Abstract
Fatty acid esters are long-chain esters, produced from the reaction of fatty acids and alcohols. They possess potential applications in cosmetic and pharmaceutical formulations due to their excellent wetting behaviour at interfaces and a non-greasy feeling when applied on the skin surfaces. This preliminary work was carried out to construct pseudo-ternary phase diagrams for oleyl laurate, oleyl stearate and oleyl oleate with surfactants and piroxicam. Then, the preparation and optimization study via ‘One-At-A-Time Approach’ were carried out to determine the optimum amount of oil, surfactants and stabilizer using low-energy emulsification method. The results revealed that multi-phase region dominated the three pseudo-ternary phase diagrams. A composition was chosen from each multi-phase region for preparing the nanoemulsions systems containing piroxicam by incorporating a hydrocolloid stabilizer. The results showed that the optimum amount (w/w) of oil for oleyl laurate nanoemulsions was 30 and 20 g (w/w) for oleyl stearate nanoemulsions and oleyl oleate nanoemulsions. For each nanoemulsions system, the amount of mixed surfactants and stabilizer needed for the emulsification to take place was found to be 10 and 0.5 g (w/w), respectively. The emulsification process via high-energy emulsification method successfully produced nano-sized range particles. The nanoemulsions systems passed the centrifugation test and freeze–thaw cycle with no phase failures, and stable for 3 months at various storage temperatures (3°C, 25°C and 45°C). The results proved that the prepared nanoemulsions system cannot be formed spontaneously, and thus, energy input was required to produce nano-sized range particles.
KEY WORDS: high-energy emulsification method, low-energy emulsification method, particle size, pseudo-ternary phase diagram, stabilizer
INTRODUCTION
Naturally occurring wax esters are expensive and limited in access; and as a result, the need to synthesize the compound has grown. The synthetic wax esters are long-chain esters, produced from the reaction of fatty acids and alcohols. Both fatty acids and alcohols have the chain lengths of 12 carbons or more. Wax esters are synthesized via chemical and enzymatic reactions. With the introduction of green chemistry, enzymatic synthesis is considered to satisfy its concept due to the environmentally friendly process with a high yield of wax ester with improving quality and purity of the product (1). The synthesis of wax esters have been carried out in mild reaction conditions, such as at normal pressure and temperatures (2–5). Wax esters possess potential applications due to their excellent wetting behaviour at interfaces and a non-greasy feeling when applied on the skin surfaces. Therefore, they have been used as important ingredients in cosmetic and pharmaceutical formulations.
In the early introduction of emulsions having droplet size in the nano metric scale, scientists reported their findings as mini-emulsions (6), submicron emulsions (7), translucent emulsions (8) and ultrafine emulsions (9). The term ‘nanoemulsions’ was introduced in 2003, and defined as emulsions having droplet sizes in the range of 20–200 nm (10,11). Nanoemulsions have been reported to have small and uniform droplet sizes, which make their appearance to be transparent (12), thermodynamically unstable, with a possible long-term physical stability (13) and stable against sedimentation or creaming (14). The small droplet size of nanoemulsions also requires less surfactant concentration for its formation (15). Nanoemulsions are applied extensively in various fields such as in health care and skin care products, food emulsions, drug delivery and fragrance delivery.
Since both wax esters and nanoemulsions possess several advantages, this work was carried out to manipulate the advantages of both wax esters and nanoemulsions as transdermal nanodelivery of the prototype drug, piroxicam (Fig. 1). Two types of non-ionic surfactants which are Pluronic F68 (Fig. 2) and Span 20 (Fig. 3) were utilized in this work. Pluronic F68 is a triblock copolymer from the A–B–A type, consisting of 75 units of ethylene oxide monomers for A-block and 30 units of propylene oxide monomers for B-block whilst Span 20 is one of the fatty acid esters of sorbitan (sorbitan monolaurate). Both Pluronic F68 and Span 20 are non-ionic surfactants. The aim of the work reported herein was, firstly, to construct the pseudo-ternary phase diagram for fatty acid esters containing piroxicam. Then, a composition from the unstable and multiphase region of the pseudo-ternary phase diagram was selected, and consequently stabilized using xanthan gum (Fig. 4). Finally, the preparation and characterization of the stabilized nanoemulsions system were investigated.
Fig. 1.
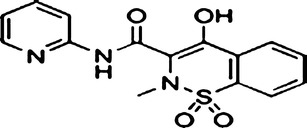
Piroxicam
Fig. 2.
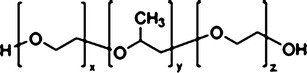
Pluronic F68
Fig. 3.
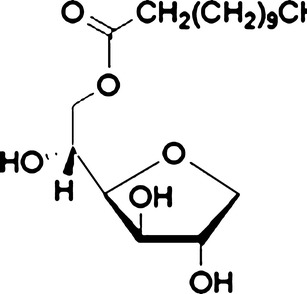
Span 20
Fig. 4.
