Abstract
This article describes the optimization of a peel-off facial mask formulation. An investigation was carried out on the parameters of the formulation that most affect the desirable characteristics of peel-off facial masks. Cereal alcohol had a significant effect on the drying time at concentrations of 1–12% (w/w). The applicability of the evaluated formulations was influenced by both carbomer (0–2.4%; w/w) and polyvinyl alcohol (PVA; 2.5–17.5%; w/w) content due to their ability to alter the formulation viscosity. Inverse concentrations of carbomer and PVA led to formulations with optimum viscosity for facial application. Film-forming performance was influenced only by the PVA concentration, achieving maximum levels at concentrations of around 11% (w/w). The optimized formulation, determined mathematically, contained 13% (w/w) PVA and 10% (w/w) cereal alcohol with no addition of carbomer. This formulation provided high levels of applicability and film-forming performance, the lowest drying time possible and excellent homogeneity of the green clay particles and aloe vera before and after drying. The preliminary stability study indicated that the optimized formulation is stable under normal storage conditions. The microbiological stability evaluation indicated that the preservative was efficient in terms of avoiding microbial growth. RSM was shown to be a useful statistical tool for the determination of the behavior of different compounds and their concentrations for the responses studied, allowing the investigation of the optimum conditions for the production of green clay and aloe vera peel-off facial masks.
Key words: aloe vera, formulation design, green clay, peel-off mask, response surface methodology
INTRODUCTION
The use of mineral-rich products has been reported since antiquity, especially with regard to dermocosmetology. The first facial masks that can be found in reports consisted mostly of different types of clays (1), and these materials have been used ever since because of their therapeutic and cosmetic properties. Nowadays, clays and mineral waters are widely employed as supplementary treatments for several dermatological pathologies. Clay minerals are mainly applied in spas and specialized dermatological clinics, while the domestic market of technologically well-made geoproducts is still a rising field in the cosmetics industry (2,3). There are several applications of mineral green clays in facial dermocosmetics, and some of the most important are astringency and physical exfoliation (4), removal of impurities, dead cells and facial skin oil (5), and accumulated water drainage (4,5).
Most clay-based products on the market consist only of dried clay powder that needs to be moistened prior to use. After facial application, the product dries naturally, forming a sandy-cracked material due to the low cohesion between the dried particles. In general, the dry aspect of this type of product leads to low acceptance by domestic users because it is hard to apply and remove. Thus, incorporating clays into cosmetic formulations, which are easy to apply and remove, is an interesting option to increase the adherence to the final product. In this context, peel-off masks are a viable alternative to promote the incorporation of active compounds into a plastic film-forming formulation that is designed to allow easy, residue-free removal.
Peel-off facial masks are known for their unique characteristics inherent to the use of film-forming polymers that, after complete drying, create a very cohesive plastic layer allowing for the manual removal of the product without leaving any residue (6). In addition, the firming action of these formulations leads to a sensation of clean skin (6,7). Moreover, it also provides slight moisturizing action and enhances the effect of the active compounds on the epithelium, especially as a result of the occlusive effect caused by the plastic polymeric layer (7).
Although formulations containing geological compounds still have remarkable commercial appeal, the high technological quality of current dermocosmetics generates the need for more complex and elaborate products in order to ensure their commercial viability, safety, and efficacy. Accordingly, the association of geoproducts with active plant compounds may be an interesting alternative to add value to the final product.
One of the most common topically applied plant products is aloe vera (Aloe barbadensis Miller). Several reports indicate that this species was used for cosmetic and therapeutic purposes in ancient Rome (8), and it has become a fundamental active compound for modern cosmetology. The most interesting effects of aloe vera in topical use are anti-inflammatory (9), antiseptic (10), antioxidant (11), and regenerative (12). It has been demonstrated that the association of green clay and aloe vera exerts a beneficial synergistic effect when it comes to developing a facial mask as a regenerative aid.
In this study, the Response Surface Methodology (RSM) was used as a tool to analyze different parameters related to the critical characteristics of peel-off masks, thus optimizing the final formulation. RSM is an advanced statistical tool that has outstanding applicability in formulation design. It fosters an understanding of the relation between several variables and their effect on the responses using a sequence of designed experiments (13). In addition, RSM provides maximum information on how the investigated factors can influence the responses with minimal consumption of time and resources. Considering all of the advantages offered by this type of experimental approach, RSM is a valuable tool in research and development for both academic and industrial purposes.
In this context, this study presents a proposal for the mathematical and technological development of an optimized green clay and aloe vera peel-off facial mask formulation in order to improve the applicability and removal of green clay and also to enhance the therapeutic properties of aloe vera. The association of these active compounds in a peel-off formulation results in a product with high commercial appeal, which links technology with practicality.
MATERIALS AND METHODS
Materials and Chemical Reagents
Chemical reagents and other materials were obtained from the following commercial sources: kaolin green clay (Dermavita, Brazil), Neolone™ PE (methylisothiazolinone and phenoxyethanol-based preservative) (The Dow Chemical Company, USA), polyvinyl alcohol PVA 224 (Pharmanostra, Brazil), aloe vera gel (A. barbadensis Miller) freeze-dried powder 200:1 AV201F (JCB Produtos Naturais, Brazil), and Rosas + SEB essence (Botanik Kosmetics, Brazil). All samples and solutions were prepared with water purified by reverse osmosis (OS20LZ, Gehaka). All other reagents and solvents were of analytical grade.
Preparation of Peel-Off Masks
The base formulation (Table I) was set according to previously published data (7,14,15), and the concentrations of the ingredients to be employed were determined experimentally.
Table I.
