Abstract
Alternative vectors to deliver viable cells of probiotics, to those conferring limited resistance to gastrointestinal conditions, still need to be sought. Therefore the main goal of the study was to develop tablets able to protect entrapped probiotic bacteria from gastric acidity, thus providing an easily manufacturing scale-up dosage form to deliver probiotics to the vicinity of the human colon. Whey protein concentrate microparticles with Lactobacillus paracasei L26 were produced by spray-drying and incorporated in tablets with cellulose acetate phthalate and sodium croscarmellose. The viability of L. paracasei L.26 throughout tableting as well as its gastric resistance and release from the tablets were evaluated. Storage stability of L. paracasei L26 tablets was also performed by evaluation of viable cells throughout 60 days at 23°C and 33% relative humidity. A decrease of approximately one logarithmic cycle was observed after the acid stage and the release of L. paracasei L26 from the tablets occurred only after 4 h in the conditions tested. Microencapsulated L. paracasei L26 in tablets revealed some susceptibility to the storage conditions tested since the number of viable cells decreased 2 log cycles after 60 days of storage. However, the viability of L. paracasei L26 after 45 days of storage did not reveal significant susceptibility upon exposure to simulated gastrointestinal conditions. The developed probiotic tablets revealed to be potential vectors for delivering viable cells of L. paracasei L26 and probably other probiotics to persons/patients who might benefit from probiotic therapy.
KEY WORDS: colonic drug delivery, Lactobacillus paracasei, microencapsulation, release studies, tableting
INTRODUCTION
During the last years, there has been an increasing interest in probiotics which have been defined by a joint expert consultation (1) as “Live microorganisms which when administered in adequate amounts confer a health benefit on the host.” Lourens-Hattingh and Viljoen (2) and Holzapfel et al. (3) considered that the benefit is due to the improvement of intestinal microbial balance. Some studies have also reported that probiotics stimulate the immune system (4–6). Members of the genus Lactobacillus are often used as probiotics (7–9). Several authors have attributed to lactic acid bacteria (LAB) therapeutic and nutritional benefits such as the control of some types of cancer (10), the reduction of cholesterol levels (11), or the prevention of diarrhea and food allergies (12,13).
LAB must remain viable and in adequate concentrations during gastrointestinal transit as a prerequisite for any beneficial action (14,15). Although there are several types of products containing probiotic strains (e.g., fermented milk, chewing gums, sachets, and capsules among others), these being incorporated in the products present limited stability and do not always survive in the harsh conditions of the gastrointestinal tract (16). Therefore, it is still necessary to develop formulations that protect LAB from gastric pH and harsh conditions found in the duodenum and the ileum. Tablets have several advantages over other dosage forms such as easiness of production and administration, accurate dosage, good acceptance, and can be developed in order to allow delivery in the colon. Probiotics must colonize the distal ileum and colon in order to exert their action (17). Previous works designed and studied probiotic tablets using lyophilization as a way to obtain concentrated probiotic powders (16). These solid forms are coated tablets obtained by double compression (18) and swelling matrix tablets (14,19). In some of those formulations, the excipients used are new and its human in vivo security is not proven (20); in other cases, some scale-up manufacturing difficulties and the deleterious contact between the gastric medium and microorganisms can originate some problems.
Encapsulation is the process of forming a continuous coating around an internal matrix that is fully contained within the wall of the capsule; immobilization refers to the capture of material within or along a matrix (21,22). Encapsulation by spray-drying is used in the food industry because in addition to being economic and flexible, it produces good quality products (22,23). Spray-drying is a potential cost-effective way to prepare large quantities of some probiotics (24). Furthermore, spray-drying proved to be a suitable method to immobilize Lactobacillus paracasei L26 in whey protein microparticles enabling good viability of the probiotic cells (>106 colony forming units per gram; CFU/g) throughout 180 days of storage (25). A disadvantage of this process is that some probiotic cells may be exposed at the surface. To overcome this possible drawback and to allow delivery in the colon, it was decided to utilize tablets as dosage form.
As far as we are aware of, there are no studies available on probiotic tablets with microencapsulated bacteria obtained via spray-drying. In general, the literature refers to examples of probiotic tablets preparation using lyophilized microorganisms. The purpose of this study was to design tablets able to protect entrapped probiotic bacteria from the gastric acidity, allowing their release near or in the colon, based on a simple and easily scale-up method. To the best of our knowledge, the use of probiotic tablets produced by such simple method, with approved excipients represents an added value consisting in a new desirable dosage form to deliver probiotics in the human colon.
