Abstract
The oncoprotein MDM2 (murine double minute 2) is often overexpressed in human tumors and thereby attenuates the function of the tumor suppressor p53. In this study, we investigated the effects of the novel MDM2-inhibitor PXN727 on p53 activation, cell proliferation, cell cycle distribution and radiosensitivity. Since the localization of heat shock protein 70 (Hsp70) exerts different effects on radioresistance of tumor cells, we investigated the impact of PXN727 on intracellular, membrane, and secreted Hsp70 levels. We could show that PXN727 exerts its effects on wildtype p53 (HCT116 p53+/+, A549) but not p53 depleted (HCT116 p53−/−) or mutated (FaDu) tumor cells. PXN727 activates p53, induces the expression of p21, reduces the proportion of cells in the radioresistant S-phase and induces senescence. Radiosensitivity was significantly increased by PXN727 in HCT116 p53+/+ tumor cells. Furthermore, PXN727 causes a downregulation of Hsp70 membrane expression and an upregulated secretion of Hsp70 in wildtype p53 tumor cells. Our data suggest that re-activation of p53 by MDM2-inhibition modulates Hsp70 membrane expression and secretion which might contribute to the radiosensitizing effect of the MDM2-inhibitor PXN727.
Keywords: Hsp70, PXN727, MDM2, p53, Radiosensitization
Introduction
Radiotherapy is one of the key treatment modalities to cure solid tumors, but its efficacy is limited by radioresistant tumor cell clones and normal tissue toxicity. Therefore, there is a high medical need to develop agents that sensitize tumor cells to ionizing radiation (IR) in a highly specific manner. However, currently no clinically applied radiosensitizers are available.
The tumor suppressor gene p53 is a transcription factor that is crucial for cellular pathways regulating DNA repair, cell cycle and apoptosis in response to irradiation. Upon activation, p53 induces the synthesis of its negative regulator MDM2 (murine double minute 2) that binds to p53 and induces its proteasomal degradation (Shangary and Wang 2008). This autoregulatory feedback loop tightly controls p53 protein levels in normal cells. However, in human tumor cells, MDM2 is frequently overexpressed and thus leads to inhibition of p53-signaling. As a result these tumor cells are impaired in inducing cell cycle arrest and apoptosis, and thus are more radioresistant (Gudkov and Komarova 2003).
Targeting the MDM2-p53 interaction by small molecule inhibitors is a promising strategy to restore the tumor suppressor and pro-apoptototic activity of p53 (Shangary and Wang 2008). Nutlin-3 is one of the first MDM2-antagonists that activates p53 and sensitizes tumor cells to IR in a non-genotoxic manner (Arya et al. 2010; Cao et al. 2006; Impicciatore et al. 2010; Supiot et al. 2008). Recently, novel isoquinolin-1-one based derivatives (PXN) have been developed as inhibitors of the p53-MDM2 interaction. They exhibit a better oral bioavailability, lower toxicity, more specific binding to the N-terminal binding site of MDM2 and an easier chemical synthesis compared to Nutlin-3 (Rothweiler et al. 2008).
In the present study, the compound PXN727 was analyzed with respect to its capacity to affect cell proliferation, cell cycle distribution, senescence, radiosensitivity and the stress response in wildtype (WT) and mutant p53 tumor cells. Similar to Nutlin-3, PXN727 significantly reduces the proportion of cells in the radioresistant S-phase, inhibits cell proliferation, induces senescence and increases the sensitivity of WT but not mutant p53 tumor cells to IR. The stress-inducible, cytoprotective heat shock protein 70 (Hsp70) is frequently overexpressed in the cytosol of radioresistant tumor cells. Different environmental stress factors (e.g., heat shock, nutrient deprivation) but also therapeutic interventions such as IR, hyperthermia, and chemotherapy further increase the synthesis of Hsp70. In contrast to normal cells, Hsp70 also can be found on the plasma membrane and in the extracellular milieu of tumor cells (Multhoff et al. 1995; Sherman and Multhoff 2007). It appeared that membrane Hsp70 contributes to radio- and chemo-resistance of tumors (Gehrmann et al. 2005), whereas extracellular located Hsp70 can promote tumor cell death (Schilling et al. 2009). In line with these results, herein, we observed a PXN727-mediated downregulation of Hsp70 on the plasma membrane, an increased release of Hsp70 into the extracellular environment and an enhanced radiosensitivity in WT but not mutant p53 tumor cells. Taken together, these results suggest that re-activation of p53 by MDM2-inhibitor PXN727 reduces membrane density of Hsp70 and increases Hsp70 secretion which might contribute to the radiosensitizing effect of PXN727.
Materials and methods
Cells and cell culture
HCT116 colon cancer cell lines with wildtype p53 (p53+/+) and depleted p53 (p53−/−) were kindly provided by Prof. Bert Vogelstein (Johns Hopkins University, Baltimore) and maintained in McCoy’s 5A medium (Invitrogen). A549 (wildtype p53) and FaDu (mutant p53) cells were maintained in DMEM medium (Invitrogen). Media were supplemented with 10 % FCS (PAA).
Treatment with MDM2-inhibitors and irradiation
Ten millimolar stock solutions of Nutlin-3 (Sigma) and PXN727 (kindly provided by Priaxon, München, Germany) were prepared in 100 % DMSO. Further dilutions were performed in PBS. As a control, DMSO diluted in PBS in the respective concentration was used in all experiments.
Tumor cells were irradiated with the indicated doses using the RT100 irradiation device (Philips) at a dose rate of 1 Gy/min. Control cells were sham irradiated at 0 Gy.
ELISA and western blot analysis
Cells were lysed in TBST buffer (1 % Triton X-100 in TBS, 1 mM PMSF, protease inhibitor cocktail) and protein content determined using the BCA™ Protein Assay Kit (Pierce). Hsp70 concentrations in cell lysates and in cell culture supernatants were determined by ELISA according to the manufacturer’s instructions (R&D).