Concentrations of Each Compound of the Base Formulation of Green Clay and Aloe Vera Peel-Off Facial Masks
| Compound | Concentrations (%;w/w) |
|---|---|
| Kaolin green clay | 5.0 |
| Aloe vera freeze-dried gel 200:1 | 0.5 |
| Polyvinyl alcohol (PVA) | 2.5–17.5 |
| Cereal alcohol (EtOH) | 0–12.0 |
| Propylene glycol | 6.0 |
| Neolone™ PE | 0.3–0.6 |
| EDTA disodium | 0.1 |
| Carbomer 940 (Carbopol) | 0–2.4 |
| Rosas + SEB Essence | 0.01 |
| Aminomethyl propanol q.e.f. | pH 7.0 |
| Water q.e.f. | 100 |
Although the concentrations of some compounds varied according to the experimental design (Table II), the preparation procedure was the same for every formulation. Initially, PVA was dispersed in 80% of the heated water (80°C) used to produce the formulation. The dispersion was constantly homogenized until total dissolution. EDTA disodium and freeze-dried aloe vera gel were dissolved in the propylene glycol and mixed with the remaining amount of water (20%). The solutions were mixed, and the remaining compounds were added. The formulation was mixed using an ultrahomogenizer (2100 Unguator®, Gako Mixing Technology, Germany) at 2,400 rpm for 6 min. The potential hydrogen (pH) was adjusted to 7.0 to enable the maximum stability of carbomer.
Table II.
Factors and Their Levels Investigated on the Experimental Design
| Levels | A: EtOH | B: PVA | C: Carbomer |
|---|---|---|---|
| (%; w/w) | (%; w/w) | (%; w/w) | |
| Minus alpha (α = −1.5) | 0 | 2.5 | 0 |
| Low | 2 | 5 | 0.4 |
| Center point | 6 | 10 | 1.2 |
| High | 10 | 15 | 2 |
| Plus alpha (α = +1.5) | 12 | 17.5 | 2.4 |
After preparation, every formulation was left to rest for 48 h before any evaluation in order to release the air incorporated into the formulation during the ultrahomogenization.
Experimental Design
An RSM design (13) was used to evaluate the factors that most influence the performance of green clay and aloe vera peel-off facial masks. A central composite design, containing axial points to determine the quadratic terms, was set up. Four replicates of the center point were performed to dampen the effect of noise and to provide the number of degrees of freedom needed for an adequate statistical evaluation of the model. The experiments were carried out, randomly, on a total of 19 formulations. The study ranges for the film-forming agent (PVA), viscosity agent (carbomer), and drying accelerator (cereal alcohol—EtOH) were determined via screening to establish the maximum and minimum concentrations in the formulation. Table II shows the full central composite design (alpha; α = 1.5) with the factors (independent variables) evaluated in this study. The responses (dependent variables) of drying time, applicability, and film-forming performance were investigated.
Regression analysis of the data was carried out by means of a statistical design software program (Design-Expert® version 8.0.6, Stat Ease, Inc, MN, USA) presuming a quadratic model with interactions among the factors. The polynomial model in terms of the coded factors can be defined as:
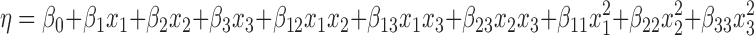 |
where η is the dependent response associated with each independent factor level combination, β0 is the mean value, and x1, x2, and x3 are the independent factors EtOH, PVA, and carbomer, respectively; x1x2, x1x3 and x2x3 represent the binary interactions among the factors; 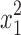 ,
, 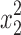 , and
, and 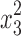 are the quadratic factors. The coefficients of the main factors, interaction factors and quadratic factors are represented as β1, β2, β3; β12, β13, β23; and β11, β22, β33, respectively.
are the quadratic factors. The coefficients of the main factors, interaction factors and quadratic factors are represented as β1, β2, β3; β12, β13, β23; and β11, β22, β33, respectively.
Analysis of variance (ANOVA) was performed to identify the significance of single factors, binary interactions, and quadratic terms in relation to the influence on the responses analyzed. These factors were considered to be significant when the coefficient p < 0.05. The terms that were not significant were removed from the model, unless they were needed to satisfy the hierarchy as a parent term of significant interactions.
The mathematical model was designed in order to maximize the formulation applicability and film-forming ability and also minimize the drying time. The optimum parameters were obtained by RSM using as a tool the desirability function and experiments were performed in order to confirm the validity of the predicted model under optimal conditions.
The desirability function is a multiple response method where an objective function is calculated, ranging from zero—outside of the limits—to one—at the goal. This function allows the combination of a good set of conditions, obtaining the optimal experimental condition that satisfies all goals. The simultaneous objective function is a geometric mean of all transformed responses (13):
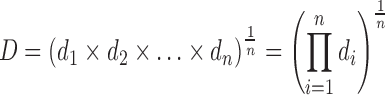 |
where D = desirability function, di = desirable ranges for each response, and n = number of responses in the measurement.
Drying Time
A modified in vitro drying time evaluation technique (14) was developed in order to estimate the time taken for the formulations to dry completely. Approximately 2.0 g of each formulation was spread over a glass plate of 60 × 60 mm forming a uniform mask layer of 55.5 mg/cm2 with a thickness of approximately 2.0 mm. The glass plate was submitted to a heated environment in the oven (37.0 ± 2.0°C) in order to simulate skin temperature. The formulations were monitored every 5 min, and the experiment only finished after the surface of the mask had dried completely. The results were expressed as the mean of three measurements.
Applicability Evaluation
The applicability of each formulation was estimated based on a sensorial score. The score is cumulative, and this approach allowed an internal comparison of the experimental design formulations. The formulations were applied on a smooth translucent glass surface and the sensorial score ranged between 0 and 5. The points observed were as follows: “easy spreadability?,” “good sensory properties?,” “user-friendly application?,” “pleasing appearance?,” and “remains on the surface without sagging?.” For each applicability-related positive response, one point was added to the score of the formulation analyzed, and the higher the score, the better the applicability of the formulation. The final value was determined as the average of the scores assigned by five observers.
