Abstract
The aim of the present study was to prepare a stable complex of doxycycline (Doxy) and hydroxypropyl-β-cyclodextrin (HPβCD) for ophthalmic delivery and investigate the inclusion mechanism and the inclusion effects on the stability of Doxy. The Doxy/HPβCD complex was prepared by solution stirring and then characterized by scanning electron microscopy and ultraviolet spectroscopy. Based on results of nuclear magnetic resonance, molecular model of Doxy/HPβCD complex was established using computational simulation of PM3 method implemented in Gaussian 03. Stabilities of Doxy/HPβCD complex in both aqueous solution and solid state at 25°C were evaluated by HPLC. Finally, in vitro antibacterial activity of the Doxy/HPβCD complex was evaluated by disk diffusion test. It was found that the stabilities of Doxy/HPβCD complex in both aqueous solution and solid state were improved obviously as compared with Doxy alone. This stability enhancement is consistent with the inclusion mechanism between HPβCD and Doxy, which showed that the unstable site of Doxy molecule at 6-CH3 was protected in the hydrophobic cavity of HPβCD, additionally, the chelation of Mg2+ provided a synergetic protection of the other unstable site of Doxy at 4-N(CH3)2. The antibacterial activity results indicated that Doxy/HPβCD complex might have potential for clinical applications.
KEY WORDS: doxycycline, hydroxypropyl-β-cyclodextrin, inclusion mechanism, molecular modeling, stability
INTRODUCTION
Doxycycline (Doxy) belongs to the tetracycline group of broad-spectrum antibiotics. Besides its common oral administration, Doxy is also recommended for ocular surface diseases, particularly for recurrent epithelial cell erosion, rosacea, corneal neovascularization, and keratitis sicca (1,2). However, the poor stability of Doxy in aqueous solution is a major challenge for pharmaceutical researchers and restricts its ophthalmic clinical application (3), and there is no ophthalmic preparation of Doxy having been marketed yet. Thus, the aim of this work was to prepare a stable formulation of Doxy for ophthalmic delivery.
Cyclodextrins (CDs) are natural cyclic oligosaccharides that are obtained through enzymatic degradation of starch. As they can complex with drugs to increase the solubility and stability of drugs and reduce bitterness and tissue irritation of drugs upon dosing, cyclodextrins have been used extensively in pharmaceutical research and development. Currently, there are over 30 marketed cyclodextrin-containing pharmaceutical products worldwide (4,5). One of the most common applications of cyclodextrins reported in the pharmaceutical literatures is their ability to enhance drug bioavailability by increasing drug solubility and permeability (6). However in this study, cyclodextrin was employed to form an inclusion complex with Doxy based on its ability to enhance drug stability (7,8). Hydroxypropyl-β-cyclodextrin (HPβCD), one of the derivates of CDs, was selected because of its great solubility and safety, and wide usage (9). In addition, divalent metal ion Mg2+ was added because it could chelate with doxycycline monohydrate to increase drug solubility and stability (10,11).
Based on the results from preliminary studies, the preparation technology of the Doxy/HPβCD complex was determined and the in vivo activity of the complex to treat corneal neovascularization on rats has been demonstrated (12). In the present study, characterizations of Doxy/HPβCD complex were carried out by scanning electron microscopy (SEM) and ultraviolet (UV) spectroscopy (13). This inclusion complex was designed as a solid formulation which could dissolve in aqueous solution before use and then keep stable for at least a week comparable to many other marketed ophthalmic preparations. Thus the stabilities of Doxy/HPβCD complex in both solid and aqueous states were evaluated. In vitro antibacterial activity of the Doxy/HPβCD complex was also detected by disk diffusion test to evaluate the bioactivities of Doxy in the inclusion system.
Although CDs have been used to enhance drug stability in several researches, the specific molecular mechanism for the stability enhancement has rarely been discussed (8,14). The present study was aimed to reveal the specific mechanism underlying this phenomenon based on the molecular modeling. Both the degradation process of Doxy and the interactions between Doxy and HPβCD molecules in the inclusion system were investigated in this study to understand the inclusion effects of HPβCD on the stability of Doxy. Molecular model of Doxy/HPβCD complex was established using computer simulation of PM3 method implemented in Gaussian 03 according to results of 1H nuclear magnetic resonance (NMR) and 2D NMR (rotation-frame nuclear Overhauser effect spectroscopy (ROESY)) studies. As magnesium chloride was added to increase the solubility of doxycycline monohydrate during the preparation of Doxy/HPβCD complex, its effects on the stability of Doxy was also investigated. Relationship between inclusion mechanism and stability enhancement was further discussed.
MATERIALS AND METHODS
Materials
Doxycycline monohydrate (doxycycline content of 98.5%) and doxycycline hydrochloride (doxycycline content of 92.5%) were kindly provided by Yancheng Suhai Pharmaceutical Co. Ltd. (Jiangsu, China). Hydroxypropyl-β-cyclodextrin (HPβCD) was provided by Roquette (Lestrem, France). Staphylococcus aureus (ATCC25923), Escherichia coli (ATCC25922), and Pseudomonas aeruginosa (ATCC27853) were supplied by the Experimental Center for Basic Medical Teaching of Sun Yat-sen University, Guangzhou, China. Double distilled and deionized water was used throughout the experiment and all other materials used were of analytical or pharmaceutical grade.
