Abstract
Heat shock proteins (hsps) have been studied in numerous cancer types, but a clear view of their clinical relevance in melanoma remains elusive. Therefore, the aim of this study was to investigate the expression of hsps in melanoma with respect to patient clinical parameters. Using Western immunoblotting, hsps 90, 70, 60, 40 and 32 were observed to be widely expressed in metastatic melanomas (n = 31), while immunofluorescence demonstrated that in the majority of samples these hsps, apart from hsp32, were increased in expression in melanoma cells compared with surrounding non-melanoma cells in situ (n = 8). Correlating hsp expression with patient clinical parameters indicated that greater hsp90 (P < 0.02) and hsp40 (P < 0.03) expression correlated with advanced stage (stage III Vs stage IV), while in the case of hsp40, this was additionally associated with reduced patient survival (P < 0.05). In contrast, higher hsp32 expression was associated with improved patient survival (P < 0.007). On the other hand, the expression of the other hsps did not correlate with any obtainable patient clinical parameters. This study provides further evidence for the importance of hsps in melanoma and for their use as therapeutic targets and biomarkers, but larger-scale follow-up studies are required to confirm these results.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-012-0363-1) contains supplementary material, which is available to authorized users.
Keywords: Metastatic melanoma, Heat shock proteins, Hsps, Patient clinical parameters, Western immunoblotting, Immunofluorescence
Introduction
Hsps play fundamental roles in tumourigenesis and contribute to the biology of cancer through multiple diverse mechanisms (Calderwood et al. 2006). Hsps are abnormally expressed in a range of cancer types, and their expression has been shown to predict patient prognosis and therapeutic response (Calderwood et al. 2006; Pick et al. 2007; Ciocca et al. 1993). The importance of hsps in human cancers is well established, and hsp inhibitors are currently under clinical evaluation for the treatment of a variety of cancer types (Clinical Trials for HSP 2012). Hsp expression has been thoroughly characterised for a number of cancer types (Ciocca and Calderwood 2005), although reports are lacking, limited or have provided conflicting data for other types of cancer (Pick et al. 2007; McCarthy et al. 2008). Inferring the role of hsps based on previous studies is thus problematic as their role and clinical relevance varies depending on the cancer type (Ciocca and Calderwood 2005). The significance of hsp expression with respect to cancer is undefined in many contexts and remains enigmatic to date.
It may well be that differences in the clinical relevance of abnormal hsp expression are products of the diverse roles that hsps perform. Hsps were originally thought to provide protection from tumour-associated stressors and to chaperone oncoproteins responsible for tumour cell proliferation, but they are now recognised to contribute to many of the characteristic features of cancer including sustained angiogenesis and apoptosis evasion (Calderwood et al. 2006). These well-recognised roles are shadowed by a second, less well-characterised role of hsps as immunomodulators. Modifying hsp expression results in biologically relevant alteration of the immunogenicity of cells (Melcher et al. 1999; Wells et al. 1997, 1998). Furthermore, cell surface hsps have been shown to act as targets for NK cells and T cells, and hsps are involved in antigen presentation whereby they can chaperone tumour antigens to APCs (Noessner et al. 2002; Multhoff et al. 1997; Harada et al. 1998). Moreover, the interplay between these two diverse roles may vary across cancer types or within tumours of the same type.
Melanoma is one such cancer type for which conflicting reports on the role of hsps exist. Melanoma is an invasive cancer with poor survival rates once metastasis occurs (Balch et al. 2001). The incidence of melanoma is increasing in many countries, and consequently, it constitutes a significant burden on human health (WHO 2008; Lucas et al. 2006; Balch et al. 2001). The validation of hsps as therapeutic targets is of particular importance because hsp inhibitors are currently being evaluated in the treatment of melanoma patients (STA-9090 in Metastatic Ocular Melanoma 2011). Studies to date have demonstrated that hsps play important roles in melanoma, but few clear relationships have been defined and conflicting data have been reported (McCarthy et al. 2008; Missotten et al. 2003; Faingold et al. 2008; Ricaniadis et al. 2001; Lazaris et al. 1995). This present study reports a preliminary assessment of hsp expression in metastatic melanoma tumour tissue with respect to patient clinical parameters.
Materials and methods
Sample acquisition and study population
Thirty-one fresh frozen tissue samples derived from metastases from 30 melanoma patients for whom clinical data were available (22 males and 8 females) were analysed by Western immunoblotting (of the two metastases from the same patient, only one was used in the evaluation of patient clinical parameters). Eight of 30 patients were stage III with the remainder stage IV. Mean patient age was 59 years, ranging from 36 to 83. Survival since analysis (survival time since removal of the metastasis that was examined in this study) and survival since diagnosis (survival time since the date of initial diagnosis) were available for all 30 patients (mean survival since analysis was 15.0 months, range 3.0–60.5 months; mean survival since diagnosis was 59.1 months, range 4.5–303 months). Twelve of 30 patients (40 %) died during the reporting period. Additionally, formalin-fixed paraffin-embedded skin metastases were sought from eight stage III melanoma patients and were used in immunofluorescence experiments. All patients were treated at the University Medical Center Tübingen and gave their written informed consent for storage and scientific analysis of tissue samples. This study was approved by the University of Tübingen Ethics Committee (approval number 140/2012BO2).
Extraction and isolation of cellular proteins from melanoma tissue
Melanoma samples (stored at −70 °C) were partially thawed and cut into small pieces. Cellular proteins were extracted from samples in a buffer containing 9 M urea (Merck, Darmstadt, Germany), 4 % (w/v) CHAPS detergent and 12.2 mM PMSF protease inhibitor (both from Sigma-Aldrich, Munich, Germany). The tissues were homogenised with a bladed electric tissue homogeniser until a solution of smooth consistency was obtained. This solution was frozen at −70 °C overnight. The following day the solutions were thawed and sonicated before being re-frozen at −70 °C. Solutions were subsequently thawed, centrifuged and the protein layer removed. Protein fractions were centrifuged until the extract was free of insoluble contaminants. All steps were performed on ice or at 4 °C.
Melanoma tissue protein extract quantification
Roti nanoquant (Carl Roth, Karlsruhe, Germany) was used according to manufacturer’s instructions to estimate the protein concentration of melanoma tissue extracts. Samples were tested in triplicate.
