Abstract
Bacterial type II toxin–antitoxins (TAs) are two-component systems that modulate growth in response to specific stress conditions, thus promoting adaptation and persistence. The major human pathogen Mycobacterium tuberculosis potentially encodes 75 TAs and it has been proposed that persistence induced by active toxins might be relevant for its pathogenesis. In this work, we focus on the newly discovered toxin–antitoxin–chaperone (TAC) system of M. tuberculosis, an atypical stress-responsive TA system tightly controlled by a molecular chaperone that shows similarity to the canonical SecB chaperone involved in Sec-dependent protein export in Gram-negative bacteria. We performed a large-scale genome screening to reconstruct the evolutionary history of TAC systems and found that TAC is not restricted to mycobacteria and seems to have disseminated in diverse taxonomic groups by horizontal gene transfer. Our results suggest that TAC chaperones are evolutionary related to the solitary chaperone SecB and have diverged to become specialized toward their cognate antitoxins.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-012-0396-5) contains supplementary material, which is available to authorized users.
Keywords: SecB chaperones, Sec translocon, Toxin–antitoxin systems, Stress response, HigBA, MqsRA, HicAB
Introduction
The human pathogen Mycobacterium tuberculosis encodes 75 putative type II toxin–antitoxin (TA) systems and it has been proposed that activated toxins could contribute to its pathogenesis (Ramage et al. 2009; Sala et al. 2013). Bacterial TA systems are stress-responsive elements generally composed of a stable toxin that forms an inactive complex with its less stable cognate antitoxin (Yamaguchi et al. 2011). Both partners are encoded within an operon autoregulated by the antitoxin, which usually carries a DNA-binding domain (Yamaguchi et al. 2011). In response to specific stress conditions, the antitoxin is degraded by stress proteases and the free active toxin subsequently targets important cellular processes such as DNA replication or protein synthesis. It is believed that such a TA-dependant growth control facilitates adaptation to stress and persistence (Makarova et al. 2009; Van Melderen 2010; Yamaguchi et al. 2011).
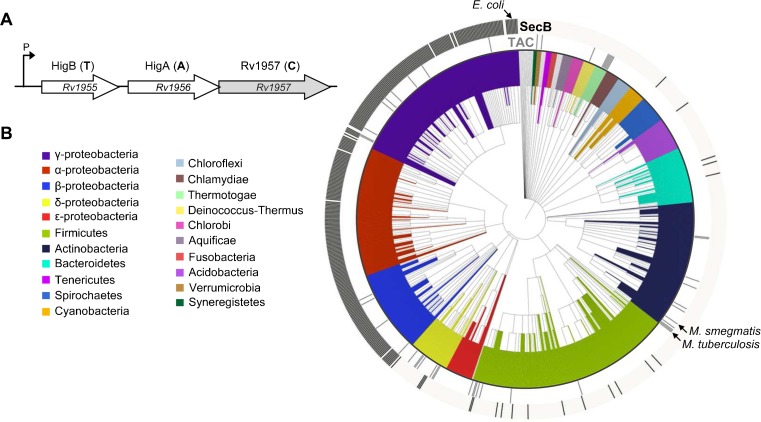
The HigB(Rv1955)–HigA(Rv1956) toxin–antitoxin system of M. tuberculosis is atypical in that its activation is specifically controlled by the SecB-like chaperone Rv1957 encoded by the same operon (Bordes et al. 2011; Sala et al. 2013; Smollett et al. 2009) (Fig. 1a). In this case, the chaperone directly interacts with the HigA antitoxin and protects it from both aggregation and degradation, thus facilitating its folding and subsequent interaction with the HigB toxin (Bordes et al. 2011). Such tripartite toxin–antitoxin–chaperone system (TAC), is significantly induced in response to relevant stresses such as heat shock (Stewart et al. 2002), hypoxia (Ramage et al. 2009), nutrient starvation (Betts et al. 2002), and persistence (Keren et al. 2011), suggesting a role for TAC in M. tuberculosis stress-adaptive response. The higBA toxin–antitoxin pair from TAC resembles the previously identified “chaperone-less” gp49-gp48 locus of the Escherichia coli N15 prophage, which consists of a toxin located upstream of an antitoxin that contains a conserved helix–turn–helix (HTH) Xre domain (Gerdes et al. 2005; Makarova et al. 2009). The mycobacterial HigB toxin belongs to the RelE-like superfamily of toxins that generally inhibit translation by cleaving processing mRNAs on ribosomes (Gerdes et al. 2005). Remarkably, overexpression of HigB severely inhibits growth of M. tuberculosis, Mycobacterium smegmatis, and E. coli, suggesting that HigB recognition motifs in mRNA are widespread or that its specific targets are well conserved among bacteria (Fivian-Hughes and Davis 2010; Gupta 2009; Ramage et al. 2009; Sala et al. 2013). In agreement with the severe toxicity of HigB, the higA antitoxin gene from M. tuberculosis TAC cannot be deleted in the presence of endogenous HigB (Fivian-Hughes and Davis 2010), while under the same condition, deletion of the chaperone exhibits a slow growth phenotype most likely due to a reduced antitoxin activity (Sassetti et al. 2003).