Film-Forming Performance
Approximately 2.0 g of each formulation was spread over a millimeter-marked glass plate of 120 × 120 mm, forming a uniform mask layer of 13.8 mg/cm2 with a thickness of approximately 1.0 mm. The mask layer in this evaluation was thinner than that usually applied in order to facilitate the detection of flaws in the film formation. The glass plate was submitted to a heated environment in the oven (37.0 ± 2.0°C) in order to simulate skin temperature. After complete drying, the surface covered with polymeric film was examined using a stereoscopic microscope (PZO, Labimex, Poland). Photomicrographs were taken using a coupled high-definition camera (NEX3, Sony, Thailand), and the total surface covered was determined using image analysis software (SizeMeter®, LCP/UFSC, Brazil). The sensorial score ranged between 0 and 5 as follows: “is there film-formation?,” “is the film formed homogeneous?,” “50% of the surface covered?,” “80% of the surface covered?,” and “100% of the surface covered?.” The score is cumulative, and this approach allowed internal comparison across the experimental design formulations. For each positive response, one point was added to the score of the formulation analyzed, and thus, the higher the score, the better the film-forming performance of the formulation. The final value was determined as the average of the scores assigned by five observers.
Physicochemical Stability
Duplicate samples of the optimized formulation were stored in opaque polyethylene bottles and submitted to the following storage conditions in order to evaluate the preliminary physicochemical stability (14–16): (1) low temperature (5.0 ± 1.0°C), (2) oven (45.0 ± 2.0°C), (3) room temperature with exposure to sunlight (22.0 ± 5.0°C), and (4) room temperature protected from sunlight (22.0 ± 2.0°C). Each sample was analyzed weekly for 28 days according to the following parameters: organoleptic characteristics, applicability, film-forming performance, drying time, pH, clay sedimentation, and total absorbance of the aloe vera ultraviolet (UV) spectrum.
Samples submitted to the UV spectrum evaluation were diluted with water in a 1:10 (w/w) ratio and centrifuged at 3,500 rpm for 15 min (4K15, Sigma); the supernatant, free of clay particles, was analyzed from 500 to 200 nm using a UV spectrophotometer (UV1800, Shimadzu, Japan). Degradation of the aloe vera was determined as the difference between the total absorbance of the spectrum after preparation (time 0) and the total absorbance of the stability test samples. The results were considered as the mean of three measurements.
Light Scattering Particle Size Analysis
The particle size distribution of the samples was evaluated using a light scattering particle size analyzer (MasterSizer 2000, Malvern, UK) equipped with an aqueous sample dispersion unit (Hydro 2000SM, Malvern, UK). Results were expressed as the median (d50%) and as the span, which is an index indicative of the particle size dispersity. Span is calculated as:
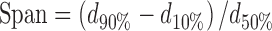 |
where dx% (x% = 10, 50, or 90%) means that the volume percentage of particles with diameters up to dx% is equal to x%, and the smaller the span, the narrower the particle size distribution.
Green clay particle size analysis was carried out on both the raw material and the supernatant of the sedimented formulations in order to evaluate the size range of the particles that remain in suspension after the formulations were submitted to the physichochemical stability conditions. The results were expressed as the mean of ten measurements.
Cryosection and Microscopic Evaluation
The dried masks were submerged in a tissue-freezing medium (Leica Microsystems, Germany), frozen in a cryochamber at −20°C, and sectioned transversely using a microtome-cryostat (CM1820 UV, Leica Microsystems, Germany) equipped with high profile blades (818, Leica Microsystems, Germany). The thickness of the cut was set at 50 μm, and the transversal-sectioned films were analyzed using a stereoscopic microscope (PZO, Labimex, Poland) in order to evaluate the thickness of the film (×40 magnification). Photomicrographs were obtained using a coupled high-definition camera (NEX3, Sony, USA). The thickness of the dried mask film was determined using image analysis software (SizeMeter®, LCP/UFSC, Brazil) as the mean of 20 measurements within the same surface section. The percentage relative standard deviation (%RSD) was used as a tool to indicate the homogeneity of the thickness of the films. The wet and dried formulation surfaces were also microscopically analyzed in order to determine their morphological characteristics.
Microbiological Stability
Duplicate samples of the optimized formulation containing 0.3 or 0.6% (w/w) Neolone™ PE (methylisothiazolinone and phenoxyethanol based preservative) were prepared in order to evaluate the microbiological stability using different concentrations of preservative. The microbiological investigation was carried out applying a direct plate count methodology (16). In order to determine the initial microbiological load of the formulation, the test was firstly conducted immediately after the formulations were prepared (time 0). The test consisted of the preparation of three dilutions of each formulation (10−1, 10−2, and 10−3) followed by the incubation of the dilutions in Petri dishes containing specific culture growth medium for bacteria and fungi. The dilutions were prepared with 10 g of each formulation and diluted in 90 mL of a phosphate buffer pH 7.2 (10−1 dilution). The 10−2 and 10−3 dilutions were prepared collecting 10 mL of the 10−1 and 10−2 dilutions, respectively, and adding them to 90 mL of phosphate buffer pH 7.2. After determination of the initial microbiological load, the formulations were stored in opaque polyethylene bottles and submitted to the following storage conditions: (1) low temperature (5.0 ± 1.0°C), (2) oven temperature (45.0 ± 2.0°C), (3) room temperature with exposure to sunlight (22.0 ± 5.0°C), and (4) room temperature protected from sunlight (22.0 ± 2.0°C). The final concentrations of methylisothiazolinone were approximately 50 and 100 ppm for the formulations containing 0.3 and 0.6% (w/w) Neolone™ PE, respectively. After 28 days, each formulation was submitted to the direct plate count method (16) in order to determine the final microbiological load after storage under different conditions.
All dilutions were plated in duplicate in 20 mL of the suitable culture medium (casein-soya agar for bacteria and Sabouraud-dextrose agar for fungi) on Petri dishes and incubated for 72 h (bacteria) or 7 days (fungi). The results were expressed as the mean of the colony-forming units per gram (CFU/g).