Western immunoblotting of melanoma tissue
Protein extracts were denatured with 5 μL of 1.66× sample buffer (5× stock consisted of 50 % glycerol, 25 % 2-mercaptoethanol (Merck), 20 % 1.5 M Tris–HCl pH 6.8 (Tris was sourced from Sigma-Aldrich and HCl from Merck), 1 mL bromophenol blue (Sigma-Aldrich) solution, all v/v and 10 % (w/v) sodium dodecyl sulphate (SDS; Serva, Heidelberg, Germany)) and heated at 95 °C for 5 min and immediately chilled to minimise residual protease activity. Based on the protein content of each sample, an adjusted volume of each was loaded and subsequently separated on 10 % acrylamide SDS-PAGE gels (5 mL ddH2O, 2.5 mL acrylamide stock solution (37.5 % (w/v) acrylamide and 2.5 % (w/v) bis-acrylamide) (Bio-Rad, Munich, Germany), 2.5 mL Tris–HCl 1.5 M pH 8.8, 100 μL 10 % SDS, 75 μL of 10 % ammonium persulphate solution (Merck), all w/v and 7.5 μL TEMED (Sigma-Aldrich)) by applying 200 V with migration buffer (3.0 g Tris, 14.4 g glycine (Carl Roth), 1.0 g SDS, per litre) until the tracking dye had migrated to the bottom of the gel. Proteins were transferred to nitrocellulose membranes (Amersham GE Life Sciences, Munich, Germany) using a wet transfer method with 110 V for 1 h in a buffer containing 3.0 g Tris, 14.4 g glycine and 20 % (v/v) methanol (AnalaR NORMAPUR (VWR International), Dublin, Ireland) per litre (freshly prepared and chilled). Following this, membranes were stained with Ponceau Red to ensure the proteins were effectively transferred to membrane. Membranes were then blocked with 5 % (w/v) skim milk powder (diluted in Tris-buffered saline-Tween (TBS-Tween) (2.42 g Tris, 8.0 g NaCl (Merck) and 1 mL Tween 20 (Bio-Rad) per litre, pH 7.6) for 90 min at room temperature before being rinsed in TBS-Tween. Primary antibody was diluted in TBS-Tween and incubated with membrane overnight at 4 °C. Membranes were rinsed and washed twice for 5 min with TBS-Tween. Appropriate horseradish peroxidase (HRP)-conjugated secondary antibody was incubated with the membrane for 1 h at room temperature. Following this, membranes were rinsed and washed twice for 5 min with TBS-Tween. Proteins were visualised by applying substrate for 1 min (0.022 % (w/v) luminol, 225 μM coumaric acid, 0.03 % (v/v) of 30 % hydrogen peroxide solution in 0.1 M Tris–HCl, pH 8.5) before reactive zones were recorded using Hyperfilm ECL (Amersham GE Life Sciences) (visualisation performed in a dark room). Incubation and washing steps were performed with gentle rocking. All expression levels were normalised with respect to the level of the β-actin house keeping protein. All experimentation and data analysis were performed in a blinded fashion.
Immunofluorescence
Formalin-fixed paraffin-embedded melanoma tissue sections were pre-chilled and 5-μm-thick slices cut and mounted on Silane-Prep slides (Sigma-Aldrich). Tissue sections were adhered to slides by incubation on a heating element at 37 °C overnight. For immunostaining, tissue sections were de-paraffinised and rehydrated by overnight incubation at 60 °C followed by xylene (AnalaR NORMAPUR) treatment for 15 min. Tissue sections were subsequently rehydrated in three ethanol solutions (100, 96 and 70 % ethanol) (SAV Liquid Production, Flintsbach a. Inn, Germany) for 5 min each and then rinsed and incubated for 30 min in ddH2O. Following deparaffinisation and rehydration, antigens were retrieved with a 2-min incubation in 10 mM citrate buffer (1.8 % (v/v) 0.1 M citric acid, 8.2 % (v/v) 0.1 M sodium citrate, pH 6) (both from Merck) in a pressurised cooking device. Tissue sections were gradually cooled, washed for 3 min with washing buffer (8.0 g NaCl, 0.2 g KCl, 1.44 g Na2HPO4, 0.24 g KH2PO4 (all from Merck) 0.25 mL Tween 20 and 0.5 mL 10 % BSA solution (Serva) per litre) and blocked in 5 % (v/v) donkey serum (Sigma-Aldrich) (diluted in washing buffer) for 30 min. Primary antibody (diluted in washing buffer) was applied to stained sections and incubated for 60 min in a humidifying chamber. Washing buffer was applied to unstained sections during primary antibody incubation to prevent dehydration. Following primary antibody incubation, slides were rinsed and washed twice for 3 min before being incubated with secondary antibody (diluted in washing buffer) for 60 min in a dark humidifying chamber. Slides were rinsed and washed twice in washing buffer before being incubated with the 4′,6-diamidino-2-phenylindole (DAPI) (Roche, Mannheim, Germany) nuclear stain (diluted 1:2,000 in washing buffer) for 15 min in a dark humidifying chamber. Slides were then rinsed and washed twice for 3 min before the addition of fluorescence mounting medium (Dako, Hamburg, Germany), topped with glass coverslip (0.08–0.12 mm). Slides were kept in the dark at 4 °C and visualised the following day. Stained tissue samples were analysed on the Carl Zeiss Axio Observer.Z1 confocal fluorescent microscope using Axiovision software. Exposure times for each fluorescence channel were adjusted using a tissue section stained with secondary antibodies in order to set an appropriate level of background fluorescence. This was performed for each tissue section. In order to quantify the fluorescence staining observed by eye, a small number of representative MelanA-positive and MelanA-negative cells were analysed using Axiovision software in a minimum of five locations in each tissue section. Secondary antibodies did not react non-specifically. All primary antibodies used in immunofluorescence experiments have been demonstrated by the manufacturer to be suitable for use with formalin-fixed paraffin-embedded tissue sections.
Antibodies used
Primary antibodies
Hsp90α/β goat polyclonal antiserum N-17, hsp70 goat polyclonal K-20, hsp60 goat polyclonal N-20 (all from Santa Cruz Biotechnology, Heidelberg, Germany), hsp40 rabbit polyclonal RB1770 (Abgent, San Diego, CA, USA), hsp32 rabbit polyclonal (Enzo Life Sciences, Lörrach, Germany), β-actin rabbit monoclonal (Cell Signaling Technology, Danvers, MA, USA) and MelanA mouse monoclonal clone A103 (Dako) were used as primary antibodies.
Secondary antibodies
DyLight488-conjugated donkey anti-goat IgG, DyLight488-conjugated donkey anti-rabbit IgG, Cy3-conjugated donkey anti-mouse IgG (all from Jackson ImmunoResearch Laboratories, West Grove, PA, USA), HRP-conjugated polyclonal goat anti-mouse, HRP-conjugated polyclonal rabbit anti-goat, HRP-conjugated polyclonal swine anti-rabbit (all from Dako) were used as secondary antibodies.
Statistical analysis
Age and time to first metastasis were dichotomised based on the median value of the study population to allow statistical evaluation. Trends across four grouping variables were assessed with two-tailed chi-squared contingency tests.
Differences between two groups were assessed with two-tailed Mann–Whitney t tests.
Survival probabilities (survival since analysis (survival time since removal of the metastasis that was examined in this study) and survival since diagnosis (survival time since the date of initial diagnosis)) were calculated by the Kaplan–Meier method. Differences were analysed by the log-rank test. The observation time was calculated from the date of the initial diagnosis or date of surgery of the analysed metastases for the survival since diagnosis or survival since analysis, respectively, and the date of last follow-up or death. Only deaths due to progressive melanoma were considered as events, whereas others were censored.
All statistics were performed with Graphad Prism software (GraphPad, San Diego, USA).
