Abstract
In this paper, two-dimensional flow field simulation was conducted to determine shear stresses and velocity profiles for bone tissue engineering in a rotating wall vessel bioreactor (RWVB). In addition, in vitro three-dimensional fabrication of tissue-engineered bones was carried out in optimized bioreactor conditions, and in vivo implantation using fabricated bones was performed for segmental bone defects of Zelanian rabbits. The distribution of dynamic pressure, total pressure, shear stress, and velocity within the culture chamber was calculated for different scaffold locations. According to the simulation results, the dynamic pressure, velocity, and shear stress around the surface of cell-scaffold construction periodically changed at different locations of the RWVB, which could result in periodical stress stimulation for fabricated tissue constructs. However, overall shear stresses were relatively low, and the fluid velocities were uniform in the bioreactor. Our in vitro experiments showed that the number of cells cultured in the RWVB was five times higher than those cultured in a T-flask. The tissue-engineered bones grew very well in the RWVB. This study demonstrates that stress stimulation in an RWVB can be beneficial for cell/bio-derived bone constructs fabricated in an RWVB, with an application for repairing segmental bone defects.
Keywords: Rotating wall vessel bioreactor, FLUENT software, Stress stimulation, Tissue-engineered bones, Animal experiment
Introduction
Currently, static culture systems that are most commonly used for in vitro amplification of seeded cells in tissue-engineered bones and in the construction of engineered tissues have many shortcoming and disadvantages (Collins et al. 1998; Volkmer et al. 2008; da Costa Goncalves et al. 2012; Zhu et al. 2010; Wang et al. 2005). Hydrodynamic advantages that are observed in bioreactor cultures of tissue-engineered bones have led to a significant assistance to this field (Rauh et al. 2011; Yeatts & Fisher 2011; Salter et al. 2012; Yu et al. 2004; Liu et al. 2012). Although many experiments and theories have shown that the functionality and quality of cultured cells and engineered tissues in bioreactors can be improved by mechanical stimulation, research on the direct impact of mechanical stimulation on cells or tissues, such as the magnitude, frequency, continuity, and cyclical changes of the mechanical stimuli, are not well characterized (Yeatts & Fisher 2011; Yu et al. 2004; Martin et al. 2004; Song et al. 2006; Hu and Athanasiou 2005; Massimo et al. 2004; Van Dyke et al. 2012). In addition, in the culture suspension, collisions with the inner and outer walls of bioreactors are inevitable especially when the density and dimensions of the cell-scaffold constructs, such as those observed in the RWVB (rotating wall vessel bioreactor), reach a certain level (Song et al. 2011; Rodrigues et al. 2011; Song et al. 2008). Such collisions could cause shear stresses that may be destructive to the cells in the engineered tissue.
In the present investigation, we seek to quantify the flow mechanics and mechanical stresses exerted on cell tissue constructs in the RWVB. To address the issue of cell collision, we devised a method for fixing cell-scaffold constructs on the outer wall of the bioreactor and rotating them at the same speed and in the same direction as the inner and outer cylinders to effectively prevent them from colliding with the wall and other scaffold constructs. Then, we evaluated the performance of the cell-scaffold construct under stress stimulation at different radial locations of the RWVB and determined the fluid field distribution around these locations. Large-scale expansion of seeded cells in a three-dimensional fabrication of tissue-engineered bones using an RWVB was evaluated in vitro and in vivo. The animal studies were performed on segmental bone defects of Zelanian rabbits and the in vitro assessments of differentiated osteoblasts in the tissue-engineered constructs were compared to static cultures. This paper describes a detailed simulation and analysis in the distribution of shear stresses and fluid field in the bioreactor using FLUENT software after cell expansion. The tissue-engineered bone construct model in RWVB was simplified in two dimensions.
Software and methods
Gambit and fluent
ANSYS Fluent software (ANSYS, Canonsburg, PA, USA) was used to calculate fluid flow distribution, and heat and mass transfer in a complex geometric system. It provides ideal grid adaptability and can solve complex fluid problems easily by an irregular grid, which is provided by complex geometry (Hou et al. 2005; Agarwal 2009). In view of the characteristics of every physical problem in fluid dynamics, a suitable numerical solution is provided by ANSYS Fluent software in order to achieve optimality in multiple aspects such as computation speed, stability, and precision (Song et al. 2010).