Preparation of Doxy/HPβCD Complex
Doxy/HPβCD complex was prepared by solution stirring (15) with an optimal formation based on the results of previous study. Firstly, 26.4 mg of doxycycline monohydrate (containing 25 mg of Doxy) was added into 5 ml of aqueous solution containing magnesium chloride (0.5%, w/w). According to the preliminary solubility test, the solubility of doxycycline monohydrate could be increased from 3.4 mmol/L in water to 7.7 mmol/L in 0.5% MgCl2 solution, thus, 0.5% MgCl2 was added to increase the solubility of doxycycline monohydrate for the following inclusion with HPβCD. Then HPβCD was added in with a Doxy: HPβCD molar ratio of 1:4, and the solution was stirred at 25°C for 2 h under protection from light degradation, and then filtered through a 0.45-μm membrane to remove insoluble ingredients, if any. After lyophilization of Doxy/HPβCD complex solution at −52°C for 24 h, the solid inclusion complex was obtained.
Characterization of Doxy/HPβCD Complex
Scanning Electron Microscopy
The morphology of solid samples was investigated using a scanning electron microscope (JSM-6330F, JEOL, Japan) operating at an accelerating voltage of 20 kV. Samples of raw materials, inclusion complex of Doxy/HPβCD, and the corresponding physical mixture were mounted onto a copper wafer via double-faced adhesive tape, and sputtered with gold for analysis.
Ultraviolet Scanning Spectroscopy
Ultraviolet (UV) scanning spectra were recorded with a UV/vis spectrophotometer at 25°C. Excess amount of doxycycline monohydrate (containing at least 25 mg of Doxy) was added into 10 ml of magnesium chloride solution (0.5%, w/w) containing different amount of HPβCD (molar ratio of HPβCD/Doxy = 0.5, 0.8, 1, 2, 3, 4, 5, and 6) and stirred for 2 h. Then the supernatants were filtered through 0.45-μm Millipore membrane followed by 1:100 dilution with double distilled water. With magnesium chloride solution (0.5%, w/w) containing the same amount of HPβCD as blank, the scanning spectra of Doxy and its inclusion complex in the wavelength range of 220–450 nm were analyzed.
Nuclear Magnetic Resonance Spectroscopy
All NMR experiments were carried out on an NMR spectrometer (Advance III, Bruker, Germany) at 400 MHz. 1H spectra of Doxy and HPβCD raw materials were obtained in CD3OD and D2O, respectively, while samples of Doxy/HPβCD complex were dissolved in both CD3OD and D2O for analysis. Extra 1H spectra were obtained while 0.5% MgCl2 was added into 0.5 ml of CD3OD containing 25 mg of Doxy. The spectra were referenced relative to the residual peak of MeOD or HOD at δ ppm. Additionally, 2D NMR spectra of Doxy/HPβCD complex were acquired in D2O.
Molecular Modeling
Molecular modeling was carried out to further elaborate the complexation mechanism of Doxy and HPβCD according to the NMR results. Since HPβCD with a substitution degree of 0.6 was employed in the present study, four 2-hydroxypropyl groups were added on the primary hydroxyl groups of β-CD at O-6 positions following approach reported in literatures (16,17). The initial structures of Doxy and HPβCD were constructed using Chembio3D ultra (Version 12.0, CambridgeSoft com., USA), and were individually optimized using PM3 method implemented in Gaussian 03 (Gaussian Inc., Wallingford, USA). After thorough energy-minimization of Doxy and HPβCD structures separately, a molecular dynamics simulation of Doxy/HPβCD complex was carried out based on PM3 optimization (18). The initial inclusion model was set up in Gaussian 03 by defining the coordinate system as placing the central plane of the HPβCD glucose unit (first, fourth, and sixth glycosidic oxygen atoms) onto the XY plane. The 2-OMe and 3-OMe groups in each glucose unit were placed pointing toward the positive Z-axis (19). The bond of C7-C6α on the benzene ring of Doxy was placed on the Z-axis as the head up orientation during the docking process. Doxy approached and passed through the cavity of HPβCD along the Z-axis, and the relative distance between the C7 of Doxy and HPβCD ranged from 8 to −8 Å with a step of 1 Å. A systematic search of energy-minimized structure of Doxy/HPβCD complex was performed at each distance point, setting at 298.15 K in vacuo. Finally, the structure with lowest heat energy at all positions was obtained as the optimal complex structure and then estabilished in Chembio3D ultra 12.0.
Stability Study
Doxy/HPβCD complex in both aqueous solution and solid state were stored at temperature of 25 ± 2°C and relative humidity of 60 ± 10% to evaluate the stability of Doxy, while Doxy·HCl aqueous solution and solid with the same Doxy content of 5 mg/ml as Doxy/HPβCD complex were employed as a control, respectively.
In addition, the accelerated tests at 40 ± 2°C were performed to evaluate the individual effects of HPβCD and Mg2+ on the stability of Doxy in the complex system. Solution of Doxy/HPβCD complex containing 5 mg/ml of Doxy and Doxy/HPβCD molar ratio of 1:4 was prepared. Additional complex samples were prepared by adding 0.5% of MgCl2 before or after the addition of HPβCD. These three solutions were all stirred at 25°C for 2 h for the complex formation, and then filtered with a 0.45-μm membrane to remove insoluble ingredients. In order to meet with the different solubility of Doxy in the three groups, samples were all diluted with double distilled water to the Doxy concentration of 1 mg/ml, then stored at 40°C for 10 days to evaluate the stability of Doxy, while Doxy·HCl aqueous solution with the same Doxy content was employed as a control.
Doxy and related substances were separated and assayed using Waters HPLC system equipped with a reversed-phase Gemini C18 column (250 × 4.6 mm, 5 μm, Phenomenex Co., USA) for separation and a UV spectrophotometer for detection. The mobile phase consisted of 0.05 M ammonium oxalate, dimethylformamide, and 0.2 M ammonium phosphate dibasic with the proportion of 65:30:5 and pH adjusted to 8.0 ± 0.2. The column oven temperature was set to 35°C. Samples of 20 μL were injected with an eluent flow rate of 1.0 ml/min for detections at 280 nm.