For Western Blot analysis, proteins were detected with monoclonal antibodies directed against Hsp70 (SPA-810, Assay Designs), p53 (BD Biosciences), p21 and MDM2 (Merck), and β-actin (Sigma). Bound antibodies were visualized using horseradish peroxidase-conjugated secondary antibodies (Promega) and a chemiluminescence developing kit (ECL, Amersham Biosciences).
Flow cytometry
Trypsinized cells (100,000) were incubated with the FITC-conjugated Hsp70 monoclonal antibody cmHsp70.1 (multimmune GmbH, Munich, Germany) or the corresponding isotype-matched control antibody (IgG1, BD Biosciences) for 30 min at 4 °C. Dead cells were stained with propidium iodide (PI, Sigma-Aldrich), and viable cells were analyzed on a FACSCalibur flow cytometer (BD Biosciences).
Proliferation assay
Cells were seeded into 96-well plates (4,000 cells/well). Twenty-four hours after seeding, various concentrations of PXN727 or Nutlin-3 were added, and cells were incubated for further 72 h. The proliferation was measured using the MTS-assay according to the manufacturer’s instructions (Promega).
Cell cycle analysis
For analysis of cell cycle distribution, cells were collected by trypsinization and fixed with ice-cold 70 % ethanol at −20 °C. After washing in PBS, cells were resuspended in 500 μl PI/RNase staining solution (Sigma-Aldrich), incubated for 60 min at room temperature and analyzed using FACSCalibur flow cytometer. The cell cycle distribution was calculated by using Modfit software (Verity software house Inc).
Clonogenic cell survival assay
Cells were treated with 10 μM PXN727 or Nutlin-3 and 1 h later irradiated. Twenty-four hours after irradiation, cells were collected by trypsinization, counted and seeded into 12-well plates. On days 6–8 after seeding, colonies were fixed in methanol, stained with crystal violet and counted. Survival curves were fitted to the linear quadratic model using Sigmaplot (Systat Software Inc). The sensitizing enhancement ratio (SER) was defined as SER = D50 (irradiation)/D50 (drug and irradiation).
β-galactosidase assay
Staining of β-galactosidase (β-gal) in tumor cells was performed using the senescence β-galactosidase staining kit (Cell Signaling Technology) according to the manufacturer’s protocol.
Statistics
Statistical analysis was performed using SPSS 18.0.2 software. The Student’s t test was used to evaluate significant differences (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001).
Results
PXN727 inhibits cell proliferation, re-activates p53 and induces a cell cycle arrest in WT p53 tumor cells
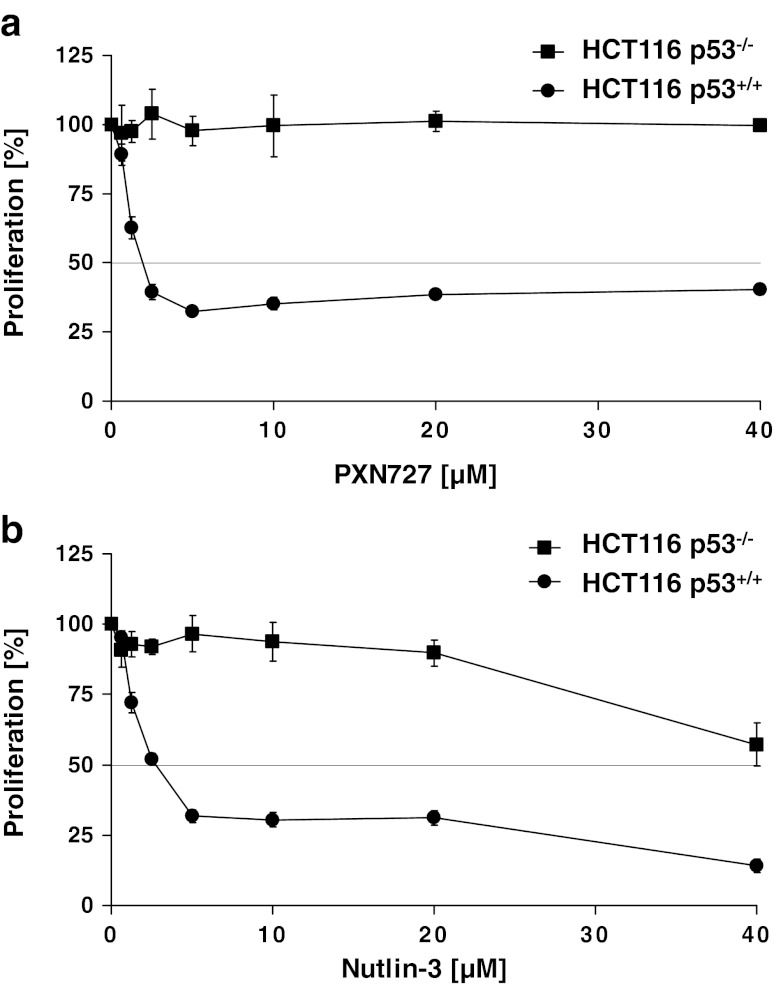
To examine the p53-dependency of PXN727, two isogenic colon cancer cell lines were chosen that differ in their p53 status; HCT116 WT p53 (p53+/+) and p53 null mutant (p53−/−). PXN727 decreased the proliferation of HCT116 p53+/+ with an IC50 value of 2 μM, but not that of the HCT116 p53−/− tumor cells (Fig. 1a). The anti-proliferative activity of PXN727 in p53+/+ tumor cells was comparable to that of Nutlin-3 (IC50: 2.5 μM) (Fig. 1b). However, at concentrations higher than 10 μM, Nutlin-3 showed a p53-independent anti-proliferative effect also in HCT116 p53−/− tumor cells. For all further experiments, PXN727 and Nutlin-3 were applied at a maximum concentration of 10 μM.
Fig. 1.
