Abstract
The aim of this study is to develop meloxicam (MX)-loaded cationic transfersomes as skin delivery carriers and to investigate the influence of formulation factors such as cholesterol and cationic surfactants on the physicochemical properties of transfersomes (i.e., particle size, size distribution, droplet surface charge and morphology), entrapment efficiency, stability of formulations and in vitro skin permeation of MX. The transfersomes displayed a spherical structure. Their size, charge, and entrapment efficiency depended on the composition of cholesterol and cationic surfactants in the formulation. Transfersomes provided greater MX skin permeation than conventional liposomes and MX suspensions. The penetration-enhancing mechanism of skin permeation by the vesicles prepared in this study may be due to the vesicle adsorption to and/or fusion with the stratum corneum. Our results suggest that cationic transfersomes may be promising dermal delivery carriers of MX.
KEY WORDS: liposomes, meloxicam, skin permeation, surfactant, transfersomes
INTRODUCTION
Liposomes, lipid-based delivery systems, have been developed as vesicles for transdermal drug delivery (TDD) because they are predominantly phospholipids bilayers similar to those found in biological membranes. Liposomes are small, spherical, and self-closing aqueous core vesicles consisting of amphiphilic lipids. Liposomes have unique properties due to the amphiphilic character of their lipids, which make them suitable for both hydrophilic (1,2) and lipophilic (3,4) drug delivery. It has become evident that conventional liposomes are of little or no value as carriers for transdermal drug delivery because they do not deeply penetrate skin, but rather they remain confined to the upper layers of the stratum corneum (5). Only specially designed vesicles such as Transfersomes® have been shown to allow transdermal delivery.
Deformable liposomes (Transfersomes®), the first generation of elastic vesicles introduced by Cevc et al. (6), were reported to penetrate intact skin carrying therapeutic concentrations of a drug but only when applied under non-occluded conditions. Transfersomes are prepared from phospholipids and edge activators. An edge activator is often a single-chain surfactant with a high radius of curvature that destabilizes the lipid bilayers of the vesicles and increases the deformability of the bilayers. Sodium cholate, sodium deoxycholate, Span 60, Span 65, Span 80, Tween 20, Tween 60, Tween 80 and dipotassium glycyrrhizinate have been employed as edge activators (7). Transfersomes were reported to improve in vitro skin permeation of various drugs (8–10). The surfactant is also the main composition of transfersomes; therefore, the type of surfactant also directly affects the physicochemical characteristics of the vesicles.
Although many studies have been performed to clarify the effect of the surfactant on the enhancement of skin permeation, the results have not been consistent. Positive (3,11–13) and negative (4,14,15) effects on skin permeation have been reported for both anionic and cationic transfersomes. Moreover, basic knowledge of surfactants was also required to understand the effect of the carbon chain length and the hydrophilic head group on the enhancement of skin permeation. The finding will provided important fundamental information for developing novel transdermal drug delivery systems. The mechanism by which liposomes and their analogs deliver drugs through the skin is not fully understood, and different mechanisms have been suggested. The mechanism may vary based on the characteristics of the liposome formulation and drug. Liposomal carriers must be designed and tested on a case-by-case basis.
Meloxicam (MX) (Fig. 1), a highly potent, non-steroidal anti-inflammatory drug (NSAID), is used for the treatment of rheumatoid arthritis, osteoarthritis and other joint diseases. However, adverse effects on the gastrointestinal (GI) tract, such as stomachache and indigestion, and patient compliance are weaknesses of oral and injectable MX administrations. Skin delivery is an alternative administration for MX that can minimize GI side effects and improve patient compliance. Transdermal dosage forms of MX such as patches (16) and gels (17,18) have been formulated and investigated. However, its low aqueous solubility (0.012 mg/mL) (18) is the limitation for preparation in topical forms.
Fig. 1.
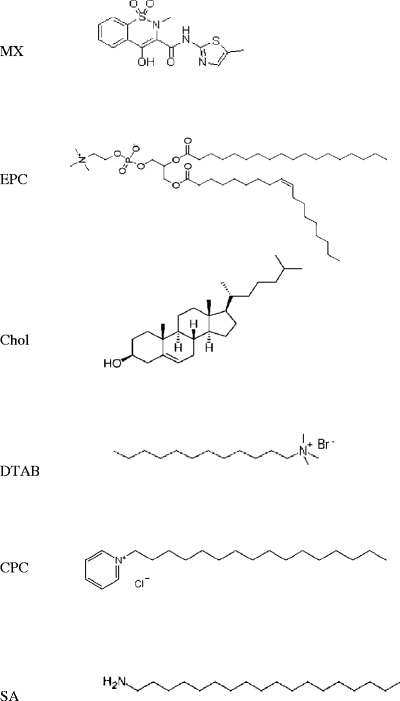
The chemical structure of meloxicam and the lipid compositions of the formulations
In this study, the various types of cationic surface were used in the comparative study for comparing the enhancement of skin permeation. The aim of the present study was to develop transfersomes as a skin delivery carriers for MX and to investigate the influence of formulation factors such as cholesterol and cationic surfactants on the physicochemical properties of the transfersomes (i.e., particle size, size distribution, droplet surface charge and morphology), entrapment efficiency, stability of formulations and in vitro skin permeation. Moreover, the possible mechanisms for enhancement of the skin permeation of transfersomes were investigated by Fourier transform infrared (FT-IR) spectroscopy and differential scanning calorimetry (DSC).