In Vitro Antibacterial Activity
Antibacterial activities of Doxy/HPβCD complex against E. coli, P. aeruginosa, and S. aureus, the three major bacteria that can cause ocular infection, were determined (20,21). Bacterial strains were cultivated on Mueller-Hinton (MH) broth medium (Hangzhou Tianhe Microorganism Reagent Co. Ltd., China) and were incubated at 37°C for 24 h prior to testing. Cell suspensions were adjusted with a sterile saline solution to obtain a concentration of 1.5 × 108 cells/mL by comparison with a 0.5 McFarland turbidity standard.
Filter paper disks (6 mm in diameter) impregnated with 30 μL of Doxy/HPβCD complex or Doxy·HCl both at Doxy concentration of 1,000 μg/ml were placed on the surface of inoculated agar plates, which contained cultured microorganisms. Plates were then incubated for 20 h at 37°C and the mean zone of inhibition around the paper disks for a particular sample was measured. Each experiment was carried out four times with a negative control of paper disk impregnated with physiological saline.
RESULTS AND DISCUSSION
This study was aimed to obtain a stable ophthalmic preparation of doxycycline. As a highly hydrophilic and strong acidic compound, doxycycline hydrochloride may cause discomfortableness or even harm to the eye surface. Besides, alkaline additives would accelerate the degradation of doxycycline. Therefore, doxycycline monohydrate, a faintly acidic and hydrophobic derivative of tetracycline which has been marketed as various oral capsules and tablets, was selected to form a drug/CD complex with HPβCD. Additionally, the hydrophobic doxycycline monohydrate compared to doxycycline hydrochloride was more favorable for the inclusion process with HPβCD. Thus, doxycycline monohydrate/HPβCD complex was more suitable for the ophthalmic delivery of doxycycline.
It was found that Doxy and HPβCD molecules were formed a 1:1 complex with an apparent stability constant K1:1 of 120.80 M−1 according to the phase-solubility study in the previous work (22). Generally, the association constants of drugs to CDs are reported in the range of 50–2,000 M−1 (6), the calculated value of K1:1 of Doxy/HPβCD complex suggested a favorable interaction occurred between Doxy and HPβCD. According to the stiochiometry between guest and host molecules in Doxy/HPβCD complex, further characterizations and molecular modeling of this inclusion complex were performed as follows.
Characterization of Doxy/HPβCD Complex
SEM Micrographs
SEM analysis was performed to investigate the particle shape and surface morphology of Doxy/HPβCD inclusion complex. Pure Doxy (Fig. 1a) appeared as club-shaped crystal, while HPβCD (Fig. 1b) exhibited as amorphous spherical or partial spherical particles. In their physical mixture (Fig. 1c), characteristic Doxy crystals and HPβCD particles were clearly observed, indicating the absence of host–guest interactions. In contrast, their inclusion complex (Fig. 1d) was in the form of irregular particles of varying sizes, which were completely different from raw materials Doxy and HPβCD, revealing the formation of inclusion complex.
Fig. 1.
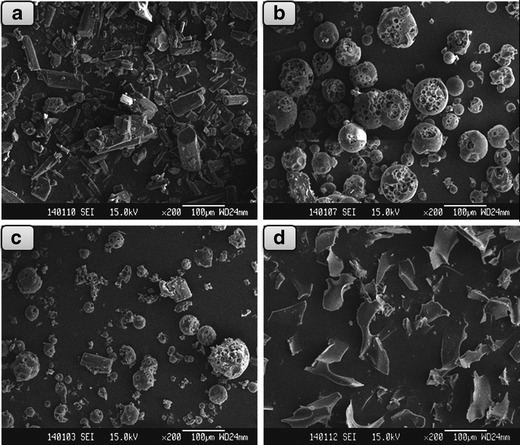
SEM images of a Doxy, b HPβCD, c Doxy/HPβCD physical mixture, and d Doxy/HPβCD inclusion complex
UV Scanning Spectra
UV scanning spectra were employed for the rudimental investigation of molecular interaction between Doxy and HPβCD in the complex. Typically, the wavelength shift in UV spectra of drug along with the addition of CD may reflect the conjugation change of the drug and the formation of drug/CD inclusion complex (23). The UV spectra of Doxy (Fig. 2a) with different ratios of HPβCD showed the solution absorption increased with the addition of HPβCD, suggesting the increase of aqueous solubility of Doxy. At the same time, it was found that the maximum absorption of Doxy shifted to a longer wavelength gradually when the ratio of HPβCD increased. In a parallel comparison of the scanning curves (Fig. 2b), two maximum absorption wavelengths of inclusion complex HPβCD/Doxy = 4 were 278 and 347 nm, indicating a redshift from absorption of Doxy alone (274 and 345 nm, respectively). This wavelength shift suggested the conjugation structural change of Doxy along the naphthalene moiety as a function of HPβCD concentration and the formation of inclusion complex (24).
Fig. 2.
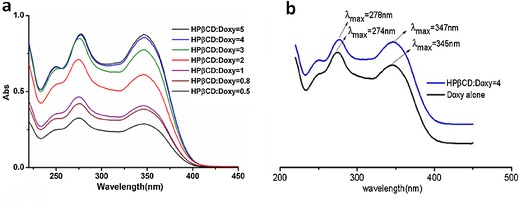
UV scanning spectra of Doxy/HPβCD complexes with different ratios
NMR Spectra
NMR analysis was performed to investigate the particular molecular mode of host–guest interactions between HPβCD and Doxy in the complex. Since HPβCD has a hydrophobic cavity which may encapsulate hydrophobic molecule Doxy, the hydrogen bonding interactions between them may provide a driving force to form Doxy/HPβCD complex. If inclusion occurs, the changes of physical and chemical environment will definitely affect the electronic density of hydrogens in Doxy and HPβCD. As the aqueous solubility of Doxy is very low, Doxy and HPβCD were dissolved in CD3OD and D2O, respectively, for obtaining high-quality NMR spectra (25). The Doxy/HPβCD complexes were dissolved in both CD3OD and D2O to perform 1H NMR experiments for comparative studies. Extra 1H NMR spectra of Doxy with addition of 0.5% MgCl2 in CD3OD was conducted to investigate the interactions between Mg2+ and Doxy before the inclusion process.