PXN727 inhibits cell proliferation of HCT116 WT p53 cells. HCT116 p53+/+ and p53−/− tumor cells were incubated with different concentrations of PXN727 or Nutlin-3 for 72 h. Inhibitory activity was evaluated in a MTS cell proliferation assay. Error bars represent SD of 4 –5 independent experiments
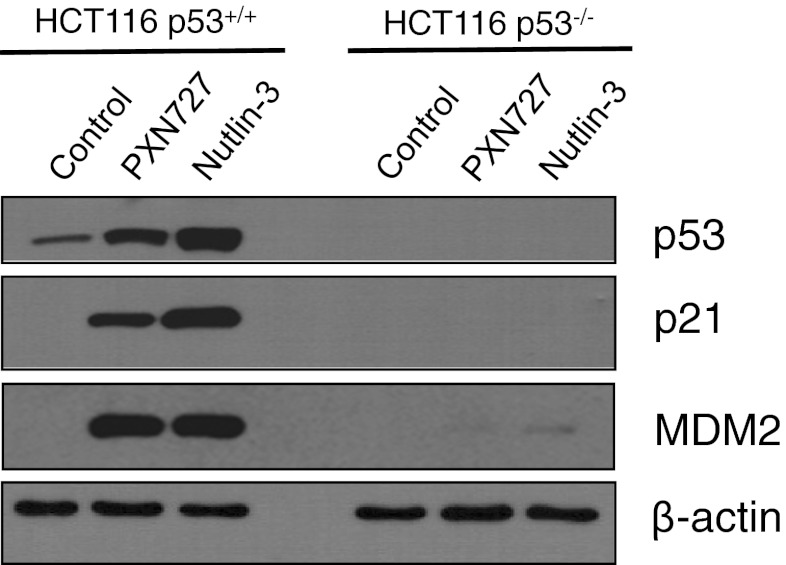
PXN727, as well as Nutlin-3, induced an accumulation of p53 and a robust upregulation of the p53 target genes p21 and MDM2 in HCT116 p53+/+ but not in p53−/− tumor cells (Fig. 2). This indicates that PXN727 is as effective as Nutlin-3 in activating the p53 pathway.
Fig. 2.
PXN727 activates p53 in HCT116 WT p53 cells. HCT116 p53+/+ and p53−/− tumor cells were treated with PXN727 or Nutlin-3 (10 μM each) for 24 h. Protein expression levels were analyzed by Western blot analysis using antibodies directed against p53, p21 and MDM2. β-actin was used as loading control
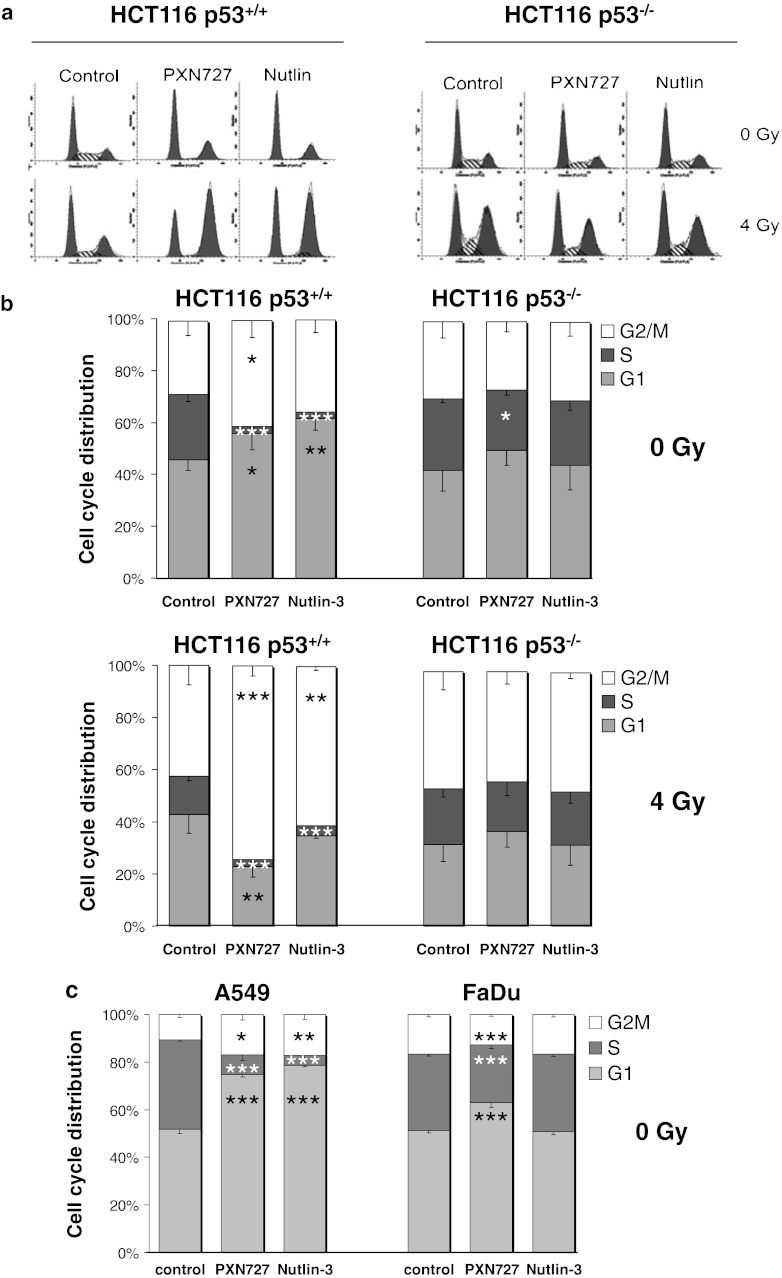
Since p53 and p21 play a major role in cell cycle regulation, we studied the PXN727-mediated effects on cell cycle distribution. In HCT116 p53+/+ tumor cells, PXN727 significantly decreased the proportion of cells in the radioresistant S-phase and induced an increase in the G1- and G2/M-phase (Fig. 3b). In line with the results of other groups (Cao et al. 2006; Miyachi et al. 2009), comparable effects on cell cycle distribution were observed when these cells were treated with Nutlin-3 (Fig. 3b). The decrease in the S-phase following treatment with both MDM2 inhibitors was confirmed in the WT p53 lung cancer cell line A549 (Fig. 3c). HCT116 p53−/− tumor cells (Fig. 3b) and the mutant p53 tumor cell line FaDu (Fig. 3c), showed a weak reduction in the proportion of cells in the S-phase after treatment with PXN727, whereas Nutlin-3 showed no alterations in the cell cycle.
