Abstract
The objective of this study was to develop a water-in-oil (w/o) microemulsion which can be utilized as a transdermal delivery for iodide ions. Several w/o microemulsion formulations were prepared utilizing Span 20, ethanol, Capryol 90®, and water. The selected formulations had 5%, 10%, 15%, 20%, and a maximum of 23% w/w water content. Potassium iodide (KI) was incorporated in all formulations at 5% w/v. Physicochemical characterizations were conducted to evaluate the structure and stability. These studies included: mean droplet size, pH, viscosity, conductivity, and chemical stability tests. In vitro human skin permeation studies were conducted to evaluate the diffusion of the iodide ion through human skin. The w/o microemulsion formulations were stable and compatible with iodide ions with water content ranging from 5% to 23% w/w. The addition of KI influenced the physicochemical properties of microemulsion as compared to blank microemulsion formulations. In vitro human skin permeation studies indicated that selected formulations improved iodide ion diffusion significantly as compared to control (KI solution; P value < 0.05). Iodide ions were entrapped within the aqueous core of w/o microemulsion. Span 20, ethanol and Capryol 90 protected the iodide ions against oxidation and formed a stable microemulsion. It is worth to note that according to Hofmeister series, iodide ions tend to lower the interfacial tension between water and oil and consequently enhance overall stability. This work illustrates that microemulsion system can be utilized as a vehicle for the transdermal administration of iodide.
KEY WORDS: iodide, microemulsion, skin permeation, transdermal
INTRODUCTION
Iodide is vital for the biosynthesis of thyroid hormones triiodothyronine (T3) and thyroxine (T4) in the thyroid gland. The deficiency of T3 and T4 leads to thyroid tissue enlargement and other metabolic and physiological problems. It has been illustrated by previous studies that iodide is massively accumulated in the thyroid gland regardless of the dosing route (1,2). This accumulation is regulated by sodium-iodide symporter which transports iodide from blood into thyroid epithelial cells (3,4). Typically, small amount of iodide is used as a daily nutritional supplement to prevent iodine deficiency (5), whereas much larger doses are used to avoid thyroid uptake of radioactive iodide following nuclear fission accidents (6,7). Iodide is present in the typical diet, primarily through the use of iodized table salt. Oral iodide can also be supplemented as potassium iodide (available in both tablet and solution dosage forms) as well as with iodine strong solution (Lugol’s solution). However, oral administration may not be ideal in certain populations at greatest risk for iodine deficiency, such as infants or patients who suffered surgical removal of GI tract. For this reason, transdermal drug delivery system may be an appropriate alternative to oral delivery, particularly when oral absorption is compromised in disease states characterized by malabsorption (e.g., short bowel syndrome). In contrast to other drug delivery systems, transdermal administration offers advantages including convenient, noninvasive, and continuous dosing and the avoidance of first-pass metabolism (8).
It is well understood that low molecular weight hydrophilic compounds including ionized compounds can permeate through skin by appendage shunt pathways such as hair follicles and sweat glands (9–11). However, the total amount of drug which can be diffused via this route is limited because of its small surface area compared to the total skin. In addition, it has been proposed that small ions can diffuse through lipid bilayer of the stratum corneum by the “aqueous” or the “pore” pathway model (12–14). In this model, pores are formed as a result of defects or imperfections in the interior structure of lipid bilayer which leads ions to travel through more rigid tortuous routes (15,16). Depending on this model, ion diffusion through stratum corneum can be improved by altering the porosity of lipid bilayer. Various types of penetration enhancers, such as water, surfactants, fatty acids, and azones, can effectively influence the porosity of the stratum corneum and further lower its resistance to chemicals (17). On the other hand, ion penetration through the skin is also influenced by ion’s permeability. A recent report demonstrated that anions have a faster diffusion rate than cations due to the presence of negatively charged phospholipid groups in the stratum corneum (18). These findings support our intent to deliver iodide ions through microemulsions.
Microemulsion is a multicomponent system composed of water, oil, surfactant, and cosurfactant (19). It has been intensively utilized as a transdermal delivery system with several advantages including low cost and simple preparation, long-term product stability, and main ingredients acting as solubilization and permeation enhancers (20–22). Microemulsion can potentially change the internal structure of the lipid bilayer in the stratum corneum and enhance compound penetration.
Our aim is to develop an iodide transdermal delivery system which has potential therapeutic uses when oral administration is not appropriate. In this study, a w/o microemulsion system with potassium iodide (KI) was developed. Several physicochemical characterizations were conducted to evaluate the system (e.g., pH, droplet size, conductivity, viscosity, and stability). Franz diffusion cells were also utilized to evaluate the penetration of the iodide ions through human skin.
MATERIALS AND METHODS
Materials
KI, Span 20, and Pyrene were purchased from Sigma Aldrich (St. Louis, MO, USA). Anhydrous ethanol was purchased from Fisher Scientific (Thermo Fisher Scientific Inc, Pittsburgh, PA, USA). Capryol® 90 was obtained from Gattefosse (Lyon, France). De-ionized water was used in this study.