MATERIALS AND METHODS
Materials
Phosphatidylcholine (PC) from eggs was purchased from Lipoid GmbH (Ludwigshafen, Germany). Cholesterol (Chol) was purchased from Carlo Erba Reagenti (Strada Rivoltana, Rodano, Italy). Dodecyltrimethylammonium bromide (DTAB), stearylamine (SA) and cetylpyridinium chloride monohydrate (CPC) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Meloxicam (MX) was supplied from Fluka (Buchs, Switzerland). All other chemicals were commercially available and of analytical and high-performance liquid chromatography (HPLC) grade.
Preparation of Meloxicam-Loaded Liposomes, Transfersomes, and Suspensions
Liposomes and transfersomes were prepared by the sonication method. Briefly, PC, Chol, DTAB, CPC, SA, and MX were dissolved in chloroform/methanol (2:1 v/v). Liposomes and transfersomes containing a fixed amount of PC and MX were prepared. The formulations were composed of a bilayer formed from PC and Chol, DTAB, CPC or SA in a molar ratio of 10:2. The MX concentration was 10.0% (w/w) of PC. To prepare MX-loaded liposomes and transfersomes, the lipid compositions were deposited in a test tube, and the solvent was evaporated with nitrogen gas. The lipid thin-film was placed in a desiccator connected to a vacuum pump for 6 h to remove the remaining organic solvent. The dried lipid thin-film was hydrated with Tris buffer pH 7.4. Following hydration, the dispersion was sonicated in a ice-bath for 30 min and then probe-sonicated for 2 cycles of 30 min. The lipid compositions of liposome and transfersome formulations utilized in this study are listed in Table I.
Table I.
The Lipid Compositions of Liposome and Transfersome Formulations
| Name (molar ratio) | Composition (%w/v) | ||||||
|---|---|---|---|---|---|---|---|
| MX | PC | Chol | DTAB | CPC | SA | Tris buffer | |
| PC (10:0) | 0.07 | 0.77 | – | – | – | – | 100 mL |
| PC/Chol (10:2) | 0.07 | 0.77 | 0.07 | – | – | – | 100 mL |
| PC/DTAB (10:2) | 0.07 | 0.77 | – | 0.06 | – | – | 100 mL |
| PC/DTAB/Chol (10:2:2) | 0.07 | 0.77 | 0.07 | 0.06 | – | – | 100 mL |
| PC/CPC (10:2) | 0.07 | 0.77 | – | – | 0.07 | – | 100 mL |
| PC/CPC/Chol (10:2:2) | 0.07 | 0.77 | 0.07 | – | 0.07 | – | 100 mL |
| PC/SA (10:2) | 0.07 | 0.77 | – | – | – | 0.05 | 100 mL |
| PC/SA/Chol (10:2:2) | 0.07 | 0.77 | 0.07 | – | – | 0.05 | 100 mL |
The MX suspension was prepared by adding an excess amount of MX (at least two times higher than the solubility) in distilled water and stirring for 24 h to ensure constant thermodynamic activity throughout the course of the permeation experiment.
Particle Size, Size Distribution and Zeta Potential of Vesicles
The particle size, size distribution and zeta potential of the liposomes and transfersomes were measured by a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK) at room temperature. One hundred microliters of the liposome and transfersome was diluted with 900 μL deionized water. At least three independent samples were prepared, each of which was measured at least three times.
Morphology of Vesicles
Morphology characterization of vesicles was performed by transmission electron microscopy (TEM) (JEM-1230, Jeol, Japan). One drop of the liposomal or transfersomal vesicles was dropped onto a copper grid; then, the excess suspension was adsorbed immediately using filter paper. The vesicles were air-dried on the copper grid. After drying, the grid was observed using TEM at 5,000-50,000 magnification with an acceleration voltage of 80 kV.