Fig. 1.
a To-scale representation of the M. tuberculosis TAC encoding operon. b Distribution of TAC/AC and SecB chaperones across the bacterial taxonomy. Presence of SecB (black, outside circle) or TAC/AC system (gray, inside circle) is depicted as a bar in front of the corresponding leaf in the circular tree of species based on the NCBI taxonomic classification. Leaves are colored according to the taxonomic group. Species discussed in the text are indicated. This representation was generated with iTol
As stated above, the Rv1957 chaperone is related to the well-characterized chaperone SecB from E. coli (Bordes et al. 2011). SecB is a homotetrameric chaperone of 69 kDa (Dekker et al. 2003) that binds nonnative precursor proteins co- or posttranslationally and specifically addresses them to the SecA motor component of the Sec translocon at the inner membrane, thus facilitating their export (Bechtluft et al. 2010; Randall and Hardy 2002). Rv1957 (~20 kDa) and SecB (~17 kDa) from E. coli share 19 % sequence identity and 31 % similarity at the amino acid level, with several key residues involved in SecB oligomerization, substrate binding, or interaction with SecA being conserved (Bordes et al. 2011). Although the tridimensional structure of Rv1957 is not known, preliminary biochemical analyses revealed that as observed for SecB, Rv1957 purifies as a homotetrameric chaperone, thus reinforcing the fact that both chaperones might be evolutionarily related (Bordes et al. 2011).
Due to its major function during protein export, SecB is generally considered a proteobacterial invention associated with the presence of an outer membrane and outer membrane proteins (van der Sluis and Driessen 2006). In agreement with such a view, SecB chaperones are present in almost all species of α-, β-, and γ-proteobacteria (van der Sluis and Driessen 2006). Nevertheless, some SecB-like genes can also be found sporadically in Gram-positive bacteria, but to date, no functional SecB-dependent secretion has been described in these cases (Bohnsack and Schleiff 2010). The fact that M. tuberculosis possesses an unusual cell wall with a well-defined outer membrane and outer membrane proteins suggests that this pathogen could use such SecB chaperone functions under certain conditions (Mah et al. 2010; Zuber et al. 2008). Supporting such a hypothesis, it was recently shown that in addition to its specific role in controlling the antitoxin of TAC, Rv1957 can efficiently replace the E. coli SecB chaperone both in vivo and in vitro (Bordes et al. 2011). Reciprocally, overexpression of the E. coli SecB stimulates inhibition of the HigB toxin by HigA, albeit less efficiently than Rv1957 (Bordes et al. 2011). Yet, the nature of the interaction of Rv1957 with its substrate(s) is unknown and it remains to be determined whether substrates wrap around Rv1957 tetramers, interacting with multiple subsites on the chaperone, as it has been shown for SecB (Lilly et al. 2009; Randall and Hardy 2002). Together, these data suggest that the TAC chaperone could represent an intimate link between protein export and activation of a stress-responsive toxin–antitoxin system.
TAC genes are conserved in the genomes of all members of the M. tuberculosis complex (MTBC). In order to study the evolutionary history of these systems and to specify their relationship with the SecB export chaperone, we searched for homologous systems in sequenced bacterial genomes.
