Abstract
Photodynamic therapy (PDT) is a regulatory-approved modality for treating a variety of malignant tumors. It induces tumor tissue damage via photosensitizer-mediated oxidative cytotoxicity. The heat shock protein 70 (HSP70-1) is a stress protein encoded by the HSPA1A gene and is significantly induced by oxidative stress associated with PDT. The aim of this study was to identify the functional region of the HSPA1A promoter that responds to PDT-induced oxidative stress and uses the stress responsiveness of HSPA1A expression to establish a rapid and cost-effective photocytotoxic assessment bioassay to evaluate the photodynamic potential of photosensitizers. By constructing luciferase vectors with a variety of hspa1a promoter fractions and examining their relative luciferase activity, we demonstrated that the DNA sequence from −218 to +87 of the HSPA1A gene could be used as a functional promoter to detect the PDT-induced oxidative stress. The maximal relative luciferase activity level of HSPA1A (HSP70-1) induced by hypericin-PDT was nearly nine times that of the control. Our results suggest that the novel reporter gene assay using a functional region of the HSP70A1A promoter has significant advantages for the detection of photoactivity in terms of both speed and sensitivity, when compared with a cell viability test based on ATP quantification and ROS levels. Furthermore, phthalocyanine zinc and methylene blue both induced significantly elevated levels of relative luciferase activity in a dose-dependent manner.
Keywords: Photodynamic therapy (PDT), HSPA1A promoter activity, Stress oxidative, Luciferase reporter, Cell viability
Introduction
Photodynamic therapy (PDT) is a regulatory-approved modality for treating a variety of malignant tumors. It uses the destructive power of reactive oxygen species (ROS) to bring about their obliteration. Photosensitizers which have accumulated in cancerous tissue/cells can generate ROS under visible light irradiation (Dolmans et al. 2003). Photosensitizers play an essential role in the course of PDT. Hence, it is necessary to develop a stable cell model with high specificity and sensitivity to assess the activity of PDT in vitro and screen antitumor lead compounds with ideal photoacitivity.
It is well established that PDT can induce tumor tissue damage via photosensitizers-mediated oxidative cytotoxicity (Kammerer et al. 2011; Ryter et al. 2007). Oxidative stress associated with PDT is able to induce genes encoding stress proteins, including those belonging to the heat shock protein (HSP) family (Gehrmann et al. 2008; Nollen and Morimoto 2002). Previous studies have reported that inducible HSP genes can be upregulated by a wide range of cellular stresses (Korbelik et al. 2005). HSPs are highly conserved molecular chaperones that play essential roles in nascent protein folding, misfolded protein refolding, or degradation (Mosser et al. 2000; Brocchieri et al. 2008). There is a strong evidence that the induction of HSPs coincides with the acquisition of tolerance to stress, which could otherwise lead to cell death (Pohlman and Harlan 2000). The HSPA (HSP70) family, the best characterized of the HSPs, contains a major stress-inducible member encoded by the HSPA1A gene (Kampinga et al. 2009).
HSPs play a positive role in cancer diagnosis and therapy. Guo et al. (2011) determined that functional HSPA1B variants are associated with lung cancer risk and survival. This finding may offer useful biomarkers to predict lung cancer risk and prognosis. Moreover, HSPs display a dual function that is protective and immunological (Schmitt et al. 2007). On the one hand, HSPs accumulate in cancer cells and are involved in their survival. Some studies have discovered that high expression of HSP70 in breast, endometrial, or gastric cancer has been associated with metastasis, poor prognosis, and resistance to chemotherapy or radiation therapy (Brondani Da Rocha et al. 2004; Vargas-Roig et al. 1998; Garrido et al. 1997). On the other hand, HSPs have been found to be important players in the process of cross-presentation of tumor-derived, antigenic peptides. On the basis of these immunological roles, patients are being injected with preparations of HSPs in order to induce a specific antitumoral immune response that is capable of rejecting the tumor.
Bioassays using reporter vectors regulated by cellular stress-responsive gene promoters have been suggested as promising tools for photocytotoxic assessment (Cheng et al. 2010). The expression of significant stress responsiveness of HSPA1A is often regarded as a potential biomarker to evaluate oxidative cytotoxicity (Demirovic and Rattan 2012; Xin et al. 2012). A recent study has shown that stable metabolically competent HepG2 cells containing a human HSPA1A promoter-driven luciferase reporter (HepG2-luciferase cells) could be used for assessing the toxicity of organic pollutants present in air (Xin et al. 2012). In addition, human cells carrying an HSPA1A promoter-driven reporter gene have been used to assess the toxicity of heavy metals, organochlorine compounds, and eluates of industrial wastes (Ait-Aissa et al. 2003). Luna et al. (2000) examined the efficiency of PDT to function as a molecular switch by initiating expression of heterologous genes ligated to the human HSP promoter. Mitra et al. (2003) used fluorescence imaging to show that the PS m-tetrahydroxyphenylchlorin mediated the induction of HSP70 in EMT6 cells that had undergone PDT. These cells were stably transfected with a plasmid that contained the gene which encodes green fluorescent protein (GFP) under the control of an HSP70 promoter; Mitra et al. (2003) observed increased GFP expression after PDT in both an in vitro and in vivo tumor model. However, few studies have yet detected PDT-induced oxidative stress by reporter gene assay using the HSPA1A promoter.
Hypericin (Hyp) is considered to be one of the most powerful photosensitizers available from nature, which makes Hyp a valuable photosensitizer for PDT (Karioti and Bilia 2010). It is derived from hypericum plants (27 out of 36 evaluated species) (Agostinis et al. 2002), more specifically the Hypericum perforatum or St. John’s wort, an erect perennial herb. Hyp exhibits potent photosensitizing characteristics, minimal dark toxicity and high singlet oxygen quantum yield.
MCF-7 is a breast cancer cell line from a pleural effusion derived from a breast carcinoma and has retained several ideal characteristics particular to the mammary epithelium. It is widely reported that the MCF-7 cell line is sensitive to PDT (Vittar et al. 2010; Li et al. 2010; Tsai et al. 2004; Xue et al. 2001).
This paper applied the MCF-7 cell line and Hyp as the preferential model cell and photosensitizer to form a reporter system. Through pilot experiments (data not shown), we determined the appropriate PDT regimen for functional promoter analysis. We gained cancer reporter cells expressing the firefly luciferase reporter under the control of the functional human HSPA1A promoter trough transient transfection, such that we could establish a rapid and cost-effective photocytotoxic assessment bioassay to determine the photodynamic potential of photosensitizers.