Loading Efficiency and Dose Uniformity
The practical loading efficiency of aloe vera in all formulations was determined spectrophotometrically. Samples were diluted, in triplicate, with water in a 1:10 (w/w) ratio and centrifuged at 3,500 rpm for 15 min (4K15, Sigma). The supernatant was analyzed at 280 nm using a UV spectrophotometer (UV1800, Shimadzu, Japan). A standard solution of 0.05% aloe vera gel powder (w/w; 1:10 dilution, corresponding to a 0.5% aloe vera solution) was prepared and also analyzed. The practical loading efficiency was determined as:
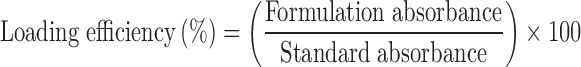 |
A modified dose uniformity assay (16) was performed for the optimized formulation in order to determine the homogeneity of the distribution of aloe vera after drying. Approximately 2.0 g of the formulation was spread over a glass plate of 60 × 60 mm forming a uniform mask layer of 55.5 mg/cm2 with a thickness of approximately 2.0 mm. After complete drying, the mask was removed from the glass plate and divided into four quadrants. Each quadrant was dissolved in 10 mL of water and centrifuged at 3,500 rpm for 15 min (4K15, Sigma). The supernatant was analyzed at 280 nm using a UV spectrophotometer (UV1800, Shimadzu, Japan). The dose homogeneity was determined according to the relative standard deviation of the absorbance values, in percentage terms (%RSD). The analysis was performed in triplicate and the result corresponds to the average %RSD.
RESULTS AND DISCUSSION
Experimental Design
Among the several factors that determine the feasibility of producing green clay and aloe vera peel-off masks, knowledge of the behavior of factors related to drying time, formulation applicability, and film-forming performance is crucial when it comes to developing high quality formulations. The drying time was taken into consideration because it is important that the formulation dries relatively quickly, allowing its fast removal. In this respect, cereal alcohol (EtOH) plays an important role because adequate concentrations of this compound may accelerate the time taken for the formulation to dry completely. Applicability is also an important parameter to be evaluated since low acceptance by domestic users may compromise the commercial viability of cosmetic products. Another response taken into consideration was the film-forming performance of each formulation because the principle of peel-off masks is based on their ability to form plastic polymeric films in order to allow easy, residueless removal. PVA and carbomer are compounds known for their capacity to alter the viscosity of formulations, which may influence applicability and film formation. Thus, the drying time, applicability, and film-forming performance were the responses evaluated in the experimental design.
The practical loading efficiency of aloe vera in all the formulations was found to be >97.5%. None of the formulation parameters significantly influenced the loading efficiency.
The values of the response variables for different experimental conditions are shown in Table III.
Table III.
Drying Time, Applicability, and Film-Forming Performance Obtained on the Experimental Design Formulations
| Experiment | Factors | Responses | ||||
|---|---|---|---|---|---|---|
| A: EtOH | B: PVA | C: Carbomer | Applicability | Film forming | ||
| (%; w/w) | (%; w/w) | (%; w/w) | Drying time (min) | |||
| 1 | 6.0 | 10.0 | 1.2 | 30 | 3 | 5 |
| 2 | 6.0 | 2.5 | 1.2 | 30 | 1 | 0 |
| 3 | 10.0 | 5.0 | 2.0 | 25 | 3 | 3 |
| 4 | 2.0 | 5.0 | 0.4 | 45 | 1 | 3 |
| 5 | 2.0 | 15.0 | 0.4 | 45 | 4 | 5 |
| 6 | 10.0 | 15.0 | 0.4 | 25 | 5 | 5 |
| 7 | 6.0 | 10.0 | 2.4 | 30 | 3 | 4 |
| 8 | 2.0 | 5.0 | 2.0 | 45 | 5 | 2 |
| 9 | 10.0 | 15.0 | 2.0 | 25 | 2 | 5 |
| 10 | 2.0 | 15.0 | 2.0 | 40 | 2 | 4 |
| 11 | 0.0 | 10.0 | 1.2 | 60 | 4 | 4 |
| 12 | 6.0 | 17.5 | 1.2 | 35 | 2 | 5 |
| 13 | 6.0 | 10.0 | 0.0 | 35 | 4 | 5 |
| 14 | 6.0 | 10.0 | 1.2 | 30 | 3 | 5 |
| 15 | 6.0 | 10.0 | 1.2 | 30 | 3 | 5 |
| 16 | 6.0 | 10.0 | 1.2 | 30 | 3 | 5 |
| 17 | 6.0 | 10.0 | 1.2 | 30 | 3 | 5 |
| 18 | 10.0 | 5.0 | 0.4 | 30 | 1 | 2 |
| 19 | 12.0 | 10.0 | 1.2 | 25 | 4 | 4 |
The results for the statistical analysis of the response variables are shown in Table IV. The quadratic model for all responses was the model maximizing the “adjusted R2” and the “predicted R2” coefficients. Adequate precisions of 25.916 (drying time), 14.931 (applicability), and 17.460 (film-forming performance) indicated an adequate signal/noise ratio (adequate precision >4.0). The ANOVA of the independent factors for each response is discussed separately below.
Table IV.
Significant Model Terms, Regression Coefficient Values, and Analysis of Variance (p Values) for the Responses of the Experimental Design
| Polynomial term | Drying time (quadratic model) | Applicabilitya (quadratic model) | Film forming (quadratic model) | |||
|---|---|---|---|---|---|---|
| Coefficient | p value | Coefficient | p value | Coefficient | p value | |
| Model | – | <0.0001 | – | <0.0001 | – | <0.0001 |
| Intercept | 30.83 | – | 1.21 | – | 4.65 | – |
| A: EtOH | −9.80 | < 0.0001 | −0.023 | 0.6687 | 0.08 | 0.6067 |
| B: PVA | −0.20 | 0.7734 | 0.22 | 0.0012 | 1.32 | <0.0001 |
| C: Carbomer | −1.40 | 0.0590 | 0.053 | 0.3286 | −0.20 | 0.2092 |
| AB | – | – | – | – | – | – |
| AC | – | – | – | – | – | – |
| BC | – | – | −0.54 | <0.0001 | – | – |
| A 2 | 4.74 | <0.0001 | – | – | – | – |
| B 2 | – | – | −0.36 | <0.0001 | −0.98 | <0.0001 |
| C 2 | – | – | – | – | – | – |
| R 2 | 0.9469 | 0.9040 | 0.8878 | |||
| Adjusted R 2 | 0.9317 | 0.8671 | 0.8557 | |||
| Predicted R 2 | 0.8745 | 0.7700 | 0.7601 | |||
| Adequate precision | 25.916 | 14.931 | 17.460 | |||
aApplicability—transform: natural log k = 0
Factors Influencing the Drying Time
The only factor that had a significant influence on the drying time was the cereal alcohol (EtOH) concentration in the formulations (Table IV). The EtOH concentration exerted a negative influence (coefficient = −9.80), indicating that an increase in the EtOH concentration leads to a decrease in the drying time. This effect can be attributed to the fact that EtOH acts as a drying enhancer because of its higher volatility in comparison to purified water. Furthermore, the quadratic term for EtOH (A2) was considered to strongly influence the drying time (p < 0.0001), which indicates a nonlinear relationship, reaching a plateau at concentrations of around 10% (Fig. 1a). This plateau indicates that the addition of over 10% (w/w) of EtOH is not recommended since the decrease in the drying time would no longer be proportional (Fig. 1a).