Distribution of TAC/AC systems in bacterial phylogeny
The M. tuberculosis TAC chaperone sequence was used in PSI-BLAST searches and neighborhood of the significant hits was analyzed in order to detect putative toxin and antitoxin pairs presenting a conserved gene organization when compared to the original TAC system, but not necessarily sharing sequence similarities with HigBA (Fig. 1a). Using this approach, we have now identified 49 complete TAC systems (Table S1). In addition, we found 11 toxin-less TAC systems, named antitoxin–chaperone (AC), which could either neutralize toxins located elsewhere on the genome or result from the loss of the toxin (Yang et al. 2010). Note that nine putative antitoxin–chaperone pairs from these AC systems do have one or more homologous pairs among the complete TAC identified in this work and thus retain the same genetic organization as TAC, except for the apparent lack of toxin. In two cases, the antitoxin–chaperone pairs from AC do not have homologous pair in complete TAC but their respective antitoxins do possess a putative DNA-binding domain, named HTH-like in this study. Moreover, these two AC retain a similar genetic organization as TAC, i.e., the antitoxin and the downstream SecB-like chaperone are present on the same operon with the two genes overlapping by few nucleotides (four nucleotides in both cases), and are localized either on a prophage region (Msme) or on a plasmid (RopA), in agreement with the proposed acquisition of TAC by horizontal gene transfer (HGT; see section below). Such criteria might be sufficient to distinguish between unrelated transcriptional regulators upstream of a solitary SecB and bona fide antitoxins among the toxin-less AC systems and thus minimize the annotation of false positives.
Bacterial phylogenetic distribution of TAC/AC systems was analyzed and compared to that of solitary SecB chaperones using a tree of bacterial species based on the NCBI taxonomy (Fig. 1b). Solitary SecB was defined as a sequence (a) which possesses the Pfam02556 domain that characterizes SecB chaperones (http://pfam.sanger.ac.uk/) and (b) which is not part of TAC or AC systems identified in this work. Accordingly, 25 Pfam02556 SecB sequences that were in fact putative TAC/AC chaperones were discarded from the 1256 Pfam02556 SecB sequences available. As expected, our search revealed that solitary SecB is conserved in almost all species of α-, β-, and γ-proteobacteria and that some SecB sequences are found sporadically in unexpected groups such as the Gram-positive Firmicutes phylum. A remarkable example is the presence of solitary SecB in all the sequenced genomes of the human pathogen Streptococcus pneumoniae. The occurrence of SecB in bacteria lacking outer membrane proteins is intriguing and suggests that in these cases, the chaperone might function as a specialized chaperone involved in the secretion or folding of one (or more) specific protein(s), or alternatively, as a generic chaperone needed under certain stress conditions. However, these sequences are very distant from the E. coli SecB and it remains to be determined whether they indeed correspond to functional SecB chaperone in these bacteria.
TAC/AC systems present a wide and sparse distribution across the taxonomy, being found in seven phyla but only in a few species of each phylum except in Thermotogae. Remarkably, among the Gamma- and Betaproteobacteria, all the species possessing a TAC also have a solitary SecB chaperone. In Actinobacteria, Thermotogae, Verrucomicrobia, and Deinococcus-Thermus all the identified SecB-like sequences are part of TAC or AC systems. Interestingly, the firmicute Caldicellulosiruptor saccharolyticus presents two other SecB homologs in addition to its TAC chaperone, all of them being part of the Pfam02556 group. We observed 2 strains containing one TAC and one AC system, 1 strain containing two TAC, 1 being on a plasmid, and 11 containing two SecB paralogs in their genomes.
The distribution profile of TAC/AC systems could hardly be explained by vertical transfer and loss events, but rather suggested HGT. Thus, we analyzed the 52 complete genomes containing TAC/AC systems with the software Alien Hunter (Vernikos and Parkhill 2006), which identifies regions with a compositional bias, and searched for other HGT signatures on TAC/AC neighborhood. Thirty-four TAC/AC systems were located in an alien region (Table S2), and potential HGT signatures were found for six additional systems. Moreover, consistent with TA-addictive properties (Van Melderen 2010), five TAC/AC systems are plasmid-borne (Table S1). Thus, at least 75 % of the analyzed systems seem to have been acquired by HGT.
