Abstract
In order to improve the bioavailability of the antidepressant drug, venlafaxine hydrochloride, in situ mucoadhesive thermoreversible gel, was formulated using Lutrol F127 (18%) as a thermo gelling polymer. Mucoadhesion was modulated by trying carbopol 934, PVP K30, HPMC K4M, sodium alginate, tamarind seed gum, and carrageenan as mucoadhesive polymers. Results revealed that as the concentration of mucoadhesive polymer increased the mucoadhesive strength increased but gelation temperature decreased. Formulation was optimized on the basis of clarity, pH, gelation temperature, mucoadhesive strength, gel strength, viscosity, drug content, diffusion through sheep nasal mucosa, histopathological evaluation of mucosa, and pharmacodynamic study in rats. Final formulation T5 containing 18% Lutrol F127 and 0.3% PVP K30 was considered as an optimized formulation. T5 released 97.86 ± 0.073% drug in 150 min with a flux of 0.1545 mg cm−2 min−1 and gelation temperature 31.17 ± 0.30°C. Histopathological evaluation of nasal mucosa revealed that T5 formulation was safe for nasal administration as it caused no damage to nasal epithelium. From the results of pharmacodynamic study, mainly forced swim test (FST), it was concluded that venlafaxine hydrochloride was more effective as an antidepressant by nasal route as in situ gel nasal drops in comparison to oral administration of equivalent dose.
Key words: lutrol F127, mucoadhesive, nasal in situ gel, thermoreversible, venlafaxine HCl
INTRODUCTION
Venlafaxine hydrochloride (VENH), an antidepressant, is used in treatment of major depressive disorder, social anxiety disorder, generalized anxiety disorder and panic disorder. Drug is extensively metabolized in the liver via CYP2D6 and so has low oral bioavailability (45%) (1). Under these circumstances, intranasal delivery appears to be an attractive alternative. However, low residence time of drug in nasal cavity affect absorption and in turn bioavailability of drug. Hence, the design of nasal dosage forms has to consider the anatomic and physiologic characteristics of nasal mucosa and more particularly the rapid mucocilliary clearance (MCC) that limits the time available for drug absorption from the applied dosage form (2,3). So, the possible strategy to decrease rapid MCC, is the use of mucoadhesive formulations to prolong the residence time at the nasal absorption site and thereby facilitate the uptake of the drug. Ordinary gels are difficult to administer, and an accurate drug dose cannot be measured while mucoadhesive powders are not highly favored products. They can cause irritation to the nasal mucosa and give a gritty feel to the tissues (4). A nasal mucoadhesive in situ gel appears very attractive since it is fluid-like prior to nasal administration and can thus easily be administered as a drop allowing accurate drug dosing. In situ gelation can be achieved by using thermo sensitive smart polymers which by sensing nasal temperature forms gel on instillation (5).
In order to formulate thermosensitive in situ gel for nasal administration, thermoreversible polymer must have gelation temperature in the nasal physiological temperature range (29°C to 34°C). Lutrol F127 (LF127) has excellent thermosensitive gelling property, (6) low toxicity and irritation, excellent water solubility, good drug release characteristics, and compatibility with other chemicals (7). It is an ABA triblock copolymer consisting of the hydrophilic polyethyleneoxide (PEO) and the hydrophobic polypropyleneoxide. The temperature-induced gelation of LF127 has been explained on the basis that the polymer exists as a mobile viscous liquid at reduced temperatures but forms a rigid semisolid gel network with an increase in temperature (8).
The objective of the present study was to develop VENH mucoadhesive thermoreversible in situ nasal gel which would enhance nasal residence time and absorption of drug across nasal mucosal membrane to improve bioavailability of the drug as compared with oral route.
MATERIALS AND METHODS
Materials
VENH was kindly gifted by Lupin Research Park, Pune India. Lutrol F127 was gifted by BASF India Ltd., Mumbai India. Carbopol 934P was gifted by Oxford Laboratory, Mumbai India. HPMC K4M was gifted by Colorcon Asia Pvt. Ltd., Goa India. PVP K30, sodium alginate, methyl paraben, and propyl paraben were gifted by Loba Chemical Pvt. Ltd., Mumbai, India. Tamarind seed gum was gifted by Arihant Industries, Barshi, India. Carrageenan was gifted by Signet Chemical Corporation, Mumbai, India.