The physical and simplified model of bioreactor flow
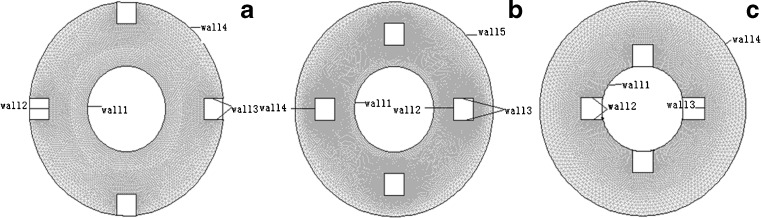
To simplify the calculation, the flow of the RWVB is treated as a two-dimensional problem in the cylindrical coordinates of r and θ in both directions. As the rotation speeds of the inner and outer cylinders used in this study were low, the calculated speed of the inner and outer cylinders were below 30 rpm, and the flow field in the bioreactor remained in the laminar flow condition. Therefore, laminar modeling in fluent could be used here for numerical simulation calculations. To calculate the shear stress distribution of the fixed cell on the wall of a hollow-fiber membrane during the cultivation process, we calculated the rate and stress of the fluid field in the bioreactor. The specific conditions and calculation objects were as follows: In order to calculate the force condition and its distribution around the flow field of the cell-scaffold construct, we set the two-dimensional section size of the constructs to 5 × 5 mm. Changes in the simulation calculations, collected from the outer cylinder wall, the middle section (between the inner and outer walls of the cylinder), and the inner cylinder wall of the reactor, were analyzed when the rotation direction and speed of the inner and outer cylinders were altered. The partition of the two-dimensional mesh and constraints that defined the physical boundaries above is shown in Fig. 1.
Fig. 1.
Partition of two-dimensional mesh and definiens of boundaries. a–c Cell-scaffold constructs fixed at the outer cylinder, middle of the chamber, and inner cylinder, respectively
Relative parameters and boundaries conditions
Parameters of the simplified two-dimensional simulation model of the RVWB are given in Table 1, and relative boundaries conditions are shown in Table 2.
Table 1.
Dimensions of bioreactor and cell-scaffold constructs
| Item | Dimensions (mm) |
|---|---|
| Inner diameter of the bioreactor | 20 |
| Outer diameter of the bioreactor | 50 |
| Sectional size of cell-scaffold constructs | 5 × 5 |
Table 2.
Parameters and boundary conditions
| Culture medium density (kg/m3) | 1,030 |
|---|---|
| Dynamic viscosity (Pa s) | 2.5×10−3 |
| Vessel rotational velocity (rpm) | 1, 10, 30, −1, −10, −30 |
| Fluent version | 2 ddp |
| Solver | Segregated, implicit |
| Pressure | Body-force weighted |
| Pressure–velocity coupling | PISO |
| Momentum | Second-order upwind |
| Flow | Laminar |
In vitro 3D fabrication of engineered bones in RWVB
Prior to cell seeding, osteoblast cells from Zelanian rabbits were transfected with green fluorescent protein and then counted and diluted with DMEM including 10 % neonatal bovine serum and 1 % antibiotics (penicillin and streptomycin) to a concentration of 2 × 106 cells/mL. The cell suspension was gently dropped onto the surface of the scaffolds, and about 100 μL of the cell suspensions permeated into the inside of each scaffold. After 3 min, the scaffolds were turned over and the cell suspensions were dropped onto the scaffolds using the same method as described previously. After allowing the seeded scaffolds to stand for 10 min, the scaffolds were put into T-flasks in an incubator at 37 °C and cultured with DMEM including 10 % neonatal bovine serum and 1 % antibiotics for 2 h, respectively. These samples were cultured for 2 h again after the appropriate culture was added. Subsequently, a fraction of the scaffolds were fixed into the outer cylinder of the RWVB. For a comparative study, some seeded scaffolds were also cultured under static conditions. For the RWVB, the outer and inner cylinders were set into rotation, driven by step motors at the same speed and direction. They were rotated at 5 rpm for the first 12 h, and the speed was then increased to 10 rpm. At the same time, the temperature control, gas control, and the circulation of medium were also initiated. The suspension samples were collected every 12 h to observe whether there were any fragments of osteoblasts in the solution. For glucose, lactic acid, and ALP determination, the supernatants samples were collected and stored at −70 °C for later analysis. No medium exchanges were performed during the 7 days of experiment.