MATERIALS AND METHODS
Materials
L. paracasei LAFTI® L26 was obtained as freeze-dried concentrated starter cultures (DELVO-PRO®, DSM, Australia). Whey protein concentrate was obtained from Formulab (Portugal), croscarmellose sodium (Ac-Di-Sol®) from FMC (Belgium), and cellulose acetate phthalate (CAP) from Eastman (USA). The other chemicals were of analytical grade.
Microorganisms Microencapsulation in Whey Protein Concentrate
L.paracasei L26 was reactivated using pre-culture in de Man–Rogosa–Sharpe (MRS) broth (from Biokar Diagnostics, France), incubated overnight at 37°C. The culture was propagated by inoculating fresh media at 10% (v/v), and incubated under appropriate conditions. The resulting culture was centrifuged at 4,000 rpm for 20 min, at 4°C. The supernatant was then discarded, and the pellet was suspended in one tenth of its original volume of aqueous 0.85% (w/v) NaCl (Panreac, Spain).
The 10% (v/v) probiotic suspension was added to a 5% (w/v) whey protein concentrate dispersion containing 50% (w/w) protein (WPC50). WPC50 culture [(6.0 ± 1.8) × 1010 CFU/mL (mean ± standard deviation)] was delivered by a peristaltic pump to a rotary atomizer (GEA Niro, Denmark), coupled to a drying chamber (Arsopi, Portugal), and spray-dried using 160°C and 75°C as inlet and outlet air temperatures, respectively, to generate the intended microparticles. The microparticles obtained were stored for a maximum of 48 h at room temperature.
Tablets Preparation
In order to evaluate the effect of compaction force on viability of the probiotic strain, tablets with 400 mg of L. paracasei L26 microparticules only, were prepared in a hydraulic press using 11-mm-diameter punches (for approximately 2 s). The compaction forces tested were 9.8, 19.6, 29.4, and 39.2 kN; three tablets (n = 3) were tested for each compaction force.
After compaction tests, tablets were produced with following composition: 400 mg of L. paracasei L26 microparticules, 20 mg of croscarmellose sodium, and 126 mg of cellulose acetate phthalate (CAP). The tablets were prepared as described above using compaction force of 9.8 kN and were submitted to (1) disintegration and hardness tests, (2) release/viability test, (3) storage stability test and, (4) resistance/susceptibility test to simulated gastrointestinal conditions. The mean tablet weight obtained was 547 mg.
For the enumeration of viable cells of L. paracasei L26, the tablets were grounded in a mortar and suspended in phosphate buffer solution (pH 6.8) in a 1:9 (g/mL) ratio. The resulting solution was then subjected to a roll and tilt mixer (Movil-Rod, from J. P. Selecta, Spain) for 2 h at room temperature. Decimal dilutions of sample—using aqueous 0.1% (w/v) peptone (Sigma-Aldrich) and 0.85% (w/v) NaCl—were afterwards plated on MRS agar (Biokar Diagnostics) in duplicate, and the viable cells of L. paracasei L26 were enumerated according to Miles and Misra method (26), following incubation at 37°C for 48 h. The results were expressed in CFU/g.
Tablets Evaluation
Tablets were submitted to disintegration and hardness tests according to the European Pharmacopeia. The disintegration time was determined using a disintegration apparatus (Electrolab ED-2 L, India). HCl 0.1 M at 37°C was used as immersion fluid for 2 h and then substituted for phosphate buffer pH 6.8 (37°C) for the remaining time. The hardness of tablets (n = 5) was evaluated using a proper apparatus (Erweka TBH 28, Germany).
Release/Viability Test
The method described by Stadler and Viernstein (14) with some modifications was used to evaluate the gastric resistance and the release of L. paracasei L26 from the tablets (n = 6) using USP dissolution apparatus II (SOTAX AT7, Switzerland) at 100 rpm. The dissolution medium (37.0 ± 0.5°C) was HCl 0.1 M (750 mL) for the first 2 h (acid stage) and phosphate buffer solution with pH 6.8 (1,000 mL) for the remaining time period (7 h; buffer stage).