Relationships between TAC/AC and solitary SecB chaperones
Despite the low sequence conservation, a multiple alignment performed on the chaperone sequences with MAFFT (Katoh et al. 2002) highlights a conserved motif present in all the solitary and TAC/AC SecB sequences (Fig. S1). This motif is located in a region corresponding to the large α-helix1 of the E. coli SecB structure, mainly involved in tetramerization (Dekker et al. 2003). As no reliable phylogenetic trees could be computed (due to the low sequence conservation), we investigated the relationships between TAC/AC and solitary SecB chaperones through a graph partitioning approach. A graph was built, in which nodes correspond to proteins and weighted edges reflect the similarity between a pair of proteins. Graph partitioning—detection of communities of highly connected nodes—was performed using the Markov clustering (MCL) program (Fig. 2). Four communities were identified, one containing almost all solitary SecB plus 14 TAC/AC chaperones and the three others containing mostly TAC/AC chaperones. SecB solitary sequences form a highly connected core that also includes five TAC chaperones, reinforcing the idea that TAC/AC and solitary SecB chaperones are evolutionarily related. MTBC TAC chaperones are found in a community comprising chaperones from diverse taxonomic groups and interestingly the solitary SecB of the Clostridium difficile phage ΦCD119. The presence of a solitary SecB in ΦCD119 suggests that this community of TAC chaperones could arise from such a phage-encoded SecB and further supports the involvement of phages in TAC/AC evolutionary history.
Fig. 2.
a MCL analysis of 60 TAC/AC chaperone sequences and 112 solitary SecB chaperone sequences corresponding to the seed sample of the Pfam02256-conserved domain (see Table S1 for abbreviations). The circles (proteins) are colored according to the four communities defined by the program. The links between proteins reflect a BLAST hit with an e-value less than or equal to 10−5. Abbreviations corresponding to plasmid-borne systems are underlined. b Antitoxin and toxin families found associated with SecB-like chaperones
SecB-like chaperones associate with diverse TA pairs
For all the TAC systems identified so far, the antitoxin is located downstream of the toxin gene, an organization thought to be unfavorable to the formation of the inactive complex because the more stable toxin is synthesized before the labile protease-sensitive antitoxin (Yamaguchi et al. 2011). The additional presence of a dedicated SecB-like chaperone could thus facilitate the co-translational assembly of an inactive toxin–antitoxin (or toxin–antitoxin–chaperone) complex by interacting with the antitoxin early during its synthesis. In this case, sensible variations in chaperone availability could rapidly trigger toxin activation in response to specific stress.
Although the genetic organization of TAC systems is conserved, inspection of the toxin–antitoxin pairs from TAC revealed that the presence of a SecB-like chaperone is not restricted to the HigBA TA family. Indeed, analysis of the TAC/AC antitoxin sequences with MCL revealed five distinct communities of antitoxins associated with a chaperone, corresponding to three canonical antitoxin families, namely HigA, MqsA, and HicB, and two other unclassified, one that we named MqsA-like because of the presence of a partial MqsA conserved domain and one that we named HTH-like because of the presence of a putative HTH domain (Fig. S2). All the HicB antitoxins identified present a ribbon–helix–helix DNA-binding domain. In E. coli, the HicB antitoxin neutralizes the HicA toxin known as a ribosome-independent ribonuclease induced by nutritional stress (Jorgensen et al. 2009). The MqsR toxin of E. coli is also a ribosome-independent ribonuclease that cleaves mRNA at GCU sites and is antagonized by the MqsA antitoxin known to modulate the general stress response via rpoS transcription regulation (Wang et al. 2011). The TAC antitoxins belonging to the MqsA family are well conserved and most of them present the two CXXCG motifs responsible for zinc binding, additionally to a HTH Xre domain (Papadopoulos et al. 2012).
Globally, for each class of antitoxin, we observed variability in the type of chaperone and toxin partners (Fig. S2). In fact, the antitoxin genes were either (a) associated with a toxin of the corresponding family, (b) toxin less, or (c) associated with an ORF of unknown function, but being part of an MCL community together with other putative TAC toxins (Fig. 2b). In this case, the latest class could thus represent new TA families. Such diversity in TA families within TAC systems suggests that several independent events of association of a TA system with a SecB-like chaperone might have occurred during evolution. These results also suggest that the control of TA systems by molecular chaperones might represent a more commonly used mechanism to modulate toxin activation in response to environmental insults. In support of such an hypothesis, four well-characterized antitoxins present in E. coli, namely MazE, RelB, MqsA, and DinJ, have been isolated as bona fide substrates of the DnaK (Hsp70) stress chaperone in vivo (Calloni et al. 2012). In addition, it was recently shown that mild overexpression of the HipA toxin of the HipAB type II TA system efficiently suppresses the severe bacterial growth and protein folding defects exhibited in the absence of the two major E. coli chaperones Trigger Factor and DnaK, by presumably restoring the coupling between protein synthesis and the downstream folding capacity of the cell (Bruel et al. 2013). Further studies are clearly needed to elucidate such a fundamental link between stress chaperones and TA activation.
