,200 mg) following resistance exercise may suppress the rates of muscle protein synthesis and protein catabolism, which may increase, decrease, or have no change on muscle accretion over time.
The maximum recommended single dosage of ibuprofen (400 mg) immediately following resistance exercise sessions for 9 months had no effect on lean tissue mass in premenopausal women [11]. Furthermore, ingesting 400 mg of ibuprofen or placebo during 6 weeks of unilateral resistance training resulted in similar gains in elbow flexor muscle thickness and biceps maximal strength in young adults [12]. Results across studies indicate that 400 mg of ibuprofen is not effective in younger populations for improving muscle mass or strength; however, postmenopausal women may be more responsive to the physiological effects of 400 mg of ibuprofen due to elevated inflammation [13] and oxidative stress [14]. Therefore, the purpose of this study was to examine the effects of low-dose ibuprofen (i.e., 400 mg) immediately following resistance exercise sessions in postmenopausal women. It was hypothesized that ibuprofen would lead to an increase in muscle mass and strength compared to placebo and training over time.
Methods
Participants
Thirty-four healthy postmenopausal women, who were not performing supervised resistance exercise, were recruited for the study. Untrained participants were chosen to potentially maximize the physiological adaptations from resistance exercise. Participants verbally confirmed that they were postmenopausal for at least 1 year. Participants were excluded if they had taken bisphosphonates, hormone replacement therapy, selective estrogen receptor modulators, parathyroid hormone, or calcitonin 12 months prior to the start of the study, if they suffered from severe osteoarthritis, if they were taking NSAIDs, and if they were smokers. Prior to group allocation, participants were given a Physical Activity Readiness Questionnaire [15], which assessed their readiness to participate in resistance exercise. This questionnaire included questions related to heart conditions, angina at rest or during physical exercise, balance, and bone or joint problems that may affect exercise performance. If the participant indicated a contraindication to exercise, they were required to get medical approval before participating in the study. Participants were also required to fill out a leisure time exercise questionnaire where the number of times on average per week strenuous (i.e., heart beats rapidly), moderate (i.e., not exhausting), and mild (i.e., minimal effort) exercise was performed [16]. Participants were instructed not to change their diet, engage in additional physical activity that was not part of their normal daily routine, or ingest other NSAIDs that were not part of the study intervention. The study was approved by the university ethics review board at the University of Regina. Participants were informed of any risks and of the purpose of the study before their written consents were obtained.
Experimental procedures
The study used a double-blind, placebo-controlled, repeated-measures design where participants were randomized to ingest either ibuprofen (400 mg) or placebo (micronized cellulose crystals) immediately following resistance exercise (3 days/week) for 9 weeks. We have previously shown that ≤8 weeks of whole-body resistance exercise is sufficient to increase muscle mass and strength [17–20]. Ibuprofen and placebo tablets were administered to each participant by a research supervisor and were similar in size, taste, and texture. Peak plasma concentrations of ibuprofen occur between 1 and 4 h after ibuprofen ingestion [21]. Inflammatory cytokines are elevated within 15 min postexercise in postmenopausal women and return to baseline within 2 h [22]. We, therefore, administered ibuprofen immediately after resistance exercise sessions so that elevated plasma concentrations of ibuprofen would coincide with increased inflammation. Participants were instructed to refrain from food or drink, excluding water, for 60 min following each training session so that a valid estimate of the effects of ibuprofen on muscle could be made.
Prior to the first visit to the laboratory for initial testing and data collection, participants were instructed to refrain from physical activity and alcohol for 24 h, caffeine for 6 h, and food and drink for 2 h. The primary dependent variables measured before and after the 9-week intervention were (1) whole-body lean tissue mass, (2) muscle thickness of the elbow and knee flexors and extensors and ankle dorsiflexors and plantar flexors, and (3) strength (leg press and chest press one-repetition maximum [1-RM]).