Methods
Preparation and Optimization of Thermoreversible PF127 Gels
The plain and drug-loaded LF127 gels were prepared by cold method described by Schmolka (9). For drug-loaded LF127 gels, drug was stirred with sufficient quantity of distilled water while for plain LF127 gels, only sufficient quantity of distilled water without drug was kept overnight at 4°C in refrigerator. LF127 was then added slowly with continuous stirring. The dispersions were then stored in a refrigerator until clear solution was obtained and finally volume was adjusted. Optimization of plain and drug-loaded LF127 gel was done by varying the concentration of LF127 and evaluating them for gelation temperature. Batch containing optimized concentration of LF127 was used for further investigation to study the effect of mucoadhesive polymers on gelation temperature and mucoadhesive strength. Three different concentrations of six mucoadhesive polymers were screened. Carbopol 934P (0.1% to 0.3%), HPMC K4M (0.5% to 1.5%), PVP K30 (0.1% to 0.5%), sodium alginate (0.1% to 0.5%), tamarind seed gum (0.1% to 0.5%), and carrageenan (0.1% to 0.5%) were tried as a mucoadhesive polymer.
Preparation of Mucoadhesive Thermoreversible Nasal Gels
VENH, mucoadhesive polymer, and methyl paraben were dissolved in distilled water by agitation at room temperature. After cooling the solution to 4°C, LF127 was added slowly with agitation. The resulting dispersion was then kept overnight at 4°C until clear transparent solution was formed. Finally volume was adjusted by using cold distilled water. Evaluation of final formulation was done, with respect to clarity, pH, gelation temperature, mucoadhesive strength, gel strength, viscosity, drug content, diffusion through sheep nasal mucosa, histopathological evaluation of mucosa, and pharmacodynamic study in rats.
Evaluation of Formulations
Clarity
The clarity of various formulations was determined by visual inspection under black and white background, and it was graded as follows: turbid, +; clear, ++; and very clear (glassy), +++.
pH of Formulation
pH of the each formulation was determined by using pH meter (Equiptronics, Model EQ-610). The pH meter was first calibrated using solutions of pH 4.5 and 7.
Gelation Temperature
Gelation temperature was measured by visual observation method and also by using Anton Paar modular compact rheometer MCR52.
Visual Observation Method
Two milliliter aliquot of gel was transferred to a test tube, immersed in a water bath. The temperature of water bath was increased slowly at a constant rate of 1°C for 2 min from room temperature to the temperature at which gel formed. The sample was then examined for gelation, which was said to have occurred when the meniscus would no longer move upon tilting the test tube through an angle of 90° (10).
Using Anton Paar Rheometer (MCR52)
The gelation temperature was determined by using Anton Paar Rheometer (model: Gmbh, 3ITT) and probe (PP25-SN 17002) using 1-ml aliquot of the sample (Fig. 1). Measurements were performed in oscillation mode using temperature sweep mode. Temperature was increased at constant rate from 10°C to 60°C. Storage modulus and loss modulus were plotted against temperature automatically using Rheoplus software to determine gelation temperature.
Fig. 1.
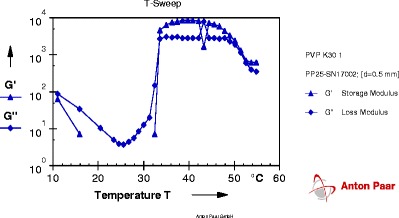
Graph of gelation temperature of formulation T5
Determination of Mucoadhesive Strength
Mucoadhesion testing was carried out using a texture analyzer (CT3, Brookfield, USA) with 50 N load cell equipped with mucoadhesive holder. Sheep nasal mucosa was utilized as the model membrane for mucoadhesive strength determination of various formulations. The tissue (about 20 × 20 mm) was equilibrated for 15 min at 37.0 ± 0.5°C before placing onto the holder stage of mucoadhesive holder. The probe was lowered at a rate of 0.5 mm/s until a contact with the membrane was made. A contact force of 1 N was maintained for 60 s, and the probe was subsequently withdrawn at a rate of 0.5 mm/s to a distance of 15 mm. By using the texture analyzer, the maximum force required to separate the probe from the tissue (i.e., maximum detachment force in grams; Fmax) could be detected directly from Texture Pro CT V1.3 Build 14 software (11–13).