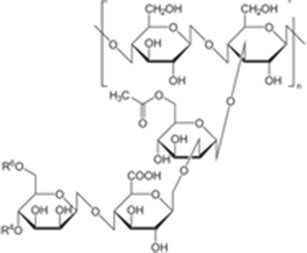
Xanthan gum
EXPERIMENTAL
Materials
Three types of oil phase, which are oleyl laurate, oleyl stearate and oleyl oleate, were synthesized in the laboratory using the optimized reaction conditions (5). Hydrophilic polymeric surfactant, Pluronic F68 and lipophilic surfactant, Span 20 were purchased from BASF, USA and Merck, Germany, respectively. A stabilizer, i.e., xanthan gum was purchased from Fluka Chemica, Switzerland whilst phenonip and solubilizer gamma 2429 were purchased from Golden Hope, Malaysia. Piroxicam was purchased from Eurochem, China.
Construction of Pseudo-Ternary Phase Diagrams Using Single Wax Ester
Pluronic F68 (5 g) was dissolved in 10 g of deionized water, and the solution was kept overnight. The solution was mixed with Span 20 with the ratio of 8:2 (w/w) and was labeled as mixed surfactants. Then, 0.5 g piroxicam was added to 100 g oleyl laurate and heated to 80°C whilst stirring using the magnetic bar. For the preparation of nanoemulsions systems, oleyl laurate/mixed surfactants were weighed at various ratios (w/w) ranging from 0:10, 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, 9:1 and 10:0. The mixtures of total weight of 0.5 g were placed in a 10-mL screw-cap glass tube. An appropriate 0.1% by weight of deionized water was added to the samples and vortexed using a vortex mixer (Uzusio VTX-3000L, Japan) for 5 min to homogenize the samples. Then, the samples were centrifuged (Eltek TC-6505, India) at 4,000 rpm for 15 min. The phase changes of the mixtures for the isotropic and anisotropic regions were examined visually through cross-polarized light. The procedures were repeated for oleyl stearate and oleyl oleate. Finally, pseudo-ternary phase diagrams were constructed for oleyl laurate, oleyl stearate and oleyl oleate.
Modification of the Nanoemulsions System Containing Piroxicam Using Stabilizer
Based on the constructed phase diagram for the nanoemulsions systems of oleyl laurate:piroxicam/mixed surfactants/deionized water and oleyl stearate:piroxicam/mixed surfactants/deionized water, one composition was selected from the two-phase regions. As for the nanoemulsions system of oleyl oleate:piroxicam/mixed surfactants/deionized water, a composition from the three-phase region was selected. The selected compositions were at low surfactant (10%) and oil (10%) contents. The other ingredients were 0.5% piroxicam, 10% solubilisant Gamma 2429, 0.5% rheological modifier (xanthan gum) and 0.5% preservative (phenonip). Deionized water was added until the total composition of the nanoemulsions systems reached 100% (w/w).
For the preparation of the oil phase, piroxicam, solubilisant Gamma 2429 and Span 20 were added into the oil (for instance, oleyl laurate). Then, for the aqueous phase, deionized water was mixed with Pluronic F68 and xanthan gum. The oil and aqueous phases were heated to 80°C and after that, were let to cool to 40°C before being mixed. Using a homogenizer (Stirrer RW16 Basic IKA®—WERKE, USA), the oil phase was stirred at 300 rpm whilst adding the aqueous phase until completed. Finally, phenonip was added to the mixture. The stirring process was performed for 4 h. The procedures were repeated for oleyl stearate nanoemulsions and oleyl oleate nanoemulsions.
Studies on Individual Effects of Various Parameters Using ‘One-At-A-Time Approach’
Following the method from “Modification of the Nanoemulsions System Containing Piroxicam Using Stabilizer” section, three factors that may affect the properties of nanoemulsions systems were selected for optimization. The three factors are percentage of oil phase (w/w), percentage of mixed surfactant (w/w) and percentage of rheology modifier (w/w). Each of the prepared sample was subjected to particle size analysis. The experimental procedures for the optimization study and analysis of samples were repeated for oleyl stearate nanoemulsions and oleyl oleate nanoemulsions.
Effect of Percentage of Oil on Nanoemulsions Formulations
The oleyl laurate nanoemulsions were prepared by varying the amount of oleyl laurate (40%, 30%, 20% and 10% (w/w)) and in the same conditions as described in “Modification of the Nanoemulsions System Containing Piroxicam Using Stabilizer” section.
Effect of Percentage of Stabilizer on Nanoemulsions Formulations
The effect of the stabilizer was investigated by varying the quantities of xanthan gum (0.5%, 1.0% and 1.5% (w/w)) and in the same conditions as described in “Modification of the Nanoemulsions System Containing Piroxicam Using Stabilizer” section.
Effect of Percentage of Mixed Surfactants on Nanoemulsions Formulations
The effect of mixed surfactants on the oleyl laurate nanoemulsions were studied by varying the concentration of mixed surfactants (10%, 8%, 6%, 4% and 2% (w/w)) and in the same conditions as described in “Modification of the Nanoemulsions System Containing Piroxicam Using Stabilizer” section.
Optimization of the Nanoemulsions Systems Using Low-Energy and High-Energy Emulsification Methods
The oleyl laurate nanoemulsions were prepared as described in “Modification of the Nanoemulsions System Containing Piroxicam Using Stabilizer” section. The mixtures of the oil phase and aqueous phase were homogenized using a high-shear homogenizer (Multimix 2003, Malaysia) at 4,000 rpm for 5 min. Phenonip was added to the formulations, and the stirring process (Stirrer RW16 Basic IKA®—WERKE, USA) was performed at a very gentle speed (100 rpm) for 1 h to ensure homogenization. The procedures were repeated for oleyl stearate nanoemulsions and oleyl oleate nanoemulsions.