Results
Western immunoblotting of melanoma tissue
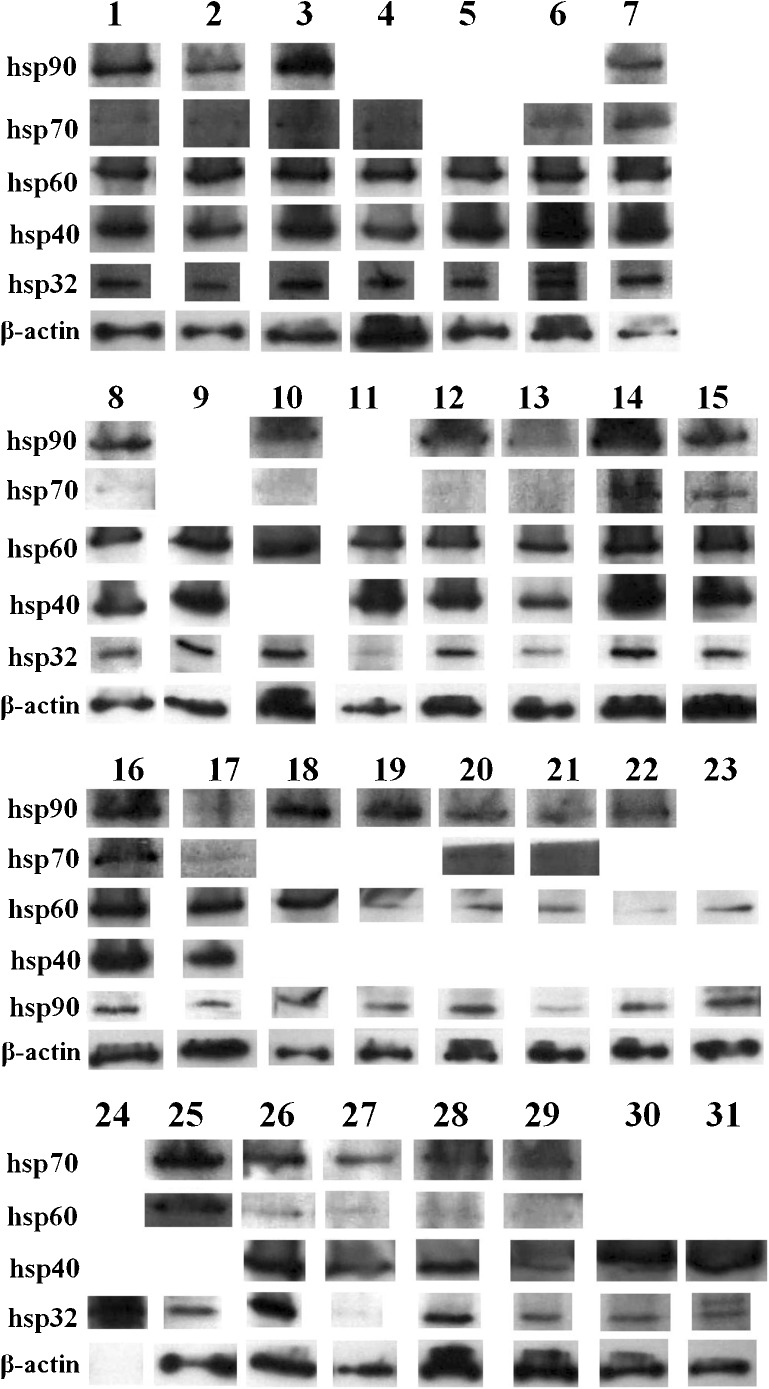
The expressions of hsp90 (HSPC), hsp70 (HSPA), hsp60 (HSPD), hsp40 (DNAJ) and hsp32 (a HSPB) were investigated with Western immunoblotting in 31 tumour samples. The vast majority of patient samples were observed to express all hsps examined (Fig. 1). Samples 9 and 10 are lymph node and ureter metastases from the same patient, respectively, and it was noteworthy that these tumours were observed to express similar levels of both hsps examined (note that only one of these metastases was used for subsequent correlation with patient clinical parameters).
Fig. 1.
Heat shock protein expression in metastatic melanoma tissue. Expression levels of hsps 90, 70, 60, 40 and 32 were assessed in 31 stage III/IV metastases using Western immunoblotting. Numbers indicate patient sample number. Samples that could not be clearly visualised on the Western immublot film were excluded
Among the tumours, a number of samples showed relative higher or lower hsp expression (Table 1). These differences in expression were investigated for association with a range of patient clinical parameters (Tables 2 and 3). High hsp90 (P < 0.02, Table 2) and hsp40 (P < 0.03, Table 3) expression correlated with advanced tumour stage (stage III to stage IV). In contrast, the expression of the other hsps was not associated with patient clinical parameters (Online resource 1).
Table 1.
Melanoma tumour categorisation according to hsp expression
| hsp90 (n = 22) | hsp70 (n = 21) | hsp60 (n = 28) | hsp40 (n = 16) | hsp32 (n = 30) | |
|---|---|---|---|---|---|
| High expression | 12 | 4 | 22 | 9 | 22 |
| Low expression | 10 | 17 | 6 | 7 | 8 |
Western immunoblots of melanoma tumours probed with hsp antibodies were categorised according to their relative level of hsp expression
Table 2.
Association between hsp90 expression and patient clinical parameters
| Clinical parameter | Patients with low hsp90 tumours | Patients with high hsp90 tumours | P value | |
|---|---|---|---|---|
| Sex (n = 22) | Male | 9 | 9 | >0.35 |
| Female | 1 | 3 | ||
| Ulceration (n = 11) | Yes | 2 | 4 | >0.12 |
| No | 4 | 1 | ||
| Breslow tumour thickness (n = 13) | 0–2 mm | 4 | 3 | >0.35 |
| >2–4 mm | 2 | 0 | ||
| >4 mm | 1 | 3 | ||
| Stage (n = 22) | III | 4 | 0 | <0.02 |
| IV | 6 | 12 | ||
| Clark Level (n = 9) | III | 0 | 1 | >0.15 |
| IV | 4 | 3 | ||
| V | 1 | 0 | ||
| Metastasis location (n = 22) | Lymph node | 5 | 4 | >0.40 |
| Other | 5 | 8 | ||
| Metastasis location (n = 22) | Skin | 3 | 7 | >0.15 |
| Other | 7 | 5 | ||
| Histological sub-type (n = 12) | SSM | 5 | 2 | >0.25 |
| Other | 2 | 3 | ||
| Time to first metastasis (n = 12) | ≤22 months | 4 | 2 | >0.20 |
| >22 months | 2 | 4 | ||
| Age (n = 22) | ≤54 | 8 | 6 | >0.12 |
| >54 | 2 | 6 | ||
Table 3.