Resistance exercise program
After a familiarization training session, participants followed the same supervised, whole-body resistance exercise program combined with ibuprofen or placebo for 9 weeks. Prior to the start of each resistance exercise session, participants performed a 5-min aerobic warm-up (i.e., stationary cycle, elliptical trainer, treadmill) at a self-selected intensity. Participants then completed three sets of 10 repetitions to muscle fatigue with a 2-min rest between sets for each exercise at an intensity corresponding to their 10-repetition maximum for each exercise. We have previously used this training protocol successfully to increase muscle mass and strength in older adults [23, 24]. Resistance exercises included leg press, chest press, lat pull-down, shoulder press, leg (knee) extension, leg curl (knee flexion), triceps extension, biceps curl, calf press, low back extension, and abdominal curl. Participants maintained daily training logs where the load and the number of sets and repetitions performed was recorded. Resistance was increased by 2–5 kg once a participant could complete three sets of 10 repetitions to muscle fatigue for an exercise. Once the resistance was increased, the participant maintained this load until a subsequent three sets of 10 repetitions to fatigue was completed.
Lean tissue mass
Whole-body lean tissue mass was assessed using dual-energy X-ray absorptiometry (Hologic Wi System, Christie Group, Winnipeg, MB, Canada) in array mode. Before scanning, participants were required to remove all objects containing metal (i.e., jewelry). Scans were performed with participants lying in a supine position along the scanning table’s centerline longitudinal axis. Feet were taped together at the toes (i.e., phalanges) to immobilize the legs, while the hands maintained a pronated position within the scanning region. The coefficient of variation was 0.54 %.
Muscle thickness
Muscle size of the elbow and knee flexors and extensors and ankle dorsiflexors and plantar flexors was measured using B-mode ultrasound (Aloka SSD-500, Tokyo, Japan). The same researcher performed the baseline and posttesting assessments. Detailed procedures for assessing muscle thickness are previously described [25]. Briefly, a 5-MHz scanning transducer head was coated with water-soluble transmission gel to provide acoustic contact with the muscle surface. When the image produced on the screen was visible, a cursor was enabled to quantify muscle thickness (in centimeters) at three sites: the proximal, the mid, and the distal, as determined by divisions (1 cm) on the monitor. Muscle thickness measurements were extrapolated from the monitor screen by measuring the distance from the bottom of the subcutaneous adipose layer to the surface of the humerus for elbow flexor and extensor muscle thickness, to the surface of the femur for knee flexor and extensor muscle thickness, and to the surface of the tibia for ankle dorsiflexor and plantar flexor muscle thickness. The coefficients of variation were 2.5 % (elbow flexors), 2.2 % (elbow extensors), 3.6 % (knee flexors), 2.1 % (knee extensors), 3.2 % (ankle plantar flexors), and 4.0 % (ankle dorsiflexors) [24, 25].
Muscle strength
Leg press and chest press strength was assessed using a 1-RM testing protocol. Following 5 min of cycling on a stationary cycle ergometer, participants performed two warm-up sets in order: one set of 10 repetitions using a light weight determined by each participant to be comfortable and another set of five repetitions using slightly increased weight. Following the warm-up sets, weight was progressively increased by approximately 10 kg for leg press and 5 kg for chest press for each subsequent 1-RM attempt with a 2-min rest interval between attempts. The 1-RM was reached in four to six trials, independent of the two warm-up sets. The leg press and chest press strength measures have coefficients of variation of 3.8 and 3.1 %, respectively [24].
Sample size calculation
Sample size was calculated using the coefficient of variation for whole-body lean tissue mass and a change in lean tissue mass that would be clinically significant [26]. A 3 % change in lean tissue mass was considered clinically significant because a 3 % reduction in muscle mass in postmenopausal women is associated with 11 % lower muscle strength compared to young premenopausal women [27]. Based on these calculations, six postmenopausal women were required per group to demonstrate a 3 % increase in lean tissue mass with a power of 0.9 and alpha of 0.05. Additional participants were recruited to account for attrition.
Statistical analyses
A 2 (groups: ibuprofen vs. placebo) × 2 (time: pretest and posttest periods) ANOVA with repeated measures on the second factor was used to determine differences between groups over time for each of the dependent variables of lean tissue mass, muscle thickness, and strength. An independent sample t test was used to determine differences in training volume between groups. Statistical analyses were carried out using SPSS version 18.0 for Windows XP (SPSS, Chicago, IL, USA). Significance was set at p < 0.05. All results are expressed as the mean ± standard deviation.