Fig. 3.
PXN727 induces S-phase depletion in WT p53 cells. HCT116 p53+/+ (p53 WT) and p53−/− (p53 null), A549 (p53 WT) and FaDu (p53 mutant) tumor cells were treated with PXN727 or Nutlin-3 (10 μM each) and after 1 h cells were irradiated with 0 or 4 Gy. 24 h after irradiation the cell cycle distribution was determined by flow cytometry. a One representative FACS analysis. b Mean values and SD of 3 (HCT116 p53+/+) and 4 (HCT116 p53−/−) independent experiments are shown. c Mean values and SD of 3 (A549) and 4 (FaDu) independent experiments are shown. Significant differences between vehicle control (DMSO) and cells treated with MDM2-inhibitors are indicated for each cell cycle phase (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001)
Several studies have shown that IR increases the expression of MDM2 in a p53-dependent manner and thereby attenuates p53 functions such as the induction of the cell cycle arrest (Impicciatore et al. 2010). Therefore, we wanted to determine how the inhibition of MDM2 in combination with IR affects the cell cycle distribution. HCT116 p53+/+ and p53−/− tumor cells were treated with either PXN727 or Nutlin-3 followed by IR with 4 Gy (Fig. 3b). IR alone induced a comparable increase of cells in the G2/M-phase in both cell lines and a slight decrease of cells in the S- and G1-phase. Interestingly, in p53+/+ cells the combination of PXN727 and IR induced a synergistic effect on the G2/M-arrest. Similar but less pronounced effects were induced by Nutlin-3. In p53−/− cells, pre-treatment of the cells with both MDM2-inhibitors did not impact the IR-induced effects.
PXN727 radiosensitizes colon cancer cells containing WT p53
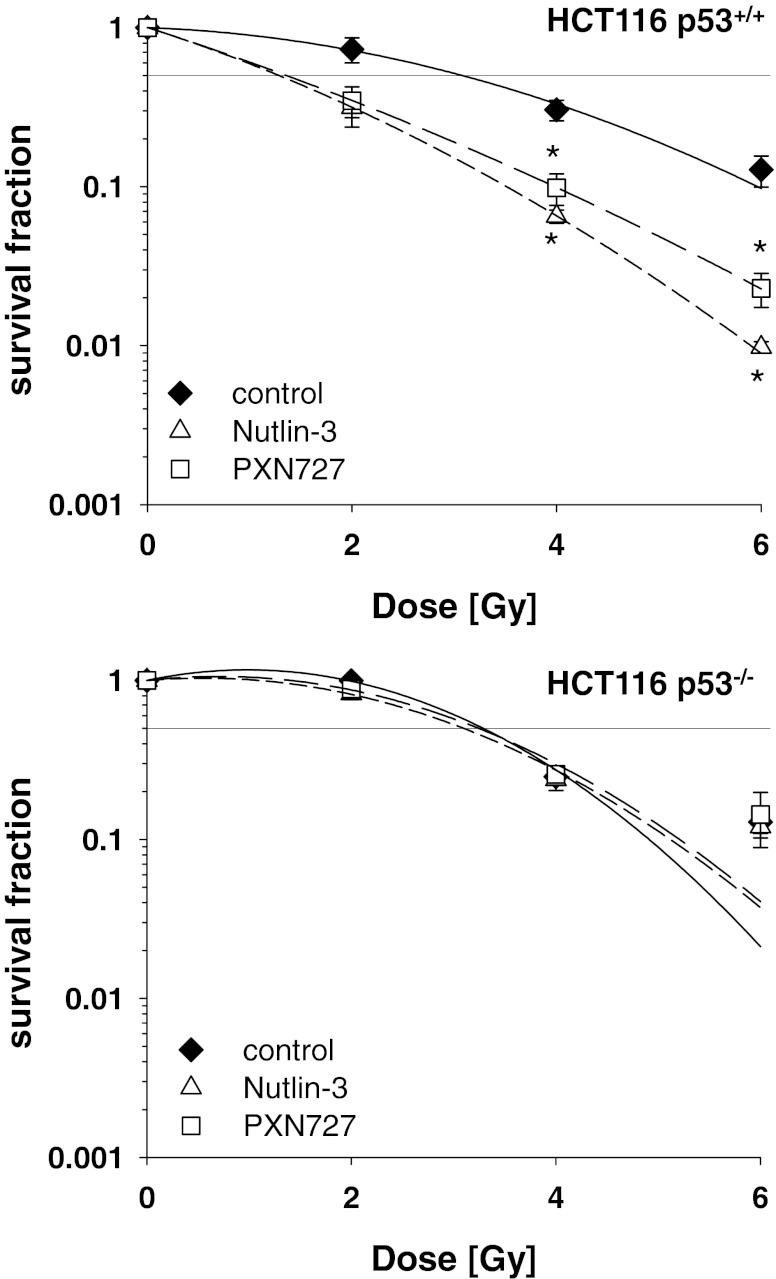
Irrespective of the p53-status, HCT116 p53+/+ and p53−/− tumor cells showed a comparable sensitivity towards IR (Fig. 4). Although PXN727 (SER = 2.3) and Nutlin-3 (SER = 2.4) significantly enhanced the radiosensitivity of HCT116 WT p53 cells, no radiosensitizing effects were observed in HCT116 tumor cells lacking p53 (SER = 1.0 for PXN727 and 1.1 for Nutlin-3). These data demonstrate for the first time that the new MDM2-inhibitor PXN727 increases the radiosensitivity of tumor cells by re-activation of WT p53.
Fig. 4.