Entrapment Efficiency
The entrapment efficiencies (EE) of MX in the formulation was determined by HPLC after disruption of the vesicles (liposomes and transfersomes) with Triton® X-100 (0.1% w/v) at a 1:1 volume ratio and appropriate dilution with PBS (pH 7.4). The vesicle/Triton® X-100 solution was centrifuged at 10,000 rpm at 4°C for 10 min. The supernatant was filtered with a 0.45-μm nylon syringe filter. The entrapment efficiencies of MX loaded in the formulation were calculated using the following equation:
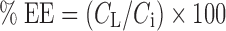 |
1 |
where CL is the concentration of MX loaded in the formulation as described in the above methods and Ci is the initial concentration of MX added into the formulation.
Stability Evaluation of Liposomes and Transfersomes
Liposomes and transfersomes were stored in glass bottles with plastic plugs at 4 ± 1°C, 25 ± 1°C (room temperature, RT) and 45 ± 1°C for 30 days to examine the stability of the formulations. The physicochemical stability of MX-loaded liposomes and transfersomes was evaluated by visual observation for sedimentation, particle size, zeta potential determination, and measurement of the MX remaining in the formulation by HPLC after 1, 7, 15, and 30 days.
In Vitro Skin Permeation Study
Shed snake skin of Naja kaouthia was used as a model membrane because it was reported to have similar permeability to human skin (19). It was donated by the Queen Saovabha Memorial Institute, Thai Red Cross Society, Bangkok, Thailand. The whole shed snake skin was obtained immediately after shedding from five to seven different snakes. Each snake skin could be divided into 10–12 pieces. The thickness of the shed snake skin was approximately 0.02–0.03 mm. It was stored at −10°C prior to use. After thawing, the skin was cut into round sections of 1 in.2 and was then immediately placed on the diffusion cell. The permeation of MX-loaded liposomes and transfersomes through the skin was determined by using a side-by-side diffusion cell. The shed snake skin was mounted between the two half-cells of a side-by-side diffusion cell with a 32°C water jacket to control the temperature. The stratum corneum side of the skin faced the donor compartment, which was filled with the formulation containing MX (3 mL). The receptor compartment was filled with 0.1 M PBS (pH 7.4) and stirred with a star-head Teflon magnetic bar driven by a synchronous motor. The sink condition in the receptor medium was obtained in this study. At appropriate intervals of 0.5, 1, 2, 4, 6, 8, 10, and 12 h, 0.5 mL aliquots of the receptor medium was withdrawn and immediately replaced with an equal volume of fresh medium. The samples from the receptor medium were analyzed by HPLC and the cumulative amount was plotted against time. The steady-state flux was determined as the slope of linear portion of the plot.
HPLC Analysis
A HPLC 1100 system (Agilent 1100 Series HPLC System, Agilent technologies, USA) was used to analyze the amount of MX. Chromatographic separation of MX was achieved using an Eclipse XDB-C18 column (particle size = 5 μm; column dimension = 4.6 × 150 mm) operating at 1 mL min−1. A mobile phase consisting of potassium dihydrogen phosphate pH 4.4, methanol, and acetonitrile (45:45:10, v/v/v) was used. The injection volume was 20 μL, and UV detector was set at 364 nm, respectively. All of the sample solutions were filtered through a polytetrafluoroethylene filter (average pore size = 0.45 μm) prior to injection. The calibration curve for MX was in the range of 1–100 μg/mL with a correlation coefficient of 0.999. The percentage recovery was found from 99.85–100.30%, and relative standard deviation for both intra-day and inter-day was less than 2%.
Characterization of Snake Skin After Skin Permeation
FT-IR Analysis of Shed Snake Skin
Following the skin permeation experiment, the shed snake skin was washed with water, blotted dry and kept in desiccators. The spectrum of the skin sample was recorded in the range of 4000–500 cm−1 using a FT-IR spectrophotometer (Nicolet 4700, Thermo Scientific, USA). The FT-IR spectrum of the treated skin with the MX suspension was also recorded and used as a control.
DSC Analysis of Shed Snake Skin
Thermal analysis of the shed snake skin after the permeation experiment prepared with the same method as used for the FT-IR analysis was performed with a DSC (Pyris Sapphire DSC, PerkinElmer instrument, USA). The skin sample (2 mg) was weighed into an aluminum crimp pan and was heated from −30 to 320°C at a heating rate of 10°C/min. All DSC measurements were collected under a nitrogen atmosphere with a flow rate of 100 mL/min. The DSC thermogram of the treated skin with MX suspension was also recorded and used as a control.
Data Analysis
The data are reported as the means ± S.D. (n = 3–6), and statistical analysis of the data was carried out using one-way ANOVA followed by an LSD post hoc test. A p value of less than 0.05 was considered to be significant.