Fig. 1.
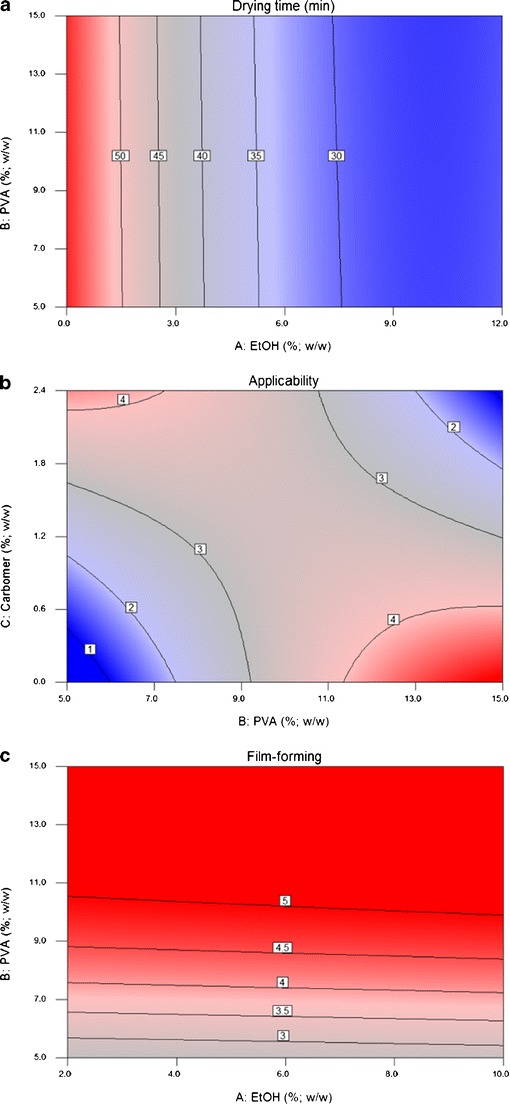
Contour graphics of a drying time, b applicability, and c film-forming performance
Factors Influencing the Applicability
Viscosity is the main applicability-related characteristic of the formulation. The quadratic term for the PVA factor (B2; p < 0.0001) was strongly significant, indicating that a nonlinear relationship was observed for this factor (Fig. 1b). However, the interaction between PVA and carbomer (BC; Table IV; p < 0.0001) was also significant, which indicates that the two factors should be evaluated concomitantly. As shown in Fig. 1b, the combinations that led to formulations with the highest applicability indexes (>4) were those combining low carbomer with high PVA concentrations or vice versa. These formulations had ideal applicability because the inverse combination of PVA and carbomer concentrations led to an optimum viscosity for facial application. Formulations containing low concentrations of both PVA and carbomer were too fluid while those containing high concentrations of both were too much like a paste, which results in low levels of applicability.
Factors Influencing the Film-Forming Performance
PVA concentration was the most important factor influencing the film-forming performance of the peel-off facial masks (Table IV; p < 0.0001). This result was to be expected since the polymeric film basically consisted of this polymer. It was verified that the quadratic term for PVA was strongly significant (B2; p < 0.0001), indicating that the influence of PVA concentration on the film-forming performance was nonlinear (Fig. 1c). A plateau was observed at concentrations above 11% (w/w), indicating that an increase in PVA concentration was not recommended above this level since the increase in film-forming performance would not be proportional.
The PVA also had an important influence on the thickness of the film after drying. Formulations containing 5, 10, and 15% PVA (w/w), with film-forming performance values of 2, 4, and 5, respectively, were transversely sectioned and the thickness of the film was determined. It was verified that the mean thicknesses were 154 ± 94 μm (%RSD = 61.23%), 217 ± 45 (%RSD = 20.74%), and 306 ± 22 (%RSD = 7.39%) for films containing 5, 10, and 15% (w/w) of PVA, respectively. The mean thickness was proportional to the PVA concentration because the increase in PVA led to a proportional increase in the thickness after drying. Nevertheless, the percentage relative standard deviation (%RSD) demonstrated that there was a high variation in the formulations containing insufficient PVA concentration due to the formation of films with flaws, where the aggregation of PVA in certain areas led to the formation of an irregular film with a highly variable thickness along the surface. The formulations containing satisfactory PVA concentration did not present high %RSD values, which indicate the formation of a dried film, which is homogeneous in relation to the thickness.
Optimized Mask Formulation
The agreement between adjusted R2 and predicted R2 values (Table IV; difference, <0.2) of the mathematical models obtained by RSM allows one to safely predict the best formulation of green clay and aloe vera peel-off facial masks, based on pre-established criteria. The numerical optimization finds a point that maximizes the desirability function. The characteristics of a goal can be altered by adjusting the weight and importance. For several responses and factors, all goals are combined into one desirability function. Desirability is an objective function that ranges from zero (outside of the limits) to one (at the goal) and its value is completely dependent on how close the set lower and upper limits are in relation to the actual optimum.
To establish these criteria, focus was directed toward the maximization of the applicability and film formation and the minimization of drying time. According to the data obtained through the statistical analysis (Table IV; Fig. 1), the objective of the optimization was to minimize the carbomer concentration because the inverse proportion of carbomer and PVA should be maintained in order for high applicability to be achieved. Moreover, the PVA concentration could not be lowered because the enhancement of the film-forming performance is also desired. Both the PVA and the EtOH concentrations were maximized with careful observation of the plateau reached at the higher concentrations of both compounds, thus avoiding a non-efficient increase in these factors without a proportional increase in the responses.