Radiosensitization of HCT116 colon cancer cells by PXN727 is dependent on p53. HCT116 p53+/+ and p53−/− tumor cells were pre-treated with PXN727 or Nutlin-3 (10 μM each) for 1 h and then cells were irradiated as indicated in the graph. Clonogenic survival fractions were calculated after normalization for cell kill by PXN727 and Nutlin-3 alone which was always below 10 %. Mean values ± SD of 4 (p53+/+) and 2 (p53−/−) independent experiments are shown. Significant differences between vehicle control (DMSO) and cells treated with MDM2-inhibitors are indicated (*p ≤ 0.05)
PXN727 induces senescence in colon cancer cells containing WT p53
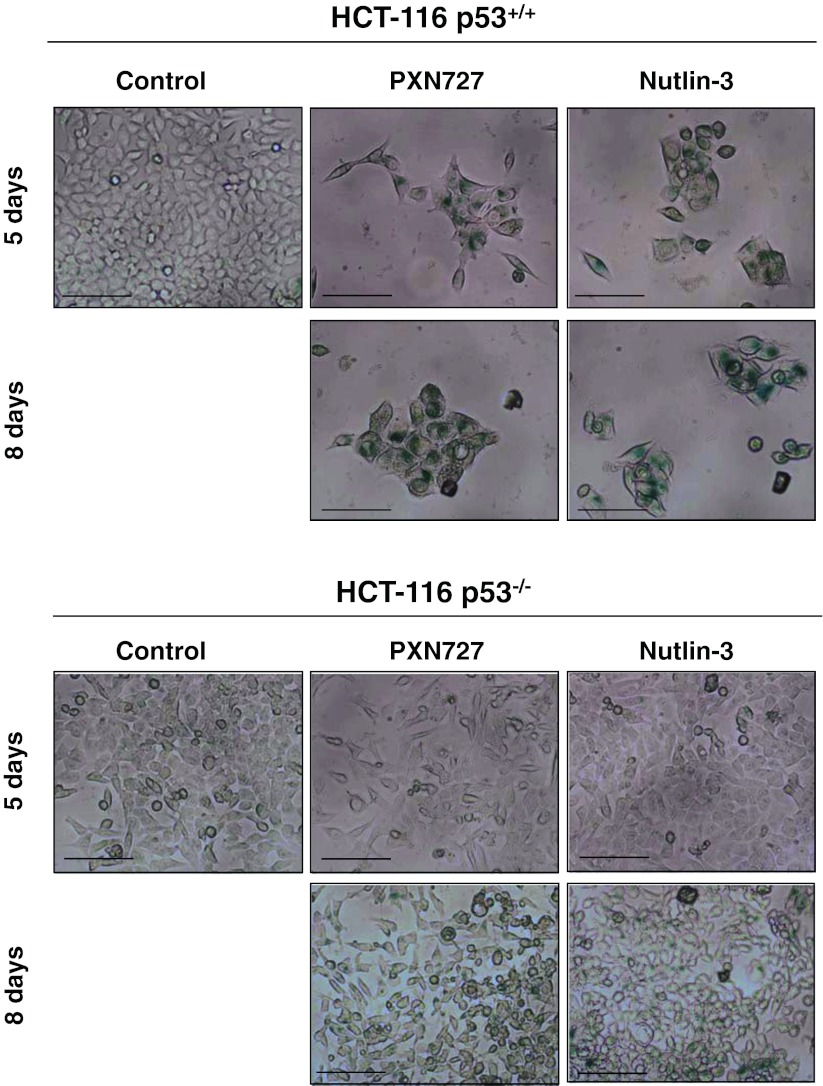
To determine the mechanism by which PXN727 mediates radiosensitization, we investigated apoptosis and cellular senescence as potential p53-dependent pathways in the isogenic HCT116 cell system. In accordance to published data (Arya et al. 2010; Lehmann et al. 2007), apoptosis was only marginally induced in HCT116 WT p53 cells by PXN727 or Nutlin-3 at a concentration of 10 μM (data not shown). However, PXN727 and Nutlin-3 caused senescence in HCT116 WT p53 cells, as shown by reduced cell proliferation, increased cell size and induction of β-galactosidase expression (Fig. 5). In contrast, no signs of senescence were observed after an identical treatment in HCT116 cells that lack p53. In line with the literature (Jackson et al. 2012), doxorubicin which was used as a positive control in our experiments, also induced cellular senescence in HCT116 WT p53 cells (data not shown).
Fig. 5.