Results
Participant characteristics and compliance
Of the original 34 participants who volunteered for the study, 28 participants (15 ibuprofen, 13 placebo; 50–68 years of age) completed the study. Six participants withdrew because of time constraints. Baseline characteristics of participants who completed the study are shown in Table 1. There were no differences among groups for any of the baseline measurements. Average resistance training compliance was 83.1 % (22 of 27 sessions performed) for the ibuprofen group and 75 % (20 of 27 sessions performed) for the placebo group.
Table 1.
Subject characteristics and physical activity performed at baseline for the ibuprofen and placebo groups
| Group | Age (years) | Body mass (kg) | Height (cm) | Strenuous activity (times per week) | Moderate activity (times per week) | Mild activity (times per week) | Total activity (times per week) |
|---|---|---|---|---|---|---|---|
| Ibuprofen (n = 15) | 57.8 (5.1) | 75.9 (9.0) | 165.9 (6.2) | 1.9 (2.5) | 2.4 (1.5) | 4.6 (2.8) | 9.0 (3.1) |
| Placebo (n = 13) | 56.5 (4.4) | 73.0 (10.4) | 163.1 (5.9) | 1.2 (1.7) | 3.7 (3.3) | 4.4 (3.6) | 9.3 (6.4) |
Values are expressed as the mean (standard deviation)
Lean tissue, muscle thickness, strength, and training volume
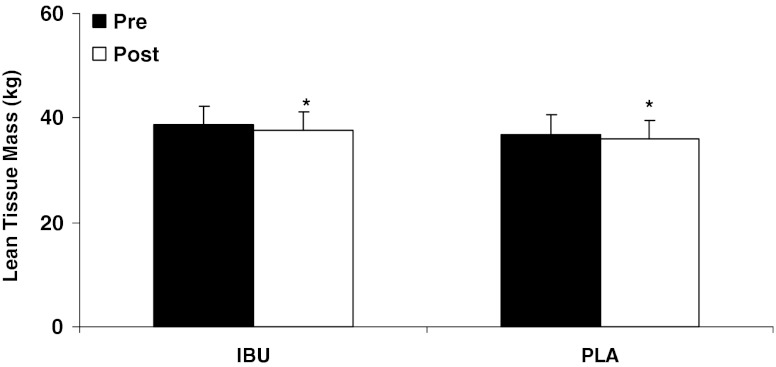
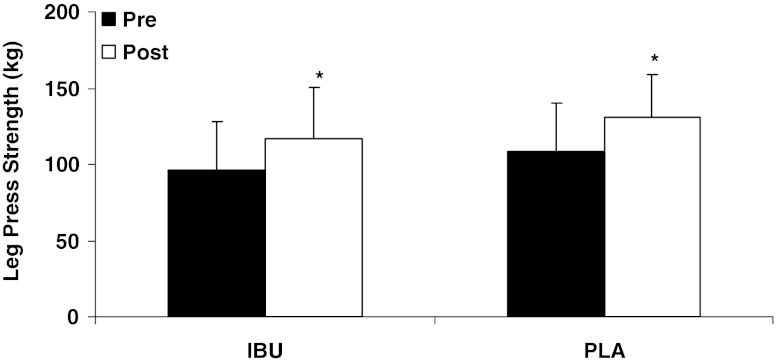
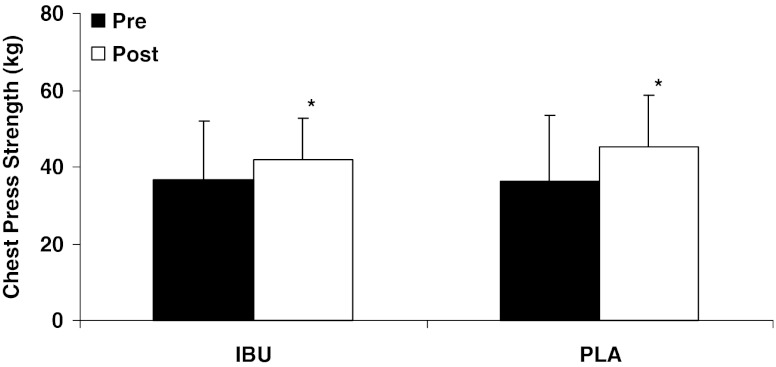
There was a significant decrease in whole-body lean tissue mass over time (IBU, −1.1 ± 1.0 kg; PLA, −0.7 ± 1.4 kg; p < 0.05; Fig. 1), with no differences between groups. There was a significant increase (p < 0.05) in muscle size of the knee extensors (IBU, 0.3 ± 0.6 cm; PLA, 0.2 ± 0.7 cm), ankle dorsiflexors (IBU, 0.5 ± 0.8 cm; PLA, 0.1 ± 0.5 cm), and ankle plantar flexors (IBU, 0.3 ± 0.9 cm; PLA, 0.5 ± 0.9 cm; Table 2), and leg press (IBU, 20.6 ± 18.0 kg; PLA, 20.0 ± 20.0 kg; Fig. 2) and chest press strength (IBU, 5.1 ± 9.5 kg; PLA, 8.1 ± 7.6 kg; Fig. 3) over 9 weeks of training, with no differences between groups.