For the enumeration of viable cells of L. paracasei L26, present in the dissolution media (HCl 0.1 M and in phosphate buffer), decimal dilutions of sample were plated on MRS agar in duplicate, according to Miles and Misra method as described above.
The evaluation of viable cells of L. paracasei L26 was also performed in the tablets after contact with the acid dissolution medium (n = 3; acid stage) and after contact with the buffer dissolution medium (n = 3; buffer stage) according to similar procedures described above for tablets produced by different compression forces. Tablets subjected to the HCl 0.1 M were first suspended in the phosphate buffer solution, in order to minimize contact of the L. paracasei L26 cells with vestigial acid solution on the tablet, where they were grounded. The results were expressed in CFU/tablet.
Storage Stability Test
For storage stability testing, tablets were kept in a perforated Petri dish in the dark at 23°C and 33% relative humidity through 60 days. These conditions were chosen based on previous results obtained by Rodrigues et al. (25). The relative humidity was achieved by a saturated MgCl2 solution inside of an anaerobic jar, where the Petri dish was being stored. The viable cells of L. paracasei L26 in the tables over storage period of 60 days were obtained as described previously.
Resistance/Susceptibility Test—Simulation of Gastrointestinal Conditions
In order to assess if storage time could affect the resistance/susceptibility of L. paracasei L26 cells in the tablets to simulated gastrointestinal conditions (SGC), tablets after 45 days of storage at 23°C and 33% relative humidity were exposed to SGC according to the procedure described by Madureira et al. (27), with modifications. Due to the lower viability of L. paracasei L26 in the tablets after 60 days of storage, these were not exposed to SGC.
The conditions prevailing in the mouth, esophagus–stomach, duodenum, and ileum were sequentially applied as follows: the mouth microenvironment was paralleled using a synthetic saliva solution prepared with 100 IU/mL of α-amylase (Sigma, USA) in CaCl2 1 mM, and pH was adjusted to 6 using NaHCO3 1 M; this simulated saliva was added at a rate of 0.6 mL/min through 2 min. For the esophagus–stomach step, 25 mg/mL pepsin (Sigma-Aldrich, USA) was prepared in HCl 0.1 M; this solution was added in equal-sized aliquots during the gastric phase, at a rate of 0.05 mL per mL or g of sample; and pH was gradually decreased to 2 using HCl 1 M. Duodenum conditions were simulated with 2 g/L pancreatin (Sigma, USA) and 12 g/L bile salts (Himedia, India), dissolved in NaHCO3 0.1 M; this solution was added at a rate of 0.25 mL per mL or g of sample. The increase of pH that takes place in the ileum was simulated by gradually adding NaHCO3 0.1 M. All enzyme solutions were freshly prepared for each experiment, and filter-sterilized through a 0.22 μm-membrane filter (from Millipore, Billerica, USA). A rotary water bath at 37°C was used to simulate the temperature and peristaltic movements that prevail during human gastrointestinal transit. Eight flasks, each one with two tablets in 25 mL of MRS broth, were submitted to the SGC procedure: (1) two flasks (two replicas) containing each two tablets were not submitted to SGC in order to evaluate the viable cells of L. paracasei L26 in the tablets; (2) two flasks were withdrawn after being exposed to mouth and esophagus–stomach conditions; (3) two flasks were withdrawn after being exposed to duodenum conditions, and (4) two flasks were withdrawn after being exposed to ileum conditions. In each sampled flask, the viable cells of L. paracasei L26 in the tablets as well as in the MRS solution were assessed.
Statistical Analysis
The results obtained in the evaluation of the effect of compaction force, the release/viability of strains, storage stability and in the SGC test were analyzed using univariate ANOVA. Whenever ANOVA showed significant differences, the Tukey HSD test was performed. All analyses were performed using PASW Statistics 18.0. Differences were accepted as statistically significant at p < 0.05.
RESULTS
The number of viable cells of L. paracasei L26 in the powder after spray-drying was of (1.05 ± 0.45) × 1010 CFU/g (mean ± standard deviation). The maximum decrease of number of viable cells due to the spray-drying process was 1 log cycle. The microparticles obtained had irregular shape, with variable dimensions (5–50 μm) (25). Due to the particle dimensions and moisture (≈9%) the powder obtained has not free flowing properties.
Effect of Compaction Force on L. paracasei Microparticles
The effect of the compaction force on the viability of probiotic bacteria in the tablets containing only microparticles of WPC50 with L. paracasei L26 are displayed in Table I.