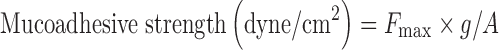 |
Where, Fmax is the maximum detachment force in grams, g is acceleration due to gravity (98°C m−1 s−2), and A is the area of tissue exposed
Drug Content
Drug content was determined by using micro pipette (20 μl). Twenty microliters of formulation was taken in 100 ml volumetric flask, added 50 ml of distilled water with gentle shaking, and final volume was adjusted to 100 ml. One-milliliter quantity from this solution was transferred into the 10-ml volumetric flask and final volume was made to 10 ml by using distilled water. Finally, the absorbance of prepared solution was measured at 226 nm by using UV–visible spectrophotometer to determine the drug content (14).
Viscosity
The viscosity of in situ gelling formulations was determined at 25°C with Anton Paar Rheometer (model: Gmbh, 3ITT) and probe (PP25-SN 17002) using 1-ml aliquot of the sample. Measurements on each value were performed in triplicate at a fixed shear rate of 50 s−1 using Rheoplus software.
Gel Strength
A sample of 50 g of the nasal gel was put in a 100-ml graduated cylinder and gelled in a thermostatically controlled water bath at 37°C. A weight of 35 g was placed onto the gel. The gel strength, which is an indication for the viscosity of the nasal gel at physiological temperature, was determined by the time in seconds required by the weight to penetrate 5 cm deep into the gel (15).
Ex vivo Permeation Studies
Fresh nasal mucosa was carefully removed from the nasal cavity of sheep obtained from the local slaughterhouse. The mucosa was stored in normal saline solution. After the removal of blood and bony cartilage from the mucosal membrane, it was ready for use (16). Franz diffusion cells were placed on six station magnetic stirring unit. Prior to study, nasal mucosa was soaked in diffusion medium (phosphate-buffered saline (PBS), pH 7.4) for 1 h and then placed on the receptor cell of the diffusion assembly. Formulation equivalent to 10.5 mg of VENH was placed in donor chamber by uniform application on membrane. Drug permeation study was carried out at 34°C for 3 h. Receptor compartment was filled with 7 ml of diffusion medium. Sampling of 0.5 ml was done at time interval of 10, 20, 30, 45, 60, 90, 120, 150, and 180 min; 0.5 ml of this sample was further diluted with distilled water to 100 ml. Absorbance of these diluted solutions was measured spectrophotometerically at 226 nm, and drug permeation was calculated by calibration curve method (17).
Permeability coefficient (P) was calculated from the slope of graph of percentage of drug transported V/S time and equations are shown as follows.
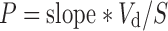 |
Where, Vd = volume of donor solution, S = surface area of tissue.
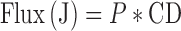 |
Where, CD is concentration of donor solution
Statistical Treatment
Values are expressed as mean ± SD. Statistical analysis of ex vivo permeation data were performed using paired t test or one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. A p value of <0.05 was considered significant (18).
Histopathological Evaluation of Mucosa
Histopathological evaluation of tissue incubated in PBS (pH 7.4) was compared with tissue incubated in the diffusion chamber with gel formulation. Tissue was fixed in 10% buffered formalin (pH 7.2), routinely processed and embedded in paraffin. Paraffin sections (7 μm) were cut on glass slides and stained with hematoxylin and eosin. Sections were examined under a light microscope to detect any damage to the tissue during in vitro permeation by a pathologist blinded to the study (19).
Pharmacodynamic Study in Rats
Rats were divided into three groups each containing four rats (n = 4). First group was treated with saline and was considered as a control. Second group was treated with VENH tablet in 4.2 mg powder equivalent to drug as an oral solution (15 mg/kg p.o.). Third group was treated with optimized thermoreversible in situ gel formulation of 20 μl containing 4.2 mg drug (15 mg/kg p.o.). Experimental animals and animal studies were approved by Institutional Animal Ethical Committee constituted for the purpose of control and supervision of experiments on animals.