Construction of Pseudo-Ternary Phase Diagrams
Pseudo-ternary phase diagrams were constructed to evaluate the miscibility of the basic components in the system at 25°C. A series of different ratios (Km) of surfactant (Span 20) to cosurfactant (ethanol) were prepared at 4:1, 1:1, 1:4, and 1:9, and then followed by the addition of oil (Capryol 90) at different weight ratios of oil to mixture of surfactant and cosurfactant of 10:0, 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, and 1:9, respectively. Water was titrated drop by drop to the three-component mixture (under constant magnetic stirring) until a transition point where transformation from transparent (optical monophase) to turbid (optical diphase) was reached. A boundary line connecting all transition points was drawn and the monophasic area AT beneath this boundary line was calculated using Origin 8 software (OriginLab Corporation, Northampton, MA, USA), which is shown in Fig. 1. AT was used to evaluate water solubilization capacity into the oil. The following solubilization capacities AT were calculated at different surfactant/cosurfactant ratio Km (Km: 4:1, AT: 16.6; Km: 1:1, AT: 28.5; Km 1:4, AT: 35.7; Km: 1:9, AT: 37.4). The pseudoternary phase diagram at a constant surfactant/cosurfactant ratio Km of 1:1 was chosen for further development because there is sufficient area in pseudoternary phase diagram which could form micromeulsion at this ratio while cosurfactant (ethanol) was kept relatively low in the formulation. A dilution line (L20) was plotted linking 100% water to a mixture of oil and surfactant/cosurfactant (S/COS) of 20% and 80% (Fig. 2). Data points on this line have a constant ratio of oil to S/COS of 1:4 (23,24). The intersection between the dilution and the boundary lines was recognized as the formulation with maximum water solubilization capacity.
Fig. 1.
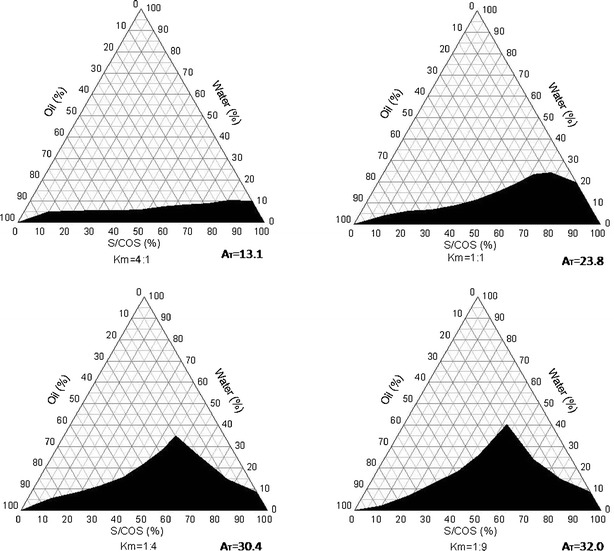
Pseudoternary phase diagrams of mixtures composed of oil (Capryol 90®), water, surfactant (Span 20), and cosurfactant (ethanol) at various S/COS ratios (K m). Shaded area the domain where the mixture system is monophasic
Fig. 2.
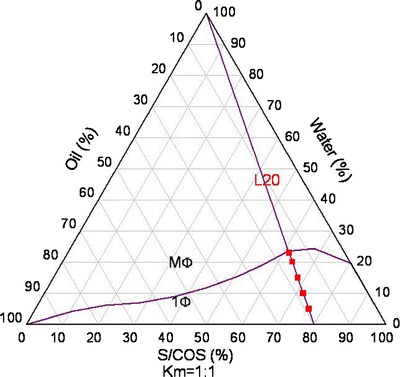
L20 is a dilution line which connects all formulations with a fixed ratio (20/80) of oil to S/COS. Below the purple boundary line, the mixture exists as one phase, which is represented by 1φ. Otherwise, the mixture is of multiple phases, which is represented by M φ
Five selected formulations (Table I) with different water contents from L20 were further tested. Blank formulations were first prepared by manually mixing Span 20, ethanol, Capryol 90, and water. Then, formulations were incorporated with KI at a constant concentration of 50 mg/mL by vortex mixing.
Table I.
Formulation composition, pH, and Z average diameter at 25°C
| Formulation | Water/Span20/Ethanol/Capryol 90® composition (w/w%) | KI (g/mL) | pH | Z-average diameter (nm) |
|---|---|---|---|---|
| A | 5/38/38/19 | None | 5.20 ± 0.01 | Size under detection limit |
| B | 10/36/36/18 | None | 5.13 ± 0.01 | 1.48 ± 0.03 |
| C | 15/34/34/17 | None | 5.00 ± 0.01 | 2.48 ± 0.39 |
| D | 20/32/32/16 | None | 4.94 ± 0.01 | 4.36 ± 0.04 |
| E | 23/30.8/30.8/15.4 | None | 4.82 ± 0.01 | 5.57 ± 0.33 |
| F | 5/38/38/19 | 0.05 | 5.68 ± 0.00 | Size under detection limit |
| G | 10/36/36/18 | 0.05 | 5.60 ± 0.01 | 1.07 ± 0.06 |
| H | 15/34/34/17 | 0.05 | 5.40 ± 0.01 | 2.19 ± 0.15 |
| I | 20/32/32/16 | 0.05 | 5.38 ± 0.01 | 2.88 ± 0.21 |
| J | 23/30.8/30.8/15.4 | 0.05 | 5.32 ± 0.01 | 4.51 ± 0.15 |
Droplet Size Measurements
The mean droplet size of selected formulations was determined by dynamic light scattering using Zetasizer Nano ZS (Malvern Instruments Inc, Westborough, MA, USA). Light was scattered at a fixed angle of 90°. Refractive index and viscosity values were inputted into the program to determine the mean droplet size accurately. All measurements were obtained at 25°C. Triplicate measurements were taken.
pH Measurements
The pH values of selected formulations were acquired using Orion 520A pH meter (Thermo Fisher Scientific Inc). The pH probe was inserted into 20 mL of liquids and values were recorded when the reading stabilized. All measurements were done in triplicate.