Animal experiment
This study was approved by the Ethics Committee of Dalian University of Technology and by an Administrative method of Experimental Animal License (2001-545). An animal experiment was performed to repair segmental bone defects of Zelanian rabbits using our tissue-engineered bone fabricated in a 3D RWVB environment. A 15-mm bone defect in the radius was induced in New Zealand white rabbits, and the models were randomized into three groups to receive implantation of tissue-engineered bone grafts constructed with bio-derived bone and osteoblast in the RWVB (experimental group), grafts of tissue-engineered bone cultured in T-flasks (control group), or grafts of exclusive bio-derived bone (blank control group), respectively. Histological changes in the tissues were sampled from the bone defect 4 and 8 weeks after operation, and the area of new bone formation was measured by image analysis.
Fluorescence microscopy analysis of double staining
Fluorescence microscopy analysis of cell death was determined by acridine orange/ethidium bromide (AO/EB) double staining with slight modifications as described by Agarwal (2009). One milliliter of dye mixture (100 lg/mL AO and 100 lg/mL EB, diluted in phosphate-buffered saline, pH 7.4) was dropped gently onto a slice of engineered bone and put onto a slide. The slice was immediately examined under a fluorescence microscope since dye uptake is very fast.
Scanning electron microscopy examination
The cell-scaffold constructs were fixed and dehydrated in increasing concentrations of ethanol solutions (50, 70, 80, 90, and 100 %) for 15 min each time and then immediately dried with hexamethyldisilazane solution for 5 min. The samples were then coated with platinum and palladium, and carbon. The preparations were viewed with SEM at 40 kV.
Statistical analysis
All experiments were performed in triplicate. Results are expressed as the mean ± SD. The statistical significance of differences within each group was evaluated by one-way analysis of variance using the software Origin7.0 (OriginLab Corporation, USA).
Results and discussion
Analysis of the cell-scaffold constructs fixed on the outer wall
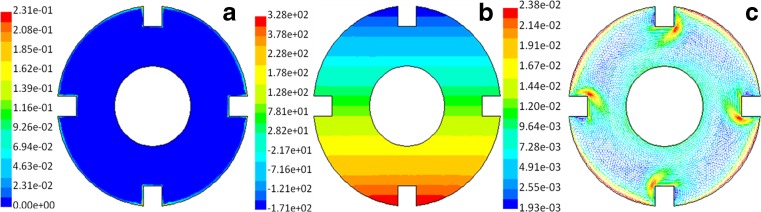
Cell-scaffold fabrications were fixed onto the outer wall of the cylinder, and the distribution of total pressure (Fig. 2b) and shear stress (Fig. 2c) of the fluid field in the bioreactor was determined. The greatest shear stresses, whose magnitudes were still very small, only occurred at the edges of cell-scaffold construction and the outer wall surfaces of the bioreactor (Fig. 2). The remaining areas inside the bioreactor had no shear stress. Total pressure was distributed symmetrically along the Y-axis. We found that these parts were inclined to become non-flow areas because of the acute angle formed between the cell-scaffold construct and the wall of bioreactor (Fig. 2). The total pressure inside the bioreactor showed no apparent relation to the rotating speed.
Fig. 2.