Fig. 1.
Change in lean tissue mass after 9 weeks of resistance exercise for ibuprofen (IBU, n = 15) and placebo (PLA, n = 13) groups. Values are expressed as the mean ± standard deviation. *p < 0.05, significant decrease over time
Table 2.
Muscle thickness measurements (in centimeters) for the elbow and knee flexors and extensors and ankle dorsiflexors and plantar flexors before and after 9 weeks of resistance exercise
| Muscle group | Ibuprofen (n = 15) | Placebo (n = 13) | ||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Elbow flexors | 3.1 (0.8) | 3.0 (0.7) | 2.5 (0.6) | 3.1 (0.6) |
| Elbow extensors | 3.6 (0.7) | 3.8 (0.8) | 3.7 (0.8) | 3.9 (0.6) |
| Knee flexors | 5.0 (0.6) | 5.1 (0.5) | 5.3 (0.5) | 5.2 (0.9) |
| Knee extensors | 3.3 (0.7) | 3.6 (0.6)* | 3.5 (0.6) | 3.7 (0.5)* |
| Ankle plantar flexors | 4.6 (0.8) | 5.0 (1.0)* | 4.2 (0.6) | 4.7 (0.8)* |
| Ankle dorsiflexors | 3.3 (0.5) | 3.7 (0.9)* | 3.3 (0.4) | 3.4 (0.6)* |
Values are expressed as the mean (standard deviation)
*p < 0.05, significant increase with training
Fig. 2.
Change in leg press strength after 9 weeks of resistance exercise for ibuprofen (IBU, n = 15) and placebo (PLA, n = 12) groups. Values are expressed as the mean ± standard deviation. *p < 0.05, significant increase over time
Fig. 3.
Change in chest press strength after 9 weeks of resistance exercise for ibuprofen (IBU, n = 15) and placebo (PLA, n = 12) groups. Values are expressed as the mean ± standard deviation. *p < 0.05, significant increase over time
Muscle size of the elbow flexors, elbow extensors, and knee flexors (Table 2) and body mass (IBU: pre, 75.9 ± 9.0 kg; post, 74.2 ± 8.1 kg; PLA: pre, 73.0 ± 10.4 kg; post, 72.4 ± 10.8 kg) did not change over time in either group. The total amount of exercise performed over the 9 weeks of training was similar between groups (IBU, 9,344 ± 1,700 kg; PLA, 10,286 ± 1,321 kg). There were no adverse effects reported from the resistance exercise program, ibuprofen, or placebo.