Characterization of Nanoemulsions Systems
In order to confirm the formation of nano-size particles and to investigate the stability of the three nanoemulsions systems, the following techniques were carried out:
-
Droplet Size Analysis
The mean droplet size and distribution of the nanoemulsions systems were determined through dynamic light scattering using a particle size analyser, Nanophox Sympatex (Germany) at 25.0 ± 0.5°C. Hydrodynamic measurements were performed at 90° scattering angle with the light source of HeNe-laser (λ = 632.8 nm); 10 mW max. The samples were diluted with deionized water (1:10, v/v) and placed in 10 × 10-mm2 disposable cuvette made of polystyrene.
-
Centrifugation Test
The centrifugation test was carried out by keeping the nanoemulsions systems in the screw cap tubes. Then, they were subjected to centrifugation (Eltek TC-6505, India) at 14,000 rpm for 15 min. After 15 min, the nanoemulsions systems were checked for phase changes.
-
Freeze–Thaw Cycle
The freeze–thaw cycle was carried out by alternately keeping the formulations at 3°C and 25°C for 24 h for 6 cycles. For each cycle, the observation was made to monitor any changes to the nanoemulsions systems such as phase separation.
-
Storage Stability
The stability of the nanoemulsions systems were carried out by observing the physical appearance of the formulation (no sedimentation, creaming, coalescence and phase separation) under different storage conditions (3°C, 25°C and 45°C) for 3 months.
RESULTS AND DISCUSSION
Construction of Pseudo-Ternary Phase Diagrams Using Single Wax Ester
Oleyl laurate, oleyl stearate and oleyl oleate were separately used as the oil phase for the emulsification process with water (aqueous phase) and mixed surfactants. The three oil phases were fatty acid esters of fatty alcohols, which had been synthesized enzymatically (5). The purpose of using these fatty acid esters of fatty alcohols as the oil phase was due to their unique properties, such as their excellent wetting behaviour and without the oily feeling. The utilization of Pluronic F68 in this study was because it is more stable and safer as a delivery agent (16).
The construction of pseudo-ternary phase diagrams is the best way to study all types of formulations that can originate from the mixing of surfactants, water and oil, and also cover the whole probabilities of mixing ratios in a more systematic way (17). Thus, nanoemulsions systems of oleyl laurate:piroxicam/mixed surfactants/deionized water, oleyl stearate:piroxicam/mixed surfactants/deionized water and oleyl oleate:piroxicam/mixed surfactants/deionized water were prepared, and three pseudo-ternary phase diagrams were constructed as shown in Figs. 5, 6 and 7, respectively.
Fig. 5.
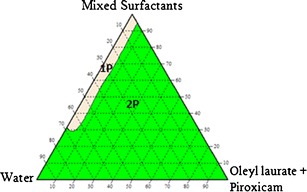
The pseudo-ternary phase diagram for the system oleyl laurate:piroxicam/mixed surfactants/water (1P = one-phase region, 2P = two-phase region)
Fig. 6.
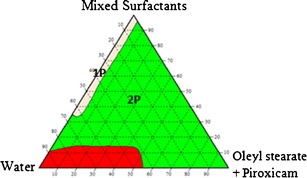
The pseudo-ternary phase diagram for the system oleyl stearate:piroxicam/mixed surfactants/water (1P = one-phase region, 2P = two-phase region, 3P = three-phase region)
Fig. 7.
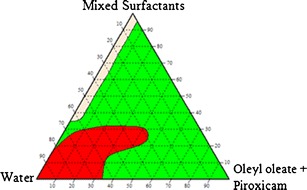
The pseudo-ternary phase diagram for the system oleyl oleate:piroxicam/mixed surfactants/water (1P = one-phase region, 2P = two-phase region, 3P = three-phase region)
The three pseudo-ternary phase diagrams showed that the formation of a one-phase region can be observed at very low concentrations of oil and high concentrations of water and surfactant mixtures. The formation of a one-phase region suggested that the surfactant mixtures were able to lower the surface tension between the aqueous phase and oil phase, hence facilitate the formation of emulsions having a milky appearance. However, due to a low amount of Span 20, it was believed that Span 20 did not play a significant role in facilitating the formation of emulsions as greatly as Pluronic F68. Pluronic F68, which is triblock polymeric surfactants having two blocks of poly(ethylene oxide) (PEO) and one block of poly(propylene oxide) (PPO) attached to the oil droplet by residing the hydrophobic PPO chain at the hydrophobic surface, whilst the other two hydrophilic PEO chains are dangling in the aqueous solution (18).
The observation made also showed that the one-phase region was a homogeneous region, which indicated that the emulsions formed were of large particle size. The large particle size may be due to the utilization of Pluronic F68 as a surfactant. This could be explained by considering the large structure of Pluronic F68, which is a triblock copolymer from the A–B–A type, consisting of 75 units of ethylene oxide monomers for A-block and 30 units of propylene oxide monomers for B-block. The hydrophilic PEO chains of Pluronic F68 were assumed to be able to form loops, which protruded out into the aqueous phase and as a result, contributed to the large particle size of the emulsions.