PXN727 induces senescence in HCT116 WT p53 cells. Representative photomicrographs of HCT116 p53+/+ (p53 WT) and p53−/−(p53 null) tumor cells stained for β-gal after a treatment with DMSO vehicle control (control), PXN727 or Nutlin-3 (10 μM each) after 5 and 8 days, respectively. Senescence is represented by a loss of tumor cells, changes in cell morphology and a blue staining which indicates the expression of β-gal. Scale bar depicts 100 μm
p53-dependent reduction of cell surface Hsp70 upon PXN727 treatment
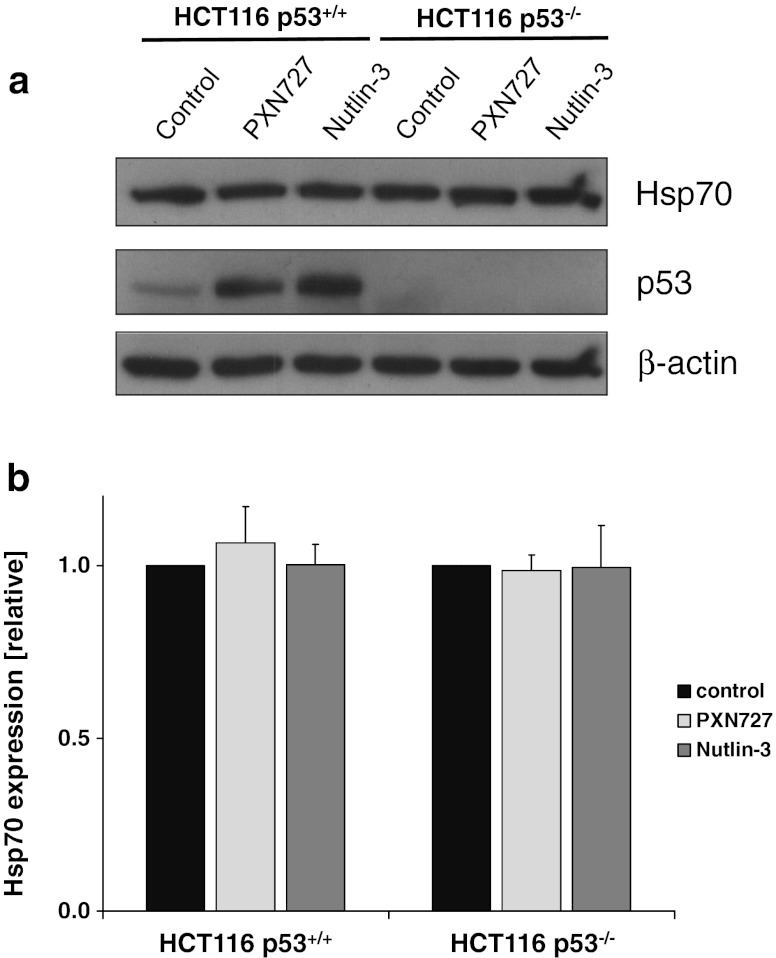
Dependent on its subcellular/extracellular localization, Hsp70 is known to exert different effects with regard to radioresistance. Therefore, we investigated whether changes in radiosensitivity upon MDM2-inhibition might be associated with altered Hsp70 levels. As shown by Western Blot analysis and ELISA the cytosolic Hsp70 levels remained unaltered upon MDM2-inhibition in both tumor sublines (Fig. 6).
Fig. 6.
PXN727 induces no changes in cellular Hsp70 protein levels. HCT116 p53+/+ and p53−/− tumor cells were treated with PXN727 or Nutlin-3 (10 μM each) for 24 h. a Intracellular Hsp70 expression was analyzed in cell lysates by Western Blot analysis using antibodies directed against Hsp70 and p53. β-actin was used as loading control. b Intracellular Hsp70 concentrations were determined by ELISA and calculated relative to the total protein content of each sample. For the calculation of relative levels, the untreated control was set at 1. Mean values ± SD of five independent experiments are shown
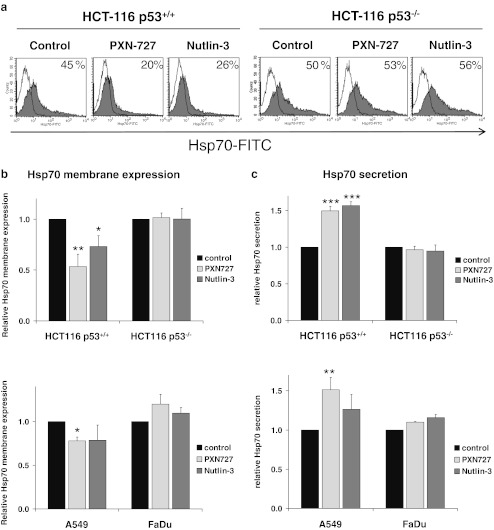
The cell surface expression of Hsp70 was measured by flow cytometry using the Hsp70-specific monoclonal antibody cmHsp70.1 (Fig. 7a+b). Under standard conditions both HCT116 sublines barely differed in their Hsp70 membrane expression (45 vs. 50 %). In HCT116 p53+/+ tumor cells, the amount of Hsp70 membrane-positive cells was significantly reduced after blocking the p53-MDM2 interaction by either PXN727 or Nutlin-3 (Fig. 7b). Similar effects on Hsp70 membrane levels were observed when A549 lung cancer cells harboring WT p53 were treated with MDM2-inhibitors (Fig. 7b). MDM2-inhibition did not influence the expression of membrane Hsp70 in p53 depleted HCT116 and mutant p53 FaDu tumor cells (Fig. 7b). To determine whether the downregulation of membrane Hsp70 by MDM2-inhibition is caused by a release of Hsp70 into the extracellular milieu, the concentration of Hsp70 was measured in cell culture supernatants. A substantial increase of extracellular Hsp70 upon MDM2-inhibition was detected in HCT116 p53+/+ and A549 cells, but not in HCT116 p53−/− and FaDu cells (Fig. 7c).
Fig. 7.
PXN727 induces a p53-dependent downregulation of membrane Hsp70 with an associated increase of released Hsp70. HCT116 p53+/+ (p53 WT) and p53−/−(p53 null), A549 (p53 WT) and FaDu (p53 mutant) tumor cells were treated with PXN727 or Nutlin-3 (10 μM each) for 24 h. a The Hsp70 cell surface expression was analyzed by flow cytometry. Representative FACS analysis showing Hsp70 cell surface staining on HCT116 tumor sublines. b Graphs show mean values ± SD of two to four independent FACS experiments. The membrane Hsp70-positivity is calculated relative to the membrane positivity of control cells. Significant differences between vehicle control (DMSO) and cells treated with MDM2-inhibitors are indicated (*p ≤ 0.05, **p ≤ 0.01). c The Hsp70 concentration in the cell culture supernatants was quantified by ELISA. Hsp70 concentrations were normalized to the number of cells and presented relative to the Hsp70 concentration in control cells. Mean values ± SD of two to three independent experiments are shown. Significant differences between vehicle control (DMSO) and cells treated with MDM2-inhibitors are marked (**p ≤ 0.01, ***p ≤ 0.001)
Discussion
The tumor suppressor gene p53 is mutated or deleted in approximately 50 % of human cancers. In WT p53 tumors, p53 function is inhibited by the negative regulatory protein MDM2 that is frequently overexpressed in tumor cells and is further increased upon radiation. An impaired p53 function is known to be associated with a reduced sensitivity of tumor cells to radiotherapy (Gudkov and Komarova 2003). Therefore, blocking the interaction of MDM2 and p53 is a promising approach to stabilize and re-activate the tumor suppressor function of p53. In this study we investigated the radiosensitizing effects of the new small molecule inhibitor PXN727 in isogenic WT and p53 depleted tumor cell lines. Similar to Nutlin-3, PXN727 increases the protein levels of p53 and its client genes p21 and MDM2 in WT but not mutant p53 tumor cells. Concomitant with the upregulation of p21, PXN727 treatment results in tumor cell growth inhibition, a G2/M-arrest and a significant reduction of cells in the radioresistant S-phase in WT p53 tumor cells. Moreover, a combined treatment of WT p53 tumor cells with PXN727 and radiation synergistically enhances the G2/M-block. This indicates that during radiation MDM2 attenuates the p53 functions in the cell cycle.