Materials and methods
Photosensitizers
Hyp, methylene blue (Me), and zinc phthalocyanine (ZnPc) were all purchased from the Food and Drug Administration (FDA, USA) and were used without any further purification. A stock solution (1 mM) was made in dimethyl sulphoxide, and then stored in the dark at 4 °C.
Cell culture
MCF-7 cells purchased from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) were cultured in Roswell Park Memorial Institute (RPMI) 1,640 medium, supplemented with 10 % heat-inactivated bovine serum, penicillin (100 U/ml), and streptomycin (100 U/ml). Cells were incubated at 37 °C in a humidified atmosphere of 95 % air and 5 % CO2.
PDT and hyperthermia treatments
Photosensitization protocols involved seeding cells into 96-well plates and incubating overnight in complete growth media for cell attachment.
For the experiment, cells were co-incubated in the dark at 37 °C with or without certain concentrations of photosensitizers. After an indicated time of incubation (Hyp, 16 h and Me and ZnPc, 24 h), the medium was changed with complete RPMI 1,640 medium. Cells were irradiated under light emitted from a 100-watt quartz-halogen lamp and harvested 4 h after irradiation. The light intensity was measured by a photo radiometer (Delta Ohm, Padua, Italy).
For Hyp treatments as a positive control of HSPA1A induction, cells were exposed to 45 °C in a temperature-controlled water bath for 20 min. The transiently transfected cells were harvested for luciferase activity assay, while the untransfected cells were harvested for the Western blot 4 h after hyperthermia treatment.
Assessment of photocytotoxicity
MCF-7 cells (1 × 104) were seeded into a 96-well plate and treated with various concentrations of Hyp for 16 h. After that, the cells were exposed to the PDT treatment described above. Cell viability was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay 4 h after PDT treatment.
Determination of cell viability
The number of viable MCF-7 luciferase cells in culture was determined using the CellTiter-Glo Luminescent Cell Viability Assay kit (Promega, Madison, WI). This assay is a homogeneous method to determine the number of viable metabolically active cells based on the quantification of ATP present. The untreated cells were the 100 % viable control.
Western blot analysis
Proteins were resolved in an SDS/PAGE gel and subjected to immunoblot analysis using monoclonal antibodies against HSPA1A or β-actin (BioWorld, Atlanta, GA). All antibodies were used at 1 mg/ml of working concentration in PBS with 5 % nonfat milk powder. The membranes were then probed with appropriate primary antibodies. Blots were then incubated with appropriate secondary antibody alkaline phosphatase (AP) conjugated and detected in 5 ml AP buffer containing 16.5 μl BCIP and 33 μl NBT at room temperature for 10–20 min, and then photographed. β-actin was used as a loading control.
Vector constructs and transient transfection
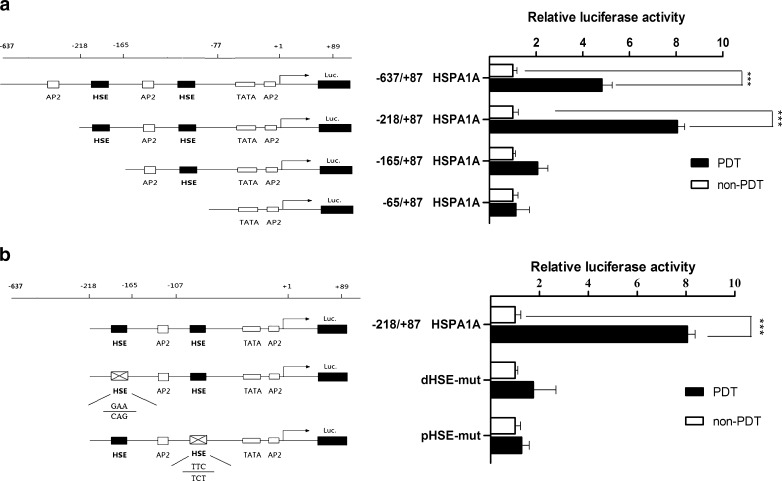
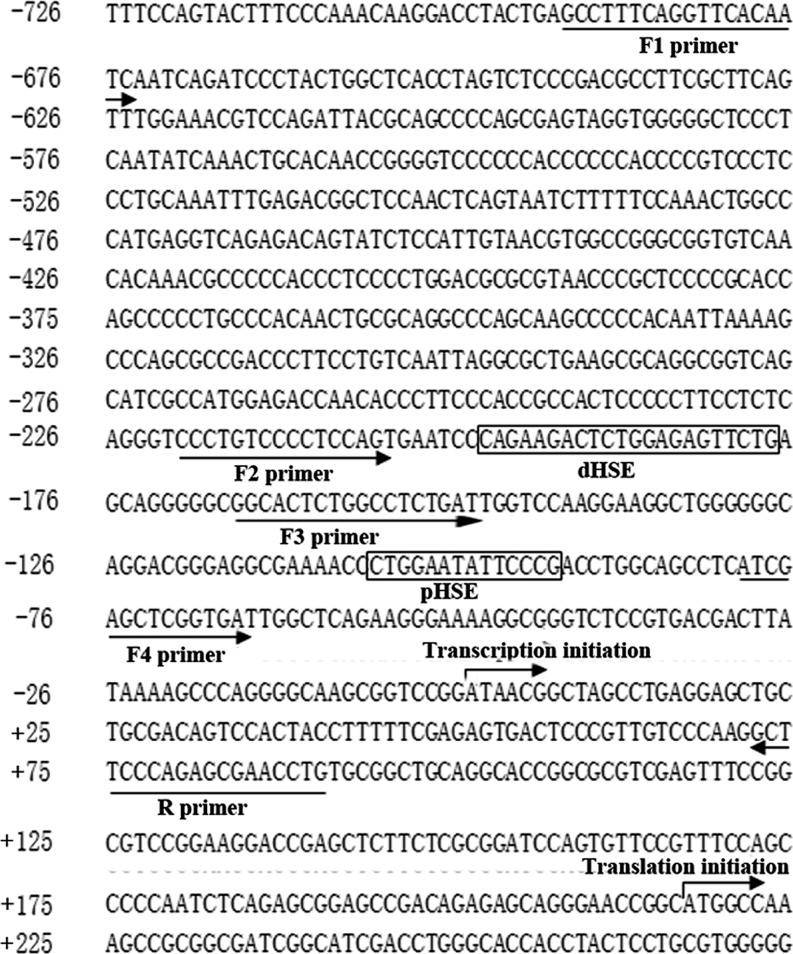
Standard polymerase chain reaction (PCR) was utilized to prepare the luciferase constructs used in this study. A 782-bp HSPA1A promoter construct (−695/+87) HSPA1A, corresponding to the sequence from −695 to +87 (relative to the transcriptional start site) of the 5′-flanking region of the human HSPA1A gene, was generated from human genomic DNA using F1 (5′-GAAGATCTGCCTTTCAGGTTCACAATC-3′) and R1 (5′-CCCAAGCTTCAATCAGCCGCTTCG-3′) for forward and reverse primers, respectively. The 5′-flanking deletion constructs of the HSPA1A promoter were similarly generated by using the −695/+87 HSPA1A construct as a template, The following oligo-nucleotides were used as the sense primers for construction of the following vectors: F2 (pHSP-218), 5′-GAAGATCTCCTGTCCCCTCCAGTGAAT-3′; F3 (pHSP-165), 5′-GAAGATCTGGCACTCTGGCCTCTGAT-3′; F4 (pHSP-77), 5′-GAAGATCT ATCGAGCTCGGTGA-3′. The anti-sense primer in all cases was 5′-CCCAAGCTTAGGTTCGCTCTGGGAAGC-3′. The underlined nucleotides represent the HindIII and BglII sites. The PCR products were cloned into the corresponding sites of pGL3-basic vector (Promega Corp., Madison, WI; Fig. 3a, left).