Viscosity Measurements
The kinematic viscosities of the selected formulations were determined using Cannon–Fenske routine viscometer (Cannon Instrument Company, State College, PA, USA) at ambient temperature. Kinematic viscosity was obtained by multiplying efflux time of sample flowing through the capillary tube of the viscometer by the viscometer constant. Thereafter, the dynamic viscosity was determined by multiplying the value of kinematic viscosity by the sample density. Triplicate measurements were performed.
Conductivity Measurements
Conductivity measurements were performed using conductivity meter FE30/FG3 (Mettler-Toledo Inc, Columbus, OH, USA) at 25°C. Conductivity diagram was obtained through drop by drop water titration to the mixture of oil and S/COS at a constant ratio of 1:4 in a beaker. The conductivity sensor was soaked in the liquid and the reading was recorded when the signal indicating the endpoint was achieved. The conductivity of the five selected formulations after the incorporation of KI was carried out using the same methodology. All measurements were carried out in triplicate.
In Vitro Permeation Studies
Skin Preparation
Human skin samples (chest and abdominal regions) were purchased from National Disease Research Interchange (Philadelphia, PA, USA). Subcutaneous fatty tissues were removed from skin using a lancet after soaking the skin in a 60°C water bath for 1 min (25). Thereafter, skin samples were washed with di-ionized water. Prior to the actual permeation study, the fat free skin was stored refrigerated at 4°C.
In Vitro Diffusion Experiments
To investigate KI formulation diffusion through the skin, Franz cells (PermeGear Inc, Hellertown, PA, USA) were utilized. The receptor volume of each cell was 5 mL and the diffusion area was 0.64 cm2. Prior to mounting the skin samples, each receptor was filled with 5 mL di-ionized water. Successively the skin samples were clamped in between the receptor (down) and donor (up) holding the stratum corneum side up. Prior to the experiment, the jacketed receptor was kept for 1 h at 37°C using a water bath with magnetic stirring. Afterwards, 1 mL of each selected formulation with 50 mg/mL KI was loaded to the donor compartment and each donor cell was sealed with Parafilm® to avoid the evaporation of formulation components. A KI solution (1 mL of 50 mg/mL solution) was used as the control. Then, 250 μL of the aqueous liquid were withdrawn from the sampling port of receptor and diluted with di-ionized water to 5 mL at different time points (0, 2, 4, 6, 8, 12, and 24 h). Simultaneously, equal volume of di-ionized water was replaced into the liquid. The diluted samples were filtered through 0.45 μm Millex® filter (Millipore, Billerica, MA, USA) and analyzed using Orion iodide selective electrode (Thermo Fisher Scientific Inc). Three replicates were carried out for each selected formulation.
The concentration of iodide in the receptor at every time point was calculated after incorporating the dilution factor. Then the cumulative amount of KI permeated across the skin per unit area (milligrams per square centimeter) was obtained by using the following equation:
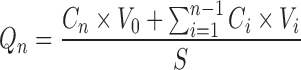 |
1 |
Where Cn is the undiluted sample concentration (liquid concentration in the receptor) at nth sampling time point, Ci is undiluted sample concentration (liquid concentration in the receptor) at ith sampling time point, V0 is the receptor volume (5 mL), Vi is the sampling volume (250 μL), and S is diffusion area (0.64 cm2). All Qn values at each time point were plotted as a function of time. The steady state flux (Jss, milligrams per square centimeter per hour) was calculated for every formulation. Jss is the slope of linear portion of cumulative iodide amounts.
Accelerated Microstructure Stability Testing
Centrifugation
Selected formulations (2 mL) with and without KI were centrifuged at 13,000 rpm (13,793 g) for 30 min using Eppendorf 5415C centrifuge.
Thermal Stability
Selected formulations (20 mL) were stored in sealed vials at a 40°C stability chamber for 4 weeks. Three replicates were carried out for each formulation.
Chemical Stability
Potassium iodide-starch test paper was utilized to test the existence of iodine. Four standard iodine solutions (2.5E-2, 2.5E-1, 5E-1, 1 mg/mL) were prepared by dissolving iodine in ethanol/water (50/50 w/w) solution. These standards were utilized as indicators for any presence of iodine in microemulsion formulations. Formulation J (23% water microemulsion with 50 mg/mL KI) was selected to observe the presence of iodine as a function of time (0, 2, and 4 weeks) at ambient temperature. The test paper was dipped into the selected microemulsion for 30 s, followed by washing with a small volume of water to provide the aqueous environment for the triiodide starch reaction.