Association between hsp40 expression and patient clinical parameters
| Clinical parameter | Patients with low hsp40 tumours | Patients with high hsp40 tumours | P value | |
|---|---|---|---|---|
| Sex (n = 16) | Male | 6 | 8 | >0.80 |
| Female | 1 | 1 | ||
| Ulceration (n = 9) | Yes | 0 | 3 | >0.12 |
| No | 3 | 3 | ||
| Breslow tumour thickness (n = 10) | 0–2 mm | 3 | 2 | >0.10 |
| >2–4 mm | 1 | 1 | ||
| >4 mm | 0 | 3 | ||
| Stage (n = 16) | III | 3 | 0 | <0.03 |
| IV | 4 | 9 | ||
| Clark level (n = 7) | III | 1 | 0 | >0.20 |
| IV | 2 | 4 | ||
| Metastasis location (n = 16) | Lymph node | 2 | 2 | >0.75 |
| Other | 5 | 7 | ||
| Metastasis location (n = 16) | Skin | 4 | 5 | >0.90 |
| Other | 3 | 4 | ||
| Histological sub-type (n = 9) | SSM | 1 | 3 | >0.60 |
| Other | 2 | 3 | ||
| Time to first metastasis (n = 11) | ≤22 months | 3 | 3 | >0.70 |
| >22 months | 2 | 3 | ||
| Age (n = 16) | ≤54 | 5 | 6 | >0.80 |
| >54 | 2 | 3 | ||
Higher hsp90 and hsp40 expressions are associated with advanced melanoma tumour stage. A range of patient clinical parameters were investigated for relationships with hsp expression. Data for hsps 70, 60 and 32 appear in Online resource 1
Melanoma patient survival analysis
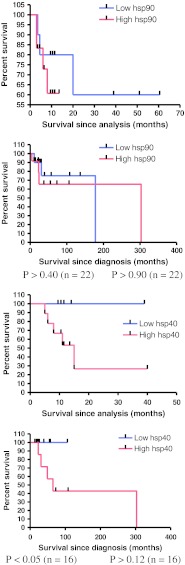
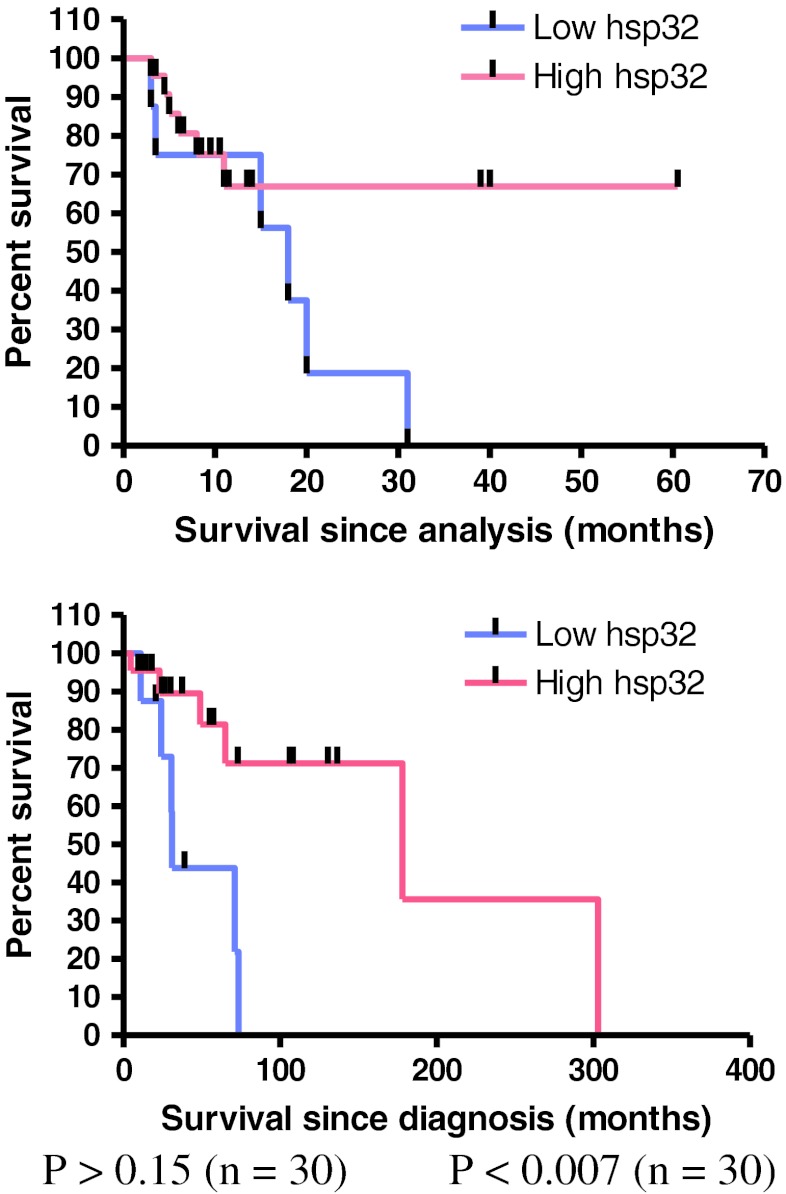
Survival analysis according to Kaplan–Meier was performed for the following endpoints: survival since analysis (survival time since removal of the metastasis that was examined in this study) and survival since diagnosis (survival time since the date of initial diagnosis). This analysis suggested that higher hsp40 expression correlated with reduced patient survival since analysis (log-rank P < 0.05, Fig. 2), while hsp32 correlated with reduced survival since diagnosis (log-rank P < 0.007, Fig. 2). In contrast, the expression of the other hsps was not associated with patient survival (Supplemantary data 2).
Fig. 2.
Hsp expression and patient survival. Hsp40 but not hsp90 was associated with reduced patient survival since analysis, while hsp32 was associated with increased patient survival since diagnosis. Survival probabilities were calculated by the Kaplan–Meier method. Data for the other hsps examined appear in Online resource 2
Fluorescence microscopy of melanoma tissue
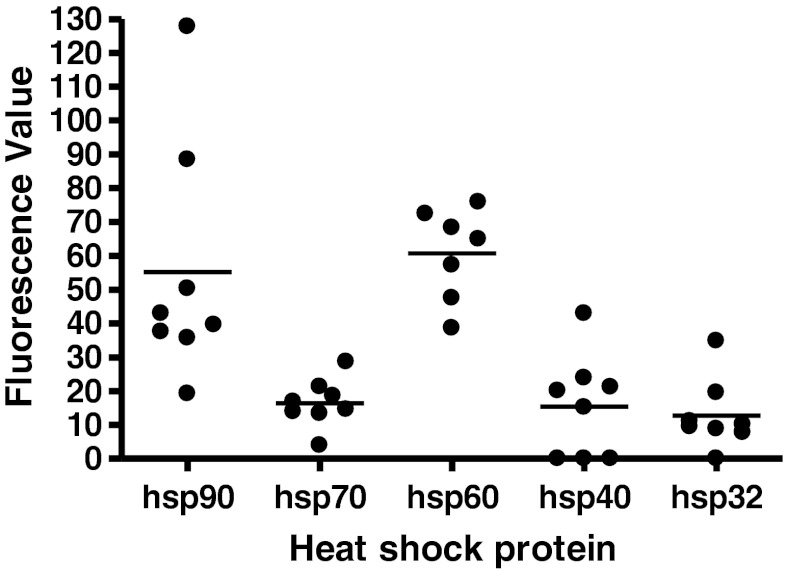
Fluorescence microscopy was used to assess hsp expression in melanoma tissue samples in order to visualise the distribution of hsp expression among melanoma cells within tumour tissue. Tissue sections from locoregional skin metastases of eight patients were analysed for the expression of hsp90, hsp70, hsp60 (n = 7), hsp40, hsp32 and the diagnostic melanoma marker, MelanA (Busam and Jungbluth 1999). In all patient tumours, positive staining for hsp90, hsp70 and hsp60 was observed, while three patients were negative for hsp40 and one negative for hsp32. Of these hsps, hsp90 and hsp60 were observed to be the most highly expressed, while hsp70, hsp40 and hsp32 were expressed at relatively lower levels. Among positive samples, the majority of melanoma cells were observed to express hsps. In order to quantify these observations, average fluorescence values were obtained and compared (Fig. 3). It was noteworthy that hsp40 was expressed in the cell nucleus, whereas the other hsps showed either weak or no nuclear staining.