Fig. 3.
Deletion and mutant analysis of the HSPA1A promoter. a Effects of PDT-induced oxidative stress on the luciferase activity of 5′deletion of the HSPA1A promoter. On the left was a schematic representation of the reporter gene constructs assayed by transient transfection of MCF-7 cells, while on the right side the bar graphs represented the relative levels of luciferase activity in each of the transfected samples. b Effects of PDT-induced oxidative stress on the mutations of the HSPA1A promoter. MCF-7 cells were transfected with different mutation HSPA1A promoter constructs or wild type HSPA1A promoter. Cells were then treated by Hyp-PDT with conditions described previously. The luciferase activity was measured 4 h post-PDT. Values are shown relative to the corresponding controls without PDT treatment. Columns, duplicate determinations representative experiment of three; bars, ±SD
The mutations in the two heat shock element (HSE) sites were generated as follows. The primers used to make the HSE1 mutant were pair 1, F1 and R2 (AGGTCGGGAATACCGcagGGTT); pair 2, F5 (5′-GAAAACCctgCGGTATTCCCG-3′) and R1. For mutating the HSE2 site, the primers used were F6 (5′-GTGAATCCCACGGGACtctGGAGAG-3′) and R3 (5′-CTCCagaGTCCCGTGGGATTCACT-3′). The first two rounds of PCR generated two fragments of DNA, with 25 bp of overlap. These two fragments were gel purified and used as templates for a third PCR with F1 and R1 primers. The mutated DNA was then cloned in pGL3-basic (Fig. 3b, left).
All primers were designed by Primer Premier5 software and obtained from Shanghai Generay Biological Engineering Technology and Services Co., Ltd. (Shanghai, China).
All luciferase constructs were verified by sequencing analysis.
Luciferase activity assay
Cells were seeded in 96-well plates and incubated overnight. Each promoter construct was transfected into subconfluent (80–90 %) monolayer cells using lipofectamine reagent LipofectamineTM2000 (Invitrogen Corporation, Carlsbad, CA) according to the manufacturer’s instructions. Each well was cotransfected with 50 ng of pRL-SV40 plasmid expressing renilla luciferase to serve as an internal control for variations in transfection efficiency. After 4 h of transfection, cells were washed by PBS and incubated with various concentrations of photosensitizer for a specific time. A luciferase activity assay was performed 4 h after PDT treatment with the dual luciferase reporter assay system (Promega, Madison, WI). The relative luciferase activity was normalized with renilla luciferase activity.
Measurement of oxidative damage
Oxidative damage was assessed by the ROS levels in the PDT-treated cells. After PDT treatment, ROS generation was measured by the DCFH-DA method. In this method, fluorogenic substrate DCFH-DA, a cell-permeable dye, is oxidized to highly fluorescent DCF by ROS, and can be used to monitor intracellular generation of ROS. The cells were treated with various concentrations of photosensitizers. DCFH-DA was added to the media at a final concentration of 20 μM and incubated at 37 °C for 30 min. After PDT treatment described previously, ROS generation was measured by fluorescence intensity at an excitation wavelength of 485 nm and an emission wavelength of 535 nm using EnSpire® Multimode Plate Reader (Perkin Elmer, Boston, MA).
Biostatistical analysis
All experiments were repeated at least three times and the results are presented as the mean of the three independent experiments. All data are described as mean ± SD. The Spearman test was used for correlation analyses. Two-tailed Student’s t tests were used to assess differences between the treated and control groups. Statistical analysis was performed using the GraphPad prism5 software to evaluate the significance of the difference between groups, considered as *p < 0.05; **p < 0.01; ***p < 0.001. All data points represented the mean of triplicates.
Results
The photocytotoxicity of Hyp
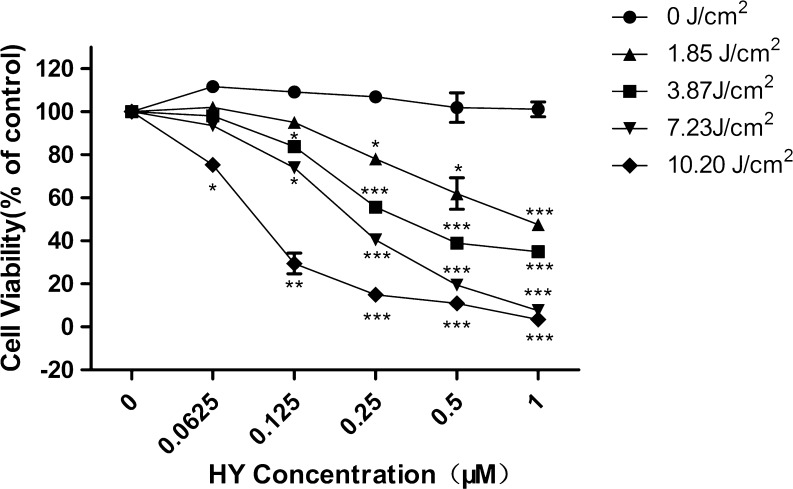
The photocytotoxic effect of Hyp in the MCF-7cells was determined by MTT assay (Fig. 1). The cells were incubated with various Hyp concentrations for 16 h in the dark and were exposed to light at different light doses (0–10.20 J/cm2). Figure 1 shows the results of the photocytotoxic effect of Hyp on MCF-7 cells assessed by MTT assay 4 h after the photosensitization. The data showed that Hyp resulted in a drug- and light-dependent cell viability. Our criterion for a sublethal dose is concentrations less than IC60 with a light dose of 3.87 J/cm2. Under these conditions, the luciferase chemiluminescence of cells in the treatment groups can be detected clearly without causing toxicity.