Discussion
To our knowledge, this is the first study to determine the effects of low-dose ibuprofen (i.e., 400 mg) after resistance exercise sessions in postmenopausal women. Contrary to our hypotheses, ibuprofen had no greater effect on muscle mass or strength compared to placebo. Previous research has shown a positive effect from ibuprofen on aging muscle. Old healthy rats (20 months of age) who were given an ibuprofen-enriched chow (700 mg/kg body mass) for 5 months experienced a significant reduction in inflammation, which corresponded with an increase in hind limb muscle mass (+1.32 g) [28]. In the postprandial state, ibuprofen-treated rats experienced a significant increase in phosphorylation of forkhead 03a (Foxo3a), a transcription factor that activates genes that express proteins such as ubiquitin-activated ligases which are involved in protein catabolism in the ubiquitin–proteasome pathway [29, 30]. Phosphorylation of Foxo3a prevents its entry into the nucleus, which inhibits muscle protein catabolism [28]. Using a double-blind, placebo-controlled, repeated-measures design, Trappe et al. [8] found that healthy older adults (n = 13, nine males, four females; 64 years) who ingested the maximal daily recommended dosage of ibuprofen (1,200 mg) during structured resistance exercise (bilateral knee extension, three sets of 10 repetitions to fatigue; 3 days/week; 12 weeks) experienced a significant increase in quadriceps muscle volume (IBU, +11 %; PLA, +8.4 %) and muscle strength (IBU, +17.5 %; PLA, +15 %). The greater increase in muscle mass from ibuprofen may be the result of a decrease in COX and PGE2 activity and muscle protein catabolism, leading to greater net protein retention and muscle accretion over time [8]. Previous findings in rats have also shown that COX inhibition reduced the production of PGE2 and suppressed muscle protein catabolism, leading to greater net protein balance [7]. In the present study, ibuprofen had no effect on muscle size of the elbow flexors and extensors or knee flexors. Perhaps a longer training period (>9 weeks) with more frequent (daily) ingestion of ibuprofen (>400 mg) is required to produce significant increases in muscle mass and strength compared to placebo in postmenopausal women.
There was a small, yet significant, decrease in whole-body lean tissue mass over the 9-week training period. While it is somewhat puzzling as to why lean tissue mass did not increase with repeated training sessions, it is possible that acute caloric deficit (i.e., dietary protein) for 1 h postexercise influenced our results. Muscle hypertrophy following resistance exercise requires net protein synthesis of myofibrillar proteins. However, in the postabsorptive state following exercise, muscle protein balance remains negative (i.e., protein catabolism > protein synthesis) until amino acids are consumed [31]. Protein ingestion following resistance exercise increases the rates of muscle protein synthesis [32–34], which may lead to muscle hypertrophy over time. In healthy older adults, protein supplementation immediately following resistance exercise for 12 weeks increased muscle cross-sectional area, mean fiber area, and muscular strength. However, delaying dietary protein intake for 2 h resulted in no beneficial effects [35]. These results imply that protein intake after exercise is crucial for muscle protein synthesis to proceed and delaying protein intake jeopardizes muscle protein accretion over time in older adults. Postmenopausal women in our study were instructed to refrain from food intake (i.e., calorie deficit) for 1 h postexercise so that a valid estimate of the effects of ibuprofen on muscle could be made. However, this postabsorptive protocol may have limited the muscle protein synthetic response from resistance exercise, which may have impaired muscle accretion over time. Unfortunately, no direct measure of muscle protein synthesis or protein catabolism was made and habitual dietary intake was not assessed, which limits our ability to determine whether ibuprofen or diet influenced muscle protein balance over time.
Conclusion
Ingestion of low-dose ibuprofen (i.e., 400 mg) in the postabsorptive state following resistance exercise does not lead to greater gains in muscle mass or strength compared to placebo in postmenopausal women. These results are in contrast to previous research showing that 1,200 mg/day of ibuprofen was effective for increasing muscle mass and strength in older men and women [8]. It is, therefore, likely that the effective ibuprofen dose for increasing muscle mass is higher than 400 mg in postmenopausal women. Future research should investigate the effects of different doses of ibuprofen (400–1,200 mg) during longer-term (>9 weeks) resistance exercise on the rates of muscle protein synthesis and protein catabolism in postmenopausal women in the postprandial state.
Acknowledgments
The study was supported by a Saskatchewan Health Research Foundation (SHRF) Establishment Grant. Summer student support was provided by the Bone Imaging Group, a SHRF-funded research group. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle [36].