Fig. 3.
Heat shock protein expression level in melanoma tumour tissue. Formalin-fixed paraffin-embedded melanoma tissue sections were stained for hsp expression using fluorescent antibodies and analysed using fluorescence microscopy. Differences in protein expression were assessed with appropriate software quantification by obtaining the mean fluorescence value of a small number of representative cells in a minimum of five regions within the tissue section. Bars indicate mean value for each group
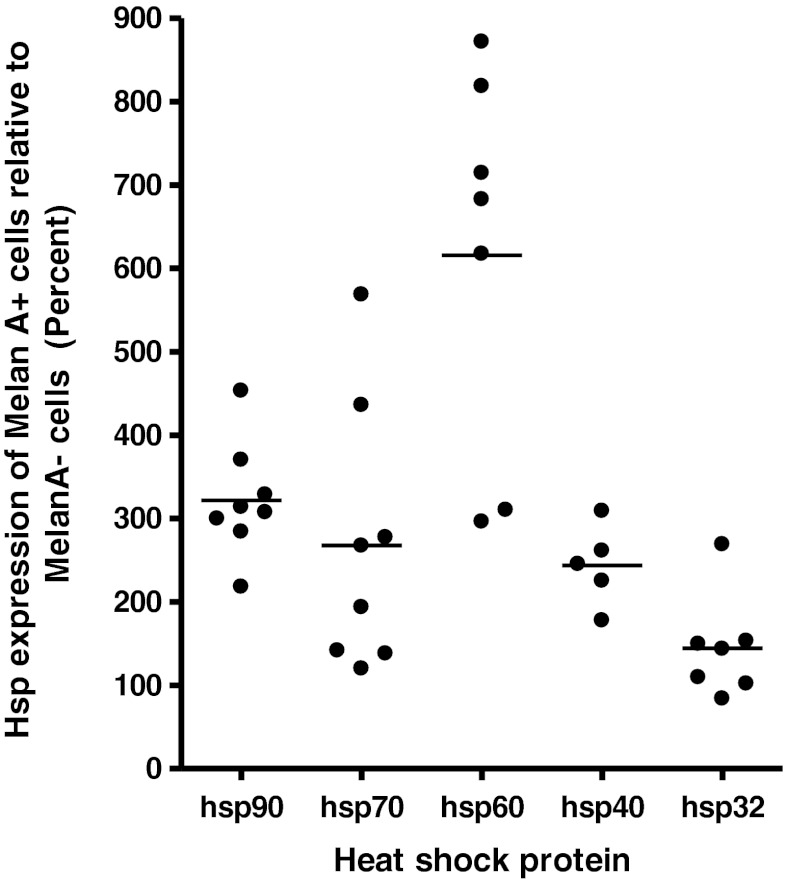
Hsp expression was visually observed to be higher in the MelanA+ cells compared with neighbouring MelanA− cells in each individual tissue section. To quantify this difference, the mean fluorescence of each hsp for a small number of representative MelanA+ and MelanA− cells within the same tissue region were compared (Fig. 4).
Fig. 4.
Fluorescence microscopy of heat shock protein expression in melanoma and non-melanoma cells within melanoma tumour tissue. Formalin-fixed paraffin-embedded melanoma tissue sections were stained for hsp expression with fluorescent antibodies and analysed using fluorescence microscopy. Differences in hsp expression between MelanA+ and MelanA− cells were quantified with appropriate software, and the difference in expression level in the MelanA+ cells compared with the MelanA− cells was expressed as a percentage. Bars indicate mean value for each group
Hsps 90 and 60 showed the highest expression in MelanA+ cells relative to MelanA− cells with between a 200–450 % and 300–900 % higher level of expression, respectively. Hsp40 showed between 150 and 300 % higher expression in MelanA+ cells. In the case of hsp70 and hsp32, protein expression in MelanA+ cells was observed to be between 100–600 % and 100–300 %, respectively, of the expression level observed in MelanA− cells. It should be noted that a few tumours did not show relative increased expression of hsp70 or hsp32. Representative images for each hsp are shown in Fig. 5.
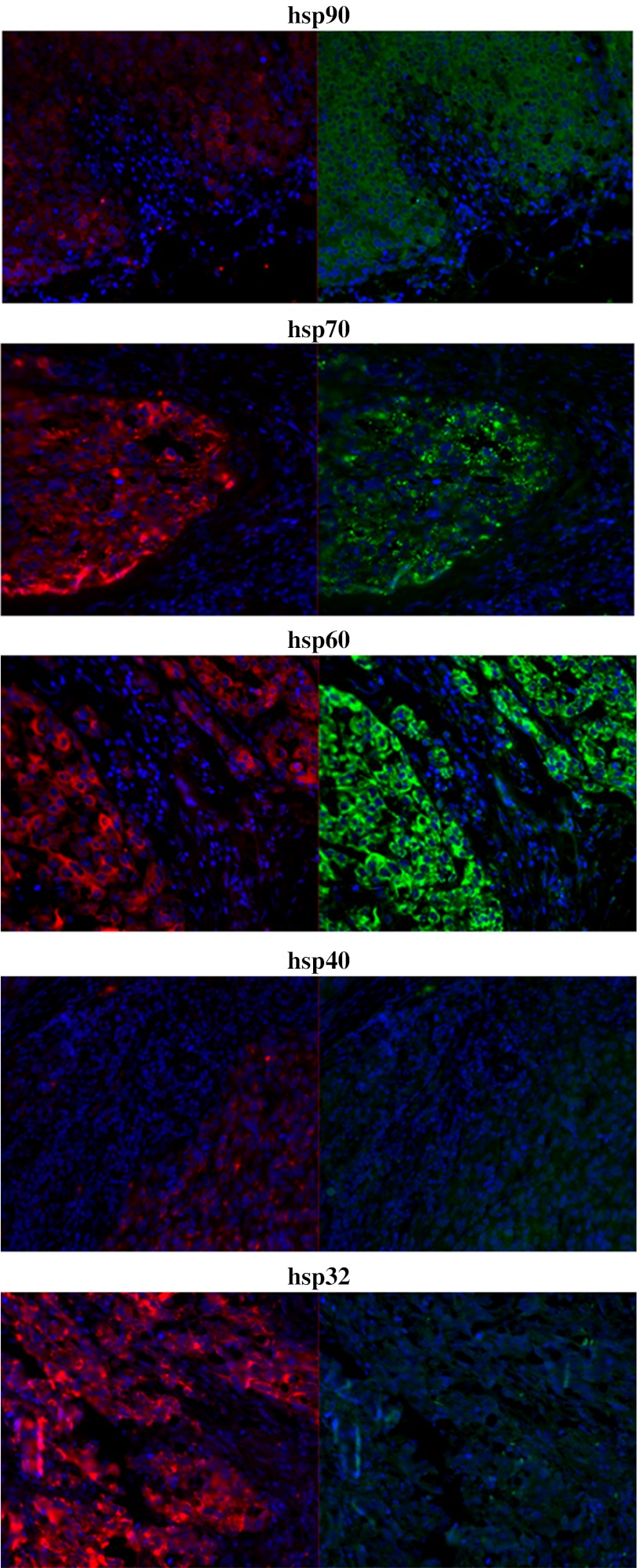
Fig. 5.