Fig. 1.
Photocytotoxicity of Hyp in the MCF-7 cells assessed by the MTT assay. Cells were incubated with various concentrations (0–1 μM) of Hyp for 16 h before photoirradiation under light emitted from a 100-watt quartz-halogen lamp with 500 to 590 long-pass filter at various light doses (0∼10.20 J/cm2)
Deletion and mutant analysis of the HSPA1A promoter
An investigation of public databases of sequence motifs revealed that there are two classical HSE elements (Fig. 2), proximal (−95 to −108) and distal (−181 to −208), within the upstream region of the HSPA1A gene (GenBank ID, NM_005345). Both of them contribute to the stress inducible elevation of the transcription. They are also involved in the interaction with the nucleus localized and polymerized HSF. The interaction will lead to release the HSPA1A transcriptional machineries which are paused under nonstress condition.
Fig. 2.
Analysis of HSPA1A promoter. The primary sequence of the two HSEs (heat shock response element, −208 to −181 and −108 to −95) was shown, respectively. Transcription start site and primers used for amplifying the complete forms and those with deletions of the HSPA1A promoter, and their orientation was also indicated
In order to identify the functional region that responds to PDT, we constructed four luciferase plasmids that carry a 5′-deleted promoter of the HSPA1A gene, following the location of each of the HSEs. The structures of the luciferase constructs and the PDT-induced stress response of cells transfected with these constructs are shown in Fig. 3a, left. The cells transfected with the pGL3−65/+87 and pGL3−122/+87 showed a similar response to oxidative stress, suggesting that the proximal HSE alone does not contribute greatly to the oxidative stress response. On the other hand, the pHSP-218 transfected cells showed a nearly fourfold higher stress response compared with that of the pGL3-65 and pGL3-122 transfected cells (Fig. 3a, right).
According to the results of 5′-deletion analysis, it seems that the deletion of dHSE abolished the PDT-mediated activation of the HSPA1A promoter reporter. Hence, we hypothesized that dHSE played an important role in the responsiveness of the HSPA1A promoter to PDT-induced oxidative stress. Site-directed mutagenesis was further performed to confirm the hypothesis (Fig. 3b, left). As shown in Fig. 3b (right), the respective mutation of the two HSEs abrogated the sensitivity of the HSPA1A promoter to PDT-induced oxidative stress, suggesting that a HSE, lying between −181 and −208, is required for regulation of the HSPA1A promoter during oxidative stress induced by PDT. The pHSE (−95 to −108) alone is insufficient.
Time course results of HSPA1A protein levels, cell viability, and intracellular ROS levels, as well as luciferase activity
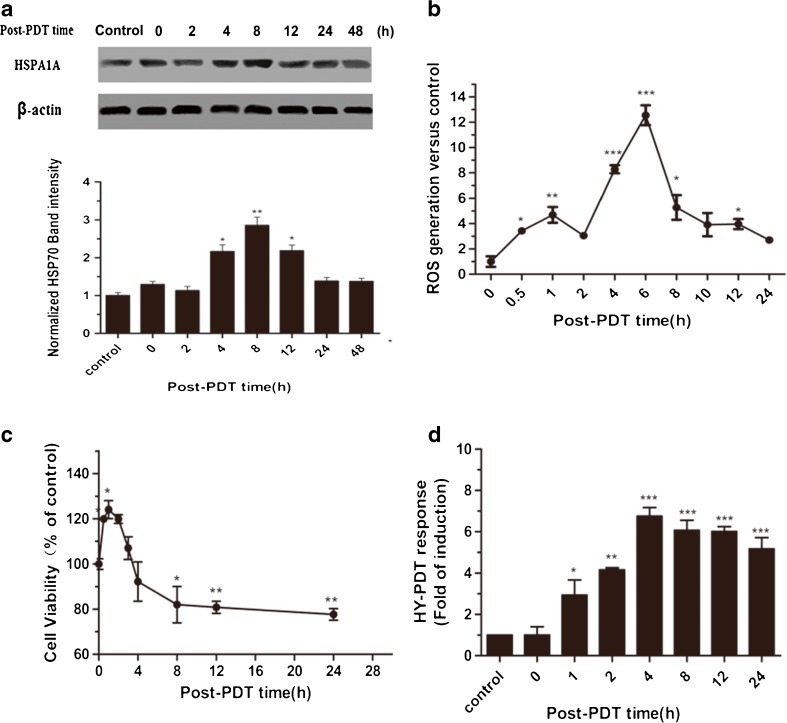
Figure 4a shows the kinetics of HSPA1A protein synthesis induced by PDT treatments. HSPA1A protein levels at various time intervals post PDT were examined by Western blot analysis. A significant increase (p < 0.01) of HSPA1A expression was observed 4 h after PDT. HSPA1A levels return to the background level at 24 h after PDT.
Fig. 4.
Time course results of HSPA1A protein level, cell viability, intracellular ROS level, and promoter activity. MCF7-luciferase cells were treated with 0.125 μM Hyp for 16 h and exposed to a light dose of 3.87 J/cm2. At the indicated time points after irradiation, cells were collected and detected HSPA1A protein level, luciferase activity, cell viability, and ROS level. a HSPA1A levels detected by Western blot after PDT and hyperthermia treatments. The untreated cells were used as a control. β-actin was used as the loading control. b The time course of ROS generation after PDT. Results are indicated as values relative to the control group. Changes in cell viability estimated by intracellular ATP quantification are shown in (c). The changes in luciferase activity of MCF7-luciferase cells are described in (d). Relative luciferase activity was measured using the luciferase assay system. In (c) and (d), all the values are shown relative to time-matched results from cells without PDT treatment. Histograms show the PDT response of MCF7-luciferase cells as folds of induction. Data represent mean ± SD of three individual experiments
In this series of experiments, we compared the time course of changes in luciferase activity linked to the functional HSPA1A promoter with cell viability estimated by ATP quantification, and the intracellular ROS levels after exposure to PDT.
ROS generation proceeded in two phases: the first increase was recorded 0.5 h after PDT (p < 0.05), with maximal levels at 1 h (p < 0.01) and a decrease after 2 h; the second increase occurred at 6 h (p < 0.001) after treatment (Fig. 4b). The initial increase of ROS generation is probably due to the reactivity of singlet oxygen (1O2) formed early after PDT and to its ability to induce the formation of other ROS species that can be detected by DCF. The late increase of ROS production, detected 6 h after PDT, is probably a downstream event associated with oxidative stress. Ouedraogo and Redmond (2003) have shown that secondary ROS can extend the initial photodamage intracellularly, while Rubio et al. (2009) and Chakraborty et al. (2009) have proved that secondary ROS can also extend the levels of oxidative stress to greater distances, reaching neighboring nontreated cells. For this reason, we believe that secondary ROS play a decisive role in PDT and are responsible for the final DNA breakdown and programmed cell death in PDT-treated cells (Kessel et al. 1997).