RESULTS
Physicochemical Characteristics of Liposomes and Transfersomes
The vesicle size of liposomes and transfersomes were in the range of 60–90 nm with the polydispersity index of 0.2–0.3, indicating that the liposomes and transfersomes were homogeneously dispersed. The zeta potential of the liposomes was between −3 and −14 mV, whereas that of the transfersomes was between 20 and 50 mV (Table II). The zeta potential of the CPC-containing formulation was higher than that of the DTAB-containing formulation, SA-containing formulation and liposome formulation. The addition of cationic surfactant increased the positive charge of the formulation. These results indicated that the zeta potential was dependent upon the type of cationic surfactant, and CPC showed a strong interaction with PC in bilayers. The entrapment efficiency of liposomes and transfersomes was in the range between 20% and 70% (Table II). The addition of cholesterol decreased the entrapment efficiency and the concentration of drug in the formulation. No significant difference (p > 0.05) was found in the vesicle formulations with and without cholesterol in this study. The incorporation of cationic surfactant (DTAB and CPC) in the transfersomes significantly (p < 0.05) increased the entrapment efficiency percentage and concentration of drug in the formulation. The two-dimensional morphology of the vesicles was further evaluated by TEM, indicating the vesicular characteristics. MX, loaded in liposomes and transfersomes, was prepared from PC and PC/CPC, respectively, was small and spherical (Fig. 2).
Table II.
The Physicochemical Characteristics of Liposomes and Transfersomes
| Sample name | Size (nm) | PDI | Zeta (mV) | %EE | Conc. (μg/ml) |
|---|---|---|---|---|---|
| PC | 69.64 ± 2.60 | 0.37 ± 0.03 | −3.70 ± 1.15 | 50.61 ± 0.72 | 391.21 ± 5.56 |
| PC/Chol | 78.72 ± 0.59 | 0.28 ± 0.01 | −14.47 ± 1.09 | 50.15 ± 1.13 | 387.69 ± 8.73 |
| PC/DTAB | 71.23 ± 0.83 | 0.32 ± 0.03 | 24.68 ± 1.77 | 70.24 ± 0.88 | 542.93 ± 6.81 |
| PC/Chol/DTAB | 67.60 ± 0.68 | 0.29 ± 0.01 | 28.31 ± 1.04 | 67.93 ± 1.06 | 525.12 ± 8.16 |
| PC/CPC | 60.92 ± 0.78 | 0.30 ± 0.03 | 52.48 ± 3.57 | 66.55 ± 1.13 | 514.41 ± 8.77 |
| PC/Chol/CPC | 66.03 ± 1.10 | 0.29 ± 0.03 | 52.23 ± 1.99 | 62.58 ± 0.85 | 483.75 ± 6.56 |
| PC/SA | 87.36 ± 0.77 | 0.36 ± 0.05 | 23.00 ± 1.61 | 23.01 ± 0.86 | 177.85 ± 6.61 |
| PC/Chol/SA | 77.88 ± 0.40 | 0.37 ± 0.01 | 22.53 ± 1.23 | 22.46 ± 0.62 | 173.80 ± 4.80 |
PDI polydispersity index
Fig. 2.
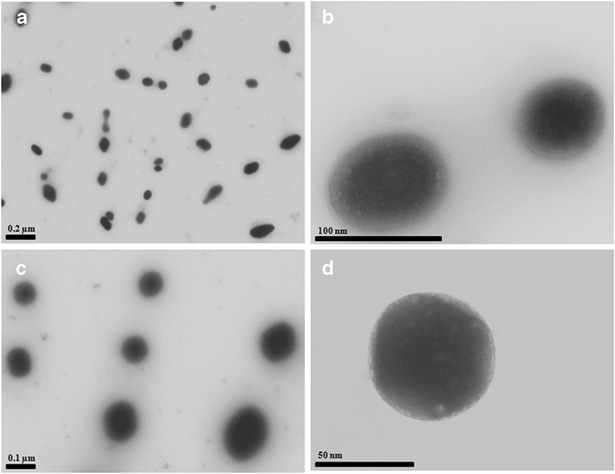
Transmission electron microscopy of MX-loaded vesicles. Visualization of MX loaded in liposomes a PC (5,000×), b PC (50,000×), c PC/CPC (5,000×), and d PC/CPC (50,000×)
Stability of Liposomes and Transfersomes
Figure 3 shows (a) size, (b) size distribution, (c) zeta potential, and (d) percentage of MX remaining of the formulation at 4°C, 25°C, and 45°C for 30 days. No sedimentation was found in any vesicle formulation after fresh preparation. After storage at 4°C for 30 days, no sedimentation was observed, and the size, size distribution and percentage of MX remaining in the formulation at 4°C for 30 days were not significantly different from the initial preparation. Similarly, after storage at 25°C and 45°C for 15 days, there was no sedimentation in any formulation, and the size, size distribution, and percentage of MX remaining in the formulation at 25°C and 45°C for 15 days were not different from the initial preparation. However, the percentage of MX remaining in the formulation was less than 80% after storage at 25°C and 45°C for 30 and 15 days, respectively.