RESULTS
Construction of Pseudo-Ternary Phase Diagrams
Nonionic surfactants are utilized in topical formulations because they are less irritating to skin and have less tendency to cause allergic reactions (20). Moreover, it has been reported that nonionic surfactants with polyethoxylated groups and/or residues may contain peroxides which accelerate the oxidation of iodide to iodine (26–28). For this reason, Span 20 (HLB 8.6) was selected as the surfactant since it contains no ethoxylated groups. In addition, Span 20 acts as a penetration enhancer which fluidizes the intercellular lipid bilayer (29). However, the emulsification capacity of Span 20 is limited since it has a relatively small polar group and a short nonpolar carbon chain. In order to obtain a stable microemulsion system at relatively high water content, ethanol was selected as a cosurfactant. Ethanol has been widely applied as a penetration enhancer (17). Capryol 90® (HLB 6), a common penetration enhancer, was selected as the oil phase (30,31). The formation of microemulsion is dependent on the assumption that Capryol 90® interacts with the hydrophobic chain of Span 20 in the presence of ethanol to lower the oil water interface. Pseudoternary phase diagrams were constructed to evaluate the miscibility of the microemulsion components (32). Mixtures of Capryol 90®, Span 20, ethanol, and water with various Km (span 20/ethanol, w/w) ratios are depicted in Fig. 1. AT represents the monophasic area (solubilization capacity) and it tends to increase as the amount of span to ethanol increases in the formulations (Km: 4:1, AT: 13.1; Km: 1:1, AT: 23.8; Km: 1:4, AT: 30.4; Km: 1:9, AT: 32.0).
Droplet Size and pH Measurements
Dynamic light scattering technique measures droplet size through direct measurement of the droplet diffusion coefficient in a dispersed medium undergoing Brownian motion then the droplet size is obtained from the Stokes–Einstein equation. In the selected formulations, surfactant, cosurfactant, and oil form the external phase, while water (aqueous core) is the internal phase. The existing boundaries between oil and aqueous core are composed of the polar parts of Span 20, the water and ethanol. The results for droplet size are depicted in Table I. Mean droplet size was not measurable for formulation containing 5% water by dynamic light scattering. These formulations may resemble cosolvent systems. It is possible that when water content in the system is low, water molecules can stay separate without the forming an aqueous droplets. As the water content increases to 10%, the average droplet size becomes more than 1 nm. It was observed that as water content further increased, droplet size of microemulsion increased also.
Surprisingly, the addition of KI to microemulsions shrinks water droplets. This is due to the salting-in effect which occurs between inorganic anionic ions such as iodide ions and water molecules. Thus, anionic ion makes water less polar and makes the organic components dissolve more readily into internal aqueous clusters (33). A schematic demonstration of microemulsion with and without KI is shown in Fig. 3. It is known that iodide, a member of Hofmeister ion series, tends to increase the solubility of nonpolar components in aqueous solvent by decreasing the surface tension between water and organic molecules (34). Thus, the polar region (water content in the core) diminishes and the existing boundary between polar and nonpolar molecules shrinks consequently, which causes the droplet size to decrease.
Fig. 3.
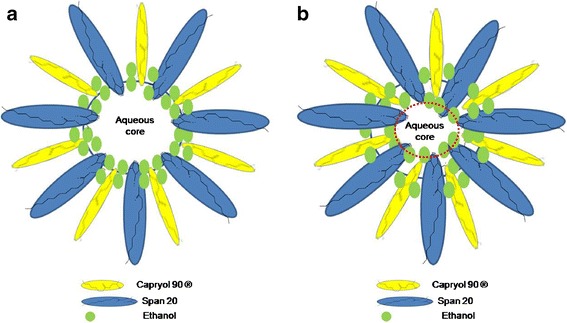
A schematic demonstration for the water interface: a microstructure of microemulsion without KI; b microstructure of microemulsion with KI. The internal domain represents the water content
The pH values of all selected formulations were physiologically acceptable for topical uses. It was observed that the pH decreased from 5.20 to 4.82 when the amount of water increased from 5% to 23% (Table I). However, after the incorporation of KI, pH values increased slightly from 5.68 to 5.32 (5–23% water content).
Viscosity Measurements
Viscosity of multicomponent systems is a polynomial function which depends on the concentrations of water, surfactant, cosurfactant, and oil in each formulation (35). Figure 4 depicts the dynamic viscosity values obtained for all tested formulations in the presence and absence of KI as it relates to water contents. The viscosity values for formulations without KI were relatively low and ranged between 9 and 11 cPoise. It was observed for the aforementioned formulations, viscosity increased as the water content increased from 5% to 23%. The observed increase in the viscosity with the water content is dependent upon the increase of the dispersant phase droplet’s volume and the increase in the frequency of collisions between the water droplets in w/o microemulsion system (36).
Fig. 4.
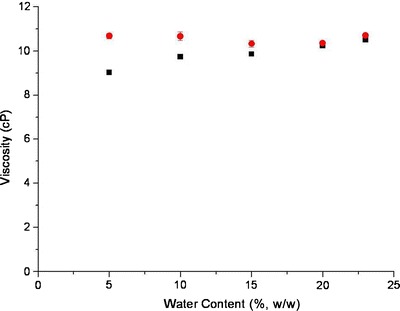
The change of dynamic viscosity as a function of water, which is the aqueous phase of microemulsions along the dilution line L20. Microemulsions without KI loading are labeled as black square; microemulsions with KI loading are labeled as red circle
It was noted that after the addition of KI the viscosity of the formulations (Fig. 4) increased slightly (10–11 cPoise). The increase of viscosity is not only due to more densed internal aqueous phase, but also due to the increase in the formation of aqueous transition clusters where iodide ions cause aqueous phase to become more hydrophobic and free to move (37).
Conductivity Measurements
The influence of water content on conductivities of selected formulations is presented in Fig. 5. The main graph relates overall ions conductivity of water content from 0% to 24.5%.
Fig. 5.