The formation of a large two-phase region was also observed for the three pseudo-ternary phase diagrams at low water content and high oil content. The results suggested that the surfactant mixtures were not sufficient to solubilize the oil phase. This suggested that the PPO portion could not provide a strong “anchor” to the oil droplet and as for Span 20, the reason was probably due to its low concentration in the nanoemulsions systems. Another reason for the inability of the surfactant mixtures to facilitate the emulsification process was probably due to no synergistic effects between the two surfactants in enhancing the surface activity (19).
The pseudo-ternary phase diagrams for the system oleyl stearate:piroxicam/mixed surfactants/water and oleyl oleate:piroxicam/mixed Surfactants/water also showed the formation of three-phase region. The three-phase region could be seen at high concentrations of water and oil, and low concentration of mixed surfactants of the two pseudo-ternary phase diagrams, which suggested that the low amount of surfactant was insufficient to emulsify the aqueous and oil phases. The formation of three-phase regions for the two emulsions systems were probably due to the high hydrophobic character of oleyl stearate and oleyl oleate as they contained longer carbon chain as compared to oleyl laurate. This suggested that higher concentration of lipophilic surfactant should be used to emulsify these two esters even though there were already hydrophobic PPO portions from Pluronic F68.
Despite the drawbacks, further investigations were carried out by choosing one composition from the two-phase region of the system oleyl laurate:piroxicam/mixed surfactants/water and oleyl stearate:piroxicam/mixed surfactants/water. A composition was also selected from the three-phase region of the system oleyl oleate:piroxicam/mixed surfactants/water. The selection of the composition was done by considering the amount of surfactant, especially when one of the objectives for the work was to produce a topically applied pharmaceutical cream. It is believe that high amount of surfactant could cause irritancy to the skin especially for transdermal application (20). Xanthan gum, which is a stabilizer, could help in stabilizing the nanoemulsions systems by modifying its rheology. Therefore, another method was considered in order to find the suitable compositions of water, oil and mixed surfactants for each nanoemulsions system.
Optimization of Nanoemulsions System by One-At-A-Time Approach
The optimization study of nanoemulsions system using ‘One-At-A-Time’ approach was conducted mainly to determine the optimum composition of oil, mixed surfactants and stabilizer. The response chosen was the particle size of the nanoemulsions systems.
Effect of Percentage of Oil on Nanoemulsions Systems
The significance of studying the amount of oil is mainly served as a guidance to the formulator in finding the optimum amount so that the cost of producing final product is minimal, as the formulation is prepared from the two-phase region. At the same time, one would suggest that the amount of oil should not exceed 40%, as it would lead to the formation of water-in-oil emulsion, as the final product would become oily, thus unfavorable by the users/patients. Therefore, the percentage of oil was set to be 10%, 20% and 30%. For oleyl laurate (OL) nanoemulsions systems, they were labeled as OL10%, OL20% and OL30%. For nanoemulsions systems of oleyl stearate (OS) and oleyl oleate (OO), they were labeled as OS10%, OS20%, OS30% and OO10%, OO20%, OO30%, respectively.
After preparing the formulations, they were subjected to a centrifugation test and storage at various temperatures to assess their stability. From the observation, the formulations passed the centrifugation test and storage at various temperatures with no phase separation detected. Table I shows the results for particle size of the nanoemulsions systems using oleyl laurate, oleyl stearate and oleyl oleate as the oil phase at different percentages (w/w). None of the formulations showed particle size in the nano-size range, i.e., 20–200 nm. From the result, it was found that OO20% has the smallest particle size (223.13 ± 0.60 nm) followed by OS20% (256.70 ± 1.80 nm) and OL30% (432.50 ± 2.20 nm). It was also observed that a small particle size could be obtained from the nanoemulsions systems of oleyl stearate and oleyl oleate as the oil phase. Both of these wax esters have long chain lengths (36 carbons), which may be the reason for the small particle size.
Table I.
The Effect of Percentage of Oil on the Particle Size (in Nanometre) of the Nanoemulsions Systems
| Formulation | Particle size (nm) |
|---|---|
| OL10% | 703.80 ± 2.50 |
| OL20% | 1284.22 ± 4.80 |
| OL30% | 432.50 ± 2.20 |
| OS10% | 310.35 ± 2.50 |
| OS20% | 256.70 ± 1.80 |
| OS30% | 262.30 ± 0.95 |
| OO10% | 394.74 ± 1.25 |
| OO20% | 223.13 ± 0.60 |
| OO30% | 257.32 ± 0.80 |
When making comparison between the particle sizes of OO20% and OS20%, it was found that the particle size of OO20% were smaller (223.13 ± 0.60 nm) than OS20% (256.70 ± 1.80 nm). It was believed that the small particle size of OO20% could be due to the presence of the two double bonds in the hydrocarbon chain. The presence of two double bonds altered the conformation of the hydrocarbon tail, thus minimizing the unfavorable interaction between the non-polar and polar molecules, and at the same time, internal forces such as bond, angle and dihedral position could also act on the interaction in the nanoemulsions systems (21). The results also showed that the particle size for the formulations of oleyl laurate was the largest of the three systems. This could be due to the shorter chain length of oleyl laurate, which explains that a higher amount of oleyl laurate was needed (30%) to enable its interaction with surfactants.