Fig. 3.
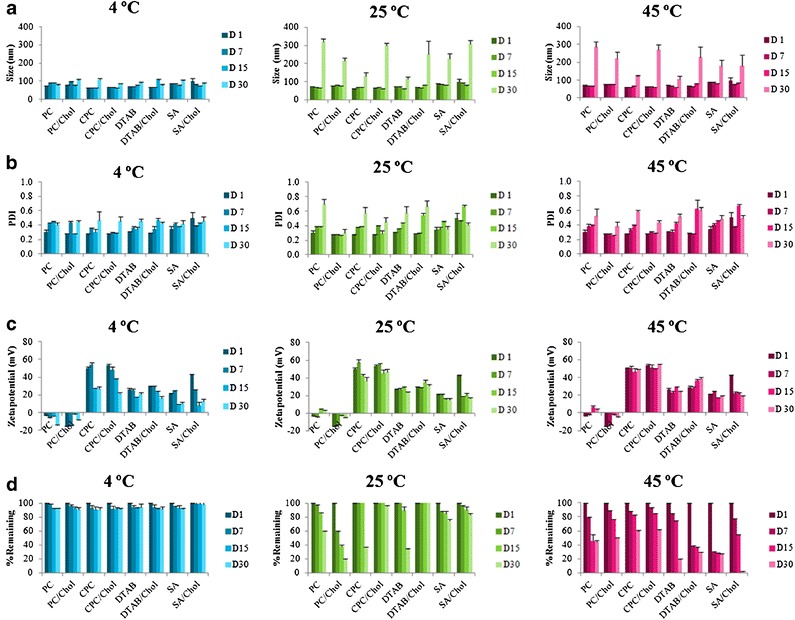
a Size, b size distribution (polydispersity index, PDI), c zeta potential and d % MX remaining in the formulation at 4°C, 25°C, and 45°C for 30 days. Each value represents the mean ± SD (n = 3)
In Vitro Skin Permeation Study
Figure 4a shows the permeation profiles of MX in the formulations, including MX suspensions (control), MX-loaded liposomes and MX-loaded transfersomes with CPC. The cumulative amount of drug increased linearly with time after a short lag time (0.5–0.8 h). This linear accumulation was also observed for other liposome and transfersome formulations in our study. Figure 4b shows the pseudosteady-state flux (F) of MX through shed snake skin in various formulations, which was determined as the slope of the linear portion of the plot. The F of MX that permeated through the skin in all vesicles formulations was significantly higher than in MX suspensions; however, the F of MX in transfersomes was significantly higher than in liposomes. The cumulative skin permeation profile of MX in the vesicles composed of cholesterol was slightly lower than vesicles without cholesterol.
Fig. 4.
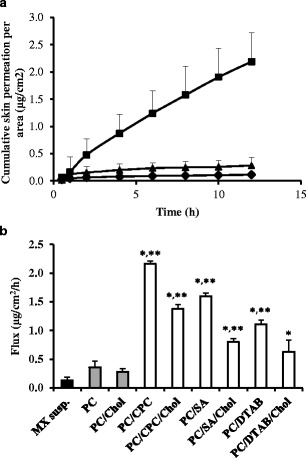
a The skin permeation profile of meloxicam from (solid circle) MX suspensions (control), (solid triangle) PC and (solid square) PC/CPC. b The fluxes of meloxicam through shed snake skin from different formulations: (solid square) control, (shaded square) liposomes, and (white square) transfersomes. Different values were statistically significant (*P < 0.05) compared with MX suspensions (control). Different values were statistically significant (*P < 0.05) compared with liposomes. Each value represents the mean ± SD (n = 3–6)
Characterization of Skin After Skin Permeation
The skin sample after skin permeation was investigated by FT-IR and DSC. The FT-IR spectra and DSC thermogram are presented in Fig. 5a,b, respectively. The FT-IR spectra of the skin sample provided a measure of fluidity of the stratum corneum (SC) lipid. The FT-IR spectra of the skin treated with the MX suspension and the skin treated with the vesicle formulation were compared. The results indicated absorption broadening for both the C–H (CH2) asymmetric stretching peak near 2920 cm−1 and the C–H (CH2) symmetric stretching peak near 2850 cm−1. The DSC thermograms of the skin treated with the MX suspension and the skin treated with the vesicle formulation were also compared. The results indicated that the thermal properties of the skin samples were shifted. The SC lipid of the skin samples exists as a solid gel state at a temperature of 229°C. The skin was treated with the vesicle formulations, which exist as liquid states at the following temperatures: PC/CPC, 226.0°C; PC/SA, 226.6°C; PC/DTAB, 227°C; PC/CPC/Chol, 227.1°C; PC/SA/Chol, 227.3°C; PC/DTAB/Chol, 228.1°C; PC, 228.2; and PC/Chol, 228.3.