Forced Swim Test
The posttreatment groups of rats were forced to swim in a vertical plastic cylinder (diameter, 21 cm; height, 50 cm) containing water of up to 25 cm maintained at 25 ± 1°C. On the 1st day of experiments, rat was forced to swim for 15 min. After 24 h, rat was re-exposed to forced swim for 6 min and onset of immobility and duration of immobility time was evaluated by two observers who were blind to the kind of treatment. A rat was judged to be immobile whenever it remained floating passively in the water in a slightly hunched but upright position with its head just above the surface. After a 5-min swim test, the animal was removed from the cylinder and returned to its home cage. The total immobility period were expressed in seconds (20).
Measurement of Locomotor Activity
Locomotor activity was measured in the open-field test. The apparatus consisted of a square arena (100 × 100 cm), with a 40-cm height. The floor was divided into 25 equal squares. The rats were individually placed in the center of the arena, and the following behavioral parameter was measured over 5 min: locomotion (number of squares crossed). A square crossed was defined as the rat placing its four paws into the quadrant and going to the adjacent quadrant. The open field was cleaned with a water–alcohol (10%) solution before behavioral testing to avoid possible bias due to odors and/or residues left by rats tested earlier. All experiments were carried out in a quiet room under controlled light conditions between 10:00 to 12:00 hours (21).
Statistical Analysis
Results were expressed as mean (in seconds) ± SEM, and the data were analyzed using one-way ANOVA where appropriate. If any statistically significant change was found, post-hoc comparisons were performed using a Dunnett’s test. Data were considered statistically significant when p < 0.05.
RESULTS AND DISCUSSION
Optimization of Concentration of LF127
Gelation temperatures for plain LF127 gels were observed for the concentration range of 16–20% (G1 to G5), and it was found that the gelation temperature of plain LF127 gels decreased with increasing concentration of LF127. When drug was added into gels, it was found that gelation temperature of formulations (G6 to G10) increased significantly for each concentration of gelling agent; however, the pattern was similar (Table I).
Table I.
Results of Optimization of Concentration of LF127
| Formulation batch | G1 | G2 | G3 | G4 | G5 | G6 | G7 | G8 | G9 | G10 |
|---|---|---|---|---|---|---|---|---|---|---|
| VENH (%, w/v) | – | – | – | – | – | 21.09 | 21.09 | 21.09 | 21.09 | 21.09 |
| Lutrol F127 (%, w/v) | 16 | 17 | 18 | 19 | 20 | 16 | 17 | 18 | 19 | 20 |
| Gelation temperature (°C) | 38.77 ± 0.37 | 34.17 ± 0.54 | 28.33 ± 0.33 | 23.33 ± 0.42 | 20.67 ± 0.84 | 41.83 ± 0.47 | 37.17 ± 0.30 | 32.33 ± 0.49 | 25.83 ± 0.47 | 23.67 ± 0.49 |
Values are expressed as mean ± SD; n = 6, p value < 0.0001. p < 0.05 considered statistically significant
From results, it was found that only 18% of LF127 gel with drug (G8) showed ability to form gel in the range of 29°C to 32°C. So, 18% (w/v) concentration of LF127 was used for further studies.
The decrease in the gelation temperature with increase in Lutrol F127 concentration may be due to the higher number and volume occupied by micelles at low temperature. As the concentration of Lutrol F127 increases, the gel structure becomes more closely packed with the arrangement in the lattice pattern and gelling occurs rapidly at low temperature. Incorporation of drug into in situ nasal gels increases the gelation temperature. This may be due to water soluble nature of VENH which may cause modification of the process of micellar association of Lutrol F127 gels thereby increasing their gelation temperature (22).
Optimization of Mucoadhesive Polymer with 18% (w/v) of LF127
Mucoadhesive polymer was optimized on the basis of mucoadhesive strength and effect of addition of mucoadhesive on gelation temperature. Formulation batches from T1 to T18 (Tables II and III) with different mucoadhesive polymers in different concentration were evaluated for mucoadhesive strength and gelation temperature.
Table II.