SecB-like chaperone from AC in M. smegmatis
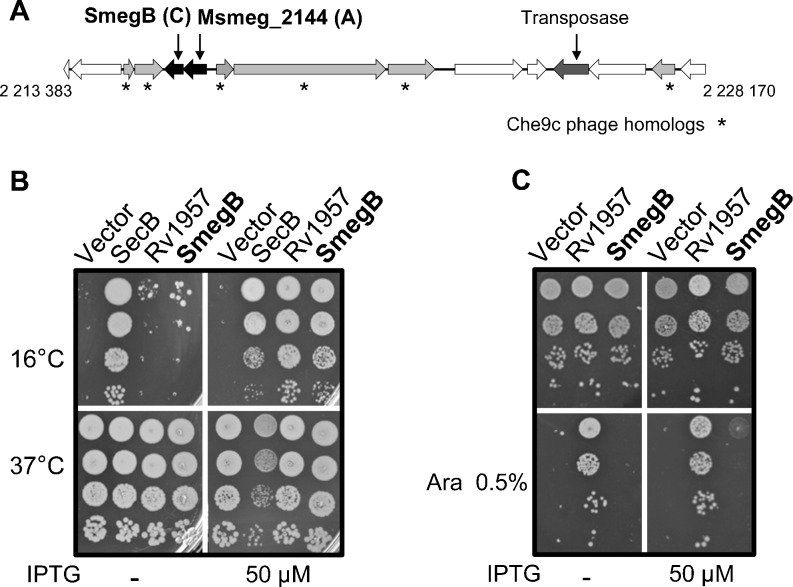
Our analysis of TAC systems revealed that the nonpathogenic bacterium M. smegmatis, which is closely related to M. tuberculosis, possesses an AC system in its genome. While TAC from M. tuberculosis is located in a large genomic island of approximately 81 kb, together with nine other TA systems and potential pathogenicity determinants (Ramage et al. 2009; Sala et al. 2013; Stinear et al. 2008), the AC system of M. smegmatis is part of a shorter genomic island of approximately 15 kb, which is not present in M. tuberculosis (Fig. 3a). Despite the fact that M. smegmatis and M. tuberculosis genomes are very close (95 % identity between 16S rRNA genes), their TAC/AC chaperones are largely distant (Fig. 2a). Primary amino acid sequence comparison of the M. smegmatis SecB-like chaperone, thereafter referred to as SmegB (M. smegmatis SecB-like chaperone SmegB), with E. coli SecB and Rv1957 reveals that SmegB is even closer to the E. coli SecB, with 37 % similarity against 31 % for Rv1957 (Fig. S3).
Fig. 3.
The M. smegmatis SecB-like chaperone. a To-scale representation of the genomic island of approximately 15 kb containing the M. smegmatis AC (Msmeg2144-SmegB) genes identified by Alien Hunter. Presence of Che9c phage and transposase genes is indicated. b SmegB replaces SecB in E. coli. Transformants of W3110 ΔsecB containing pSE (vector), pSE-SecB, pSE-Rv1957, or pSE-SmegB were grown to midlog phase, serially diluted and spotted on lysogeny broth (LB)–ampicillin with or without isopropyl β-d-1-thiogalactopyranoside (IPTG), and incubated at the indicated temperature. c SmegB does not replace Rv1957 in TAC control. Midlog phase cultures of W3110 ΔsecB containing the pK6-HigBA1 plasmid and pSE (vector), pSE-Rv1957, or pSE-SmegB were serially diluted and spotted on LB–ampicillin–kanamycin agar plates with or without arabinose and IPTG inducers as indicated. Plates were incubated at 37 °C overnight
The fact that these two closely related mycobacteria retain independently acquired SecB-like chaperones in their genomes is intriguing and suggests that both proteins could also function as generic chaperones in their respective hosts. The gene encoding SmegB was cloned on a plasmid and tested for its ability to replace the solitary SecB chaperone from E. coli in vivo (Bordes et al. 2011; Sakr et al. 2010; Ullers et al. 2007). Note that the E. coli ΔsecB mutant presents a strong cold-sensitive phenotype associated with a general defect in protein secretion (Marani et al. 2006; Pogliano and Beckwith 1993; Sakr et al. 2010; Ullers et al. 2004). As shown in Fig. 3b, overexpression of SmegB fully complements the cold-sensitive phenotype of the E. coli ΔsecB, in a manner comparable to that of SecB and Rv1957, even at the stringent temperature of 16 °C (Fig. 3b). This suggests that SmegB is indeed capable of performing a generic chaperone function. Since Rv1957 and SmegB are associated with antitoxins that most likely belong to distinct groups (i.e., HigA and HTH-like, respectively), we next asked