Fig. 5.
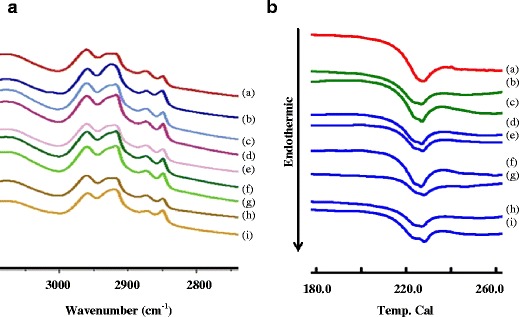
a FT-IR spectra and b DSC thermogram of the shed skin after 24 h skin permeation. Each shed skin was treated with (a) MX suspension, (b) PC, (c) PC/Chol, (d) PC/CPC, (e) PC/CPC/Chol, (f) PC/DTAB, and (g) PC/DTAB/Chol (h) PC/SA, and (i) PC/SA/Chol
DISCUSSION
Several researchers have outlined that the physicochemical properties of a vesicle can be influenced by modifying the surface charge of vesicle (15). Comparing liposomes consisting of phospholipid alone and transfersomes consisting of phospholipid and edge activator (surfactant), the vesicles of liposomes were different in physicochemical characteristics such as size, charge, morphology and % EE than those of transfersomes. The presence of surfactant led to modification of the surface charge of the vesicle, which may affect the vesicular characteristics (9). No significant difference (p > 0.05) was found in the vesicle size between liposomes and transfersomes both with and without cholesterol. However, the previous study (14) reported that the neutralization between the different charges of the cationic drug and anionic vesicles can reduce the repulsive forces between the bilayers, thus resulting in a decrease in vesicle size of niosomes compared to the case when the drug and vesicles have the same charge, which results in a larger vesicle size from repulsive forces between the cationic drug and cationic niosomes. The vesicle size of those containing cholesterol was significantly different compared to vesicles without cholesterol because the cholesterol may cause the bilayer to become more compact (20).
The surface charge of transfersomes containing cationic surfactant was significantly higher than the conventional liposomes. The presence of cationic surfactant directly affects the positive charge of transfersomes due to the intrinsic properties of the cationic surfactant. However, the surface charge of each transfersome formulation was significantly different because the hydrophilic head group of each cationic surfactant is different (Fig. 1).
The entrapment efficiency of transfersomes containing cationic surfactant was significantly higher than that of the conventional liposomes. These results might be attributed to the intrinsic properties of the cationic surfactant (such as a solubilizer) and the interactions among the surfactants (CPC and DTAB), MX and lipid bilayer. When a surfactant, such as sodium stearate, was incorporated into the phosphatidylethanolamine vesicles, the entrapment efficiency of the drug was significantly increased (21).
In the stability study, the absence of cholesterol and/or cationic surfactant did not lead to any significant differences in physicochemical stability at the storage age. In our expectation, the formulations should be stable during the storage age, and all physicochemical characteristics (size, charge, % MX remaining in the formulation) should not be significantly different. However, the higher temperature caused the physicochemical instability of vesicles containing MX. Temperature is the major factor affecting the stability of the vesicle formulation (22). In this study, the temperature recommended for storing liposomes and transfersomes was 4°C and 25°C for 30 days.
The in vitro skin permeation flux (F) of MX-loaded cationic transfersomes was significantly higher than liposomes and MX suspensions. The F of MX permeation into the skin from transfersomes with different cationic surfactants are ranked as follows: CPC (C21) > SA (C18) > DTAB (C15). The higher the carbon chain length of the cationic surfactant in the formulation, the higher the skin permeation of MX. The surfactants consisting of different carbon chains showed different surfactant properties (23). C15–C21 might be a suitable structure and carbon chain length range for skin permeation of MX-loaded transfersomes. These results suggested that the cationic surfactant in transfersomes directly affected the skin permeation. The transfersomes containing cationic surfactant may affect the lipids of SC and produce an enhancing effect by disturbing the SC lipids. The significantly lower skin permeation of MX suspensions than the MX-loaded vesicle formulation indicated that the free-drug mechanism was significantly smaller or had no more effect than the mechanism of vesicle. Comparing the vesicles composed of or lacking cholesterol, the results indicated that cholesterol might be a factor affecting the enhancement in skin permeation of MX. An increase in cholesterol led to an increase in the stability and rigidity and a decrease in the permeability of the lipid bilayers (20). Our results indicated that the incorporation of cholesterol caused lower skin permeation of the drug because of the intrinsic properties of cholesterol that may affect the physicochemical properties of the vesicle. In particular, an effect on the elasticity of the vesicles may directly affect the intact vesicular skin permeation mechanism (24). However, cholesterol was the main lipid in the composition, which was important for the stability of the vesicle formulation, and therefore the optimum ratio of cholesterol needs to be further studied.