In this way, under the experimental conditions studied, it was possible to establish the optimized formulation as that with 13.0% (w/w) of PVA, 10.0% (w/w) of EtOH, and no addition of carbomer (Fig. 2; Table V). The combined desirability function of the formulation was 0.976 and the drying time, applicability and film-forming performance predicted by this model were 27 min, 4.5, and 5.5, respectively. The individual desirability values for each factor were 1.0000, 0.9997, and 0.9999 for EtOH, PVA, and carbomer, respectively, which indicates that the model was capable of fulfilling the optimization requirements for each one of the factors. The individual desirability values for each response were 0.9216, 0.9806, and 1.0000 for drying time, applicability, and film-forming performance, respectively, which indicates that the model was able to optimize all of the responses simultaneously until they reached an adequate level according to the requirements (individual desirability higher than 0.9 is recommended).
Fig. 2.
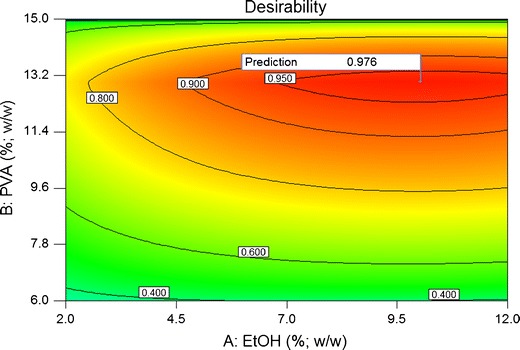
Contour graphic of desirability of the optimized peel-off mask (no addition of carbomer)
Table V.
Point Prediction Information of the Optimized Formulation
| Factors | Responses | |||||
|---|---|---|---|---|---|---|
| A: EtOH | B: PVA | C: Carbomer | Drying time (min) | Applicability | Film forming | |
| (%; w/w) | (%; w/w) | (%; w/w) | ||||
| Predicted formulation | 10.0 | 13.0 | 0 | 27 | 4.5 | 5.5 |
| Confirmation runs (n = 3) | 10.0 | 13.0 | 0 | 25 | 5 | 5 |
| 30 | 5 | 5 | ||||
| 25 | 5 | 5 | ||||
| Individual desirability | 1 | 0.9997 | 0.9999 | 0.9216 | 0.9806 | 1 |
| Combined desirability | 0.976 | |||||
| 95% Confidence interval (CI) | 24.6–30.8 | 3.7–6.4 | 4.7–6.1 | |||
| 95% Prediction interval (PI) | 21.7–33.7 | 3.0–7.9 | 4.1–6.8 | |||
The conditions obtained mathematically were applied experimentally by preparing confirmation runs (n = 3). The outcome results of the confirmation experiments are within both the 95% prediction interval (PI) and the 95% confidence interval (CI; Table V), indicating good predictability of the model for all responses analyzed, confirming the high robustness of the established model for the responses observed in the RSM design.
The optimized formulation presented satisfactory visual homogeneity of the green clay particles before (Fig. 3d) and after (Fig. 3e) drying. The aloe vera dose uniformity assay for this formulation indicated a mean %RSD of only 3.7 ± 0.6%, which confirms an excellent homogeneity of the aloe vera after drying.
Fig. 3.
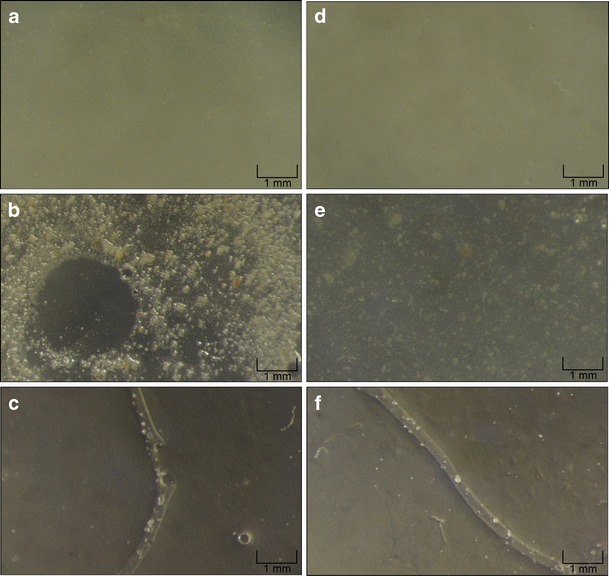
Photomicrographs of the a/d wet and b/e dried surface and c/f transversal cryosection of the experiment 8 and the optimized mask respectively
Physicochemical Stability
Physicochemical parameters were evaluated weekly in order to verify signs of instability in the formulations under different conditions. The results are shown in Table VI. It was verified that the optimized formulations stored at room temperature protected from sunlight (22 ± 2°C) and at low temperature (5 ± 1°C) did not show signs of instability in any of the parameters evaluated. Nevertheless, the storage at room temperature with exposure to sunlight (22 ± 5°C) led to a small decrease in the applicability of the formulations and an increase in the drying time of 30% compared to the time zero formulations. Exposure to sunlight leads to higher variations in temperature, which may accelerate the evaporation of the cereal alcohol in the formulations, thereby increasing the drying time and concentrating the formulation. Due to the concentrated state, the formulation had greater viscosity, inducing a decrease in its applicability. A slight sedimentation of the compounds suspended in the formulation was observed, and this may occur as a result of an increase in the temperature after exposure to sun light, accelerating the natural sedimentation of the insoluble green clay particles.
Table VI.