Fluorescence microscopy of heat shock protein expression in melanoma tumour tissue. Hsps 90, 70, 60, 40 and 32 were increased in expression in MelanA+ cells relative to MelanA− cells. Formalin-fixed paraffin-embedded melanoma tissue sections were stained for hsp expression with fluorescent antibodies and analysed using fluorescence microscopy. Representative images shown. Red MelanA (marker for melanoma cells), green hsp (marker for hsp), blue DAPI DNA stain (marker for cell nucleus), 20× magnification
Discussion
The results of this pilot study suggest that hsps are widely expressed in melanoma tumours and that their expression correlates with certain clinical parameters of the patient. Using immunofluorescence to examine hsp expression within tumour tissue showed that hsps were expressed at higher levels in melanoma cells relative to neighbouring non-melanoma cells. These observations might have a number of important implications.
This study presents preliminary findings that hsp90 and hsp40 expression levels may be associated with advanced tumour stage and hsp40 expression levels with reduced survival in metastatic melanoma patients (Tables 2 and 3; Fig. 2). These relationships suggest that these hsps play a role in the progression of metastatic melanoma and thus may act as biomarkers of poor patient prognosis or as therapeutic targets. These relationships may be due to the role that hsp90 plays in stabilising multiple signalling pathways exploited by proliferating cancer cells (Neckers 2007). Hsp40 is a recognised hsp90 co-chaperone (Whitesell and Lindquist 2005) and thus may facilitate melanoma progression through a similar mechanism. These findings contribute to the proposal of hsp90 and hsp40 as valid therapeutic targets in the treatment of melanoma and to the understanding of the biology of melanoma tumours.
Previous studies have suggested that higher hsp expression levels in melanoma cells is associated with increased immunogenicity (Melcher et al. 1999; Wells et al. 1997, 1998). Therefore, if hsps are involved in activating the immune system against melanoma tumours, higher hsp90 and hsp40 expression would not be expected to correlate with markers of disease progression as observed in this study, although the possibility of tumour-immune escape cannot be excluded. Therefore, these data suggest that these hsps are not involved in activating the immune system against melanoma tumours; on the contrary, increased hsp expression may even protect against tumour directed immune system attack (Jäättelä et al. 1989).
Similar studies using melanoma tissue have previously reported that hsp90 and hsp70 are expressed in a minority of tumour cells and that hsp90 shows nuclear localisation (Missotten et al. 2003; Westekemper et al. 2011), but the results from the present study are not in accordance with these earlier reports. Although a number of studies have shown that the expressions of hsp70 and hsp90 are up-regulated in melanoma tissue relative to melanocytic naevi or melanocytes (McCarthy et al. 2008; Becker et al. 2004; Deichmann et al. 2004; Farkas et al. 2003), to the best of our knowledge, this is the first report that the expressions of hsp90, hsp70, hsp60 and hsp40 proteins are increased in melanoma cells relative to neighbouring non-transformed cells. Hsp32, the inducible form of the haeme oxygenase enzyme, was observed to be widely expressed in melanoma tissue as analysed by Western blotting, but MelanA+ cells by comparison with MelanA− cells using immunofluorescence did not show significantly higher expression in the majority of samples when compared with MelanA− cells using immunofluorescence. This contrasts with a previous study using immunohistochemistry which indicated that hsp32 was up-regulated in prostate cancer tissue (Maines and Abrahamsson 1996). However, because it was observed to be widely expressed it could act as a pro-survival protein, as it may in mastocytosis (Kondo et al. 2007). Up-regulation of hsp32 expression has been shown to result in increased proliferation of melanoma cell lines and, compared with wild type cell lines, to significantly reduce survival time in mice (Was et al. 2006). This evidence fits with the proposition of hsp32 as a tumour-promoting protein (Jozkowicz et al. 2007). The potential role of hsp32 as a tumour growth mediator may be facilitated through biochemical or immunological mechanisms. For example, hsp32-specific CD8+ regulatory T cells have been identified in the blood and tumour of melanoma patients. These T cells were demonstrated to suppress the cytotoxic activity and proliferation of other T cells, and to render target cells more resistant to T cell lysis (Andersen et al. 2009), thereby potentially aiding the growth of the tumour. Conversely, since a relationship between higher hsp32 expression and improved survival since diagnosis (P < 0.007) (Fig. 2) and a possible trend between higher hsp32 expression and improved survival since analysis was observed (P = 0.17) (Fig. 2), this protein may be associated with improved patient outcome. This might occur through a biochemical mechanism whereby hsp32 directly or indirectly affects the growth of tumour cells. Although hsp32 up-regulation has been reported to result in increased cell proliferation in melanoma (Was et al. 2006), the opposite has been demonstrated for other cancer types (Jozkowicz et al. 2007). Studies performed to date on the role of hsp32 in melanoma have not been extensive and so it is unclear whether its effect on tumour cell proliferation may vary across melanoma patient sub-groups. Thus, it is not excluded that hsp32 plays a role in tumour suppression. Alternatively, the potential role of hsp32 as a tumour suppressor may be mediated through other mechanisms such as the immune system. In renal carcinoma, an MHC-bound peptide derived from hsp32 was preferentially expressed in the tumour tissue compared with the healthy tissue of the same patient. This peptide was demonstrated to stimulate CD8+ T cells with cytolytic activity, lending support to the notion that the immune system may be activated against hsp32-expressing tumours (Flad et al. 2006). The roles that these hsps play in cancer may thus vary depending on the biological context, as previously demonstrated for other cancer types (Ciocca and Calderwood 2005).
A number of relationships bordering on statistical significance with other patient parameters were observed in the present study, but, in contrast to previous studies (Kalogeraki et al. 2006; Ricaniadis et al. 2001; Lazaris et al. 1995; McCarthy et al. 2008), hsp90 was not associated with Breslow tumour thickness or Clark level and hsp70 expression was not associated with Clark level, patient survival or stage of disease (Online resources 1 and 2). On the other hand, in accordance with previous studies, hsp90 expression was not associated with patient age or gender, tumour ulceration or patient survival, and hsp70 expression did not relate to Breslow thickness or patient gender (McCarthy et al. 2008; Kalogeraki et al. 2006). Although hsp70 and hsp60 were widely expressed in melanoma tumours, there was no obvious correlation with disease progression or patient parameters. Discrepancies between previous studies and the results presented here are possibly related to differences in the patient sample groups or due to differences between primary and metastatic melanomas or may be due to the small sample sizes of this and of previous studies. These inconsistencies and potential relationships require more comprehensive larger-scale follow-up studies in order to conclusively define the role of hsps in melanoma. However, such attempts may be hampered by several factors. A potential limitation to studies of this type is that tumours are a dynamic, evolving tissue mass (Hanahan and Weinberg 2011). This is an important characteristic when considering hsp expression determined experimentally in biopsy tissue samples. The degree of hsp expression observed in such tissue samples is representative of the tumour at the time of excision only and does not take into account the variation of hsp expression that may occur throughout in vivo tumour development. Further, it is known that hsps are regulated at the post-translational level (Wandinger et al. 2008). Thus, the experimental quantification of hsp levels may not be indicative of their biological importance across different experimental samples due to the potential for variation in the activity and functionality induced post-translationally. These factors should be considered when evaluating data from studies using tumour tissue.