After the in vitro skin permeation study, the shed snake skin samples were investigated by FT-IR and DSC. In the FT-IR spectra, the absorption broadening for both the C–H (CH2) asymmetric stretching and the C–H (CH2) symmetric stretching of the skin samples that were treated with the vesicle formulation indicated that the vesicles might affect fluidity of the SC lipids by disruption them. The DSC thermogram indicated that the vesicles may disrupt the SC lipids, which also directly affects the barrier function of the SC. The FT-IR spectra and the DSC thermogram were consistent with the previous study (25). Our results indicated that the mechanisms of the skin permeation enhancement of the vesicles might be vesicle adsorption to and/or fusion with the SC.
CONCLUSION
MX-loaded cationic transfersomes were successfully prepared using a sonication method. The incorporation of cationic surfactants resulted in small size, positive charge and high entrapment efficiency. Cationic transfersomes provided greater MX skin permeation than conventional liposomes and MX suspensions. The results indicated that the barrier function of the SC was affected by the physicochemical characteristics of the vesicle systems (size, charge, and %EE) and lipid composition (cholesterol and surfactant). A penetration-enhancing mechanism of transfersomes, such as the vesicle adsorption to and/or fusion with the SC, was observed. In our study, CPC showed the greatest skin permeation among other surfactants. This study suggests that cationic transfersomes have the potential to be dermal delivery carriers of MX.
Acknowledgments
The authors wish to thank the Silpakorn University Research and Development Institute, Graduate School of Silpakorn University and the Thailand Research Funds through the Golden Jubilee Ph.D. Program (Grant No. PHD/0141/2550) for financial support.
Declaration of Interest
The authors declare no conflicts of interest.
References
- 1.Pérez-Cullell N, Coderch L, Maza A, Parra JL, Estelrich J. Influence of the fluidity of liposome compositions on percutaneous absorption. Drug deliv. 2000;7:7–13. doi: 10.1080/107175400266731. [DOI] [PubMed] [Google Scholar]
- 2.Verma DD, Verma S, Blume G, Fahr A. Liposomes increase skin penetration of entrapped and non-entrapped hydrophilic substances into human skin: a skin penetration and confocal laser scanning microscopy study. Eur J Pharm Biopharm. 2003;55:271–277. doi: 10.1016/S0939-6411(03)00021-3. [DOI] [PubMed] [Google Scholar]
- 3.Montenegro L, Panico AM, Ventimiglia A, Bonina FP. In vitro retinoic acid release and skin permeation from different liposome formulations. Int J Pharm. 1996;133:89–96. doi: 10.1016/0378-5173(95)04422-1. [DOI] [Google Scholar]
- 4.Sinico C, Manconi M, Peppi M, Lai F, Valenti D, Fadda AM. Liposomes as carriers for dermal delivery of tretinoin: in vitro evaluation of drug permeation and vesicle–skin interaction. J Control Release. 2005;103:123–136. doi: 10.1016/j.jconrel.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Touitou E, Dayan N, Bergelson L, Godin B, Eliaz M. Ethosomes—novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J Control Release. 2000;65:403–418. doi: 10.1016/S0168-3659(99)00222-9. [DOI] [PubMed] [Google Scholar]
- 6.Cevc G, Blume G. Lipid vesicles penetrate into intact skin owing to the transdermal osmotic gradients and hydration force. Biochim Biophy Acta. 1992;1104(1):226–232. doi: 10.1016/0005-2736(92)90154-E. [DOI] [PubMed] [Google Scholar]
- 7.Elsayed MMA, Abdallah OY, Naggar VF, Khalafallah NM. Deformable liposomes and ethosomes: mechanism of enhanced skin delivery. Int J Pharm. 2006;322:60–66. doi: 10.1016/j.ijpharm.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 8.El Maghraby GM, Williams AC, Barry BW. Skin delivery of oestradiol from deformable and traditional liposomes: mechanistic studies. J Pharm Pharmacol. 1999;51:1123–1134. doi: 10.1211/0022357991776813. [DOI] [PubMed] [Google Scholar]