Generally, the smallest particle size was obtained at the optimum amount of oil. This observation could correlate to the amount of surfactants that were added to each nanoemulsions system as there would be interactions between the hydrophobic portions of the surfactants and the hydrophobic tail of the oil. Therefore, below the optimum amount of oil, the particle size was found to be larger as there were insufficient hydrophobic sites available for the interactions to happen, which resulted in the dangling of the hydrophobic portions of Pluronic F68 in the aqueous phase. For the amount of oil that was beyond the optimum amount, one would like to postulate that sufficient hydrophobic interactions occurred, but there were the leftovers of oil that might have been attached to the emulsions particles, which finally were observed as the enlargement of the particle size.
Effect of Percentage of Rheology Modifier on Nanoemulsions Systems
In this study, xanthan gum (XG) was added to the nanoemulsions system as the composition chosen from the ternary phase diagrams were of the multiphase regions. As a stabilizer, xanthan gum contributed an important role as it modified the rheology of the nanoemulsions system, thus increasing its stability. Generally, a stabilizer is added in small amounts into the emulsions system. Therefore, it was decided to study the effect of percentage of stabilizer (w/w) at 0.5%, 1.0% and 1.5%. The formulations for nanoemulsions systems using oleyl laurate (OL) as the oil phase were labeled as OLXG0.5%, OLXG1.0% and OLXG1.5%. The formulations for the nanoemulsions systems using oleyl stearate (OS) and oleyl oleate (OO) as the oil phases, were labeled as OSXG0.5%, OSXG1.0%, OSXG1.5% and OOXG0.5%, OOXG1.0%, OOXG1.5%, respectively.
It is essential to evaluate the optimum percentage of xanthan gum for each nanoemulsions system and most importantly, to enhance the stability of the system. All formulations were in the stable form (one phase), although initially they were prepared from the two-phase and three-phase regions (Table II). With the addition of xanthan gum to the nanoemulsions systems, all formulations were observed to be stable with no phase separation after performing the centrifugation test. They also survived the stability test when stored at various temperatures (3°C, 25°C and 45°C) for 3 months. The addition of polysaccharide could enhance protein adsorption at interfaces and therefore, increasing emulsion stability (22).
Table II.
The Effect of Percentage of Rheology Modifier on the Particle Size (in Nanometre) of the Nanoemulsions Systems
| Formulation | Phase behaviour | Particle size (nm) |
|---|---|---|
| OLXG0.5% | 1 phase | 740.14 ± 0.85 |
| OLXG1.0% | 1 phase | 810.30 ± 0.40 |
| OLXG1.5% | 1 phase | 1003.68 ± 2.80 |
| OSXG0.5% | 1 phase | 389.71 ± 0.45 |
| OSXG1.0% | 1 phase | 437.45 ± 0.60 |
| OSXG1.5% | 1 phase | 558.07 ± 2.30 |
| OOXG0.5% | 1 phase | 331.12 ± 0.60 |
| OOXG1.0% | 1 phase | 414.47 ± 0.90 |
| OOXG1.5% | 1 phase | 520.93 ± 1.50 |
Table II also shows the results of particle size (nm) for all the formulations prepared. It was found that the size of the particles for oleyl laurate nanoemulsions, oleyl stearate nanoemulsions and oleyl oleate nanoemulsions were directly proportional to the amount of xanthan gum. The smallest particle size could be obtained when using 0.5% of xanthan gum. This could be due to the structure of the xanthan gum, which attributes to the enlargement of the droplet size.
Effect of Percentage of Mixed Surfactants on Nanoemulsions System
This study was conducted by preparing the nanoemulsions system with various amounts of surfactants (S) ranging between 2% and 10%. Table III shows that the amount of surfactant is inversely proportional to the particle size of each nanoemulsions system using oleyl laurate (OL), oleyl stearate (OS) and oleyl oleate (OO) as the oil phase. The smallest particle size was observed for OSS10% (325.96 ± 1.10 nm) followed by OOS10% (388.43 ± 2.55 nm) and finally, OLS10% (757.21 ± 5.50 nm). The results also showed that a large particle size was observed when using a 2% surfactant. This showed that surfactants also help in stabilizing the emulsions system along with xanthan gum, but due to low amount of surfactants, the emulsions were found to have bigger particle sizes
Table III.
The Effect of Percentage of Mixed Surfactants on the Particle Size (in Nanometre) of the Nanoemulsions Systems
| Formulation | Particle size (nm) |
|---|---|
| OLS2% | 4882.65 ± 16.10 |
| OLS4% | 2507.32 ± 8.80 |
| OLS6% | 2390.84 ± 10.50 |
| OLS8% | 1464.63 ± 13.00 |
| OLS10% | 757.21 ± 5.50 |
| OSS2% | 918.65 ± 5.00 |
| OSS4% | 750.65 ± 3.60 |
| OSS6% | 527.27 ± 6.70 |
| OSS8% | 407.08 ± 3.20 |
| OSS10% | 325.96 ± 1.10 |
| OOS2% | 983.13 ± 4.00 |
| OOS4% | 775.14 ± 2.50 |
| OOS6% | 657.92 ± 4.00 |
| OOS8% | 537.86 ± 1.00 |
| OOS10% | 388.43 ± 2.55 |
As mentioned earlier, a 2–10% surfactant was insufficient to form a stable formulation with a nano-sized particle even though xanthan gum was added to each nanoemulsions system. It is suggested that a higher amount of surfactant, which was more than 10% (w/w) should be used, as a higher amount of surfactant may produce a smaller particle size, probably in the nano-size range. Microemulsions are reported to require a high surfactant concentration, usually in the region of 20% and higher (15). Therefore, the amount of surfactant is kept below 20% (w/w), as one of the main objectives of this study is to produce nanoemulsions systems, not microemulsions systems. All formulations were found to be stable with no phase separation after conducting the centrifugation test and storage at various temperatures (3°C, 25°C and 45°C).