The finding that PXN727 radiosensitizes WT p53 tumor cells but not p53-depleted tumor cells confirmed the dependency of PXN727 on WT p53. Furthermore, we hypothesize that the radiosensitizing effect of PXN727 on WT p53 tumor cells is mainly mediated by senescence but not by apoptosis. An individualized tumor therapy requires determination of the p53 status in tumors to select for those patients who might profit from the radiosensitizing effect of PXN727.
Similar radiosensitizing effects have previously been demonstrated for the MDM2-inhibitor Nutlin-3, which was used as a control in our experiments (Arya et al. 2010; Cao et al. 2006). Due to its lower toxicity and better bioavailability, PXN727 might be preferable. Furthermore, Nutlin-3 can induce mutations in WT p53 tumor cells which result in a reduced radiosensitivity and an increased drug resistance (Michaelis et al. 2011). The induction of p53 mutations is not a general problem of MDM2-inhibitors, since RITA, another MDM2 inhibitor did not reveal these adverse side effects (Michaelis et al. 2012). With respect to PXN727, further examinations are warranted to investigate long-term effects on the p53-status and radioresistance of tumor cells.
The p53-dependent radiosensitization of HCT116 colon cancer cells by MDM2-inhibitors is associated with a reduced Hsp70 membrane expression. This finding was not expected, since most clinically applied anti-cancer drugs have been shown to increase the Hsp70 membrane expression on tumor cells (Gehrmann et al. 2002). High Hsp70 membrane levels have been associated with an unfavorable prognosis and increased resistance towards radiochemotherapy (Gehrmann et al. 2003; 2005). Therefore, the downregulation of membrane Hsp70 by MDM2-inhibition might be beneficial regarding the therapeutic response. Moreover, the reduction of membrane Hsp70 by PXN727 was associated with an increased secretion of Hsp70 from tumor cells. Since we have demonstrated previously that extracellular Hsp70 can increase the radiosensitivity of tumor cells by inducing tumor cell death (Schilling et al. 2009), this effect might contribute to the radiosensitizing effect of PXN727.
The majority of Hsp70 has been shown to be released from tumor cells not as a free protein but rather in membrane vesicles (Gastpar et al. 2005). Due to detergents in the dilution buffer, the ELISA detecting Hsp70 does not distinguish between free and vesicular Hsp70. Therefore, it is not possible to address the question whether MDM2-inhibition induces the release of free or vesicular Hsp70. However, we assume that externalized Hsp70 is derived from the plasma membrane, since cytosolic Hsp70 levels remained unaltered upon MDM2-inhibition. p53 activation has been shown to enhance the secretion of membrane vesicles from human WT p53 cancer cells (Lehmann et al. 2008; Yu et al. 2006). In addition, p53 activation was reported to elicit an endosomal clearance of growth factor receptors from the plasma membrane with the consequence of an attenuated cell growth (Yu et al. 2009). The observed reduction of cell surface Hsp70 levels and increased Hsp70 release upon MDM2-inhibition in WT p53 tumor cells fits into the model of a p53-dependent downregulation of plasma membrane-localized proteins and vesicular secretion.
Since the secretion of Hsp70 was found to be associated with p53-activation and radiosensitization of tumor cells, we speculate that extracellular Hsp70 levels might serve as a biomarker for monitoring and predicting the outcome of patients receiving p53-activating therapeutics. Future experimental approaches using different tumor cell lines and xenograft models are required to confirm this assumption.
Acknowledgment
The authors would like to thank Michaela Wagner, Caroline Hagedorn and Nathalie Lippmann for excellent technical assistance, Peter Vaupel and Christine Bayer for helpful discussion and Khashayar Fakhrian for clinical advice. This work was supported by the Helmholtz Zentrum München (Clinical Cooperation Group - “Innate Immunity in Tumor Biology”), Deutsche Forschungsgemeinschaft (SFB 824/1; Cluster of Excellence 158: Munich-Centre of Advanced Photonics; INST 95/980-1 FUGG, Gulmay irradiation device), BMBF (MOBITUM, 01EZ0826; Kompetenzverbund Strahlenforschung, 03NUK007E; Leading-Edge Cluster m4—Personalized Medicine and Targeted Therapies, 01EX1021C).
Conflict of interest
None
Footnotes
Daniela Schilling and Michael Düwel contributed equally.