Table I.
Effect of Compaction Force on Probiotics Viability in Tablets Containing Only L. paracasei L26 Microparticles
| Compaction force (kN) | Viable cellsa (CFU/g) |
|---|---|
| 0.0 | (1.48 ± 0.96) × 109 |
| 9.8 | (1.74 ± 0.09) × 108 |
| 19.6 | (1.53 ± 0.85) × 108 |
| 29.4 | (1.49 ± 0.30) × 108 |
| 39.2 | (1.94 ± 0.58) × 108 |
aMean ± standard deviation
A decrease of 1 log cycle was observed after the compaction with a 9.8-kN force; however, the number of viable cells for different compaction forces was of the same order of magnitude showing no increase of detrimental effects for compaction forces higher than 9.8 kN (p > 0.05).
Tablets Evaluation: Disintegration and Hardness Tests
Tablets resisted for 2 h in acidic medium and disintegrated only after 2 h in phosphate buffer pH 6.8. The hardness of tablets was 314.8 ± 0.4 N (mean ± standard deviation). The compaction force selected for tablets preparation (9.8 kN) caused a decrease of 1 log cycle, from 6.00 × 109 to 6.17 × 108 CFU/g.
Release/Viability Test
Figure 1, displays the number of viable cells of L. paracasei L26 in the tablets as well as in the phosphate buffer medium, obtained in the release/viability test. No significant decrease of the number of probiotic viable cells in the tablets was observed after the 2 h of the acidic stage (p = 0.854). After the intestinal stage (buffer stage), the decrease of viable cells of L. paracasei L26 in tablets was approximately 1 logarithmic cycle, it reduced from 6.96 to 5.88 log CFU/g (p = 0.036).
Fig. 1.
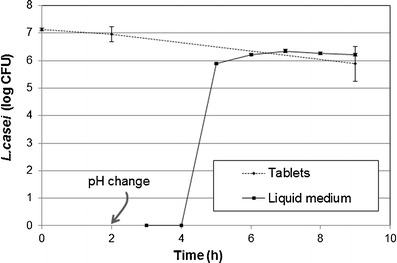
Evaluation of the release/viability of L. paracasei L26 cells (dash line represents the number of L. paracasei L26 in tablets at the beginning, after acid stage and in the end; bold line represents the number of L. paracasei L26 released to the phosphate medium)
Storage Stability and Resistance/Susceptibility of the Probiotic Tablets Throughout Storage
The evolution of viable cells of L. paracasei L26 in the tablets throughout 60 days at 23°C and 33% of relative humidity is displayed in Fig. 2, whereas the viability of L. paracasei L26 in the tablets upon 45 days of storage and exposed to SGC test is tabulated in Table II. Over the period of storage tested, the number of viable cells decreased from 6.17 × 108 to 1.33 × 106 CFU/g (p < 0.01). The number of viable cells of L. paracasei L26 in the tablets did not change significantly upon exposure to the SGC throughout 4 h (between 0 and 240 min; p = 0.512).
Fig. 2.
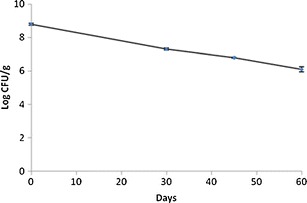
Storage stability of the tablets at 23°C and 33% of relative humidity
Table II.
Variation of L. paracasei L26 Viable Cells (Mean ± Standard Deviation) in Tablets after 45 Days of Storage at 23°C/33% of Relative Humidity and in MRS Solution Throughout the Simulated Gastrointestinal Conditions
| Gastrointestinal compartment | Sampling (min) | Tablets (CFU/g) | MRS solution (CFU/mL) |
|---|---|---|---|
| Initial | 0 | (6.26 ± 0.40) × 106 | NDa |
| Mouth (pH 6, 37°C, 200 rpm) | 90 | (3.24 ± 0.53) × 106 | NDa |
| Esophagus–stomach (ΔpH 6 to 2, 37°C, 130 rpm) | |||
| Duodenum (ΔpH 2 to 5, 37°C, 45 rpm) | 120 | (4.05 ± 0.88) × 106 | NDa |
| Ileum (ΔpH 5 to 6.5, 37°C, 45 rpm) | 240 | (7.72 ± 0.17) × 106 | (1.34 ± 0.30) × 105 |
aNo viable cells detected in first decimal dilution
DISCUSSION
Microencapsulation of microorganisms has frequently been used to impart protection against stressful environmental factors. Spray-drying is one of the most used methods of encapsulation based on dehydration which allows maintenance of microbial biomass viability. This technology is able to produce high rates of dry and stable powders at relatively low costs. In this work, microencapsulation was used to study an alternative technological way to obtain a probiotic concentrate able to be incorporated in the tablets. According to Rodrigues et al., (25) dry microcapsules of L. paracasei L26 in WPC50via spray-drying proved to be a vector for 6-months storage of probiotic bacteria under regular room conditions.