Preparation of Nanoemulsions System Using Optimum Amount of Mixed Surfactants, Oil and Xanthan Gum Via Low-Energy and High-Energy Emulsification Methods
Table IV shows the optimum % (w/w) of mixed surfactants, oil and xanthan gum for oleyl laurate nanoemulsions, oleyl stearate nanoemulsions and oleyl oleate nanoemulsions. Using the compositions from Table IV, two formulations for each nanoemulsions system were prepared via low-energy emulsification and high-energy emulsification methods. This was done to investigate whether the three nanoemulsions systems require high energy to produce nano-sized particles.
Table IV.
The Percentage of Composition (w/w) of Mixed Surfactants, Oil and Xanthan Gum with Respect to Optimum Particle Size and Surface Charge of the Nanoemulsions Systems
| Nanoemulsions system | Factors (%,w/w) | ||
|---|---|---|---|
| Mixed surfactant | Oil | Xanthan gum | |
| OL1 | 10.00 | 30.00 | 0.50 |
| OS1 | 10.00 | 20.00 | 0.50 |
| OO1 | 10.00 | 20.00 | 0.50 |
The particle size for each nanoemulsions system prepared via low-energy emulsification method and high-energy emulsification method are shown in Table V. OS1b showed the smallest particle size (107.28 ± 2.00 nm), followed by OO1b (124.80 ± 3.50 nm) and OL1b (147.47 ± 1.15 nm). For the formulations prepared via the low-energy emulsification method, the particle size was observed to be between 242.33 nm and 454.64 nm. The results proved that the prepared nanoemulsions system cannot be formed spontaneously, and thus, energy input was required using high-energy emulsification methods, such as high-shear stirring, high-pressure homogenizers and ultrasound generators (14).
Table V.
The Particle Sizes for Formulations Prepared Using Different Emulsification Methods
| Low-energy emulsification method | High-energy emulsification method | ||
|---|---|---|---|
| Nanoemulsions system | Particle size (nm) | Nanoemulsions system | Particle size (nm) |
| OL1 | 454. 64 ± 3.50 | OL1b | 147.47 ± 1.15 |
| OS1b | 242.33 ± 2.00 | OS1b | 107.28 ± 2.00 |
| OO1b | 273.88 ± 2.50 | OO1b | 124.80 ± 3.50 |
Characterizations of Nanoemulsions Systems
Stability Study
Tables VI, VII and VIII show the results for the stability study of OL1b, OS1b and OO1b. For the centrifugation test, the prepared nanoemulsions formulations showed no phase separation after performing centrifugation at 14,000 rpm for 15 min. By using centrifugation test, the stability of the emulsion is directly proportional to the gravitational force (23). Therefore, by performing centrifugation test, the destabilization of the nanoemulsions can be accelerated, thus stimulating its ageing period (24). It is also accepted that shelf-life under normal storage conditions can be predicted by observing the creaming or coalescence of the dispersed phase when the emulsion is exposed to centrifugation.
Table VI.
Centrifugation Test
| Nanoemulsions system | Observation |
|---|---|
| OL1b | Stable |
| OS1b | Stable |
| OO1b | Stable |
Table VII.
Freeze–Thaw Cycle
| Duration (days) | 1 (5°C) | 2 (RT) | 3 (5°C) | 4 (RT) | 5 (5°C) | 6 (RT) |
|---|---|---|---|---|---|---|
| Nanoemulsions | ||||||
| OL1b | Stable | Stable | Stable | Stable | Stable | Stable |
| OS1b | Stable | Stable | Stable | Stable | Stable | Stable |
| OO1b | Stable | Stable | Stable | Stable | Stable | Stable |
Table VIII.
Storage Stability
| Day(s) | 1 | 30 | 60 | 90 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 3 | 25 | 45 | 3 | 25 | 45 | 3 | 25 | 45 | 3 | 25 | 45 |
| OL1b | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| OS1b | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| OO1b | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
✓ stable (no phase separation)
For the freeze–thaw cycling test, phase separation was accelerated by applying thermal treatment (25). Table VII shows that the nanoemulsions formulations were found to be in one-phase, even when the temperatures were drastically changed, with no growth of mold. These observations show that OL1b, OS1b and OO1b are physically stable, even though they have to undergo some freezing and thawing processes during their storage, which is probably due to the incorporation of Pluronic F68 and xantham gum into the nanoemulsions systems.
Table VIII shows the physical stability of OL1b, OS1b and OO1b over a period of 90 days at various storage temperatures. The utilization of Pluronic F68 has led to the formation of a three-dimensional cross-linked network in the external aqueous phase and contribute to the particle stabilization through the formation of thick steric barrier at the droplet interface (26). On the other hand, xanthan gum which consists of 1,4-linked β-d-glucose residues, with a trisaccharide side chain attached to alternate d -glucosyl residues would form a three-dimensional network formed by the associated chains making it as an efficient stabilizer for suspensions and emulsions (27). Additionally, the small size of the particles could reduce the creaming movements of droplets and the high viscosity of OL1b, OS1b and OO1b which was contributed by xanthan gum could also restrain the Brownian motion (28).