whether SmegB could replace Rv1957 in controlling the HigBA TA system form M. tuberculosis. To do so, we expressed both the toxin HigB and the antitoxin HigA in the presence of overexpressed SmegB and monitored the HigB-dependent growth inhibition as described previously (Bordes et al. 2011). The results presented in Fig. 3c show that in contrast with Rv1957, co-expression of SmegB and HigA in E. coli does not efficiently suppress the growth inhibition mediated by HigB. In agreement with the bacterial growth data and unlike Rv1957, the overexpression of SmegB is not capable of protecting the antitoxin of M. tuberculosis TAC from degradation in vivo (Fig. S3). Taken together, these results suggest that although SmegB efficiently performs solitary SecB chaperone function in E. coli, it does not replace Rv1957 in the specific control of HigBA from M. tuberculosis.
Concluding remarks
In this work, we have identified 60 systems in which a SecB-like chaperone is associated with either a TA or an isolated antitoxin, whose evolutionary history seems to be complex and largely mediated by HGT. In addition, we found that well-distinct TA pairs can be associated with SecB-like chaperones, suggesting that independent events of association might have occurred during evolution. The observation that TAC chaperones from closely related mycobacteria may not be interchangeable suggests a specificity of partnership with the antitoxins, whose determinants remain to be identified. Nevertheless, the fact that the two chaperones tested so far can efficiently replace SecB in E. coli is intriguing and one of the main issues for future investigations will be to determine whether these SecB-like chaperones have conserved their ability to perform generic chaperone functions in their natural hosts and whether they are indeed capable of interacting with the Sec translocon. In addition, more work will be needed to shed light on the mechanism of activation of the toxin and the precise role played by the SecB-like chaperone, and possibly by stress-induced proteases, in this process. Indeed, it is generally believed that activation of toxins from type II TA systems relies on the degradation of their cognate antitoxins by the stress proteases Lon and/or Clp (Gerdes et al. 2005). Although it is not known yet whether proteases participate in the HigB toxin activation cascade, the additional presence of a SecB-like chaperone that assists the antitoxin in the case of TAC suggests a more competitive mode of regulation. Finally, another challenging perspective will be to identify the cellular processes targeted by HigB and to examine its impact on M. tuberculosis physiology and virulence.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 540 kb)
Acknowledgments
We thank Anne-Marie Cirinesi for technical support, Thomas Wood for critical reading of the manuscript, Wladimir Malaga for the kind gift of M. smegmatis genomic DNA, and members of the Genevaux and Fichant laboratories for insightful discussions. This work was supported by an ATIP-CNRS grant to PG and MENRT fellowships to AS and VC.