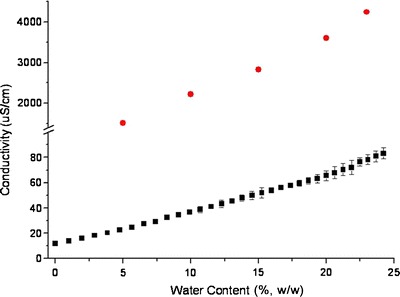
Conductivity of microemulsion formulations along dilution line L20 versus water content. Microemulsions without KI loading are labeled as black square; microemulsions with KI loading are labeled as red circle
In general, when water molecules are dispersed in an oil phase at a small volume fraction, droplets are separated from each other and exhibits minimum interactions and liquid conductivity is low.
Further addition of water increases the total number of aqueous droplets which can increase the formation and deformation dynamics of the transient clusters (aggregation of water molecules) to increase liquid conductivity. The cluster formation and deformation process is described in three steps: fusion, mass transfer, and fission (38). Transient clusters have significant influence on increasing the conductivity. The transient collisions of water droplets provide water channels where ions hop from one droplet to the other (39). Therefore, a rapid increase in conductivity up to 23% water content was also observed. This observation is consistent with other researchers who described similar conductivity behavior in w/o microemulsion systems (40).
Remarkably, the addition of KI into the selected formulations enhanced conductivities more than 50-fold as compared to microemulsions without KI. This observation is consistent with the quantitative charge fluctuation model where the aqueous channels created by the transient clusters contain more dissociated ions which are able to facilitate the overall conductivity tremendously (41).
In Vitro Microemulsion Skin Permeation Studies
Cumulative amounts of iodide that permeated through human skin over 24 h for selected formulations are depicted in Fig. 6. Results indicated that at the end of 24 h all KI formulations with different water contents (5%, 10%, 15%, 20%, and 23%) had significantly better iodide permeation through skin as compared to the control sample (KI solution; Student’s paired t test, P < 0.05).
Fig. 6.
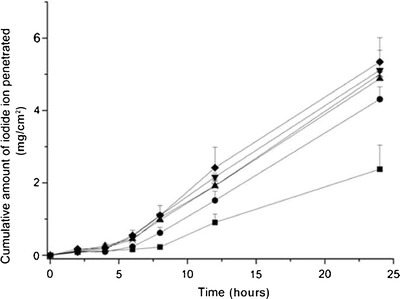
Permeation profiles of KI formulations. Black square control (KI solution), black circle formulation F (5% water), cross formulation G (10% water), black down-pointing triangle formulation H (15% water), black up-pointing triangle formulation I (20% water), black diamond formulation J (23% water)
Formulation F exhibited the lowest cumulative amount of iodide that permeated the skin at the end of 24 h out of the five selected formulations (F to J). It permeated about two times the amount of KI as compared to the solution after 24 h. On the other hand, formulation J was the most effective formulation for iodide permeation study (about 2.5 times of KI solutions) after 24 h. Statistically, iodide permeation at the end of 24 h for formulation J (23% water content) was significantly higher (Student’s paired t test, P < 0.05) as compared to formulation F (5% water content), but had no significant difference compared to formulation G (10% water content), H (15% water content), and I (20% water content; Student’s paired t test, P > 0.05).
Values of steady-state flux and cumulative amounts of iodide that permeated are listed in Table II. Jss indicates that selected formulations (F to J) had a significant better permeation of iodide compared to KI solution. These results indicate that organic components (Span 20, Capryol 90, and ethanol) in microemulsion formulations act as penetration enhancers. They potentially modify the lipid structure within the stratum corneum and make it looser and more porous for iodide permeation. Likewise, larger amount of water in w/o microemulsion could influence iodide permeability to a higher extent since Formulation J (23% water content) had the highest flux rate 0.266 ± 0.037 mg/cm2/h. In the presence of water, skin is hydrated and exists in a swollen state, thus more void spaces within the skin create wider diffusion channels (42). In summary, the permeation profile of iodide within microemulsion formulations is affected by a combination of factors including permeation enhancement and skin hydration.
Table II.
Cumulative permeated iodide (Q 24) and flux at steady-state (J ss; milligrams per square centimeter per hectare) of selected formulations
| Formulation (50 mg/mL KI) | Cumulative permeated iodide (Q24) (mg/cm2) | Flux at steady-state (Jss) (mg/cm2/h) |
|---|---|---|
| Solution | 2.38 ± 0.66 | 0.127 ± 0.036 |
| F | 4.31 ± 0.34 | 0.228 ± 0.014 |
| G | 5.00 ± 0.66 | 0.252 ± 0.038 |
| H | 5.11 ± 0.29 | 0.254 ± 0.014 |
| I | 4.88 ± 0.50 | 0.245 ± 0.029 |
| J | 5.35 ± 0.53 | 0.266 ± 0.037 |
Accelerated Microstructure Stability Testing
Centrifugational forces accelerate physical instability of microemulsions and lead to turbidity and phase separation (43). Brownian motion maintains droplets’ kinetic energy which causes irregular movements of small droplets, so it prevents droplet settling. Additionally, low interfacial tension and droplets kinetic energy lead to inhibition of creaming, sedimentation, flocculation, and coalescence (44).