Based on preliminary release/viability studies (data not shown) with tablets containing microparticles of WPC50 with L. casei and several excipients (5% of sodium croscarmellose, or 5% of sodium croscarmellose and 20% sodium alginate, or 5% of sodium croscarmellose and 20% CAP) the composition of the tablets was selected due to their higher resistance to acid, conferring protection to probiotic bacteria in tablets. Additionally, CAP was included in the composition of the tablets because it is generally used to produce enteric tablets due to its pH solubility dependence (pH > 6). Enteric coatings based on CAP are resistant to acidic gastric fluids, but easily soluble in mildly basic medium of the intestine (28). The main function of croscarmellose sodium (approximately 4%) in the tablets is to help the disintegration of probiotic tablets after passage through duodenum. CAP and croscarmellose sodium are excipients approved for oral administration by FDA and generally regarded as nontoxic (28,29) and therefore chosen to be the excipients in L. paracasei L26 tablets. Whey protein concentrate in turn is generally recognized as safe-GRAS (30).
The produced probiotic tablets were of monolithic matrix type where the microparticles containing the probiotic bacteria were evenly distributed through it. The production of monolithic matrix tablets was very simple and can be easily scaled up.
Effect of Compaction Force
The decrease of the void spaces and some microparticles fragmentation, which occurred during the compaction, may justify the decrease of L. paracasei L26 viability due to damages on the bacterial cells, (18) that possibly did not increase for higher compaction forces.
As expected, a decrease of 1 log cycle in the number of viable cells of L. paracasei L26 in the tablets (from 6.0 × 109 to 6.2 × 108 CFU/g), was also observed in the tablets containing the probiotic microparticles and excipients compressed with 9.8 kN. Brachkova (31) produced several formulas of mini-tablets with or without microcrystalline cellulose and inulin (2.5 mm diameter) and several strains of Lactobacillus by applying compaction forces of 1, 2, and 5 kN and also reported decreases in the viability of probiotic bacteria (<2 log units); a more negative impact in comparison to the results reported herein.
Evaluation of Release/Viability
Stadler and Viernstein (14) have considered that a decreased of 1 log unit after 2 h of acid stage is a good achievement. No significant decrease in the number of viable cells of L. paracasei L26 were observed through the acid stage; only after intestinal stage it was observed significant (p = 0.036), but rather small decrease of approximately 1 logarithmic cycle in the tablets.
The release of L. paracasei L26 from the tablets to the phosphate buffer medium occurred only after 4 h. Tablets for colonic delivery should protect the bioactive agent from gastric acidity but also prolong their release in the small intestine (20). In order to assure the delivery near the colon, a dosage form that retards the release of probiotics in intestinal stage is considered adequate suitable choice. The tablets resisted for 2 h in acidic medium and only disintegrated after 2 h in phosphate buffer pH 6.8. This fact may be attributed not only to CAP but also to the tablets hardness. Klayraung et al. (16) considered a disintegration time of approximately 5 h (2 h in acidic medium and 3 h in phosphate buffer pH 6.8) suitable for a probiotic formulation.