The 1H NMR spectra are shown in Fig. 3 and the proton shifts of Doxy and HPβCD in free and complex states were analyzed and summarized in Table I(26). A significant cleavage of 4′-N(CH3)2 peak of Doxy around 2.9 ppm, as identified in Fig. 3a, appeared in the presence of 0.5% MgCl2 (Fig. 3b). And the resonances of H7′, H8′, H9′, and 6′-CH3 of Doxy shifted upon complex formation, from δ of 6.95, 7.48, 6.84, and 1.55 ppm in the free compound (Fig. 3a) to δ of 6.98, 7.53, 6.87, and 1.58 ppm in the inclusion system in CD3OD, respectively. On the other hand, changes in the chemical shift of H1, H2, H3, H4, H5,6, and 9-CH3 protons on the glucose unit of HPβCD molecule were also observed. The signals from H3 and H5,6 of HPβCD resonated at δ of 3.90 and 3.74 ppm, respectively (Fig. 3c), while in the inclusion complex, the corresponding peaks shifted to δ of 3.91 and 3.77 ppm in D2O (Fig. 3d; 27,28). These signal displacements indicated that the environment around these protons of host and guest molecules changed through complexing.
Fig. 3.
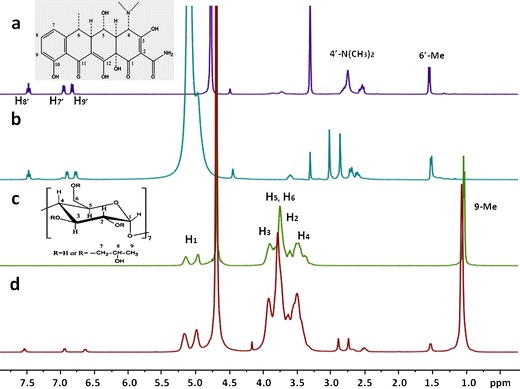
1H NMR spectra of a Doxy, b Doxy with addition of MgCl2, c HPβCD, and d Doxy/HPβCD inclusion complex, with the insert picture for proton identification
Table I.
1H NMR Chemical Shifts of Doxy and HPβCD in Doxy/HPβCD Complex
| Hydrogen | δ Doxy | δ HPβCD | δ Doxy/HPβCD | δ Doxy/HPβCD–δ free |
|---|---|---|---|---|
| 7′ | 6.95 | 6.98 | 0.03 | |
| 8′ | 7.48 | 7.53 | 0.05 | |
| 9′ | 6.84 | 6.87 | 0.03 | |
| 6′-CH3 | 1.55 | 1.58 | 0.03 | |
| 1 | 5.05 | 5.06 | 0.01 | |
| 3 | 3.90 | 3.91 | 0.01 | |
| 5,6 | 3.74 | 3.77 | 0.03 | |
| 2 | 3.61 | 3.63 | 0.02 | |
| 4 | 3.47 | 3.49 | 0.02 | |
| 9-CH3 | 1.02 | 1.05 | 0.03 |
It is known that two protons closely locating in space can produce a nuclear Overhauser effect (NOE) cross-correlation in NOE spectroscopy (NOESY) or ROESY (25,29). Therefore, 2D ROESY experiment was carried out on the Doxy/HPβCD complex to gain the information on the spatial proximity of the molecules. The contour plot shown in Fig. 4 revealed that the signals of 6′-CH3 and H9′ on Doxy (δ at 1.58 and 6.87, respectively) have intense intermolecular cross-peaks with H3 (δ at 3.91) or H5 and H6 (δ at 3.77) protons of HPβCD. And from the partial dilated picture on the top-left, H8′ of Doxy (δ at 7.53) also showed interaction with H5 and H6 of HPβCD. In addition, a weaker correlation between H7′ of Doxy (δ at 6.98) and H5 and H6 of HPβCD could be observed by a further magnification. These associations indicated atoms near 6′-CH3 side of the aromatic group on Doxy molecule docked into the cavity of HPβCD. The encapsulation of the aromatic moiety, the major hydrophobic group of Doxy molecule, is in agreement with the conjecture that hydrophobic interaction between guest and host molecules is the driving force for formation of inclusion complex.
Fig. 4.
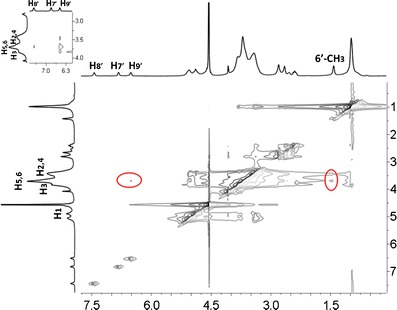
Contour plot of the two-dimensional ROESY spectra of Doxy/HPβCD inclusion complex in D2O
Molecular Modeling
Molecular structure of the inclusion complex was established using computational modeling technology to visually illustrate the formation of Doxy/HPβCD complex. PM3 method was adopted to search for the lowest energy structure for the inclusion system of Doxy into HPβCD cavity. The binding energy (BE) of each structure obtained in the optimization process was calculated by the following formula (30):
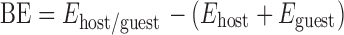 |
where Ehost/guest, Ehost, and Eguest represent the heats of Doxy/HPβCD inclusion complex, free Doxy, and free HPβCD, respectively.