Distribution of shear stress (a), total pressure (b), and velocity (c) within the flow field of the bioreactor (cell-scaffold constructs). Rotating speeds of outer cylinder and inner cylinder were both at 10 rpm
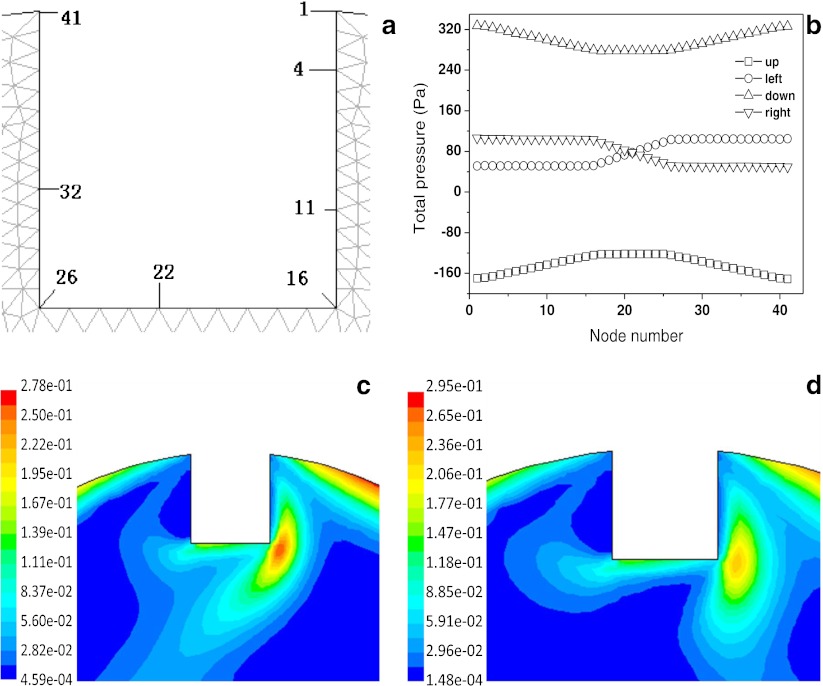
Figure 3 shows the distribution of wall shear stress on the surface of the cell-scaffold constructs’ node within the flow field of the bioreactor at different rotating directions and speeds. The shear stress profiles show that the change in shear stresses on the surface of the node of the construct is interrelated with the change of rotating speed of the outer cylinder (E, F→A, B→C, D). Because the construct is far from the inner cylinder, shear stress on the surface of the node of the construct is not related with the rotating directions of the inner cylinder; it was also found that shear stress on the surface of the same construct changed with different positions, but it did not change on the same node when the reactor was revolving. The distribution of dynamic pressure around the walls of cell-scaffold constructs within the flow field of the bioreactor under rotation with different directions and speeds, when the construct was on the wall of the outer cylinder, was determined (Fig. 4). It was found that on either side of the construct, dynamic pressures were obviously different and could result in the formation of dynamic pressure gradients. Thus, when hollow fiber membrane assembly or the engineered bone tissues with different sizes were installed, scaffold placement on the wall of the outer cylinder could greatly improve the efficiency of mass transfer by convection.
Fig. 3.
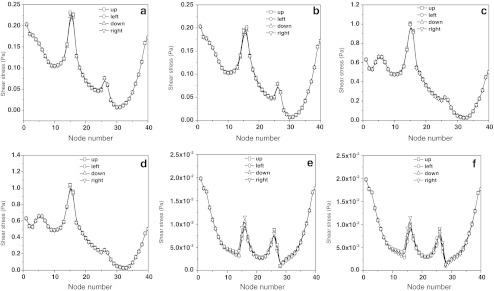
Distribution of wall shear stress on the cell-scaffold constructs within the flow field of the bioreactor with different rotating directions and speeds. Rotating speeds of outer cylinder and inner cylinder are a 10 and 10 rpm, b 10 and −10 rpm, c 30 and 30 rpm, d 30 and −30 rpm, e 1 and 1 rpm, and f 1 and −1 rpm, respectively
Fig. 4.
Node distribution of constructs wall (a), total pressure (b), and dynamic pressure distribution of wall on constructs at different locations in bioreactor. Rotating speeds of inner and outer cylinder are 10 and 10 rpm (c) and 10 and −10 rpm (d)
Figure 5 shows the choice of different positions and the distribution of velocities around constructs. Our simulation results showed that when the rotating speeds of both the outer cylinder and inner cylinder were increased(F,G→B,C→D,E), the velocity of flow obviously increased. However, it was also found that the velocity of the flow in the same construct differed at different positions.