Figure 4c shows the results of changes in cell viability as evaluated by the quantification of intracellular ATP. MCF-7 cells showed 1.2- to 1.3-fold (p < 0.05) higher cell viability compared with time-matched controls during the first 2 h post-PDT treatment. It has been previously reported that in a certain range of ROS levels, ROS generation is capable of triggering beneficial effects, which is known as hormesis (Radak et al. 2005). We hypothesize that the increased cell viability observed in the present study may be attributed to hormesis from low levels of ROS production.
Figure 4d shows that cells-transfected pGL3-218 were exposed to PDT (Hyp, 0.125 μM and light dose, 3.87 J/cm2). Maximum luciferase activity was observed at 4 h post-PDT treatment (p < 0.001) and remained elevated for 24 h post-PDT. From this perspective, it suggests that the activation of the HSPA1A gene promoter induced by PDT-mediated oxidative stress occurs prior to the secondary ROS.
The results mentioned above indicate that the reporter gene assays using the promoter of the HSPA1A gene are able to rapidly detect the cytotoxicity of PDT in comparison to ROS and ATP detection.
Correlations between relative luciferase activity and HSPA1A protein
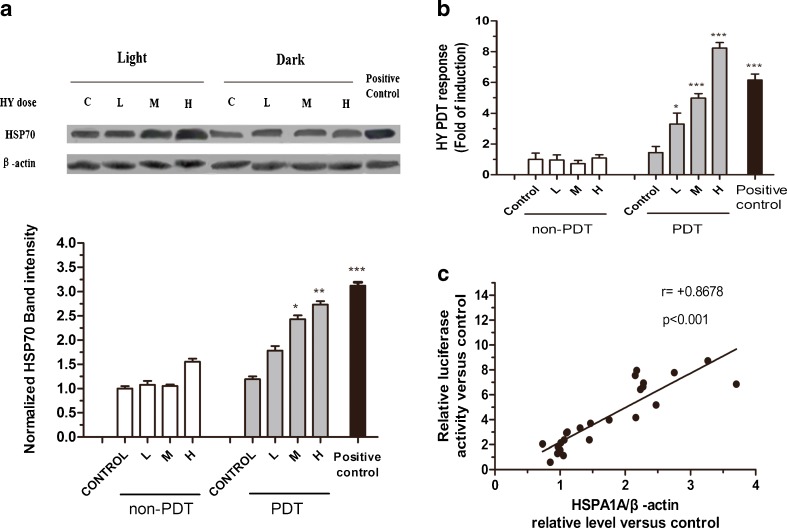
The MCF-7 cells were treated with a nonlethal dose of PDT, and the classic inducer of the heat shock response, heat shock treatment, was performed as a positive control. The nonlethal PDT induced a significant increase in the abundance of HSPA1A and relative luciferase activity at 4 h of recovery (Fig. 5a, b). Both the endogenous HSPA1A and the transfected HSPA1A promoter-driven luciferase reporter responded to Hyp-PDT with maximal protein levels of HSPA1A (nearly three times the control level, p < 0.001) and luciferase activity (greater than eight times the control level, p < 0.001). It suggests that the HSPA1A promoter activity is more sensitive than its protein expression in response to Hyp-PDT. These findings show that the newly developed HSPA1A promoter-driven luciferase reporter is superior to the method of Western blot in oxidative stress evaluation. The Spearman rank test showed that the relative luciferase activity was positively correlated with the protein level of HSPA1A (Fig. 5c).
Fig. 5.
MCF-7 luciferase cells respond to PDT mediated by various concentration Hyp with increased levels of HSPA1A and relative luciferase activity. H high dose, M middle dose, L low dose. The MCF-7 luciferase cells were treated by PDT mediated by various concentration Hyp (H, 0.125 μM; M, 0.0625 μM; L, 0.03125 μM) with a light dose of 3.87 J/cm2, and then recovered at 37 °C for the indicated times. The untreated cells were used as control. Heat shock treatment was performed as positive control. a HSPA1A levels detected by Western analysis after PDT treatment. β-actin was used as the loading control. Levels of HSPA1A and β-actin were determined by densitometric analysis. b Relative luciferase activity of MCF-7 luciferase cells treated by PDT mediated by various concentration Hyp was measured using the luciferase assay system. c Correlation between relative luciferase activity and relative level of HSPA1A was evaluated using the Spearman rank test. Data represent mean ± SD of three individual experiments
Validation of the reporter cells
Hyp, ZnPc (Vittar et al. 2010; Haywood-Small et al. 2006), and Me (Wagner et al. 2012; Song et al. 2011; Orth et al. 2000) are all well-studied photosensitizers with defined photochemical properties, for which reason we applied them to verify the sensitivity and reliability of our luciferase reporter.
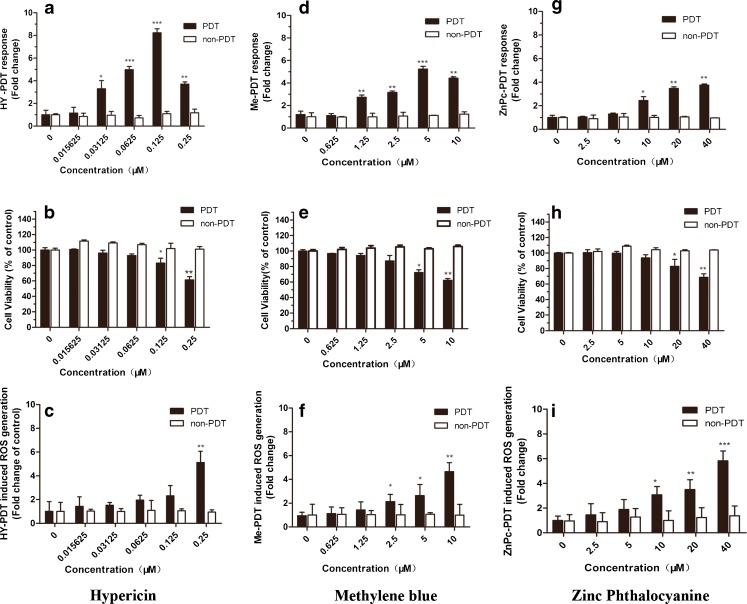
The reporter cells were treated by PDT under a specific light fluency (3.87 J/cm2) with various positive compounds concentration (Hyp, 0∼0.25 μM; Me, 0∼10 μM; and ZnPc, 0∼40 μM).