Typically, the more negative the binding energy is, the stronger the interaction between host and guest molecules is built. After PM3 optimization, an energy-minimized complex structure with a heat energy of −8,093.41 kJ/mol was obtained at the initial docking position of −2 Å from Doxy to HPβCD. With the heat energy of optimal structure of free Doxy and HPβCD being −1,135.05 and −6,872.45 kJ/mol, respectively, the binding energy of optimized structure for Doxy/HPβCD complex was calculated according to the formula above as −85.91 kJ/mol. This negative binding energy indicated that the inclusion process was energetically favorable.
As shown in Fig. 5, in the optimal inclusion system, Doxy molecule partially docked into the HPβCD cavity rather than completely penetrating. The primarily inserted group of Doxy was the aromatic ring on the 6-CH3 side, with the distances of (H9Doxy–H5 HPβCD) and (6-CH3 Doxy–H3 HPβCD) being 1.89 and 1.72 Å, respectively. It is noteworthy that this kind of encapsulation had been recorded in literatures, which demonstrated insertions of the naphthalene ring of 6-chloro-5-(1-naphthyloxy)-2-(trifluoromethyl)-1H-benzimidazole and the chromene ring of Morin into the HPβCD cavity, respectively (29,31). The suitable size (approximate length of 6 Å) and hydrophobic property of the aromatic moiety together were suggested to provide synergetic effects for the guest molecule docking into the cavity of HPβCD (approximate inner diameter of 8 Å in the middle) (32).
Fig. 5.
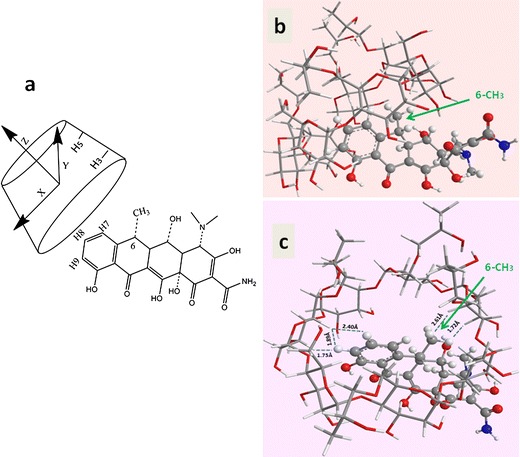
a Docking procedure for Doxy into HPβCD; and Energy-minimized structures of Doxy/HPβCD b viewed from the side wall of the HPβCD and c viewed from the wide edge of the HPβCD cavity
Stability of Doxy/HPβCD Complex and the Effects of Inclusion on Stability
In the present study, the Doxy/HPβCD complex was designed as a solid preparation which could dissolve in aqueous solution before use and then keep stable for at least a week. Thus stabilities of Doxy/HPβCD complex in both aqueous solution and solid state were evaluated at 25°C and 60 ± 10% RH for up to 6 months. Contents of Doxy and related substances were detected and analyzed during the storage. According to the criteria for doxycycline hyclate tablets in Chinese pharmacopoeia version 2010, the practical amount of doxycycline should be 93–107% of the labeled amount, while the total related substances should be no more than 5%. As shown in Table II, the preparation of Doxy/HPβCD inclusion complex could keep stable in solid state for at least 2 months and in solution for 2 weeks. Compared with Doxy·HCl, Doxy/HPβCD complex in both solid and solution states showed much more stable Doxy content, and generated less related substances of 6-epidoxycycline, metacycline, and oxytetracycline during the storage. The differences at all time points were significant with P < 0.05 by Student’s t test, indicating that stabilities of Doxy were improved obviously in the inclusion complex at both solid and solution states.
Table II.
Stabilities of Doxy/HPβCD Complex in Aqueous Solution and Solid State at 25°C (n = 3)
| State | Time | Doxy/HPβCD complex | Doxy·HCl (Control) | ||
|---|---|---|---|---|---|
| Doxy content (%) | Related substance (%) | Doxy content (%) | Related substance (%) | ||
| Solution | 0 day | 100.00 ± 1.21 | 0.89 ± 0.34 | 100.00 ± 1.44 | 0.39 ± 0.05 |
| 7 day | 98.65 ± 1.01 | 1.23 ± 0.38 | 92.49 ± 3.25 | 3.57 ± 0.07 | |
| 14 day | 95.32 ± 2.05 | 2.07 ± 0.06 | 89.67 ± 2.34 | 5.54 ± 0.57 | |
| Solid | 0 month | 100.00 ± 1.21 | 0.89 ± 0.34 | 100.00 ± 1.44 | 0.39 ± 0.05 |
| 2 month | 98.71 ± 2.63 | 1.15 ± 0.21 | 91.50 ± 1.94 | 2.95 ± 0.45 | |
| 6 month | 93.63 ± 3.05 | 1.39 ± 0.63 | 83.53 ± 4.67 | 4.54 ± 0.57 | |
Additionally, a series of accelerated tests were performed to evaluate the influence of HPβCD and Mg2+on the stability of Doxy individually. As shown in Fig. 6, it was found that the relative amount of Doxy in the HPβCD solution was 81.16% after 10 days of storage at 40°C, higher than that of 77.98% in Doxy·HCl solution. With the addition 0.5% of MgCl2, the relative concentration of Doxy in HPβCD solution was even increased to 84.42%, while reversing the addition sequence of Mg2+ and HPβCD did not make significant difference on the content of Doxy (P > 0.05). Thus, both Mg2+ and HPβCD showed positive effects on the stability improvement of Doxy.
Fig. 6.