Fig. 5.
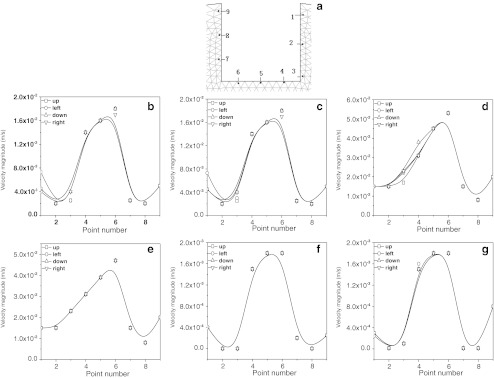
Distribution of velocities around the cell-scaffold constructs within the flow field of the bioreactor under the rotation with different directions and speeds. Position of sample points (a); rotating speeds of outer cylinder and inner cylinder are b 10 and 10 rpm, c 10 and −10 rpm, d 30 and 30 rpm, e 30 and −30 rpm, f 1 and 1 rpm, and g 1 and −1 rpm, respectively
Analysis on cell-scaffold material complex at other locations in the chamber
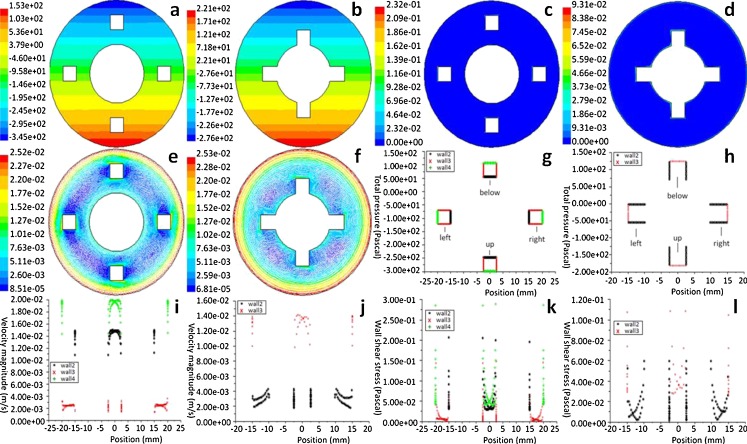
In Fig. 6g–l, the x-axis is the abscissa of the constructions. Figure 6 shows that the total pressure, velocity, and shear stress around the spot in the same construct changed when they were placed at different locations. The total pressure around the surface of the construct changed periodically at different locations; the fields of velocity showed that fluid velocity in the radial position from the outside to inside and the wall of the construct would lessen. The shear stress around the surface of construct was affected by the velocities on the outer-and-inner wall of the vessel.
Fig. 6.
Distribution of total pressure, wall shear stress, and velocities around the cell-scaffold constructs located at the different radial locations (g–l) and within the flow field (a–f) of the bioreactor. Cell-scaffold constructs in the middle of the inner and outer cylinders (a, c, e) and near the inner cylinder (b, d, f); around the cell-scaffold constructs wall, in the middle of the inner, and outer cylinders (g, i, k) and near the inner cylinder (h, j, l), rotating speeds of the outer and inner cylinders are 10 and 10 rpm
Morphology of cells and scaffolds
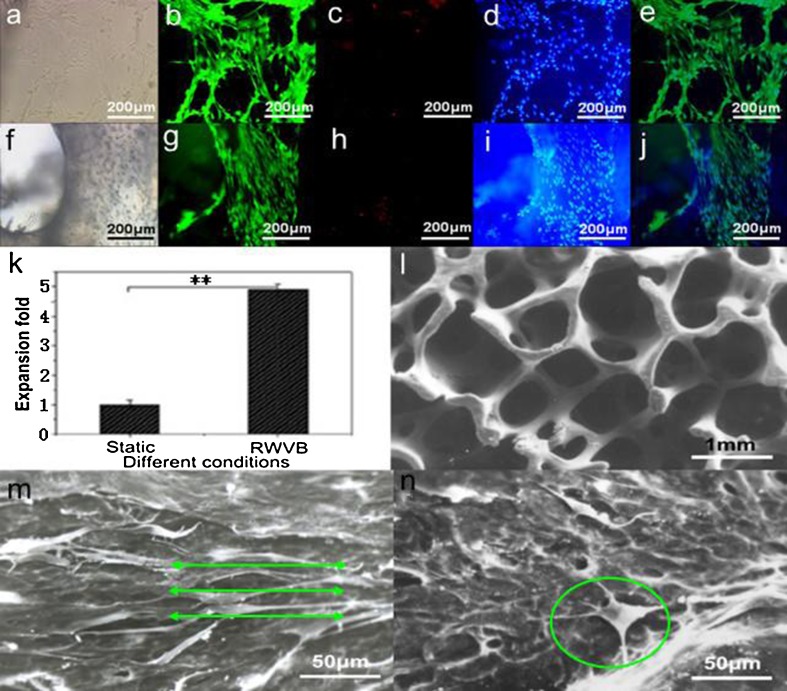
As shown in Figs. 7a–j, the osteoblasts were biologically active and showed typical osteoblast phenotypes. The cells were evaluated with an inverted microscope (Fig.7a, f). We found that the cell membrane was unbroken, cells had several synapses, and the profile of the nucleolus was clear and unbroken. Figure 7b–d showed that the seeded osteoblasts maintained very high cellular viability. The osteoblasts uniformly adhered to the inner and outer surfaces of scaffolds and the biomaterial used for the scaffold construct showed good biocompatibility with the cells (Fig. 7g–i). The in vitro experiments showed that the number of cells cultured in RWVB was five times higher than those cultured in static T-flasks, as shown in Fig. 7k. Based on a morphological evaluation, the surfaces of the inner