Abbreviations
- TA
Toxin–antitoxin
- TAC
Toxin–antitoxin–chaperone
- AC
Antitoxin–chaperone
- IPTG
Isopropyl β-d-1-thiogalactopyranoside
- MTBC
Mycobacterium tuberculosis complex
- MCL
Markov clustering
- HTH
Helix–turn–helix
References
- Bechtluft P, Nouwen N, Tans SJ, Driessen AJ. SecB—a chaperone dedicated to protein translocation. Mol Biosyst. 2010;6:620–627. doi: 10.1039/b915435c. [DOI] [PubMed] [Google Scholar]
- Betts JC, Lukey PT, Robb LC, McAdam RA, Duncan K. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol Microbiol. 2002;43:717–731. doi: 10.1046/j.1365-2958.2002.02779.x. [DOI] [PubMed] [Google Scholar]
- Bohnsack MT, Schleiff E. The evolution of protein targeting and translocation systems. Biochim Biophys Acta. 2010;1803:1115–1130. doi: 10.1016/j.bbamcr.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Bordes P, Cirinesi AM, Ummels R, Sala A, Sakr S, Bitter W, Genevaux P. SecB-like chaperone controls a toxin-antitoxin stress-responsive system in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2011;108:8438–8443. doi: 10.1073/pnas.1101189108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruel N, Castanie-Cornet M, Cirinesi A, Koningstein G, Georgopoulos C, Luirink J,Genevaux P (2013) Hsp33 controls elongation factor-Tu stability and allows Escherichia coli growth in the absence of the major DnaK and Trigger Factor chaperones. J Biol Chem http://www.jbc.org/cgi/doi/10.1074/jbc.M112.418525 [DOI] [PMC free article] [PubMed]
- Calloni G, Chen T, Schermann SM, Chang HC, Genevaux P, Agostini F, Tartaglia GG, Hayer-Hartl M, Hartl FU. DnaK functions as a central hub in the E. coli chaperone network. Cell Rep. 2012;1:251–264. doi: 10.1016/j.celrep.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Dekker C, de Kruijff B, Gros P. Crystal structure of SecB from Escherichia coli. J Struct Biol. 2003;144:313–319. doi: 10.1016/j.jsb.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Fivian-Hughes AS, Davis EO. Analyzing the regulatory role of the HigA antitoxin within Mycobacterium tuberculosis. J Bacteriol. 2010;192:4348–4356. doi: 10.1128/JB.00454-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes K, Christensen SK, Lobner-Olesen A. Prokaryotic toxin-antitoxin stress response loci. Nat Rev Microbiol. 2005;3:371–382. doi: 10.1038/nrmicro1147. [DOI] [PubMed] [Google Scholar]
- Gupta A. Killing activity and rescue function of genome-wide toxin-antitoxin loci of Mycobacterium tuberculosis. FEMS Microbiol Lett. 2009;290:45–53. doi: 10.1111/j.1574-6968.2008.01400.x. [DOI] [PubMed] [Google Scholar]
- Jorgensen MG, Pandey DP, Jaskolska M, Gerdes K. HicA of Escherichia coli defines a novel family of translation-independent mRNA interferases in bacteria and archaea. J Bacteriol. 2009;191:1191–1199. doi: 10.1128/JB.01013-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren I, Minami S, Rubin E, Lewis K. Characterization and transcriptome analysis of Mycobacterium tuberculosis persisters. mBio. 2011;2:e00100–e00111. doi: 10.1128/mBio.00100-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly AA, Crane JM, Randall LL. Export chaperone SecB uses one surface of interaction for diverse unfolded polypeptide ligands. Protein Sci Publ Protein Soc. 2009;18:1860–1868. doi: 10.1002/pro.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah N, Perez-Iratxeta C, Andrade-Navarro MA. Outer membrane pore protein prediction in mycobacteria using genomic comparison. Microbiology. 2010;156:2506–2515. doi: 10.1099/mic.0.040089-0. [DOI] [PubMed] [Google Scholar]
- Makarova KS, Wolf YI, Koonin EV. Comprehensive comparative-genomic analysis of type 2 toxin-antitoxin systems and related mobile stress response systems in prokaryotes. Biol Direct. 2009;4:19. doi: 10.1186/1745-6150-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marani P, Wagner S, Baars L, Genevaux P, de Gier JW, Nilsson I, Casadio R, von Heijne G. New Escherichia coli outer membrane proteins identified through prediction and experimental verification. Protein Sci Publ Protein Soc. 2006;15:884–889. doi: 10.1110/ps.051889506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos E, Collet JF, Vukojevic V, Billeter M, Holmgren A, Graslund A, Vlamis-Gardikas A. Solution structure and biophysical properties of MqsA, a Zn-containing antitoxin from Escherichia coli. Biochimica et Biophysica Acta. 2012;1824(12):1401–1408. doi: 10.1016/j.bbapap.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Pogliano KJ, Beckwith J. The Cs sec mutants of Escherichia coli reflect the cold sensitivity of protein export itself. Genetics. 