Even though the use of nonionic polymers was not able to decrease the interfacial tension to much lower as compared to that of surfactants, they could still stabilize the emulsion by steric repulsions, by forming a polymeric layer at the particle surface upon adsorption (29). The addition of water-soluble polymers, such as gums, are effective enough to protect emulsions against coalescence by raising the viscosity of the bulk phase, reducing the kinetic energy of the particles, and therefore, decreasing the probability of collisions (29).
The nanoemulsions formulations were found to be physically stable when they were stored at 5°C and 45°C. At 5°C, the nanoemulsions formulations were found to have good physical stability because the storage temperature was almost reaching freezing temperature, whereby all particles were believed to be in a nearly frozen state, by preserving all the three-dimensional cross-link networks of Pluronic F68 and xanthan gum, and also slowing down the movement (kinetic energy) of the particles. When stored at 45°C, water vapour could be observed at the inner wall of the sealed glass tube, which probably due to the evaporation of water from the continuous phase. However, the nanoemulsions formulations were found to show excellent physical stability, which is also believed to be the results of incorporating Pluronic F68 and xanthan gum into the systems. Evaporation of water from the continuous phase could actually disrupt the interfacial tension, electrostatic and static repulsions as well as the viscosity of the outer phase.
CONCLUSIONS
The phase behaviour of the systems: oleyl laurate:piroxicam/mixed surfactants/water, oleyl stearate:piroxicam/mixed surfactants/water and oleyl oleate:piroxicam/mixed surfactants/water were successfully constructed which showed the domination of multiphase region. The incorporation of xanthan gum into the systems was found to be successful for converting the multi-phase and unstable emulsions to one phase and stable emulsions. The preparation of the emulsions system was carried out via high-energy emulsification method and particle sizes between 50 and 200 nm with good physical stability were successfully produced.
ACKNOWLEDGEMENTS
This project is funded by National Biotechnology Directorate (project number 5487707), Ministry of Science, Technology and Innovation (MOSTI), Malaysia, UPM, UiTM and Ministry of Higher Learning (MOHE) grant for the scholar, Nursyamsyila Mat Hadzir.
Contributor Information
Nursyamsyila Mat Hadzir, Phone: +60-4-9882251, FAX: +60-4-9882484, Email: nursyamsyila@perlis.uitm.edu.my.
Mahiran Basri, Phone: +60-3-89467266, FAX: +60-3-89466997, Email: mahiran@science.upm.edu.my.
REFERENCES
- 1.Syamsul KMW, Salina MR, Siti SO, Hanina MN, Basyaruddin MAR, Kamaruzaman J. Green synthesis of lauryl palmitate via lipase-catalyzed reaction. WASJ. 2010;11(4):401–7. [Google Scholar]
- 2.Trani M, Ergan F, Andre G. Lipase-catalyzed production of wax esters. JAOCS. 1991;68(1):20–3. doi: 10.1007/BF02660302. [DOI] [Google Scholar]
- 3.Chen JP, Wang JB. Wax esters synthesis by lipase-catalyzed esterification with fungal cells immobilized on cellulose biomass support particles. Enzyme Microb Technol. 1997;18:615–22. doi: 10.1016/S0141-0229(96)00209-8. [DOI] [Google Scholar]
- 4.Gunawan ER, Basri M, Rahman MBA, Salleh AB, Rahman RNZA. Lipase-catalyzed synthesis of palm-based wax esters. J Oleo Sci. 2004;53:471–7. doi: 10.5650/jos.53.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mat Radzi S, Basri M, Salleh AB, Arbakariya A, Rosfarizan M, Abdul Rahman MB, Abdul Rahman RNZ. High performance enzymatic synthesis of oleyl oleate using immobilised lipase from Candida antartica. Electron J Biotechnol. 2005;8:292–8. doi: 10.2225/vol8-issue3-fulltext-4. [DOI] [Google Scholar]
- 6.El-Aasser MS, Lack CD, Vanderhoff JW, Fowkes FM. The mini-emulsification process-different form of spontaneous emulsification. Colloids Surf. 1988;29(1):103–18. doi: 10.1016/0166-6622(88)80174-4. [DOI] [Google Scholar]
- 7.Benita S, Levy MY. Submicron emulsions as colloidal drug carriers for intravenous administration: comprehensive physicochemical characterization. J Pharm Sci. 1993;82:1069–79. doi: 10.1002/jps.2600821102. [DOI] [PubMed] [Google Scholar]
- 8.Sing AJF, Gracia A, Lachaise J, Brochette P, Salager JL. Interactions and coalescence of nanodroplets in translucent O/W emulsions. Colloids Surf A. 1999;152(1–2):31–9. doi: 10.1016/S0927-7757(98)00622-0. [DOI] [Google Scholar]
- 9.Nakajima H. Microemulsions in cosmetics. In: Solans C, Kunieda H, editors. Industrial applications of microemulsions. New York: Marcel Dekker; 1997. pp. 175–97. [Google Scholar]