and outer pores of vacant scaffolds were smooth and glossy, showing excellent three-dimensional reticular configuration (Fig.7l). A mass of cells was observed to attach and spread within the pores and spread across smaller pores inside the scaffolds fabricated in the RWVB (Fig. 7m). Moreover, osteoblasts in the fabricated construct in Fig. 7m presented typical and regular osteoblast phenotypes after 1 week of culture, with a high level of cellular connectivity as cells attached to the scaffolds with filopodia, and formed bridges to each other with bunchy fibrillar collagen that was synthesized and organized by the seeded osteoblasts. These results indicate that hydrodynamic stimulation provided by the RWVB directly affected the cell framework and caused tactical alterations that were not observed in the static culture, as shown in Fig. 7n.
Fig. 7.
Live/dead assay of passaged osteoblasts at day 7 and cell viability, expansion, and morphology of osteoblast/bio-derived bone scaffold constructs after 7 days of fabrication. a Bright field, b live cells stained with calcein AM, c dead cells stained with PI, d blue nuclei of live cells stained with Hoechst 33342, e overlayed by b–d; f bright field, g live cells stained with calcein AM, h dead cells stained with PI, i blue nuclei of live cells stained with Hoechst 33342, j overlayed by g–i; a–j ×100. k Comparison of cell expansion for osteoblasts in different culture fashions. l SEM micrographs of vacant scaffold. SEM micrographs of scaffolds cultured with osteoblasts in RWVB (m, green lines showed the cellular direction for osteoblast spreading affected by hydrodynamic stimulation) and static T-flask (n, osteoblast marked with green circle did not show typical phenotype) after 1 week of fabrication
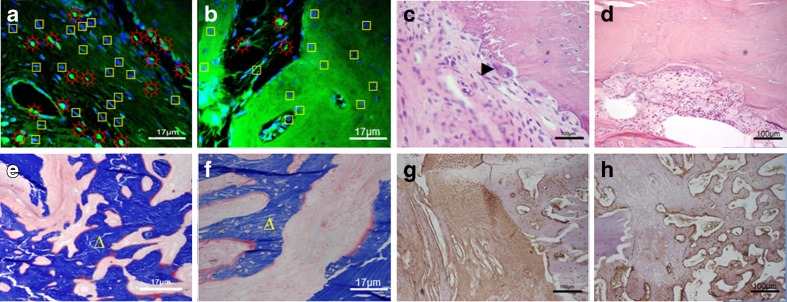
Histological sections
As shown in Fig. 8a, the osteoprogenitor cells and bio-derived bone scaffolds combined and healed better compared to those in the static repair group (Fig. 8b). Figure 8c, d showed that bone remodeling and fusion of the material and the host bone tissues were observed in tissue after 8 weeks of transplantation and that surfaces of the implant material had adsorption depressions of Conical bone, which contained osteoclasts. A mass of chondrocytes, much more collagen and new bone, were observed in the tissue-engineered construct compared with the static repair group Fig. 8e, f. Figure 8g showed stronger type I collagen expressions of osteoblasts than that in the static repair group (Fig. 8h).