Hyp-PDT induced a dose-dependent increase in relative luciferase activity up to more than eight times the control level (p < 0.001) at 0.125 μM (Fig. 6a), where the cell viability declined to about 83 % (p < 0.05) of the control (Fig. 6b). The increases which Hyp-PDT treatment brought about in intracellular ROS levels in this tested concentration range are not statistically significant except at 0.25 μM (p < 0.01) (Fig. 6c).
Fig. 6.
MCF-7 luciferase cells respond in a dose-dependent manner to three positive photosensitive compounds. Compounds tested were Hyp, methylene blue, and ZnPc. The luciferase activity in MCF-7 luciferase cells was measured and expressed as relative luciferase activity versus control (a, d, and g). Cell viability was evaluated using the CellTiter-Glo Assay kit (b, e, and h). Oxidative damage was measured by ROS assay (c, f, and i). Data represent mean ± SD of three individual experiments
Me-PDT produced a strong dose-dependent induction of relative luciferase activity up to about five times (p < 0.001) the control level at 5 μM (Fig. 6d), while the corresponding cell viability decreased to 72 % (p < 0.05) of the control (Fig. 6e). At the same time, Me-PDT treatment resulted in slight increases in ROS levels (Fig. 6f).
ZnPc-PDT led to a 3.7 times (p < 0.01) induction of relative luciferase activity at 40 μM. It was effective at 10 μM (p < 0.05) (Fig. 6g), with a cell viability of 93 % (p > 0.05) of the control at this dose (Fig. 6h). The increases in intracellular ROS levels showed dose-dependent relationships with ZnPc concentration (Fig. 6i).
The decrease of relative luciferase activity at the highest tested concentrations of Hyp and Me may be attributable to a decrease in cell viability caused by PDT.
Discussion
In the present study, we discovered the functional promoter region of HSPA1A. We thus established a rapid and highly sensitive HSPA1A promoter-driven luciferase reporter system to assess oxidative stress associated with low-dose PDT. The reporter cells transfected with the HSPA1A promoter-driven recombinant plasmid had a dose-dependent increase in relative luciferase activity induced by a group of positive photoactive compounds mediated by PDT, suggesting their suitability as a screening tool for photosensitizers. In contrast with the previous in vitro/in vivo methods/models, the reporter system that we have established in this study is based on the inducing factor of photocytotoxicity, oxidative stress. We successfully associated the promoter activity and the protein expression level of HSPA1A with PDT-induced oxidative stress, forming a quantitative indicator to evaluate PDT-mediated oxidative stress at the molecular level, which thus indirectly reflects the efficacy of PDT. Based on this, the comprehensive photoreaction process is simplified into a relatively mature method of dual luciferase reporter gene assays. Moreover, because of the broad anticancer spectrum of PDT, we used transient transfection to form the reporter cells. When evaluating the photocytotoxicity in a specific cancer cells, we could choose the corresponding cell line to establish a preferential model. In other words, the transiently transfected model is more flexible.
Previous studies have demonstrated that HSP genes are upregulated in response to PDT, suggesting that the reporters of HSP genes can be used to evaluate the photoreaction in cells (Korbelik 2006; Kalmar and Greensmith 2009). Comer et al. first reported that photosensitizer-mediated oxidative stress can activate the heat shock factor as well as increase HSP-70 mRNA and protein levels in mouse RIF-1 cells (Gomer et al. 1996). It has previously been shown that HSPA1A was the most dominantly induced gene subtype in response to stress (Kammerer et al. 2011), suggesting that the HSPA1A promoter is extremely sensitive to cellular stress. Therefore, we believe that this method can also feasibly detect oxidative stress associated with PDT mediated by various photodynamic compounds.
It is well documented that the induction of HSP70 genes is mediated by the heat shock transcription factor (HSF) which binds to the heat shock element (HSE) (Walther and Stein 2009). It comprises multiple adjacent inverted arrays of the binding site (5′-nGAAn-3′) (Bouchier-Hayes et al. 2010), interaction of which with its cognate transcription factors (HSFs) after polymerization and nucleus-entering lead to both elevation of the transcription initiation and to abolishing pausing of the engaged RNA polymerase II in the stressed cells (+25 to +45 at downstream of the transcription initiation site) (Mason and Lis 1997).
In this article, we performed 5′-deletion analysis of the HSPA1A gene promoter to locate the functional region. The results have shown that the DNA fragment from −218 to +87 of the human HSPA1A gene contains a functional promoter responding to PDT-induced oxidative stress. Furthermore, through site-directed mutagenesis, we confirmed that both of the two HSEs played an important role in stress responsiveness. Consequently, the HSPA1A promoter fraction used to construct recombinant plasmid is thought to contain both dHSE and pHSE.
Intriguingly, in reporter cells, the endogenous HSPA1A promoter is driving the expression of the endogenous HSPA1A gene and the transfected plasmid containing the HSPA1A promoter is driving the luciferase reporter. We observed that the luciferase activity of reporter cells is more sensitive than the protein expression level in response to oxidative stress associated with PDT.
Hyp, ZnPc, and Me have been widely studied for PDT applications. They exhibit excellent photochemical properties characterized by a high quantum yield of intersystem crossing and 1O2 generation and can produce radical species in the presence of reducing agents. These photosensitizers can mediate PDT, which induces tumor tissue damage by causing oxidative stress. Our results indicated that the pGL3-218 transfected cells detected oxidative stress at low concentrations of PDT (<IC60), which did not affect cell viability as monitored by quantification of intracellular ATP. The relative luciferase activity diminished when the cells were treated with PDT mediated by the highest concentration of photosensitizers. This is qualitatively consistent with a report by Luna et al., who observed that CAT expression, under the control of an HSP70 promoter, initially increased with increasing NPe6-PDT doses and then decreased. The authors attributed the decrease in CAT expression to the PDT cytotoxic response (Luna et al. 2000).
The lowest effective concentrations of Hyp (0.03125 μM), ZnPc (10 μM), and Me (1.25 μM) which increased ROS levels and decreased intracellular ATP (Table 1) slightly, also increased relative luciferase activity. This suggests that relative luciferase activity is a sensitive indicator of oxidative stress associated with PDT mediated by these compounds. These findings validate the relative luciferase activity of reporter cells as a sensitive and responsive indicator of oxidative stress induced by photosensitizers.
Table 1.