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91:1123S–27S. doi: 10.3945/ajcn.2010.28608A. [DOI] [PubMed] [Google Scholar]
- 2.Short KR, Nair KS. Muscle protein metabolism and the sarcopenia of aging. Int J Sport Nutr Exerc Metab. 2001;11:S119–27. doi: 10.1123/ijsnem.11.s1.s119. [DOI] [PubMed] [Google Scholar]
- 3.Rolland YM, Perry HM, Patrick P, Banks WA, Morley JE. Loss of appendicular muscle mass and loss of muscle strength in young postmenopausal women. J Gerontol A Biol Sci Med Sci. 2007;62:330–35. doi: 10.1093/gerona/62.3.330. [DOI] [PubMed] [Google Scholar]
- 4.Boirie Y. Physiopathological mechanism of sarcopenia. J Nutr Health Aging. 2009;13:717–23. doi: 10.1007/s12603-009-0203-x. [DOI] [PubMed] [Google Scholar]
- 5.Holm L, Esmarck B, Suetta C, Matsumoto K, Doi T, Mizuno M, Miller BF, Kjaer M. Postexercise nutrient intake enhances leg protein balance in early postmenopausal women. J Gerontol A Biol Sci Med Sci. 2005;60:1212–8. doi: 10.1093/gerona/60.9.1212. [DOI] [PubMed] [Google Scholar]
- 6.Forbes SC, Little JP, Candow DG. Exercise and nutritional interventions for improving aging muscle health. Endocrine. 2012. doi:10.1007/s12020-012-9676-1. [DOI] [PubMed]
- 7.Rodemann HP, Goldberg AL. Arachidonic acid, prostaglandin E2 and F2 alpha influence rates of protein turnover in skeletal and cardiac muscle. J Biol Chem. 1982;257:1632–8. [PubMed] [Google Scholar]
- 8.Trappe TA, Carroll CC, Dickinson JM, Lemoine JK, Haus JM, Sullivan BE, et al. Influence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol Regul Integr Comp Physiol. 2011;300:R655–62. doi: 10.1152/ajpregu.00611.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trappe TA, White F, Lambert CP, Cesar D, Hellerstein M, Evans WJ. Effect of ibuprofen and acetaminophen on postexercise muscle protein synthesis. Am J Physiol Endocrinol Metab. 2002;282:E551–6. doi: 10.1152/ajpendo.00352.2001. [DOI] [PubMed] [Google Scholar]
- 10.Trappe TA, Fluckey JD, White F, Lambert CP, Evans WJ. Skeletal muscle PGF(2)(alpha) and PGE(2) in response to eccentric resistance exercise: influence of ibuprofen acetaminophen. J Clin Endocrinol Metab. 2001;86:5067–70. doi: 10.1210/jc.86.10.5067. [DOI] [PubMed] [Google Scholar]
- 11.Kohrt WM, Barry DW, Van Pelt RE, Jankowski CM, Wolfe P, Schwartz RS. Timing of ibuprofen use and bone mineral density adaptations to exercise training. J Bone Miner Res. 2010;25:1415–22. doi: 10.1002/jbmr.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krentz JR, Quest B, Farthing JP, Quest DW, Chilibeck PD. The effects of ibuprofen on muscle hypertrophy, strength, and soreness during resistance training. Appl Physiol Nutr Metab. 2008;33:470–5. doi: 10.1139/H08-019. [DOI] [PubMed] [Google Scholar]
- 13.Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119:526.e9–17. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 14.Maltais ML, Desroches J, Dionne IJ. Changes in muscle mass and strength after menopause. J Musculoskelet Neuronal Interact. 2009;9:186–97. [PubMed] [Google Scholar]
- 15.Thomas S, Reading J, Shephard RJ. Revision of the Physical Activity Readiness Questionnaire (PAR-Q) Can J Sport Sci. 1992;17:338–45. [PubMed] [Google Scholar]
- 16.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–6. [PubMed] [Google Scholar]
- 17.Candow DG, Chilibeck PD, Burke DG, Mueller KD, Lewis JD. Effect of different frequencies of creatine supplementation on muscle size and strength in young adults. J Strength Cond Res. 2011;25:1831–8. doi: 10.1519/JSC.0b013e3181e7419a. [DOI] [PubMed] [Google Scholar]
- 18.Candow DG, Burke DG. Effect of short-term equal-volume resistance training with different workout frequency on muscle mass and strength in untrained men and women. J Strength Cond Res. 2007;21:204–7. doi: 10.1519/00124278-200702000-00037. [DOI] [PubMed] [Google Scholar]