Fig. 8.
Laser confocal micrograph, HE stained, Masson stained and type I collagen assay of histological sections of rabbit radial defects repaired with tissue-engineered bone constructs after 8 weeks. Laser confocal microscope picture in the RWVB repair group (a) and the static repair group (b): sun with rays ectogenous osteoblasts, square endogenous osteoblasts, 600×; HE stains in the RWVB repair group (c) and the static repair group (d), right-pointing pointer osteoclasts, 100×; Masson stains in the RWVB repair group (e) and the static repair group (f), triangle new bones, 600×; type I collagen assays in the RWVB repair group (g) and the static repair group (h), 100×
Conclusions
Simulation studies performed in the RWVB showed that the total pressure, velocity, and shear stress around the surface of a hollow-fiber membrane and cell-scaffold construct periodically changed with its locations. Despite the fact that shear stress within the flow field is relatively low, cell-scaffold constructs located at the wall of the outer cylinder would be subjected to the biggest dynamic pressure gradients, so that the efficiency of mass transfer by convection could be improved. Furthermore, our in vitro studies showed the hydrodynamicity and the three-dimensional culture environment provided by the RWVB improved proliferation and differentiation of osteoblast cells in scaffold constructs and significantly shortened the time for cell construct organization. The ability to repair defective bones using organized tissue-engineered bones constructed by the RWVB is superior to results achieved in culture bottles and static flask cultures.
Acknowledgments
This work was supported by the Fok Ying Tung Education Foundation (132027), the National Science Foundation of China (31170945/30700181), the Fundamental Research Funds for the Central Universities (DUT11SM09/DUT12JB09), and the SRF for ROCS, SEM.
Contributor Information
Kedong Song, Phone: +86-411-84706360, Email: Kedongsong@dlut.edu.cn.
Tianqing Liu, Phone: +86-411-84706360, Email: liutq@dlut.edu.cn.
References
- Agarwal P (2009) Simulation of heat transfer phenomenon in furnace using fluent-gambit. Thesis, National Institute of Technology, Rourkela.
- Collins PC, Miller WM, Papoutsakis ET. Stirred culture of peripheral and cord blood hematopoietic cells offers advantages over traditional static systems for clinically relevant applications. Biotechnol Bioeng. 1998;59(5):534–543. doi: 10.1002/(SICI)1097-0290(19980905)59:5<534::AID-BIT2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- da Costa Goncalves F, da Rosa Paz AH, Priscila Schmidt L, et al. Dynamic culture improves MSC adhesion on freeze-dried bone as a scaffold for bone engineering. World journal of stem cells. 2012;4(2):9–16. doi: 10.4252/wjsc.v4.i2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q, Pan HL, Fen QB (2005) Research of optimizing fluid field of agitator by fluent. Mach Des Res 21(3):78–82
- Hu JC, Athanasiou KA. Low-density cultures of bovine chondrocytes: effects of scaffold material and culture system. Biomaterials. 2005;26(14):2001–2012. doi: 10.1016/j.biomaterials.2004.06.038. [DOI] [PubMed] [Google Scholar]
- Liu CX, Abedian R, Meister R, et al. Influence of perfusion and compression on the proliferation and differentiation of bone mesenchymal stromal cells seeded on polyurethane scaffolds. Biomaterials. 2012;33(4):1052–1064. doi: 10.1016/j.biomaterials.2011.10.041. [DOI] [PubMed] [Google Scholar]