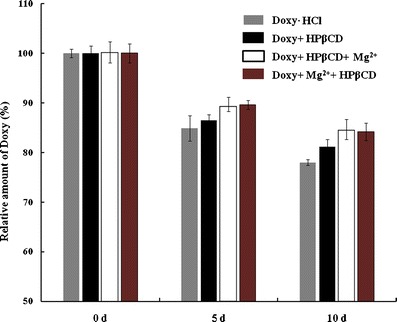
Stability of Doxy solution with addition of HPβCD and magnesium chloride at 40°C (n = 3)
In order to understand the factors that could influence the stability of Doxy, the degradation process of Doxy was analyzed. The potential epimers and degradation products of Doxy are 4-epidoxycycline, 4, 6-epidoxycycline, 6-epidoxycycline, metacycline (6-dehydro-doxycycline), and oxytetracycline (6-oxido-doxycycline) (Fig. 7; 33). Potential degradations of Doxy may occur at the sites of 4-N(CH3)2 and 6-CH3. Degradation products of Doxy at 6-CH3, 6-epidoxycycline, metacycline, and oxytetracycline, are three related substances that are limited to a certain amount (total amount no more than 4%) in Doxy preparations per many pharmacopoeias such as USP, EUP, and CP. In the present study, the primary susceptible site on Doxy molecule was identified as 6-CH3, and related substances including 6-epidoxycycline, metacycline, and oxytetracycline were detected.
Fig. 7.
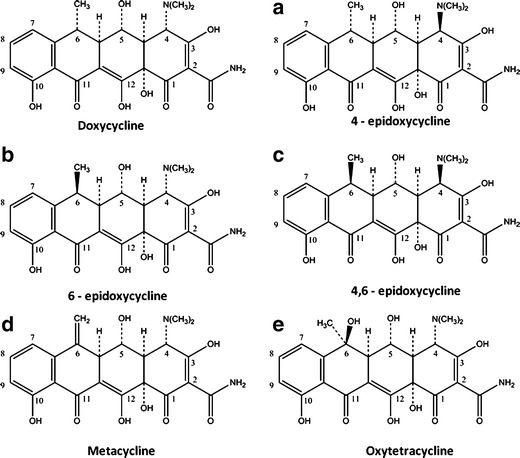
Epimers and degradation products of doxycycline
Based on the molecular model of Doxy/HPβCD complex, it was found that 6-CH3 located on the hydrophobic aromatic ring of Doxy, which docked into the hydrophobic cavity of HPβCD through inclusion. In addition, results of 1H NMR and 2D ROESY studies showed that the proton shift of 6-CH3 in Doxy obviously changed after the inclusion process, indicating intermolecular interactions such as hydrogen bonding and/or van der Waals force occurred at the site of 6-CH3 in the inclusion system. These interactions could competitively inhibit the epimerization and degradations at the 6-CH3 site, while the cavity of HPβCD could exert a steric effect to the attacking reagents thus improve the stability of Doxy (14). The molecular model in Fig. 5 could graphically illustrate the protective effects of HPβCD on Doxy.
As MgCl2 was added to increase the solubility of doxycycline monohydrate for the following inclusion with HPβCD, its effects on the stability of Doxy was also considered. Since Mg2+ could chelate with Doxy between the 11-carbonyl group and the 12-enol functional group leading to a more delocalized system (34), interestingly, it was found that the stability of Doxy/HPβCD inclusion complex was further improved with the addition of Mg2+ based on the accelerated tests. The particular interactions between Doxy and Mg2+ were investigated by 1H NMR experiment. A significant cleavage of 4-N(CH3)2 peak of Doxy around 2.9 ppm was observed in the presence of Mg2+ (Fig. 3a and b), indicating the chelation of the second metal ion to 4-N and 3-O of Doxy, as proposed by other authors for tetracycline (35,36). Since 4-N(CH3)2 is another potential degradation site of Doxy, the chelation effects with Mg2+ at this site could further stabilize Doxy. The stabilility enhancement of Doxy could be due to the synergistic effects of inclusion of HPβCD and chelation of Mg2+.
In Vitro AntiBacterial Activity
The antibacterial activities of Doxy/HPβCD complex against E. coli, P. aeruginosa, and S. aureus, the three major bacteria that can cause ocular infection, were determined using a disk diffusion test. The diameters of visible inhibition zones with 30 μL of Doxy/HPβCD complex or Doxy·HCl at the same Doxy concentration of 1,000 μg/ml were measured (Table III). According to the National Committee for Clinical Laboratory Standards, diameter of doxycylcine antibacterial zone is classified into three categories: ≥ 16, 13–15, and ≤12, which respectively represent the degree that the pathogen reacts to this antibiotic as: susceptible, intermediate, and resistant. Thus, E. coli and S. aureus with mean diameters of antibacterial zone of 19.32 and 28.25 mm showed strong sensitivities, while P. aeruginosa with a mean diameter of antibacterial zone of 12.20 mm exhibited intermediate susceptibility to Doxy/HPβCD complex. There was no significant difference in the antibacterial activities between Doxy·HCl and Doxy/HPβCD complex groups (Student’s t test, P > 0.05). These results indicated that the in vitro antibacterial activities of Doxy were not weakened in the inclusion complex. Besides, the in vivo activity of the Doxy/HPβCD complex to treat corneal neovascularization on rats has been demonstrated in a related research (12).
Table III.