Hsp90 inhibiting drugs are under clinical assessment for the treatment of cancer. It is noteworthy that the present study indicated that melanoma cells express higher levels of hsp90 relative to non-melanoma cells. These observations therefore support the clinical use of hsp90 inhibitors in the treatment of melanoma. Given that hsps 70, 60 and 40 were also observed to be increased in expression in melanoma cells, these hsps may make useful therapeutic targets in the treatment of melanoma as well, although it should be noted that the expressions of hsps 70 and 60 were not associated with patient clinical parameters. These data further suggest that the chaperoning activity of these hsps is under greater demand in these cells. This is likely due to the many roles that these proteins play in supporting cancer progression, such as providing protection from tumour-associated stressors and chaperoning overexpressed oncoproteins. Indeed, the mutated B-Raf oncoprotein V600E is dependent on hsp90 for stability in melanoma (Grbovic et al. 2006). Nonetheless, in immunofluorescence experiments, a number of samples were observed to be negative for hsps, suggesting that not all tumours exploit the hsp molecular chaperone system. These hsp-negative tumours may not have experienced such a degree of tumour-associated stressors and/or may be driven by a set of proteins that do not require the assistance of the hsps investigated in this study. The identity of the MelanA-negative cells is unknown, but they may be tumour-associated fibroblasts that support the growth of the tumour (Hanahan and Weinberg 2011; Liao et al. 2009). Despite the widespread apparent increased hsp expression in MelanA+ cells with respect to neighbouring MelanA− cells, considerable variation was observed both between individuals and between hsps in any individual. Therefore, melanoma patient sub-groups that do not exhibit up-regulated hsp expression or that have reduced or a lack of hsp expression within their tumours should be carefully screened and as they may be unsuitable for treatment with hsp inhibiting drugs due to the likelihood of low efficacy and the selection of hsp-negative melanoma cells.
In agreement with previous studies, the current data demonstrated that hsps play important roles in melanoma. It is evident that larger-scale follow-up studies are required to confirm these results and to provide definitive evidence of the significance of hsp expression with respect to patient clinical parameters. Furthermore, the results presented in this study are indicative of a key role for hsps in melanoma tumours as they were observed to be widely expressed and were related to patient clinical parameters and point to the putative clinical application of hsp inhibitors for the treatment of cancer. Furthermore, these results indicate that hsp inhibitors may not be an efficacious form of therapy in all patients and that their use should be accompanied with prior assessment of intra-tumoural levels of hsp expression.
Electronic supplementary material
(DOC 94 kb)
Kaplan–Meier evaluation of patient survival since analysis. In addition, t tests were performed on other continuous patient clinical variables such as age, time to first metastasis, stage III to IV progression time, but no significant relationships were observed (data not shown). (DOC 78 kb)
Acknowledgements
We are grateful to Markus Claus for assistance in the preparation of melanoma tissue protein extracts. Christopher Shipp is the recipient of an Australian Postgraduate Award and a University of New England Strategic Doctoral Scholarship.
References
- Andersen MH, Sorensen RB, Brimnes MK, Svane IM, Becker JC, thor Straten P. Identification of heme oxygenase-1-specific regulatory CD8+ T cells in cancer patients. J Clin Invest. 2009;119(8):2245–2256. doi: 10.1172/JCI38739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton A, Jr, Kirkwood JM, McMasters KM, Mihm MF, Morton DL, Reintgen DS, Ross MI, Sober A, Thompson JA, Thompson JF. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19(16):3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- Becker B, Multhoff G, Farkas B, Wild PJ, Landthaler M, Stolz W, Vogt T. Induction of Hsp90 protein expression in malignant melanomas and melanoma metastases. Exp Dermatol. 2004;13:27–32. doi: 10.1111/j.0906-6705.2004.00114.x. [DOI] [PubMed] [Google Scholar]
- Busam KJ, Jungbluth AA. Melan-A, a new melanocytic differentiation marker. Adv Anat Pathol. 1999;6(1):12–18. doi: 10.1097/00125480-199901000-00002. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2006;31(3):164–172. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperon. 2005;10(2):86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocca DR, Clark GM, Tandon AK, Fuqua SA, Welch WJ, McGuire WL. Heat shock protein hsp70 in patients with axillary lymph node-negative breast cancer: prognostic implications. J Natl Cancer I. 1993;85(7):570–574. doi: 10.1093/jnci/85.7.570. [DOI] [PubMed] [Google Scholar]
- Clinical Trials for HSP (2012) Clinical trials feeds. http://clinicaltrialsfeeds.org/clinical-trials/results/term=HSP?recr=Open. Accessed 25 Jan 2012
- Deichmann M, Polychronidis M, Benner A, Kleist C, Thome M, Kahle B, Helmke BM. Expression of the heat shock cognate protein HSP73 correlates with tumour thickness of primary melanomas and is enhanced in melanoma metastases. Int J Oncol. 2004;25(2):259–268. [PubMed] [Google Scholar]
- Faingold D, Marshall J-C, Antecka E, Di Cesare S, Odashiro AN, Bakalian S, Fernandes BF, Burnier MN. Immune expression and inhibition of heat shock protein 90 in uveal melanoma. Clin Cancer Res. 2008;14(3):847–855. doi: 10.1158/1078-0432.CCR-07-0926. [DOI] [PubMed] [Google Scholar]
- Farkas B, Hantschel M, Magyarlaki M, Becker B, Scherer K, Landthaler M, Pfister K, Gehrmann M, Gross C, Mackensen A, Multhoff G. Heat shock protein 70 membrane expression and melanoma-associated marker phenotype in primary and metastatic melanoma. Melanoma Res. 2003;13(2):147–152. doi: 10.1097/00008390-200304000-00006. [DOI] [PubMed] [Google Scholar]
- Flad T, Mueller L, Dihazi H, Grigorova V, Bogumil R, Beck A, Thedieck C, Mueller GA, Kalbacher H, Mueller CA. T cell epitope definition by differential mass spectrometry: identification of a novel, immunogenic HLA-B8 ligand directly from renal cancer tissue. Proteomics. 2006;6(1):364–374. doi: 10.1002/pmic.200500099. [DOI] [PubMed] [Google Scholar]