1993;133:763–773. doi: 10.1093/genetics/133.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage HR, Connolly LE, Cox JS. Comprehensive functional analysis of Mycobacterium tuberculosis toxin-antitoxin systems: implications for pathogenesis, stress responses, and evolution. PLoS Genet. 2009;5:e1000767. doi: 10.1371/journal.pgen.1000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall LL, Hardy SJ. SecB, one small chaperone in the complex milieu of the cell. Cell Mol life Sci: CMLS. 2002;59:1617–1623. doi: 10.1007/PL00012488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakr S, Cirinesi AM, Ullers RS, Schwager F, Georgopoulos C, Genevaux P. Lon protease quality control of presecretory proteins in Escherichia coli and its dependence on the SecB and DnaJ (Hsp40) chaperones. J Biol Chem. 2010;285:23506–23514. doi: 10.1074/jbc.M110.133058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala A, Bordes P, Fichant G, Genevaux P. Toxin-antitoxin loci in Mycobacterium tuberculosis. In: Gerdes K, editor. Prokaryotic toxin–antitoxins. Berlin: Springer; 2013. pp. 295–314. [Google Scholar]
- Sassetti CM, Boyd DH, Rubin EJ. Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol. 2003;48:77–84. doi: 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- Smollett KL, Fivian-Hughes AS, Smith JE, Chang A, Rao T, Davis EO. Experimental determination of translational start sites resolves uncertainties in genomic open reading frame predictions—application to Mycobacterium tuberculosis. Microbiology. 2009;155:186–197. doi: 10.1099/mic.0.022889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart GR, Wernisch L, Stabler R, Mangan JA, Hinds J, Laing KG, Young DB, Butcher PD. Dissection of the heat-shock response in Mycobacterium tuberculosis using mutants and microarrays. Microbiology. 2002;148:3129–3138. doi: 10.1099/00221287-148-10-3129. [DOI] [PubMed] [Google Scholar]
- Stinear TP, Seemann T, Harrison PF, Jenkin GA, Davies JK, Johnson PD, Abdellah Z, Arrowsmith C, Chillingworth T, Churcher C, et al. Insights from the complete genome sequence of Mycobacterium marinum on the evolution of Mycobacterium tuberculosis. Genome Res. 2008;18:729–741. doi: 10.1101/gr.075069.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullers RS, Ang D, Schwager F, Georgopoulos C, Genevaux P. Trigger factor can antagonize both SecB and DnaK/DnaJ chaperone functions in Escherichia coli. Proc Natl Acad Sci U S A. 2007;104:3101–3106. doi: 10.1073/pnas.0608232104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullers RS, Luirink J, Harms N, Schwager F, Georgopoulos C, Genevaux P. SecB is a bona fide generalized chaperone in Escherichia coli. Proc Natl Acad Sci U S A. 2004;101:7583–7588. doi: 10.1073/pnas.0402398101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluis EO, Driessen AJ. Stepwise evolution of the Sec machinery in Proteobacteria. Trends Microbiol. 2006;14:105–108. doi: 10.1016/j.tim.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Van Melderen L. Toxin-antitoxin systems: why so many, what for? Curr Opin Microbiol. 2010;13:781–785. doi: 10.1016/j.mib.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Vernikos GS, Parkhill J. Interpolated variable order motifs for identification of horizontally acquired DNA: revisiting the Salmonella pathogenicity islands. Bioinformatics. 2006;22:2196–2203. doi: 10.1093/bioinformatics/btl369. [DOI] [PubMed] [Google Scholar]
- Wang X, Kim Y, Hong SH, Ma Q, Brown BL, Pu M, Tarone AM, Benedik MJ, Peti W, Page R, et al. Antitoxin MqsA helps mediate the bacterial general stress response. Nat Chem Biol. 2011;7:359–366. doi: 10.1038/nchembio.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Park JH, Inouye M. Toxin-antitoxin systems in bacteria and archaea. Annu Rev Genet. 2011;45:61–79. doi: 10.1146/annurev-genet-110410-132412. [DOI] [PubMed] [Google Scholar]
- Yang M, Gao C, Wang Y, Zhang H, He ZG. Characterization of the interaction and cross-regulation of three Mycobacterium tuberculosis RelBE modules. PLoS One. 2010;5:e10672. doi: 10.1371/journal.pone.0010672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber B, Chami M, Houssin C, Dubochet J, Griffiths G, Daffe M. Direct visualization of the outer membrane of mycobacteria and corynebacteria in their native state. J Bacteriol. 2008;190:5672–5680. doi: 10.1128/JB.01919-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 540 kb)