- 19.Burke DG, Chilibeck PD, Parise G, Candow DG, Mahoney D, Tarnopolsky M. Effect of creatine and weight training on muscle creatine and performance in vegetarians. Med Sci Sports Exerc. 2003;35:1946–55. doi: 10.1249/01.MSS.0000093614.17517.79. [DOI] [PubMed] [Google Scholar]
- 20.Pinkoski C, Chilibeck PD, Candow DG, Esliger D, Ewaschuk JB, Facci M, et al. The effects of conjugated linoleic acid supplementation during resistance training. Med Sci Sports Exerc. 2006;38:339–48. doi: 10.1249/01.mss.0000183860.42853.15. [DOI] [PubMed] [Google Scholar]
- 21.Davies NM. Clinical pharmacokinetics of ibuprofen. The first 30 years. Clin Pharmacokinet. 1998;34:101–54. doi: 10.2165/00003088-199834020-00002. [DOI] [PubMed] [Google Scholar]
- 22.Phillips MD, Flynn MG, McFarlin BK, Stewart LK, Timmerman KL. Resistance training at eight-repetition maximum reduces the inflammatory milieu in elderly women. Med Sci Sports Exerc. 2010;42:314–25. doi: 10.1249/MSS.0b013e3181b11ab7. [DOI] [PubMed] [Google Scholar]
- 23.Candow DG, Little JP, Chilibeck PD, Abeysekara S, Zello GA, Kazachkov M, et al. Low-dose creatine combined with protein during resistance training in older men. Med Sci Sports Exerc. 2008;40:1645–52. doi: 10.1249/MSS.0b013e318176b310. [DOI] [PubMed] [Google Scholar]
- 24.Candow DG, Chilibeck PD, Facci M, Abeysekara S, Zello GA. Protein supplementation before and after resistance training in older men. Eur J Appl Physiol. 2006;97:548–56. doi: 10.1007/s00421-006-0223-8. [DOI] [PubMed] [Google Scholar]
- 25.Candow DG, Chilibeck PD. Differences in size, strength, and power of upper and lower body muscle groups in young and older men. J Gerontol A Biol Sci Med Sci. 2005;60:148–56. doi: 10.1093/gerona/60.2.148. [DOI] [PubMed] [Google Scholar]
- 26.Chilibeck P, Calder A, Sale DG, Webber C. Reproducibility of dual-energy x-ray absorptiometry. Can Assoc Radiol J. 1994;45:297–302. [PubMed] [Google Scholar]
- 27.Lynch NA, Metter EJ, Lindle RS, Fozard JL, Tobin JD, Roy TA, et al. Muscle quality. I. Age-associated differences between arm and leg muscle groups. J Appl Physiol. 1999;86:188–194. doi: 10.1152/jappl.1999.86.1.188. [DOI] [PubMed] [Google Scholar]
- 28.Rieu I, Magne H, Savary-Auzeloux I, Averous J, Bos C, Peyron MA, et al. Reduction of low grade inflammation restores blunting of postprandial muscle anabolism and limits sarcopenia in old rats. J Physiol. 2009;15:5483–92. doi: 10.1113/jphysiol.2009.178319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greig CA, Atherton PJ, Rennie MJ. Can an NSAID a day keep muscle wasting away? J Physiol. 2009;15:5799–800. doi: 10.1113/jphysiol.2009.184416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng SJ, Yu LJ. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci. 2010;11:1509–26. doi: 10.3390/ijms11041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breen L, Phillips SM. Nutrient interaction for optimal protein anabolism in resistance exercise. Curr Opin Clin Nutr Metab Care. 2012;15:226–32. [DOI] [PubMed]
- 32.Burd NA, Yang Y, Moore DR, Tang JE, Tarnopolsky MA, Phillips SM. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. Br J Nutr. 2012;31:1–5. doi: 10.1017/S0007114511006271. [DOI] [PubMed] [Google Scholar]
- 33.Churchward-Venne TA, Burd NA, Mitchell CJ, West DW, Philp A, Marcotte GR, Baker SK, Baar K, Phillips SM. Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J Physiol. 2012. doi:10.1113/jphysiol.2012.228833. [DOI] [PMC free article] [PubMed]
- 34.West DW, Burd NA, Coffey VG, Baker SK, Burke LM, Hawley JA, et al. Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. Am J Clin Nutr. 2011;94:795–803. doi: 10.3945/ajcn.111.013722. [DOI] [PubMed] [Google Scholar]
- 35.Esmarck B, Andersen JL, Olsen S, Richter EA, Mizuno M, Kjaer M. Timing of postexercise protein intake is important for muscle hypertrophy with resistance training in elderly humans. J Physiol. 2001;535:301–11. doi: 10.1111/j.1469-7793.2001.00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7–8. doi: 10.1007/s13539-010-0003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]