All selected formulations (A–J) in Table I in the presence and the absence of KI had no phase separation and clarity change by the end of 30 min under high centrifugational forces (13,000 rpm, 13,793 g) which is a sign of the strong physical stability of formulations. Thermal stability testing under 40°C showed no turbidity by the end of 3 weeks, thus lending further support to the physical stability of the microemulsion under thermal stress.
Chemical stability was performed by exploring the presence of degradation product iodine. Iodine reacts with starch in the presence of iodide and expresses blue–black color. So we utilized iodide–starch test paper as an indicator to detect the existence of iodine. A series of iodine solution standards with different concentrations (Fig. 7) were prepared at 0.05% (2.5E-2 mg/mL iodine), 0.5% (2.5E-1 mg/mL iodine), 1% (5E-1 mg/mL iodine), and 2% (1 mg/mL iodine). The rational for preparing these four iodine standards was related to the assumption that if the percentages of degradation of the initial concentration of potassium iodide in microemulsion (50mg/mL) were 0.05%, 0.5%, 1% and 2% then the concentrations of iodine that should be formed will be 2.5E-2, 2.5E-1, 5E-1 and 1 mg/mL respectively. We observed that with increasing iodine concentration the blue–black color became more intense. Samples were collected over 1 month period (Fig. 7). At the end of 1 month, the absence of blue–dark color in iodide–starch test paper indicates the percentage of degradation product iodine is much less than 0.5%. Thus, we report with confidence that KI microemulsion with 23% water is chemically stable for at least 1 month.
Fig. 7.

Test papers from left to right: sample at 0 week, sample at 2 weeks, sample at 4 weeks, sample with 2.5E-2 mg/mL iodine, sample with 2.5E-1 mg/mL iodine, sample with 5E-1 mg/mL iodine, and sample with 1 mg/mL iodine
DISCUSSION
When oral or parental route of administration is inapplicable or encountered with insufficient patient compliance, alternative routes such as transdermal delivery is pursued.
Transdermal delivery has been utilized for several decades and considered as a noninvasive route of administration. Although, transdermal delivery is utilized in several clinical situations, the main challenge for this delivery route has always been the drug dose and the skin permeability since skin function as a barrier to prevent external invasions. Nanotechnology has been utilized in transdermal drug delivery to improve permeation; and microemulsion is considered the second most popular colloidal system which has been used for dermal applications (21). Here, we mention several advantages of microemulsions: First, the procedures for preparing microemulsions are simple, inexpensive, and rapid. Second, microemulsions have shown to enhance the permeability of hydrophobic and hydrophilic compounds (20) either by decreasing the skin resistance or by increasing the concentration gradient of the drug diffusion (17). In addition, the nanosize droplets of microemulsion are more likely to adhere to the skin surface and penetrate into the skin (20). In addition, microemulsions as compared to regular emulsions (microns size), are thermodynamically stable during storage, packaging, and transportation due to the lack of fixed curvature and the low interfacial tension. Since, the dose requirement for iodide is 150 μg per day, a transdermal delivery of microemulsion iodide is feasible.
In this study, we explored a w/o microemulsion as a vehicle which could potentially improve the permeation of iodide ion through skin. In this system, Capryol® 90, Span 20, ethanol, and water were combined at appropriate ratios. Despite the fact that other surfactants with polyoxyethylene groups have better solubilization capacity than Span 20, these types of surfactants are not selected since the residues of peroxide originated from polyoxyethylene groups may lead to the oxidation of iodide. Thus to stabilize the microemulsion, a cosurfactant is necessary to further decrease the interfacial tension between the oil phase and the aqueous phase. We have selected ethanol as the cosurfactant for this system since it is relatively polar and miscible with water and considered safe at a major content of less than 40% w/w. The addition of potassium iodide did not show any evidence of microemulsion destabilization. Iodide ion disrupts water–water interactions and makes water more hydrophobic thus forcing water dissolve more organic components including ethanol, Span 20, and Capryol® 90 into the aqueous phase. As a result, the internal droplet size of water decreases. This phenomenon was also explained by other authors who illustrated that iodide ions adsorb and interact to the inter-phase (33). On the other hand, cationic ions such as potassium have subtle effect on microemulsion system.
A perspective application for this iodide microemulsion is envisioned to be in the therapy for specialized patients who suffer from iodide deficiency or who suffer from short bowel syndrome where oral absorption is limited. Currently, patients with this illness can only rely on parental routes since the majority of nutritional elements including iodide may be difficult to get absorbed by GI tract. Therefore, a satisfactory transdermal delivery system may provide a new advent for the therapy of this disease. Transdermal delivery of iodide could serve as the first front for the transdermal delivery of nutrients. Although microemulsion is a nontoxic and noninvasive colloidal formulation, there are still limitations for topical use because of the poor adherence on the skin. A patch system of microemulsion will provide the solution for this problem. Such a patch will maintain the microemulsion entrapped behind the adhesive layer and the membrane and will control the release of the ions.
CONCLUSION
In summary, several w/o microemulsion formulations were prepared and fully characterized and evaluated. The physicochemical characterizations included: pH, droplet size, viscosity, conductivity, and stability. These studies indicated that the selected formulations were stable and compatible with iodide ions. The permeation studies in human skin showed that microemulsion enables the iodide ions to diffuse through the skin. Further development of this work will be to incorporate the microemulsions into a patch which can be applied more conveniently in clinical treatment.
ACKNOWLEDGMENTS
The authors cordially thank M. Liu for the professional assistance.