The lowest effective concentrations of positive compounds detected by HSPA1A promoter-driven luciferase reporter gene assay and other in vitro toxicity tests
| Tested positive compounds | Luciferase reporter gene assay | Cell viability assay | ROS level |
|---|---|---|---|
| Hypericin(μM) | 0.03125 | 0.125 | 0.25 |
| Methylene Blue(μM) | 1.25 | 5 | 2.5 |
| Zinc Phthalocyanine(μM) | 10 | 20 | 10 |
In conclusion, our results have demonstrated that using the luciferase assay linked to the functional HSPA1A promoter region could perform the rapid and highly sensitive detection of oxidative stress mediated by PDT. We have also successfully applied the method in two other cancer cells (HepG2 and Hela) for detecting oxidative stress (data not shown). We believe that our method can feasibly evaluate the photocytotoxicity of various photosensitizers which act through causing oxidative stress in cancer cells. Compared with other in vitro toxicity tests examined in the present study, the bioassays have higher sensitivity, reproducibility, and lower costs, and facilitate rapid screening of leading compounds of photosensitizers.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (NSFC project number: 81173224), the Shanghai Municipal Committee of Science and Technology(No. 11ZR1437000), and the Scholar of Longhua Hospital (D-11).
Abbreviations
- HSP
Heat shock protein
- PDT
Photodynamic therapy
- ROS
Reactive oxygen species
- DMSO
Dimethyl sulfoxide
- Hyp
Hypericin
- Me
Methylene blue
- ZnPc
Zinc phthalocyanine
- μM
Micromoles per liter
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
Contributor Information
Jianhui Tian, Phone: +86-21-64252044, FAX: +86-21-64252044, Email: hawk7150@hotmail.com.
Jianwen Liu, Phone: +86-21-64252044, FAX: +86-21-64252044, Email: liujian@ecust.edu.cn.
References
- Agostinis P, Vantieghem A, Merlevede W, de Witte PA. Hypericin in cancer treatment: more light on the way. Int J Biochem Cell Biol. 2002;34(3):221–241. doi: 10.1016/S1357-2725(01)00126-1. [DOI] [PubMed] [Google Scholar]
- Ait-Aissa S, Pandard P, Magaud H, Arrigo AP, Thybaud E, Porcher JM. Evaluation of an in vitro HSP70 induction test for toxicity assessment of complex mixtures: comparison with chemical analyses and ecotoxicity tests. Ecotoxicol Environ Saf. 2003;54(1):92–104. doi: 10.1016/S0147-6513(02)00026-X. [DOI] [PubMed] [Google Scholar]
- Bouchier-Hayes L, McBride S, van Geelen CM, Nance S, Lewis LK, Pinkoski MJ, Beere HM. Fas ligand gene expression is directly regulated by stress-inducible heat shock transcription factor-1. Cell Death Differ. 2010;17(6):1034–1046. doi: 10.1038/cdd.2010.4. [DOI] [PubMed] [Google Scholar]
- Brocchieri L, Conway de Macario E, Macario AJ. HSP70 genes in the human genome: conservation and differentiation patterns predict a wide array of overlapping and specialized functions. BMC Evol Biol. 2008;8:19. doi: 10.1186/1471-2148-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brondani Da Rocha A, Regner A, Grivicich I, Pretto Schunemann D, Diel C, Kovaleski G, Brunetto De Farias C, Mondadori E, Almeida L, Braga Filho A, Schwartsmann G. Radioresistance is associated to increased Hsp70 content in human glioblastoma cell lines. Int J Oncol. 2004;25(3):777–785. [PubMed] [Google Scholar]
- Chakraborty A, Held KD, Prise KM, Liber HL, Redmond RW. Bystander effects induced by diffusing mediators after photodynamic stress. Radiat Res. 2009;172(1):74–81. doi: 10.1667/RR1669.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Guerasimova A, Manke T, Rosenstiel P, Haas S, Warnatz HJ, Querfurth R, Nietfeld W, Vanhecke D, Lehrach H, Yaspo ML, Janitz M. Screening of human gene promoter activities using transfected-cell arrays. Gene. 2010;450(1–2):48–54. doi: 10.1016/j.gene.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Demirovic D, Rattan SI (2012) Establishing cellular stress response profiles as biomarkers of homeodynamics, health and hormesis. Exp Gerontol [DOI] [PubMed]
- Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3(5):380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- Garrido C, Ottavi P, Fromentin A, Hammann A, Arrigo AP, Chauffert B, Mehlen P. HSP27 as a mediator of confluence-dependent resistance to cell death induced by anticancer drugs. Cancer Res. 1997;57(13):2661–2667. [PubMed] [Google Scholar]
- Gehrmann M, Radons J, Molls M, Multhoff G. The therapeutic implications of clinically applied modifiers of heat shock protein 70 (Hsp70) expression by tumor cells. Cell Stress Chaperones. 2008;13(1):1–10. doi: 10.1007/s12192-007-0006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomer CJ, Ryter SW, Ferrario A, Rucker N, Wong S, Fisher AM. Photodynamic therapy-mediated oxidative stress can induce expression of heat shock proteins. Cancer Res. 1996;56(10):2355–2360. [PubMed] [Google Scholar]
- Guo H, Deng Q, Wu C, Hu L, Wei S, Xu P, Kuang D, Liu L, Hu Z, Miao X, Shen H, Lin D, Wu T. Variations in HSPA1B at 6p21.3 are associated with lung cancer risk and prognosis in Chinese populations. Cancer Res. 2011;71(24):7576–7586. doi: 10.1158/0008-5472.CAN-11-1409. [DOI] [PubMed] [Google Scholar]
- Haywood-Small SL, Vernon DI, Griffiths J, Schofield J, Brown SB. Phthalocyanine-mediated photodynamic therapy induces cell death and a G0/G1 cell cycle arrest in cervical cancer cells. Biochem Biophys Res Commun. 2006;339(2):569–576. doi: 10.1016/j.bbrc.2005.11.046. [DOI] [PubMed] [Google Scholar]
- Kalmar B, Greensmith L. Induction of heat shock proteins for protection against oxidative stress. Adv Drug Deliv Rev. 2009;61(4):310–318. doi: 10.1016/j.addr.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Kammerer R, Buchner A, Palluch P, Pongratz T, Oboukhovskij K, Beyer W, Johansson A, Stepp H, Baumgartner R, Zimmermann W. Induction of immune mediators in glioma and prostate cancer cells by non-lethal photodynamic therapy. PLoS One. 2011;6(6):e21834. doi: 10.1371/journal.pone.0021834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14(1):105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karioti A, Bilia AR. Hypericins as potential leads for new therapeutics. Int J Mol Sci. 2010;11(2):562–594. doi: 10.3390/ijms11020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel D, Luo Y, Deng Y, Chang CK. The role of subcellular localization in initiation of apoptosis by photodynamic therapy. Photochem Photobiol. 1997;65(3):422–426. doi: 10.1111/j.1751-1097.1997.tb08581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbelik M. PDT-associated host response and its role in the therapy outcome. Lasers Surg Med. 2006;38(5):500–508. doi: 10.1002/lsm.20337. [DOI] [PubMed] [Google Scholar]