Tablets containing probiotics, as previously referred, have already been developed. In the coated tablets obtained by double compression (18), some scale-up difficulties may be expected in result of the more complex manufacturing procedure proposed (tablet in tablet system). In the swelling matrix tablets containing non-encapsulated/immobilized probiotics (14,19,20), the deleterious contact between the gastric medium and microorganisms was not avoidable only diminished due to the fact that the swelling of the polymer permitted the slow entrance of the dissolution liquid. The release of the probiotic bacteria was in the form of sustained release and was not pH dependent. These problems can be, at least reduced, with the CAP matrix tablets developed. Besides, CAP is described in the major pharmacopeias (28). The protecting effect of the probiotic bacteria was probably consequence of the low diffusion of the gastric dissolution medium into the tablets, due to CAP. The diffusion of the aqueous medium into the tablets was probably superficial and the tablets interior remained almost dry during this phase. The lower specific area of the tablets, in comparison to the microparticles, also reduced the contact between the bacteria and the dissolution medium which contributed to the protecting effect. According to this scenario, only the bacteria cells in the outer region of the microparticles located at tablet surface were exposed to this deleterious effect (Fig. 3). The release mechanism of the probiotic cells at the buffer stage (pH 6.8) were due to the visible erosion of the matrix, and swelling was not noticed. The release of the probiotic bacteria was pH dependent, in the form of retarded release (Fig. 3), only allowing the release of viable cells in intestinal regions with pH > 6, such as in the colon.
Fig. 3.
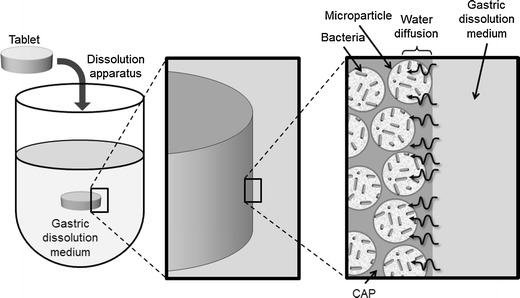
Schematic representation of phenomena occurring in the release/viability test, focusing on the low diffusion of gastric dissolution medium and the exposure of probiotic cells in the outer region of the microparticles located at tablet surface
Storage Stability
Microencapsulated L. paracasei L26 in tablets revealed some susceptibility to the storage conditions over the period of storage tested. According to Rodrigues et al. (25), L. paracasei was the strain less susceptible to the parameters under scrutiny presenting values above 106 CFU/g of viable cells throughout 180 days of storage at 22°C, irrespective of relative humidity, presence/absence of oxygen and of presence/absence of l-cysteine-HCL in the WPC50 microparticles produced by the same method used in this research work (spray-drying). The values of viable cells (log CFU per gram) versus storage time followed a zero order model (linear model): y = −0.0444x + 8.7468, R2 = 0.9962, (p < 0.01). This linear model demonstrates that further research should be done in order to optimize L. paracasei L26 viability over longer storage periods. A decreasing tendency of viable cells of Lactobacillus fermentum incorporated in tablets through 6 months of storage at 30°C was also reported by Klayraung et al. (16). Lower values of relative humidity could probably extend the storage time; storage at 22°C and under 12% relative humidity promoted the highest survival rates throughout 180 days of storage for L. paracasei L26 in WPC50 microparticles, with viable numbers above 107 CFU/g (25).
The presence of viable cells of L. paracasei L26 in MRS solution (Table II) was only detected after exposure to simulated ileum conditions demonstrating that the developed tablets with CAP and croscarmellose sodium, were in fact suitable to protect L. paracasei L26 from gastric pH and harsh conditions in duodenum and ileum enabling the delivery of viable cells in the colon.
CONCLUSION
This study shows the potential of tablets based on a combined CAP and croscarmellose sodium matrix to deliver viable cells of L. paracasei L26 in simulated GI fluids namely in the colon with very good extension of cell survival. Microencapsulation of L. paracasei L26 in WPC50via spray-drying revealed to be a potential alternative way to obtain cells concentrate able to be incorporated in the tablets. The storage stability of the tablets was acceptable at 23°C for 60 days; however, further research should be done in order to optimize L. paracasei L26 viability over long storage periods. Lower values of relative humidity could also probably extend storage period. According to these results, the authors consider that the developed probiotic tablets are a potential way to deliver probiotics to persons/patients who might benefit from probiotic therapy with oral solid dosage forms.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Formulab and DSM for providing the whey protein concentrate and probiotic strain, respectively. This work was funded by FEDER under the Operational Program for Competitiveness Factors—COMPETE and by National funds via FCT—Fundação para a Ciência e a Tecnologia within the framework of project PROBIOCAPS—references PTDC/AGR-ALI/71051/2006 and FCOMP-01-0124-FEDER-008792, and through individual research grants (SFRH/BPD/73781/2010, SFRH/BD/77647/2011 and SFRH/BPD/65410/2009) by FCT under QREN–POPH funds, co-financed by the European Social Fund and Portuguese National Funds from MCTES.