REFERENCES
- 1.Morgan DJ, Morgan A. Studies on the retention and metabolism of inhaled methyl iodide-I: retention of inhaled methyl iodide. Heal Phys. 1967;13:1055–65. doi: 10.1097/00004032-196710000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Risher JF, Keith LS. Iodine and inorganic iodides: human health aspects. 1. Geneva: WHO Press; 2009. pp. 14–6. [Google Scholar]
- 3.Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature. 1996;379:458–60. doi: 10.1038/379458a0. [DOI] [PubMed] [Google Scholar]
- 4.Smanik PA, Ryu K-Y, Theil KS, Mazzaferri EL, Jhiang SM. Expression, exon–intron organization, and chromosome mapping of the human sodium iodide symporter. Endocrinology. 1997;138:3555–8. doi: 10.1210/en.138.8.3555. [DOI] [PubMed] [Google Scholar]
- 5.Delange F. The disorders induced by iodine deficiency. Thyroid. 1994;4:107–28. doi: 10.1089/thy.1994.4.107. [DOI] [PubMed] [Google Scholar]
- 6.Blum M, Eisenbud M. Reduction of thyroid irradiation from 131I by potassium iodide. J Am Med Assoc. 1967;200:1036–40. doi: 10.1001/jama.1967.03120250070012. [DOI] [PubMed] [Google Scholar]
- 7.Zanzonico PB, Becker DV. Effects of time of administration and dietary iodine levels on potassium iodide (KI) blockade of thyroid irradiation by 131I from radioactive fallout. Heal Phys. 2000;78:660–7. doi: 10.1097/00004032-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 8.McNeill SC, Potts RO, Francoeur ML. Local enhanced topical delivery (LETD) of drugs: does it truly exist? Pharm Res. 1992;9:1422–7. doi: 10.1023/A:1015854728278. [DOI] [PubMed] [Google Scholar]
- 9.Wallace SM, Barnett G. Pharmacokinetic analysis of percutaneous absorption; evidence of parallel penetration pathways for methotrexate. J Pharmacokinet Pharmacodyn. 1978;6:315–25. doi: 10.1007/BF01060095. [DOI] [PubMed] [Google Scholar]
- 10.Keister JC, Kasting GB. The use of transient diffusion to investigate transport pathways through skin. J Control Release. 1986;4:111–7. doi: 10.1016/0168-3659(86)90046-5. [DOI] [Google Scholar]
- 11.Edwards DA, Langer R. A linear theory of transdermal transport phenomena. J Pharm Sci. 1994;83:1315–34. doi: 10.1002/jps.2600830925. [DOI] [PubMed] [Google Scholar]
- 12.Pechtold LARM, Abraham W, Potts RO. The Influence of an electric field on ion and water accessibility to stratum corneum lipid lamellae. Pharm Res. 1996;13:1168–73. doi: 10.1023/A:1016099800770. [DOI] [PubMed] [Google Scholar]
- 13.Boddé HE, Kruithof MAM, Brussee J, Koerten HK. Visualisation of normal and enhanced HgCl2 transport through human skin in vitro. Int J Pharm. 1989;53:13–24. doi: 10.1016/0378-5173(89)90356-6. [DOI] [Google Scholar]
- 14.Cornwell PA, Barry BW. The routes of penetration of ions and 5-fluorouracil across human skin and the mechanisms of action of terpene skin penetration enhancers. Int J Pharm. 1993;94:189–94. doi: 10.1016/0378-5173(93)90023-9. [DOI] [Google Scholar]
- 15.Costigan SC, Booth PJ, Templer RH. Estimations of lipid bilayer geometry in fluid lamellar phases. Biochim Biophys Acta (BBA) Biomembr. 2000;1468:41–54. doi: 10.1016/S0005-2736(00)00220-0. [DOI] [PubMed] [Google Scholar]
- 16.Mitragotri S. Modeling skin permeability to hydrophilic and hydrophobic solutes based on four permeation pathways. J Control Release. 2003;86:69–92. doi: 10.1016/S0168-3659(02)00321-8. [DOI] [PubMed] [Google Scholar]
- 17.Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliv Rev. 2004;56:603–18. doi: 10.1016/j.addr.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 18.Zhang K, Chen M, Scriba GKE, Abraham MH, Fahr A, Liu X. Human skin permeation of neutral species and ionic species: extended linear free-energy relationship analyses. J Pharm Sci. 2012;101:2034–44. doi: 10.1002/jps.23086. [DOI] [PubMed] [Google Scholar]
- 19.Danielsson I, Lindman B. The definition of microemulsion. Colloids Surf. 1981;3:391–2. doi: 10.1016/0166-6622(81)80064-9. [DOI] [Google Scholar]
- 20.Kogan A, Garti N. Microemulsions as transdermal drug delivery vehicles. Adv Colloid Interf Sci. 2006;123:369–85. doi: 10.1016/j.cis.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Cevc G, Vierl U. Nanotechnology and the transdermal route: a state of the art review and critical appraisal. J Control Release. 2010;141:277–99. doi: 10.1016/j.jconrel.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev. 2000;45:89–121. doi: 10.1016/S0169-409X(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 23.Hathout RM, Woodman TJ, Mansour S, Mortada ND, Geneidi AS, Guy RH. Microemulsion formulations for the transdermal delivery of testosterone. Eur J Pharm Sci. 2010;40:188–96. doi: 10.1016/j.ejps.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Monzer F. Phase behavior, transport, diffusion and structural parameters of nonionic surfactants microemulsions. J Mol Liq. 2008;139:14–22. doi: 10.1016/j.molliq.2007.10.005. [DOI] [Google Scholar]