- Korbelik M, Sun J, Cecic I. Photodynamic therapy-induced cell surface expression and release of heat shock proteins: relevance for tumor response. Cancer Res. 2005;65(3):1018–1026. [PubMed] [Google Scholar]
- Li B, Chu X, Gao M, Li W. Apoptotic mechanism of MCF-7 breast cells in vivo and in vitro induced by photodynamic therapy with C-phycocyanin. Acta Biochim Biophys Sin (Shanghai) 2010;42(1):80–89. doi: 10.1093/abbs/gmp104. [DOI] [PubMed] [Google Scholar]
- Luna MC, Ferrario A, Wong S, Fisher AM, Gomer CJ. Photodynamic therapy-mediated oxidative stress as a molecular switch for the temporal expression of genes ligated to the human heat shock promoter. Cancer Res. 2000;60(6):1637–1644. [PubMed] [Google Scholar]
- Mason PB, Jr, Lis JT. Cooperative and competitive protein interactions at the hsp70 promoter. J Biol Chem. 1997;272(52):33227–33233. doi: 10.1074/jbc.272.52.33227. [DOI] [PubMed] [Google Scholar]
- Mitra S, Goren EM, Frelinger JG, Foster TH. Activation of heat shock protein 70 promoter with meso-tetrahydroxyphenyl chlorin photodynamic therapy reported by green fluorescent protein in vitro and in vivo. Photochem Photobiol. 2003;78(6):615–622. doi: 10.1562/0031-8655(2003)078<0615:AOHSPP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Mosser DD, Caron AW, Bourget L, Meriin AB, Sherman MY, Morimoto RI, Massie B. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol. 2000;20(19):7146–7159. doi: 10.1128/MCB.20.19.7146-7159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollen EA, Morimoto RI. Chaperoning signaling pathways: molecular chaperones as stress-sensing ‘heat shock’ proteins. J Cell Sci. 2002;115(Pt 14):2809–2816. doi: 10.1242/jcs.115.14.2809. [DOI] [PubMed] [Google Scholar]
- Orth K, Beck G, Genze F, Ruck A. Methylene blue mediated photodynamic therapy in experimental colorectal tumors in mice. J Photochem Photobiol B. 2000;57(2–3):186–192. doi: 10.1016/S1011-1344(00)00105-6. [DOI] [PubMed] [Google Scholar]
- Ouedraogo GD, Redmond RW. Secondary reactive oxygen species extend the range of photosensitization effects in cells: DNA damage produced via initial membrane photosensitization. Photochem Photobiol. 2003;77(2):192–203. doi: 10.1562/0031-8655(2003)0770192SROSET2.0.CO2. [DOI] [PubMed] [Google Scholar]
- Pohlman TH, Harlan JM. Adaptive responses of the endothelium to stress. J Surg Res. 2000;89(1):85–119. doi: 10.1006/jsre.1999.5801. [DOI] [PubMed] [Google Scholar]
- Radak Z, Chung HY, Goto S. Exercise and hormesis: oxidative stress-related adaptation for successful aging. Biogerontology. 2005;6(1):71–75. doi: 10.1007/s10522-004-7386-7. [DOI] [PubMed] [Google Scholar]
- Rubio N, Rajadurai A, Held KD, Prise KM, Liber HL, Redmond RW. Real-time imaging of novel spatial and temporal responses to photodynamic stress. Free Radic Biol Med. 2009;47(3):283–290. doi: 10.1016/j.freeradbiomed.2009.04.024. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal. 2007;9(1):49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- Schmitt E, Gehrmann M, Brunet M, Multhoff G, Garrido C. Intracellular and extracellular functions of heat shock proteins: repercussions in cancer therapy. J Leukoc Biol. 2007;81(1):15–27. doi: 10.1189/jlb.0306167. [DOI] [PubMed] [Google Scholar]
- Song D, Lindoso JA, Oyafuso LK, Kanashiro EH, Cardoso JL, Uchoa AF, Tardivo JP, Baptista MS. Photodynamic therapy using methylene blue to treat cutaneous leishmaniasis. Photomed Laser Surg. 2011;29(10):711–715. doi: 10.1089/pho.2010.2915. [DOI] [PubMed] [Google Scholar]
- Tsai T, Hong RL, Tsai JC, Lou PJ, Ling IF, Chen CT. Effect of 5-aminolevulinic acid-mediated photodynamic therapy on MCF-7 and MCF-7/ADR cells. Lasers Surg Med. 2004;34(1):62–72. doi: 10.1002/lsm.10246. [DOI] [PubMed] [Google Scholar]
- Vargas-Roig LM, Gago FE, Tello O, Aznar JC, Ciocca DR. Heat shock protein expression and drug resistance in breast cancer patients treated with induction chemotherapy. Int J Cancer. 1998;79(5):468–475. doi: 10.1002/(SICI)1097-0215(19981023)79:5<468::AID-IJC4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Vittar NB, Awruch J, Azizuddin K, Rivarola V. Caspase-independent apoptosis, in human MCF-7c3 breast cancer cells, following photodynamic therapy, with a novel water-soluble phthalocyanine. Int J Biochem Cell Biol. 2010;42(7):1123–1131. doi: 10.1016/j.biocel.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Wagner M, Suarez ER, Theodoro TR, Machado Filho CD, Gama MF, Tardivo JP, Paschoal FM, Pinhal MA. Methylene blue photodynamic therapy in malignant melanoma decreases expression of proliferating cell nuclear antigen and heparanases. Clin Exp Dermatol. 2012;37(5):527–533. doi: 10.1111/j.1365-2230.2011.04291.x. [DOI] [PubMed] [Google Scholar]
- Walther W, Stein U. Heat-responsive gene expression for gene therapy. Adv Drug Deliv Rev. 2009;61(7–8):641–649. doi: 10.1016/j.addr.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Xin L, Li X, Deng H, Kuang D, Dai X, Huang S, Wang F, He M, Currie RW, Wu T. Development of stable HSPA1A promoter-driven luciferase reporter HepG2 cells for assessing the toxicity of organic pollutants present in air. Cell Stress Chaperones. 2012;17(5):567–576. doi: 10.1007/s12192-012-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue LY, Chiu SM, Oleinick NL. Photodynamic therapy-induced death of MCF-7 human breast cancer cells: a role for caspase-3 in the late steps of apoptosis but not for the critical lethal event. Exp Cell Res. 2001;263(1):145–155. doi: 10.1006/excr.2000.5108. [DOI] [PubMed] [Google Scholar]