References
- Arya AK, El-Fert A, Devling T, Eccles RM, Aslam MA, Rubbi CP, Vlatkovic N, Fenwick J, Lloyd BH, Sibson DR, Jones TM, Boyd MT. Nutlin-3, the small-molecule inhibitor of MDM2, promotes senescence and radiosensitises laryngeal carcinoma cells harbouring wild-type p53. Br J Cancer. 2010;103:186–195. doi: 10.1038/sj.bjc.6605739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C, Shinohara ET, Subhawong TK, Geng L, Woon KK, Albert JM, Hallahan DE, Lu B. Radiosensitization of lung cancer by nutlin, an inhibitor of murine double minute 2. Mol Cancer Ther. 2006;5:411–417. doi: 10.1158/1535-7163.MCT-05-0356. [DOI] [PubMed] [Google Scholar]
- Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, Multhoff G. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005;65:5238–5247. doi: 10.1158/0008-5472.CAN-04-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrmann M, Pfister K, Hutzler P, Gastpar R, Margulis B, Multhoff G. Effects of antineoplastic agents on cytoplasmic and membrane-bound heat shock protein 70 (Hsp70) levels. Biol Chem. 2002;383:1715–1725. doi: 10.1515/BC.2002.192. [DOI] [PubMed] [Google Scholar]
- Gehrmann M, Schmetzer H, Eissner G, Haferlach T, Hiddemann W, Multhoff G. Membrane-bound heat shock protein 70 (Hsp70) in acute myeloid leukemia: a tumor specific recognition structure for the cytolytic activity of autologous NK cells. Haematologica. 2003;88:474–476. [PubMed] [Google Scholar]
- Gehrmann M, Marienhagen J, Eichholtz-Wirth H, Fritz E, Ellwart J, Jaattela M, Zilch T, Multhoff G. Dual function of membrane-bound heat shock protein 70 (Hsp70), Bag-4, and Hsp40: protection against radiation-induced effects and target structure for natural killer cells. Cell Death Differ. 2005;12:38–51. doi: 10.1038/sj.cdd.4401510. [DOI] [PubMed] [Google Scholar]
- Gudkov AV, Komarova EA. The role of p53 in determining sensitivity to radiotherapy. Nat Rev Cancer. 2003;3:117–129. doi: 10.1038/nrc992. [DOI] [PubMed] [Google Scholar]
- Impicciatore G, Sancilio S, Miscia S, Di PR. Nutlins and ionizing radiation in cancer therapy. Curr Pharm Des. 2010;16:1427–1442. doi: 10.2174/138161210791033932. [DOI] [PubMed] [Google Scholar]
- Jackson JG, Pant V, Li Q, Chang LL, Quintas-Cardama A, Garza D, Tavana O, Yang P, Manshouri T, Li Y, El-Naggar AK, Lozano G. p53-mediated senescence impairs the apoptotic response to chemotherapy and clinical outcome in breast cancer. Cancer Cell. 2012;21:793–806. doi: 10.1016/j.ccr.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann BD, McCubrey JA, Jefferson HS, Paine MS, Chappell WH, Terrian DM. A dominant role for p53-dependent cellular senescence in radiosensitization of human prostate cancer cells. Cell Cycle. 2007;6:595–605. doi: 10.4161/cc.6.5.3901. [DOI] [PubMed] [Google Scholar]
- Lehmann BD, Paine MS, Brooks AM, McCubrey JA, Renegar RH, Wang R, Terrian DM. Senescence-associated exosome release from human prostate cancer cells. Cancer Res. 2008;68:7864–7871. doi: 10.1158/0008-5472.CAN-07-6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis M, Rothweiler F, Barth S, Cinatl J, van Rikxoort M, Loschmann N, Voges Y, Breitling R, von Deimling A, Rodel F, Weber K, Fehse B, Mack E, Stiewe T, Doerr HW, Speidel D, Cinatl J., Jr Adaptation of cancer cells from different entities to the MDM2 inhibitor nutlin-3 results in the emergence of p53-mutated multi-drug-resistant cancer cells. Cell Death Dis. 2011;2:e243. doi: 10.1038/cddis.2011.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis M, Rothweiler F, Agha B, Barth S, Voges Y, Loschmann N, von Deimling A, Breitling R, Wilhelm DH, Rodel F, Speidel D, Cinatl J., Jr Human neuroblastoma cells with acquired resistance to the p53 activator RITA retain functional p53 and sensitivity to other p53 activating agents. Cell Death Dis. 2012;3:e294. doi: 10.1038/cddis.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyachi M, Kakazu N, Yagyu S, Katsumi Y, Tsubai-Shimizu S, Kikuchi K, Tsuchiya K, Iehara T, Hosoi H. Restoration of p53 pathway by nutlin-3 induces cell cycle arrest and apoptosis in human rhabdomyosarcoma cells. Clin Cancer Res. 2009;15:4077–4084. doi: 10.1158/1078-0432.CCR-08-2955. [DOI] [PubMed] [Google Scholar]
- Multhoff G, Botzler C, Wiesnet M, Muller E, Meier T, Wilmanns W, Issels RD. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer. 1995;61:272–279. doi: 10.1002/ijc.2910610222. [DOI] [PubMed] [Google Scholar]
- Rothweiler U, Czarna A, Krajewski M, Ciombor J, Kalinski C, Khazak V, Ross G, Skobeleva N, Weber L, Holak TA. Isoquinolin-1-one inhibitors of the MDM2-p53 interaction. Chem Med Chem. 2008;3:1118–1128. doi: 10.1002/cmdc.200800025. [DOI] [PubMed] [Google Scholar]
- Schilling D, Gehrmann M, Steinem C, De MA, Pockley AG, Abend M, Molls M, Multhoff G. Binding of heat shock protein 70 to extracellular phosphatidylserine promotes killing of normoxic and hypoxic tumor cells. FASEB J. 2009;23:2467–2477. doi: 10.1096/fj.08-125229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shangary S, Wang S. Targeting the MDM2-p53 interaction for cancer therapy. Clin Cancer Res. 2008;14:5318–5324. doi: 10.1158/1078-0432.CCR-07-5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman M, Multhoff G. Heat shock proteins in cancer. Ann N Y Acad Sci. 2007;1113:192–201. doi: 10.1196/annals.1391.030. [DOI] [PubMed] [Google Scholar]
- Supiot S, Hill RP, Bristow RG. Nutlin-3 radiosensitizes hypoxic prostate cancer cells independent of p53. Mol Cancer Ther. 2008;7:993–999. doi: 10.1158/1535-7163.MCT-07-0442. [DOI] [PubMed] [Google Scholar]
- Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66:4795–4801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- Yu X, Riley T, Levine AJ. The regulation of the endosomal compartment by p53 the tumor suppressor gene. FEBS J. 2009;276:2201–2212. doi: 10.1111/j.1742-4658.2009.06949.x. [DOI] [PubMed] [Google Scholar]