- Grbovic OM, Basso AD, Sawai A, Ye Q, Friedlander P, Solit D, Rosen N. V600E B-Raf requires the Hsp90 chaperone for stability and is degraded in response to Hsp90 inhibitors. P Natl Acad Sci USA. 2006;103(1):57–62. doi: 10.1073/pnas.0609973103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Harada M, Kimura G, Nomoto K. Heat shock proteins and the antitumor T cell response. Biotherapy. 1998;10:229–235. doi: 10.1007/BF02678301. [DOI] [PubMed] [Google Scholar]
- Jäättelä M, Saksela K, Saksela E. Heat shock protects WEHI-164 target cells from the cytolysis by tumor necrosis factors alpha and beta. Eur J Immunol. 1989;19(8):1413–1417. doi: 10.1002/eji.1830190810. [DOI] [PubMed] [Google Scholar]
- Jozkowicz A, Was H, Dulak J. Heme oxygenase-1 in tumors: is it a false friend? Antioxid Redox Sign. 2007;9(12):2099–2117. doi: 10.1089/ars.2007.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalogeraki A, Garbagnati F, Darivianaki K, Delides GS, Santinami M, Stathopoulos EN, Zoras O. HSP-70, C-myc and HLA-DR expression in patients with cutaneous malignant melanoma metastatic in lymph nodes. Anticancer Res. 2006;26(5A):3551–3554. [PubMed] [Google Scholar]
- Kondo R, Gleixner KV, Mayerhofer M, Vales A, Gruze A, Samorapoompichit P, Greish K, Krauth MT, Aichberger KJ, Pickl WF, Esterbauer H, Sillaber C, Maeda H, Valent P. Identification of heat shock protein 32 (Hsp32) as a novel survival factor and therapeutic target in neoplastic mast cells. Blood. 2007;110(2):661–669. doi: 10.1182/blood-2006-10-054411. [DOI] [PubMed] [Google Scholar]
- Lazaris AC, Theodoropoulos GE, Aroni K, Saetta A, Davaris PS. Immunohistochemical expression of C-myc oncogene, heat shock protein 70 and HLA-DR molecules in malignant cutaneous melanoma. Virchows Arch. 1995;426(5):461–467. doi: 10.1007/BF00193169. [DOI] [PubMed] [Google Scholar]
- Liao D, Luo Y, Markowitz D, Xiang R, Reisfeld RA (2009) Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS ONE 4(11):e7965 [DOI] [PMC free article] [PubMed]
- Lucas R, McMichael T, Smith W, Armstrong B (2006) Solar ultraviolet radiation—global burden of disease from solar ultraviolet radiation. World Health Organization, Geneva
- Maines MD, Abrahamsson PA. Expression of heme oxygenase-1 (HSP32) in human prostate: normal, hyperplastic, and tumor tissue distribution. Urology. 1996;47(5):727–733. doi: 10.1016/S0090-4295(96)00010-6. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Pick E, Kluger Y, Gould-Rothberg B, Lazova R, Camp RL, Rimm DL, Kluger HM. HSP90 as a marker of progression in melanoma. Ann Oncol. 2008;19(3):590–594. doi: 10.1093/annonc/mdm545. [DOI] [PubMed] [Google Scholar]
- Melcher A, Murphy S, Vile R. Heat shock protein expression in target cells infected with low levels of replication-competent virus contributes to the immunogenicity of adenoviral vectors. Hum Gene Ther. 1999;10(9):1431–1442. doi: 10.1089/10430349950017770. [DOI] [PubMed] [Google Scholar]
- Missotten GS, Journée-de Korver JG, Wolff-Rouendaal D, Keunen JE, Schlingemann RO, Jager MJ. Heat shock protein expression in the eye and in uveal melanoma. Invest Ophth Vis Sci. 2003;44:3059–3065. doi: 10.1167/iovs.02-1038. [DOI] [PubMed] [Google Scholar]
- Multhoff G, Botzler C, Jennen L, Schmidt J, Ellwart J, Issels R. Heat shock protein 72 on tumor cells: a recognition structure for natural killer cells. J Immunol. 1997;158(9):4341–4350. [PubMed] [Google Scholar]
- Neckers L. Heat shock protein 90: the cancer chaperone. J Bioscience. 2007;32(3):517–530. doi: 10.1007/s12038-007-0051-y. [DOI] [PubMed] [Google Scholar]
- Noessner E, Gastpar R, Milani V, Brandl A, Hutzler PJ, Kuppner MC, Roos M, Kremmer E, Asea A, Calderwood SK, Issels RD. Tumor-derived heat shock protein 70 peptide complexes are cross-presented by human dendritic cells. J Immunol. 2002;169(10):5424–5432. doi: 10.4049/jimmunol.169.10.5424. [DOI] [PubMed] [Google Scholar]
- Pick E, Kluger Y, Giltnane JM, Moeder C, Camp RL, Rimm DL, Kluger HM. High HSP90 expression is associated with decreased survival in breast cancer. Cancer Res. 2007;67(7):2932–2937. doi: 10.1158/0008-5472.CAN-06-4511. [DOI] [PubMed] [Google Scholar]
- Ricaniadis N, Kataki A, Agnantis N, Androulakis G, Karakousis CP. Long-term prognostic significance of HSP-70, c-myc and HLA-DR expression in patients with malignant melanoma. Eur J Surg Oncol. 2001;27:88–93. doi: 10.1053/ejso.1999.1018. [DOI] [PubMed] [Google Scholar]
- STA-9090 in Metastatic Ocular Melanoma (2011) Clinical trials feeds. http://clinicaltrialsfeeds.org/clinical-trials/show/NCT01200238. Accessed 14 June 2011
- Wandinger SK, Richter K, Buchner J. The Hsp90 chaperone machinery. J Biol Chem. 2008;283(27):18473–18477. doi: 10.1074/jbc.R800007200. [DOI] [PubMed] [Google Scholar]
- Was H, Cichon T, Smolarczyk R, Rudnicka D, Stopa M, Chevalier C, Leger JJ, Lackowska B, Grochot A, Bojkowska K, Ratajska A, Kieda C, Szala S, Dulak J, Jozkowicz A. Overexpression of heme oxygenase-1 in murine melanoma: increased proliferation and viability of tumor cells, decreased survival of mice. Am J Pathol. 2006;169(6):2181–2198. doi: 10.2353/ajpath.2006.051365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AD, Rai SK, Salvato MS, Band H, Malkovsky M. Restoration of MHC class I surface expression and endogenous antigen presentation by a molecular chaperone. Scand J Immunol. 1997;45(6):605–612. doi: 10.1046/j.1365-3083.1997.d01-436.x. [DOI] [PubMed] [Google Scholar]
- Wells AD, Rai SK, Salvato MS, Band H, Malkovsky M. Hsp72-mediated augmentation of MHC class I surface expression and endogenous antigen presentation. Int Immunol. 1998;10:609–617. doi: 10.1093/intimm/10.5.609. [DOI] [PubMed] [Google Scholar]
- Westekemper H, Karimi S, Süsskind D, Anastassiou G, Freistühler M, Steuhl K-P, Bornfeld N, Schmid K-W, Grabellus F (2011) Expression of HSP 90, PTEN and Bcl-2 in conjunctival melanoma. Brit J Ophthalmol 95:853–858 [DOI] [PubMed]
- Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5(10):761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- WHO (2008) World cancer report 2008. International Agency for Research on Cancer, Geneva
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 94 kb)
Kaplan–Meier evaluation of patient survival since analysis. In addition, t tests were performed on other continuous patient clinical variables such as age, time to first metastasis, stage III to IV progression time, but no significant relationships were observed (data not shown). (DOC 78 kb)