REFERENCES
- 1.FAO/WHO. Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria Report of a Joint Expert Consultation. Córdoba-Argentina2001 1–4 October 2001.
- 2.Lourens-Hattingh A, Viljoen BC. Yogurt as probiotic carrier food. Int Dairy J. 2001;11(1–2):1–17. doi: 10.1016/S0958-6946(01)00036-X. [DOI] [Google Scholar]
- 3.Holzapfel WH, Haberer P, Geisen R, Bjorkroth J, Schillinger U. Taxonomy and important features of probiotic microorganisms in food and nutrition. Am J Clin Nutr. 2001;73(2 Suppl):365S–373S. doi: 10.1093/ajcn/73.2.365s. [DOI] [PubMed] [Google Scholar]
- 4.Cukrowska B, Motyl I, Kozakova H, Schwarzer M, Gorecki RK, Klewicka E, et al. Probiotic Lactobacillus strains: in vitro and in vivo studies. Folia Microbiol (Praha) 2009;54(6):533–537. doi: 10.1007/s12223-009-0077-7. [DOI] [PubMed] [Google Scholar]
- 5.Pagnini C, Saeed R, Bamias G, Arseneau KO, Pizarro TT, Cominelli F. Probiotics promote gut health through stimulation of epithelial innate immunity. Proc Natl Acad Sci USA. 2010;107(1):454–459. doi: 10.1073/pnas.0910307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirjavainen PV, ElNezami HS, Salminen SJ, Ahokas JT, Wright PF. Effects of orally administered viable Lactobacillus rhamnosus GG and Propionibacterium freudenreichii subsp. shermanii JS on mouse lymphocyte proliferation. Clin Diagn Lab Immunol. 1999;6(6):799–802. doi: 10.1128/cdli.6.6.799-802.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin PW, Nasr TR, Berardinelli AJ, Kumar A, Neish AS. The probiotic Lactobacillus GG may augment intestinal host defense by regulating apoptosis and promoting cytoprotective responses in the developing murine gut. Pediatr Res. 2008;64(5):511–516. doi: 10.1203/PDR.0b013e3181827c0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naruszewicz M, Johansson ML, Zapolska-Downar D, Bukowska H. Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. Am J Clin Nutr. 2002;76(6):1249–1255. doi: 10.1093/ajcn/76.6.1249. [DOI] [PubMed] [Google Scholar]
- 9.Cremonini F, Di Caro S, Nista EC, Bartolozzi F, Capelli G, Gasbarrini G, et al. Meta-analysis: the effect of probiotic administration on antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2002;16(8):1461–1467. doi: 10.1046/j.1365-2036.2002.01318.x. [DOI] [PubMed] [Google Scholar]
- 10.Ohashi Y, Nakai S, Tsukamoto T, Masumori N, Akaza H, Miyanaga N, et al. Habitual intake of lactic acid bacteria and risk reduction of bladder cancer. Urol Int. 2002;68(4):273–280. doi: 10.1159/000058450. [DOI] [PubMed] [Google Scholar]
- 11.Tok E, Aslim B. Cholesterol removal by some lactic acid bacteria that can be used as probiotic. Microbiol Immunol. 2010;54(5):257–264. doi: 10.1111/j.1348-0421.2010.00219.x. [DOI] [PubMed] [Google Scholar]
- 12.Goldin BR, Gorbach SL. Clinical indications for probiotics: an overview. Clin Infect Dis. 2008;46(Suppl 2):S96–S100. doi: 10.1086/523333. [DOI] [PubMed] [Google Scholar]
- 13.Hilton E, Kolakowski P, Singer C, Smith M. Efficacy of Lactobacillus GG as a diarrheal preventive in travelers. J Travel Med. 1997;4(1):41–43. doi: 10.1111/j.1708-8305.1997.tb00772.x. [DOI] [PubMed] [Google Scholar]
- 14.Stadler M, Viernstein H. Optimization of a formulation containing viable lactic acid bacteria. Int J Pharm. 2003;256(1–2):117–122. doi: 10.1016/S0378-5173(03)00068-1. [DOI] [PubMed] [Google Scholar]
- 15.Del Piano M, Morelli L, Strozzi GP, Allesina S, Barba M, Deidda F, et al. Probiotics: from research to consumer. Dig Liver Dis. 2006;38(Supplement 2):S248–S255. doi: 10.1016/S1590-8658(07)60004-8. [DOI] [PubMed] [Google Scholar]