- Martin I, Wendt D, Heberer M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004;22(2):80–86. doi: 10.1016/j.tibtech.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Massimo P, Nicola L, Alberto C, et al. Modeling of engineered cartilage growth in rotating bioreactors. Chem Eng Sci. 2004;59:5035–5040. doi: 10.1016/j.ces.2004.07.101. [DOI] [Google Scholar]
- Rauh J, Milan F, Günther KP, et al. Bioreactor systems for bone tissue engineering. Tissue Eng Part B Rev. 2011;17(4):263–280. doi: 10.1089/ten.teb.2010.0612. [DOI] [PubMed] [Google Scholar]
- Rodrigues CAV, Fernandes TG, Diogo MM, et al. Stem cell cultivation in bioreactors. Biotechnol Adv. 2011;29(6):815–829. doi: 10.1016/j.biotechadv.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Salter E, Goh B, Hung B, et al. Bone tissue engineering bioreactors: a role in the clinic? Tissue Engineering Part B-Reviews. 2012;18(1):62–75. doi: 10.1089/ten.teb.2011.0209. [DOI] [PubMed] [Google Scholar]
- Song KD, Yang ZM, Liu TQ, et al. Fabrication and detection of tissue-engineered bones with bio-derived scaffolds in a rotating bioreactor. Biotechnol Appl Biochem. 2006;45:65–74. doi: 10.1042/BA20060045. [DOI] [PubMed] [Google Scholar]
- Song KD, Liu TQ, Cui ZF, et al. Three-dimensional fabrication of engineered bone with human bio-derived bone scaffolds in a rotating wall vessel bioreactor. J Biomed Mater Res A. 2008;86A(2):323–332. doi: 10.1002/jbm.a.31624. [DOI] [PubMed] [Google Scholar]
- Song AP, Gao S, Wu WW (2010) The mesh characteristics of arch cylindrical gear and its digitization analysis. In: Proceedings of the 2010 International Conference on Digital Manufacturing & Automation, vol 2, pp 720–724
- Song KD, Liu Y, Wang H, et al. Ex vivo expansion of human umbilical cord blood hematopoietic stem/progenitor cells with support of microencapsulated rabbit mesenchymal stem cells in a rotating bioreactor. Tissue Engineering and Regenerative Medicine. 2011;8(3):334–345. [Google Scholar]
- Van Dyke WS, Sun XH, Richard AB, et al. Novel mechanical bioreactor for concomitant fluid shear stress and substrate strain. J Biomech. 2012;45(7):1323–1327. doi: 10.1016/j.jbiomech.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Volkmer E, Drosse I, Otto S, et al. Hypoxia in static and dynamic 3D culture systems for tissue engineering of bone. Tissue Eng Part A. 2008;14(8):1331–1340. doi: 10.1089/ten.tea.2007.0231. [DOI] [PubMed] [Google Scholar]
- Wang D, Liu W, Han B, et al. The bioreactor: a powerful tool for large-scale culture of animal cells. Curr Pharm Biotechnol. 2005;6(5):397–403. doi: 10.2174/138920105774370580. [DOI] [PubMed] [Google Scholar]
- Yeatts AB, Fisher JP. Bone tissue engineering bioreactors: dynamic culture and the influence of shear stress. Bone. 2011;48:171–181. doi: 10.1016/j.bone.2010.09.138. [DOI] [PubMed] [Google Scholar]
- Yu XJ, Botchwey EA, Levine EM, et al. Bioreactor-based bone tissue engineering: the influence of dynamic flow on osteoblast phenotypic expression and matrix mineralization. Pans. 2004;101:11203–11208. doi: 10.1073/pnas.0402532101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YX, Liu TQ, Ye H, et al. Enhancement of adipose-derived stem cell differentiation in scaffolds with IGF-I gene impregnation under dynamic microenvironment. Stem cells and development. 2010;19(10):1547–1556. doi: 10.1089/scd.2010.0054. [DOI] [PubMed] [Google Scholar]