- 9.El Maghraby GM, Williams AC, Barry BW. Oestradiol skin delivery from ultradeformable liposomes: refinement of surfactant concentration. Int J Pharm. 2000;196:63–74. doi: 10.1016/S0378-5173(99)00441-X. [DOI] [PubMed] [Google Scholar]
- 10.W-s Z, X-q F, L-l W, Y-j Z. Preparation and quality assessment of itraconazole transfersomes. Int J Pharm. 2012;436(1–2):291–298. doi: 10.1016/j.ijpharm.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Song Y-K, Kim C-K. Topical delivery of low-molecular-weight heparin with surface-charged flexible liposomes. Biomaterials. 2006;27:271–280. doi: 10.1016/j.biomaterials.2005.05.097. [DOI] [PubMed] [Google Scholar]
- 12.Mohammed AR, Weston N, Coombes AGA, Fitzgerald M, Perrie Y. Liposome formulation of poorly water soluble drugs: optimisation of drug loading and ESEM analysis of stability. Int J Pharm. 2004;285:23–34. doi: 10.1016/j.ijpharm.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Katahira N, Murakami T, Kugai S, Yata N, Takano M. Enhancement of topical delivery of a lipophilic drug from charged multilamellar liposomes. J Drug Target. 1999;6(6):405–414. doi: 10.3109/10611869908996847. [DOI] [PubMed] [Google Scholar]
- 14.Manosroi A, Khanrin P, Lohcharoenkal W, Werner RG, Götz F, Manosro W, et al. Transdermal absorption enhancement through rat skin of gallidermin loaded in niosomes. Int J Pharm. 2010;392:304–310. doi: 10.1016/j.ijpharm.2010.03.064. [DOI] [PubMed] [Google Scholar]
- 15.Gillet A, Compère P, Lecomte F, Hubert P, Ducat E, Evrard B, et al. Liposome surface charge influence on skin penetration behaviour. Int J Pharm. 2011;411:223–231. doi: 10.1016/j.ijpharm.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 16.Young-Chang A, Jin-Kyu C, Yang-Kyu C, Han-Moi K, Joon-Ho B. A novel transdermal patch incorporating meloxicam: in vitro and in vivo characterization. Int J Pharm. 2010;385:12–19. doi: 10.1016/j.ijpharm.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Gupta SK, Bansal P, Bhardwaj RK, Jaiswai J, Velpandian T. Comparison of analgesic and anti-inflammatory activity of meloxicam gel with dichlofenac and piroxicam gel in animal models: pharmacokinetic parameters after topical application. Skin Pharmacol Appl Skin Phys. 2002;15:105–111. doi: 10.1159/000049397. [DOI] [PubMed] [Google Scholar]
- 18.Jantharaprapap R, Stagni G. Effects of penetration enhancers on in vitro permeability of meloxicam gels. Int J Pharm. 2007;343:26–33. doi: 10.1016/j.ijpharm.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Ngawhirunpat T, Panomsuk S, Opanasopit P, Rojanarata T, Hatanaka T. Comparison of the percutaneous absorption of hydrophilic and lipophilic compounds in shed snake skin and human skin. Pharmazie. 2006;61:331–336. [PubMed] [Google Scholar]
- 20.Tavano L, Muzzalupo R, Cassano R, Trombino S, Ferrarelli T, Picci N. New sucrose cocoate based vesicles: preparation characterization and skin permeation studies. Colloid Surface B. 2010;75:319–322. doi: 10.1016/j.colsurfb.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Fang Y-P, Tsai Y-H, Wu P-C, Huang Y-B. Comparison of 5-aminolevulinic acid-encapsulated liposome versus ethosome for skin delivery for photodynamic therapy. Int J Pharm. 2008;356:144–152. doi: 10.1016/j.ijpharm.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 22.Duangjit S, Opanasopit P, Rojanarata T, Ngawhirunpat T. Characterization and in vitro skin permeation of meloxicam-loaded liposomes versus transfersome. J Drug Deliv. 2010. doi:10.1155/2011/418316. [DOI] [PMC free article] [PubMed]
- 23.Smith EW, Maibach HI. Percutaceous penetration enhancement. 2. Boca Raton: Taylor and Francis; 2006. [Google Scholar]
- 24.El Maghraby GM, Barry BW, Williams AC. Liposomes and skin: from drug delivery to model membranes. Eur J Pharm Sci. 2008;34:203–222. doi: 10.1016/j.ejps.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Obata Y, Utsumi S, Watanabe H, Suda M, Tokudome Y, Otsuka M, et al. Infrared spectroscopic study of lipid interaction in stratum corneum treated with transdermal absorption enhancers. Int J Pharm. 2010;389:18–23. doi: 10.1016/j.ijpharm.2010.01.007. [DOI] [PubMed] [Google Scholar]