Diameters of Antibacterial Zones of Doxy/HPβCD Complex and Doxy·HCl (n = 4)
| Doxy/HPβCD | Doxy·HCl (control) | |
|---|---|---|
| Pseudomonas aeruginosa | 12.20 ± 0.48 mm | 11.90 ± 1.06 mm |
| Escherichia coli | 19.32 ± 1.64 mm | 19.55 ± 1.21 mm |
| Staphylococcus aureus | 28.25 ± 1.03 mm | 27.20 ± 1.01 mm |
CONCLUSIONS
The formation of inclusion complex with HPβCD improved the stability of Doxy obviously. This improvement can be explained by the molecular model and inclusion mechanism analysis of Doxy/HPβCD complex, which indicated that the unstable site 6-CH3 of Doxy molecule was protected in the hydrophobic cavity of HPβCD and the other susceptible site of 4-N(CH3)2 was chelated with Mg2+ for protection. Doxy/HPβCD complex with good in vitro antibacterial activities may have potential values for clinical applications.
REFERENCES
- 1.Ferreri AJ, Ponzoni M, Guidoboni M, Resti AG, Politi LS, Cortelazzo S, et al. Bacteria-eradicating therapy with doxycycline in ocular adnexal MALT lymphoma: a multicenter prospective trial. J Natl Cancer Inst. 2006;98(19):1375–1382. doi: 10.1093/jnci/djj373. [DOI] [PubMed] [Google Scholar]
- 2.Smith VA, Cook SD. Doxycycline-a role in ocular surface repair. Br J Ophthalmol. 2004;88(5):619–625. doi: 10.1136/bjo.2003.025551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libinson GS, Ushakova TA. Doxycycline. Stability in solutions. Pharm Chem J. 1976;10(8):1076–1078. doi: 10.1007/BF00758100. [DOI] [Google Scholar]
- 4.Carrier RL, Miller LA, Ahmed M. The utility of cyclodextrins for enhancing oral bioavailability. J Control Release. 2007;123(2):78–99. doi: 10.1016/j.jconrel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 5.Loftsson T, Duchene D. Cyclodextrins and their pharmaceutical applications. Int J Pharm. 2007;329(1–2):1–11. doi: 10.1016/j.ijpharm.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 6.Brewster ME, Loftsson T. Cyclodextrins as pharmaceutical solubilizers. Adv Drug Deliv Rev. 2007;59(7):645–666. doi: 10.1016/j.addr.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Mady FM, Abou-Taleb AE, Khaled KA, Yamasaki K, Iohara D, Taguchi K, et al. Evaluation of carboxymethyl-beta-cyclodextrin with acid function: improvement of chemical stability, oral bioavailability and bitter taste of famotidine. Int J Pharm. 2010;397(1–2):1–8. doi: 10.1016/j.ijpharm.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Stella VJ, Rao VM, Zannou EA, Zia V. Mechanisms of drug release from cyclodextrin complexes. Adv Drug Deliv Rev. 1999;36(1):3–16. doi: 10.1016/S0169-409X(98)00052-0. [DOI] [PubMed] [Google Scholar]
- 9.Cal K, Centkowska K. Use of cyclodextrins in topical formulations: practical aspects. Eur J Pharm Biopharm. 2008;68(3):467–478. doi: 10.1016/j.ejpb.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong WW, Neck M. Oxytetracycline compositions. US patent. 1976;646,295.
- 11.Nosworthy MM, Ferry G. Doxycycline parenteral compositions. US patent. 1976;477,703.
- 12.Su WR, Li ZR, Lin ML, Li YP, He ZX, Wu CB, et al. The effect of doxycycline temperature-sensitive hydrogel on inhibiting the corneal neovascularization induced by BFGF in rats. Graefes Arch Clin Exp Ophthalmol. 2011;249(3):421–427. doi: 10.1007/s00417-010-1539-y. [DOI] [PubMed] [Google Scholar]
- 13.Cho HJ, Balakrishnan P, Shim WS, Chung SJ, Shim CK, Kim DD. Characterization and in vitro evaluation of freeze-dried microparticles composed of granisetron-cyclodextrin complex and carboxymethylcellulose for intranasal delivery. Int J Pharm. 2010;400(1–2):59–65. doi: 10.1016/j.ijpharm.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 14.Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J Pharm Sci. 1996;85(10):1017–1025. doi: 10.1021/js950534b. [DOI] [PubMed] [Google Scholar]
- 15.de Paula WX, Denadai AML, Santoro MM, Braga ANG, Santos RAS, Sinisterra RD. Supramolecular interactions between losartan and hydroxypropyl-beta-CD: ESI mass-spectrometry, NMR techniques, phase solubility, isothermal titration calorimetry and anti-hypertensive studies. Int J Pharm. 2011;404(1–2):116–123. doi: 10.1016/j.ijpharm.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Mura P, Bettinetti G, Melani F, Manderioli A. Interaction between naproxen and chemically modified [beta]-cyclodextrins in the liquid and solid state. Eur J Pharm Sci. 1995;3(6):347–355. doi: 10.1016/0928-0987(95)00025-X. [DOI] [Google Scholar]
- 17.Yap KL, Liu X, Thenmozhiyal JC, Ho PC. Characterization of the 13-cis-retinoic acid/cyclodextrin inclusion complexes by phase solubility, photostability, physicochemical and computational analysis. Eur J Pharm Sci. 2005;25(1):49–56. doi: 10.1016/j.ejps.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Xing SK, Zhang C, Ai HQ, Zhao Q, Zhang Q, Sun DZ. Theoretical study of the interactions of beta-cyclodextrin with 2 ′-hydroxyl-5 ′-methoxyacetophone and two of its isomers. J Mol Liq. 2009;146(1–2):15–22. doi: 10.1016/j.molliq.2009.01.005. [DOI] [Google Scholar]