Results of the Physicochemical Stability Evaluation of the Optimized Formulation for Each Storage Condition
| Parameter | Time (week) | |||||||
|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th | |
| 22 ± 2°C—protected from sunlight | 22 ± 5°C—exposed to sunlight | |||||||
| Color | N | N | N | N | N | N | N | N |
| Odor | N | N | N | N | N | N | N | ↓ |
| Applicability | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 4 |
| Drying time (min) | 30 | 30 | 30 | 35 | 30 | 30 | 35 | 40 |
| Film forming | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| pH | 5.36 ± 0.01 | 5.31 ± 0.08 | 5.34 ± 0.12 | 5.30 ± 0.04 | 5.39 ± 0.05 | 5.29 ± 0.10 | 5.35 ± 0.12 | 5.33 ± 0.06 |
| Sedimentation? | NS | NS | NS | NS | NS | NS | NS | MIS |
| UV spectrum | ND | ND | ND | ND | ND | ND | ND | ND |
| 5 ± 1°C—low temperature | 45 ± 2°C—oven | |||||||
| Color | N | N | N | N | ↓ | ↓↓ | ↓↓ | ↓↓ |
| Odor | N | N | N | N | ↑ | ↑↑ | ↑ | ↓ |
| Applicability | 5 | 5 | 5 | 5 | 3 | 3 | 3 | 3 |
| Drying time (min) | 30 | 30 | 30 | 30 | 40 | 50 | 50 | 55 |
| Film forming | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| pH | 5.34 ± 0.02 | 5.32 ± 0.05 | 5.33 ± 0.08 | 5.33 ± 0.07 | 5.15 ± 0.09 | 5.03 ± 0.03 | 4.85 ± 0.06 | 4.42 ± 0.10 |
| Sedimentation? | NS | NS | NS | NS | MAS | MAS | MAS | MAS |
| UV spectrum | ND | ND | ND | ND | ND | −12.07% | −30.40% | −55.12% |
N normal, NS no sedimentation, MIS minor sedimentation, MAS major sedimentation, ND no significant degradation
The formulations are highly unstable when submitted to high temperature conditions (oven; 45 ± 2°C). After 28 days, the drying time was considerably increased (by approximately 85%), which was probably due to evaporation of the cereal alcohol, and this also led to a concentration of the formulation and an increase in the final viscosity. After storage, these formulations were too dense for facial application, which was indicated by the decrease in the applicability from 5 to 3 (Table VI). Alterations in the odor of the formulations were noted, with an initial increase in the smell of the essence followed by a decrease in the final week, characterizing loss/degradation of its aromatic compounds. The UV spectra of the formulations were obtained in order to verify possible degradation. The UV spectrum was consistent with that expected for an aloe vera gel (17), indicating that no other compound of the formulation interfered in this analysis. However, an important time-dependent spectrum absorbance reduction (−55.12% after 28 days) was verified, suggesting that aloe vera compounds are unstable when submitted to high temperature conditions. Major sedimentation of the suspended particles was also noted after 28 days, which is attributable to the fact that an increase in the temperature leads to a temporary decrease in the viscosity of PVA-based formulations and accelerates the natural sedimentation process of the insoluble particles of green clay. The loss of color in these formulations occurred as a result of particle sedimentation because its green color is due to the suspended green clay particles. In general, all of the formulations were visually homogeneous while still wet (Fig. 3a, d), but the sedimentation phenomenon, which occurred under unstable storage conditions led to a distribution gradient where larger particles were concentrated at the bottom of the storage bottle, leading to a loss of the homogeneity of the formulations. After drying, an interesting behavior was noted. The formulations that contained insufficient PVA concentration (film-forming performance, <4) presented an important loss of the homogeneity of the green clay particles on the dried surface, probably due to the highly hydrophilic profile of PVA, which leads to a tendency towards aggregation during the drying process, dragging the clay particles. This also explains why the formulations containing insufficient concentrations of PVA tend to form a film full of perforations and irregular thickness, as can clearly be seen in Fig. 3b and c, which represents experiment 8 (film-forming performance of 2).
The formulations containing satisfactory PVA concentration (film-forming performance, >4) did not present loss of homogeneity. The formation of small agglomerates of green clay was observed (Fig. 3e), due to the total loss of the water and ethanol in the formulation, but these agglomerates were still homogeneously distributed in the film because there was no dragging effect due to the absence of PVA aggregation in these formulations.
Light scattering particle size analysis was carried out, and it was verified that the raw green clay material presented a high particle size disparity (span = 2.272; median = 26.2 μm), containing particles within the range of approximately 1– 100 μm (Fig. 4a). Analysis of the supernatant of the formulations where sedimentation occurred was carried out, and it was observed that the particles that remained in suspension even after the temperature variations showed a narrower particle size range of approximately 1– 10 μm (span = 1.853; median = 1.9 μm; Fig. 4b).
Fig. 4.
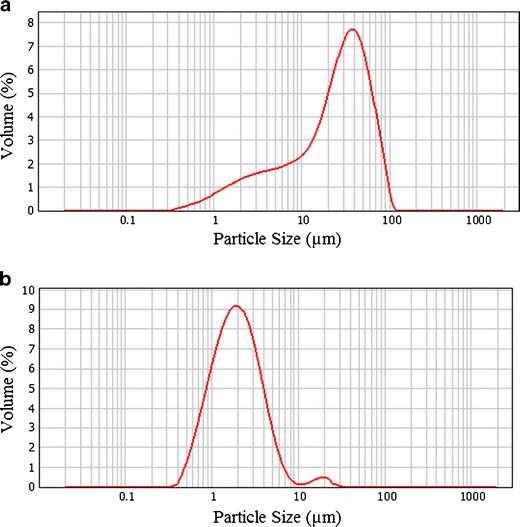
Light scattering particle size analysis of a green clay raw material and b unsedimented fraction of the formulations
In order to avoid instability issues, storage conditions must be carefully selected because the evaporation of cereal alcohol leads to a considerable increase in the drying time and decrease in the stability. The results led to the conclusion that the use of polyethylene bottles for this type of formulation is not recommended, and instead, tight-sealing containers should be used for storage. The use of refined-particle-size clay supplies is also preferable in order to eliminate the possibility of sedimentation as a result of slight changes in temperature.
Microbiological Stability
The results obtained from the microbiology analysis indicated that all of the samples submitted to the plate count test (time 0 and those submitted to different storage conditions for 28 days) did not show colony growth in fungal or bacterial media, which indicates that the microbial load was <1 × 101 CFU/g.