- 10.Solans C, Esquena J, Forgiarini AM, Usón N, Morales D, Izquierdo P, Azemar N, Garcia-Celma MJ. Nano-emulsions: formation, properties and applications. Surfactant Sci Ser. 2003;109:525–54. [Google Scholar]
- 11.Morales D, Gutierrez JM, Garcia-Celma MJ, Solans C. A study of the relation between bicontinuous microemulsions and oil/water nano-emulsion formation. Langmuir. 2003;19(18):7196–200. doi: 10.1021/la0300737. [DOI] [Google Scholar]
- 12.Aubrun OS, Simonnet JT, Alloret FL. Nanoemulsion: a new vehicle for skincare products. Adv Colloid Interface Sci. 2004;108–109:145–9. doi: 10.1016/j.cis.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Sole I, Maestro A, Pey CM, González C, Solans C, Gutiérrez JM. Nano-emulsions preparation by low energy methods in an ionic surfactant system. Colloids Surf A. 2006;288:138–43. doi: 10.1016/j.colsurfa.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Solans C, Izquierdo P, Nolla J, Azemar N, Garcia-Celma MJ. Nano-emulsions. Curr Opin Colloid Interface Sci. 2005;10:102–10. doi: 10.1016/j.cocis.2005.06.004. [DOI] [Google Scholar]
- 15.Tadros T, Izquierdo P, Esquena J, Solans C. Formation and stability of nano-emulsions. Adv Colloid Interface Sci. 2004;108/109:303–18. doi: 10.1016/j.cis.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 16.Chiappetta DA, Sosnik A. Poly(ethylene oxide)-poly(propylene oxide) block copolymer micelles as drug delivery agents: Improved hydrosolubility, stability and bioavailability of drugs. Eur J Pharm Biopharm. 2007;66:303–17. doi: 10.1016/j.ejpb.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Abdulkarim MF, Abdullah GZ, Sakeena MHF, Chitneni M, Yam MF, Mahdi ES, Salman IM, Ameer OZ, Munavvar AS, Basri M, Noor AM. Study of pseudoternary phase diagram behaviour and the effect of several Tweens and Spans on palm oil esters characteristics. Int J Drug Deliv. 2011;3:95–100. doi: 10.5138/ijdd.2010.0975.0215.03058. [DOI] [Google Scholar]
- 18.Tadros T. Polymeric surfactants in disperse systems. Adv Colloid Interface Sci. 2009;147–148:281–99. doi: 10.1016/j.cis.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Tadros T. Applied surfactants: principal and applications. Weinheim: Wiley; 2005. [Google Scholar]
- 20.Shakeel F, Ramadan W. Transdermal delivery of anticancer drug caffeine from water-in-oil nanoemulsions. Colloids Surf B. 2010;75:356–62. doi: 10.1016/j.colsurfb.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Peltonen L, Hirvonen J, Yliruusi J. The behavior of sorbitan surfactants at the water–oil interface: straight-chained hydrocarbons from pentane to dodecane as an oil phase. J Colloid Interface Sci. 2001;240:272–6. doi: 10.1006/jcis.2001.7612. [DOI] [PubMed] [Google Scholar]
- 22.Grinberg VY, Tolstoguzov VB. Thermodynamic incompatibility of proteins and polysaccharides in solutions. Food Hydrocoll. 1997;11(2):145–58. doi: 10.1016/S0268-005X(97)80022-7. [DOI] [Google Scholar]
- 23.Salim N, Basri M, Rahman MBA, Abdullah DK, Basri H, Salleh AB. Phase behaviour, formation and characterization of palm-based esters nanoemulsion formulation containing ibuprofen. J Nanomedic Nanotechnol. 2011;2(4):1–5. doi: 10.4172/2157-7439.1000113. [DOI] [Google Scholar]
- 24.Ng SH, Basri M, Rahman MBA, Rahman RNZA, Salleh AB, Ismail Z. Phase behavior and formulation of palm oil esters o/w nanoemulsions stabilized by hydrocolloid gums for cosmeceuticals application. J Dispers Sci Technol. 2011;32:1428–33. doi: 10.1080/01932691.2010.513301. [DOI] [Google Scholar]
- 25.Vilasau J, Solans C, Gomez MJ, Dabrio J, Mujika-Garai R, Esquena J. Stability of oil-in-water paraffin emulsions prepared in a mixed ionic/non-ionic surfactant system. Colloids Surf A. 2011;389:222–9. doi: 10.1016/j.colsurfa.2011.08.023. [DOI] [Google Scholar]
- 26.Capek I. Degradation of kinetically-stable o/w emulsions. Adv Colloid Interface Sci. 2004;107:125–55. doi: 10.1016/S0001-8686(03)00115-5. [DOI] [PubMed] [Google Scholar]
- 27.Katzbauer B. Properties and applications of xanthan gum. Polym Degrad Stab. 1998;59(1–3):81–4. doi: 10.1016/S0141-3910(97)00180-8. [DOI] [Google Scholar]
- 28.Mou D, Chen H, Du D, Mao C, Wan J, Xu H, Yang X. Hydrogel-thickened nanoemulsion system for topical delivery of lipophilic drugs. Int J Pharm. 2008;353:270–6. doi: 10.1016/j.ijpharm.2007.11.051. [DOI] [PubMed] [Google Scholar]
- 29.Sadtler VM, Imbert P, Dellacherie E. Ostwald ripening of oil-in-water emulsions stabilized by phenoxy-substituted dextrans. J Colloid Interface Sci. 2002;254:355–61. doi: 10.1006/jcis.2002.8624. [DOI] [PubMed] [Google Scholar]