- 16.Klayraung S, Viernstein H, Okonogi S. Development of tablets containing probiotics: effects of formulation and processing parameters on bacterial viability. Int J Pharm. 2009;370(1–2):54–60. doi: 10.1016/j.ijpharm.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Albertini B, Vitali B, Passerini N, Cruciani F, Di Sabatino M, Rodriguez L, et al. Development of microparticulate systems for intestinal delivery of Lactobacillus acidophilus and Bifidobacterium lactis. Eur J Pharm Sci. 2010;40(4):359–366. doi: 10.1016/j.ejps.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Chan ES, Zhang Z. Encapsulation of probiotic bacteria Lactobacillus acidophilus by direct compression. Food Bioprod Process. 2002;80(2):78–82. doi: 10.1205/09603080252938708. [DOI] [Google Scholar]
- 19.Poulin JF, Caillard R, Subirade M. Beta-Lactoglobulin tablets as a suitable vehicle for protection and intestinal delivery of probiotic bacteria. Int J Pharm. 2011;405(1–2):47–54. doi: 10.1016/j.ijpharm.2010.11.041. [DOI] [PubMed] [Google Scholar]
- 20.Calinescu C, Mulhbacher J, Nadeau T, Fairbrother JM, Mateescu MA. Carboxymethyl high amylose starch (CM-HAS) as excipient for Escherichia coli oral formulations. Eur J Pharm Biopharm. 2005;60(1):53–60. doi: 10.1016/j.ejpb.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Vidhyalakshm R, Bhakyaraj R, Subhasree R. Encapsulation the future of probiotics—a review. Adv Biol Res. 2009;3:96–103. [Google Scholar]
- 22.Kailasapathy K. Microencapsulation of probiotic bacteria: technology and potential applications. Curr Issues Intest Microbiol. 2002;3(2):39–48. [PubMed] [Google Scholar]
- 23.Burgain J, Gaiani C, Linder M, Scher J. Encapsulation of probiotic living cells: from laboratory scale to industrial applications. J Food Eng. 2011;104(4):467–483. doi: 10.1016/j.jfoodeng.2010.12.031. [DOI] [Google Scholar]
- 24.Gardiner GE, O’Sullivan E, Kelly J, Auty MAE, Fitzgerald GF, Collins JK, et al. Comparative survival rates of human-derived probiotic Lactobacillus paracasei and L. salivarius strains during heat treatment and spray drying. Appl Environ Microbiol. 2000;66(June):2605–2612. doi: 10.1128/AEM.66.6.2605-2612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodrigues D, Sousa S, Rocha-Santos T, Silva JP, Lobo JMS, Costa P, et al. Influence of l-cysteine, oxygen and relative humidity upon survival throughout storage of probiotic bacteria in whey protein-based microcapsules. Int Dairy J. 2011;21(11):869–876. doi: 10.1016/j.idairyj.2011.05.005. [DOI] [Google Scholar]
- 26.Miles AA, Misra SS, Irwin JO. The estimation of the bactericidal power of the blood. J Hyg (Lond) 1938;38(6):732–749. doi: 10.1017/S002217240001158X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madureira AR, Amorim M, Pintado ME, Gomes AMP, Malcata FX. Protective effect of whey cheese upon probiotic strains exposed to simulated gastrointestinal conditions. Food Res Int. 2011;4(1):465–470. doi: 10.1016/j.foodres.2010.09.010. [DOI] [Google Scholar]
- 28.Rowe RC, Sheskey PJ, Weller PJ, editors. Handbook of pharmaceutical excipients. 4. London, Chicago: Pharmaceutical Press American Pharmaceutical Association; 2003. [Google Scholar]
- 29.US FDA. Inactive Ingredient Search for Approved Drug Products. http://www.accessdata.fda.gov/scripts/cder/iig/index.cfm. Accessed 15 Mar 2012.
- 30.US FDA. CFR—Code of Federal Regulations Title 21. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=184.1979c. Accessed 15 Mar 2012.
- 31.Brachkova MI, Duarte A, Pinto JF. Evaluation of the viability of Lactobacillus spp. After the production of different solid dosage forms. J Pharm Sci-Us. 2009;98(9):3329–3339. doi: 10.1002/jps.21609. [DOI] [PubMed] [Google Scholar]