- 19.Shi JH, Ding ZJ, Hu Y. Theoretical study on chiral recognition mechanism of methyl mandelate enantiomers on permethylated beta-cyclodextrin. J Mol Model. 2012;18(2):803–813. doi: 10.1007/s00894-011-1118-0. [DOI] [PubMed] [Google Scholar]
- 20.Chen MW, Pan X, Wu HM, Han K, Xie XB, Wedge DE, et al. Preparation and anti-bacterial properties of a temperature-sensitive gel containing silver nanoparticles. Pharmazie. 2011;66(4):272–277. [PubMed] [Google Scholar]
- 21.Rios JL, Recio MC, Villar A. Screening methods for natural products with antimicrobial activity: a review of the literature. J Ethnopharmacol. 1988;23(2–3):127–149. doi: 10.1016/0378-8741(88)90001-3. [DOI] [PubMed] [Google Scholar]
- 22.He ZX, Wang ZH, Zhang HH, Pan X, Su WR, Liang D, et al. Doxycycline and hydroxypropyl-β-cyclodextrin complex in poloxamer thermal sensitive hydrogel for ophthalmic delivery. Acta Pharmacol Sin B. 2011;1(4):254–260. doi: 10.1016/j.apsb.2011.10.004. [DOI] [Google Scholar]
- 23.Gibaud S, Ben ZS, Mutzenhardt P, Fries I, Astier A. Melarsoprol-cyclodextrins inclusion complexes. Int J Pharm. 2005;306(1–2):107–121. doi: 10.1016/j.ijpharm.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Zhao M, Wang H, Yang B, Tao H. Identification of cyclodextrin inclusion complex of chlorogenic acid and its antimicrobial activity. Food Chem. 2010;120(4):1138–1142. doi: 10.1016/j.foodchem.2009.11.044. [DOI] [Google Scholar]
- 25.Yang B, Lin J, Chen Y, Liu Y. Artemether/hydroxypropyl-beta-cyclodextrin host-guest system: characterization, phase-solubility and inclusion mode. Bioorg Med Chem. 2009;17(17):6311–6317. doi: 10.1016/j.bmc.2009.07.060. [DOI] [PubMed] [Google Scholar]
- 26.Torri G, Bertini S, Giavana T, Guerrini M, Puppini N, Zoppetti G. Inclusion complex characterization between progesterone and hydroxypropyl-beta-cyclodextrin in aqueous solution by NMR study. J Incl Phenom Macrocycl Chem. 2007;57(1–4):317–321. doi: 10.1007/s10847-006-9180-4. [DOI] [Google Scholar]
- 27.Jullian C, Cifuentes C, Alfaro M, Miranda S, Barriga G, Olea-Azar C. Spectroscopic characterization of the inclusion complexes of luteolin with native and derivatized beta-cyclodextrin. Bioorg Med Chem. 2010;18(14):5025–5031. doi: 10.1016/j.bmc.2010.05.079. [DOI] [PubMed] [Google Scholar]
- 28.Li JS, Xiao HN, Li JH, Zhong YP. Drug carrier systems based on water-soluble cationic beta-cyclodextrin polymers. Int J Pharm. 2004;278(2):329–342. doi: 10.1016/j.ijpharm.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 29.Rojas-Aguirre Y, Yepez-Mulia L, Castillo I, Lopez-Vallejo F, Soria-Arteche O, Hernandez-Campos A, et al. Studies on 6-chloro-5-(1-naphthyloxy)-2-(trifluoromethyl)-1H-benzimidazole/2-hydroxypropyl-beta-cyclodextrin association: characterization, molecular modeling studies, and in vivo anthelminthic activity. Bioorg Med Chem. 2011;19(2):789–797. doi: 10.1016/j.bmc.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 30.Kollman PA, Massova I, Reyes C, Kuhn B, Huo S, Chong L, et al. Calculating structures and free energies of complex molecules: combining molecular mechanics and continuum models. Acc Chem Res. 2000;33(12):889–897. doi: 10.1021/ar000033j. [DOI] [PubMed] [Google Scholar]
- 31.Jullian C, Orosteguis T, Perez-Cruz F, Sanchez P, Mendizabal F, Olea-Azar C. Complexation of morin with three kinds of cyclodextrin. A thermodynamic and reactivity study. Spectrochim Acta A Mol Biomol Spectrosc. 2008;71(1):269–275. doi: 10.1016/j.saa.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 32.Szejtli J. Introduction and general overview of cyclodextrin chemistry. Chem Rev. 1998;98(5):1743–1754. doi: 10.1021/cr970022c. [DOI] [PubMed] [Google Scholar]
- 33.Skulason S, Ingolfsson E, Kristmundsdottir T. Development of a simple HPLC method for separation of doxycycline and its degradation products. J Pharm Biomed Anal. 2003;33(4):667–672. doi: 10.1016/S0731-7085(03)00316-9. [DOI] [PubMed] [Google Scholar]
- 34.Newman EC, Frank CW. Circular dichroism spectra of tetracycline complexes with Mg+2 and Ca+2. J Pharm Sci. 1976;65(12):1728–1732. doi: 10.1002/jps.2600651209. [DOI] [PubMed] [Google Scholar]
- 35.Lambs L, Venturim M, Révérend BDL, Kozlowski H, Berthon G. Metal ion-tetracycline interactions in biological fluids: part 8. Potentiometric and spectroscopic studies on the formation of Ca (II) and Mg (II) complexes with 4-dedimethylamino-tetracycline and 6-desoxy-6-dem. J Inorg Biochem. 1988;33(3):193–209. doi: 10.1016/0162-0134(88)80049-7. [DOI] [PubMed] [Google Scholar]
- 36.Silva PP, Guerra W, Silveira JN, Ferreira AMC, Bortolotto T, Fischer FL, et al. Two new ternary complexes of copper(II) with tetracycline or doxycycline and 1,10-phenanthroline and their potential as antitumoral: cytotoxicity and DNA cleavage. Inorg Chem. 2011;50(14):6414–6424. doi: 10.1021/ic101791r. [DOI] [PubMed] [Google Scholar]