Since the green clay is a geologically active compound, its intrinsic microbiological load may become an issue regarding the conservation of the formulation under specific conditions. This study evaluated the final microbiological load of the formulations after production and after 28 days submitted to extreme storage conditions. The results obtained indicate that both concentrations of the Neolone™ PE preservative, 0.3 and 0.6% (w/w), were able to maintain the optimized formulation microbiologically stable under different storage conditions, which means that the use of a concentration of only 0.3% (w/w) is sufficient to prevent microbial growth even under propitious conditions. The final microbial load of the formulations is considered satisfactory for facial application products according to international applied standards (16).
CONCLUSIONS
In this study, the parameters that most affect the desirable characteristics of peel-off facial masks were investigated. It was observed that cereal alcohol had a significant effect on the drying time. The applicability of the formulations was considerably influenced by both the carbomer and PVA concentrations as a result of their ability to alter the formulation viscosity. The film-forming performance was only influenced by the PVA concentration. The optimized formulation of the peel-off facial mask was mathematically determined as that containing 13% (w/w) PVA, 10% (w/w) cereal alcohol, with no addition of carbomer, achieving high levels of applicability and film-forming performance and the shortest possible drying time. The preliminary stability study indicated that the optimized formulation is stable under normal storage conditions. The preservative demonstrated efficiency in avoiding microbial growth under the storage conditions evaluated (microbial charge <1 × 101 CFU/g for both fungi and bacteria). The Response Surface Methodology was found to be an efficient statistical tool to determine the behavior of different compounds and their concentrations based on the responses studied, allowing the investigation of the optimum conditions to produce green clay and aloe vera peel-off facial masks. This paper provides background information on the application of peel-off formulations as a vehicle for several pharmaceutical ingredients.
ACKNOWLEDGMENTS
This study was financially supported by Dermus Farmácia Dermatológica e Cosmética, Florianópolis, Brazil. The authors express their appreciation to Dr. Siobhan Wiese for assistance with the English correction of the manuscript.
REFERENCES
- 1.Wilkinson JB, Moore RJ. Harry’s cosmetology. 7. London: Longman Group; 1982. [Google Scholar]
- 2.Carretero MI, Lagaly G. Clays and health: an introduction. Appl Clay Sci. 2009;36:1–3. [Google Scholar]
- 3.Ghersetich I, Lotti TM. Immunologic aspects: immunology of water spas. Clin Dermatol. 1996;14:563–566. doi: 10.1016/S0738-081X(96)00085-5. [DOI] [PubMed] [Google Scholar]
- 4.Zague V, Santos DA, Baby AR, Velasco MV. Argilas: natureza nas máscaras faciais. Cosmet Toiletries. 2007;19:64–66. [Google Scholar]
- 5.Carretero MI. Clay minerals and their beneficial effects upon human health. A review. Appl Clay Sci. 2002;21:155–163. doi: 10.1016/S0169-1317(01)00085-0. [DOI] [Google Scholar]
- 6.DeNaverre MG. The chemistry and manufacture of cosmetics. 2. Orlando: Continental; 1975. [Google Scholar]
- 7.Baby AR, Zague V, Maciel CPM, Kaneko TM, Consiglieri VO, Valesco MVR. Development of cosmetic mask formulations. Rev Bras Ciênc Farm. 2004;40:159–161. [Google Scholar]
- 8.Crosswhite FS, Crosswhite CD. Aloe vera, plant symbolism and the threshing floor. Desert Plants. 1984;6:43–50. [Google Scholar]
- 9.Vijayalakshmi D, Dhandapani R, Jayaveni S, Jithendra P, Rose C, Mandal AB. In vitro anti inflammatory activity of Aloe vera by down regulation of MMP-9 in peripheral blood mononuclear cells. J Ethnopharmacol. 2012;141(1):542–546. doi: 10.1016/j.jep.2012.02.040. [DOI] [PubMed] [Google Scholar]
- 10.Yun N, Lee C, Lee S. Protective effect of Aloe vera on polymicrobial sepsis in mice. Food Chem Toxicol. 2009;47:1341–1348. doi: 10.1016/j.fct.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Hu Q, Hu Y, Xu J. Free radical-scavenging activity of Aloe vera (Aloe barbadensis Miller) extracts by supercritical carbon dioxide extraction. Food Chem. 2005;91(1):85–90. doi: 10.1016/j.foodchem.2004.05.052. [DOI] [Google Scholar]
- 12.Olsen DL, Raub W, Bradley C, Johnson M, Macias JL, Love V, et al. The effect of aloe vera gel/mild soap versus mild soap alone in preventing skin reactions in patients undergoing radiation therapy. Oncol Nurs Forum. 2001;28(3):543–547. [PubMed] [Google Scholar]
- 13.Myers RH, Montgomery DC, Anderson-Cook CM. Response surface methodology: process and product optimization using designed experiments. 3. New York: Wiley; 2009. [Google Scholar]
- 14.Vieira RP, Fernandes AR, Kaneko TM, Consiglieri VO, Pinto CASO, Pereira CSC, et al. Physical and physicochemical stability evaluation of cosmetic formulations containing soybean extract fermented by Bifidobacterium animalis. Braz J Pharm Sci. 2009;45(3):515–525. doi: 10.1590/S1984-82502009000300018. [DOI] [Google Scholar]
- 15.Nishikawa DO, Zague V, Pinto CASO, Vieira RP, Kaneko TM, Velasco MVR, et al. Avaliação da estabilidade de máscaras faciais peel-off contendo rutina. Rev Ciências Farm Básica Apl. 2007;28:227–232. [Google Scholar]
- 16.United States Pharmacopeia. USP 31/National Formulary 26. 12601 Twinbrook Parkway, Rockville, MD, USA; 2008.
- 17.Ravi S, Kabilar P, Velmurugan S, Kumar RA, Gayathiri M. Spectroscopy studies on the status of aloin in Aloe vera and commercial samples. J Exp Sci. 2011;2(8):10–13. [Google Scholar]