- 25.Lee PJ, Ahmad N, Langer R, Mitragotri S, Prasad Shastri V. Evaluation of chemical enhancers in the transdermal delivery of lidocaine. Int J Pharm. 2006;308:33–9. doi: 10.1016/j.ijpharm.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 26.Jaeger J, Sorensen K, Wolff SP. Peroxide accumulation in detergents. J Biochem Biophys Methods. 1994;29:77–81. doi: 10.1016/0165-022X(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 27.Lever M. Peroxides in detergents as interfering factors in biochemical analysis. Anal Biochem. 1997;83:274–84. doi: 10.1016/0003-2697(77)90536-X. [DOI] [PubMed] [Google Scholar]
- 28.Mancuso JR, McClements DJ, Decker EA. Ability of iron to promote surfactant peroxide decomposition and oxidize α-tocopherol. J Agric Food Chem. 1999;47:4146–9. doi: 10.1021/jf990453r. [DOI] [PubMed] [Google Scholar]
- 29.López A, Llinares F, Cortell C, Herráez M. Comparative enhancer effects of Span®20 with Tween®20 and Azone® on the in vitro percutaneous penetration of compounds with different lipophilicities. Int J Pharm. 2000;202:133–40. doi: 10.1016/S0378-5173(00)00427-0. [DOI] [PubMed] [Google Scholar]
- 30.Azeem A, Ahmad F, Khar R, Talegaonkar S. Nanocarrier for the transdermal delivery of an antiparkinsonian drug. AAPS PharmSciTech. 2009;10:1093–103. doi: 10.1208/s12249-009-9306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh A, Chaurasiya A, Singh M, Upadhyay S, Mukherjee R, Khar R. Exemestane loaded self-microemulsifying drug delivery system (SMEDDS): development and optimization. AAPS PharmSciTech. 2008;9:628–34. doi: 10.1208/s12249-008-9080-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gwak HS, Chun IK. Effect of vehicles and penetration enhancers on the in vitro percutaneous absorption of tenoxicam through hairless mouse skin. Int J Pharm. 2002;236:57–64. doi: 10.1016/S0378-5173(02)00009-1. [DOI] [PubMed] [Google Scholar]
- 33.Kabalnov A, Olsson U, Wennerstroem H. Salt effects on nonionic microemulsions are driven by adsorption/depletion at the surfactant monolayer. J Phys Chem. 1995;99:6220–30. doi: 10.1021/j100016a068. [DOI] [Google Scholar]
- 34.Zhang Y, Cremer PS. Interactions between macromolecules and ions: the Hofmeister series. Curr Opin Chem Biol. 2006;10:658–63. doi: 10.1016/j.cbpa.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 35.Yuan JS, Ansari M, Samaan M, Acosta EJ. Linker-based lecithin microemulsions for transdermal delivery of lidocaine. Int J Pharm. 2008;349:130–43. doi: 10.1016/j.ijpharm.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 36.Djordjevic L, Primorac M, Stupar M, Krajisnik D. Characterization of caprylocaproyl macrogolglycerides based microemulsion drug delivery vehicles for an amphiphilic drug. Int J Pharm. 2004;271:11–9. doi: 10.1016/j.ijpharm.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 37.Gradzielskin M, Hoffmann H. Rheological properties of microemulsions. In: Kumar P, Mittal KL, editors. Handbook of micromulsion science and technology. 1. New York: Marcel Dekker; 1999. pp. 357–86. [Google Scholar]
- 38.Li X, He G, Zheng W, Xiao G. Study on conductivity property and microstructure of TritonX-100/alkanol/n-heptane/water microemulsion. Colloid Surf A. 2010;360:150–8. doi: 10.1016/j.colsurfa.2010.02.026. [DOI] [Google Scholar]
- 39.Mathew C, Patanjali PK, Nabi A, Maitra A. On the concept of percolative conduction in water-in-oil microemulsions. Colloid Surf. 1998;30:253–63. [Google Scholar]
- 40.Jian X, Ganzuo L, Zhiqiang Z, Guowei Z, Kejian J. A study of the microstructure of CTAB/1-butanol/octane/water system by PGSE-NMR, conductivity and cryo-TEM. Colloid Surf A. 2001;191:269–78. doi: 10.1016/S0927-7757(01)00689-6. [DOI] [Google Scholar]
- 41.Eicke HF, Borkovec M, Das-Gupta B. Conductivity of water-in-oil microemulsions: a quantitative charge fluctuation model. J Phys Chem. 1989;93:314–7. doi: 10.1021/j100338a062. [DOI] [Google Scholar]
- 42.Yuan Y, Li S-M, Mo F-K, Zhong D-F. Investigation of microemulsion system for transdermal delivery of meloxicam. Int J Pharm. 2006;321:117–23. doi: 10.1016/j.ijpharm.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 43.Jain J, Fernandes C, Patravale V. Formulation development of parenteral phospholipid-based microemulsion of etoposide. AAPS PharmSciTech. 2010;11:826–31. doi: 10.1208/s12249-010-9440-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Gennes PG, Taupin C. Microemulsions and the flexibility of oil/water interfaces. J Phys Chem. 1982;86:2294–304. doi: 10.1021/j100210a011. [DOI] [Google Scholar]