Formulation Table for Batches T1 to T9
| Composition (%, w/v) | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 |
|---|---|---|---|---|---|---|---|---|---|
| VENH | 21.09 | 21.09 | 21.09 | 21.09 | 21.09 | 21.09 | 21.09 | 21.09 | 21.09 |
| Lutrol F127 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 |
| Carbopol 934 | 0.1 | 0.2 | 0.3 | – | – | – | – | – | – |
| PVP K30 | – | – | – | 0.1 | 0.3 | 0.5 | – | – | – |
| HPMC K4M | – | – | – | – | – | – | 0.5 | 1 | 1.5 |
| Methyl paraben | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Propyl paraben | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Distilled water (ml) | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. |
Table III.
Formulation Table for Batches T10 to T18
| Composition (%, w/v) | T10 | T11 | T12 | T13 | T14 | T15 | T16 | T17 | T18 |
|---|---|---|---|---|---|---|---|---|---|
| VENH | 21.09 | 21.09 | 21.09 | 21.09 | 21.09 | 21.09 | 21.09 | 21.09 | 21.09 |
| Lutrol F127 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 |
| Sodium alginate | 0.1 | 0.2 | 0.3 | – | – | – | – | – | – |
| Tamarind seed gum | – | – | – | 0.1 | 0.3 | 0.5 | – | – | – |
| Carrageenan | – | – | – | – | – | – | 0.5 | 1 | 1.5 |
| Methyl paraben | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Propyl paraben | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Distilled water (ml) | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. |
As concentrations of mucoadhesive polymer increased, there was significant decrease in gelation temperature and increase in mucoadhesive strength of formulations (Table IV). From the results, it was found that 0.2% carbopol 934 (T2), 0.3% PVP K30 (T5), 0.3% tamarind seed gum (T14), and 0.3% carrageenan (T17) showed optimum results in terms of mucoadhesive strength and gelation temperature. Hence, these concentrations of mucoadhesive polymers were selected for further study.
Table IV.
Optimization of Mucoadhesive Polymer with 18% (w/v) of LF127
| Formulation batch No. | Gelation temperature (°C) | Mucoadhesive strength (dyn/cm2) |
|---|---|---|
| G8 | 32.33 ± 0.494 | 591.3 ± 4.421 |
| T1 | 29 ± 0.258 | 865 ± 5.364 |
| T2 | 28.17 ± 0.307 | 1,160 ± 5.207 |
| T3 | 28.27 ± 0.315 | 1,806 ± 4.410 |
| T4 | 31.83 ± 0.30 | 666 ± 5.487 |
| T5 | 31.17 ± 0.307 | 981 ± 4.333 |
| T6 | 29.83 ± 0.60 | 1,505 ± 3.480 |
| T7 | 30.17 ± 0.307 | 882 ± 5.207 |
| T8 | 28 ± 0.258 | 1,704 ± 9.82 |
| T9 | 27.33 ± 0.421 | 2,505 ± 8.66 |
| T10 | 31.17 ± 0.307 | 620 ± 5.812 |
| T11 | 29.83 ± 0.307 | 768 ± 6.692 |
| T12 | 29 ± 0.3651 | 826 ± 5.812 |
| T13 | 32.33 ± 0.210 | 773 ± 3.93 |
| T14 | 31.33 ± 0.421 | 904 ± 6.96 |
| T15 | 33.17 ± 1.70 | 1,483 ± 7.055 |
| T16 | 30.83 ± 0.307 | 667 ± 6.429 |
| T17 | 30.17 ± 0.47 | 1,017 ± 5.548 |
| T18 | 29.50 ± 0.42 | 1,273 ± 6.119 |
Values are expressed as mean ± SD; n = 6 for gelation temperature and n = 3 for mucoadhesive strength, p < 0.05 considered statistically significant, optimized batches are shown in bold
Lowering effect on gelation temperature of mucoadhesive polymer could be explained by their ability to bind to PEO chains present in the Lutrol F127 molecules promoting dehydration and causing an increase in entanglement of adjacent molecules with more extensive intermolecular hydrogen bonding (23).
Mucoadhesive strength increases with increasing concentration of mucoadhesive polymer. The mechanism of mucoadhesion may be related to hydrogen bonding between gel formulation and mucosal membrane (24). Mucoadhesive strength depends on mechanism of mucoadhesion and strength of bonding of polymer with membrane which varies polymer to polymer; therefore, difference in mucoadhesive strength of different polymers was observed.
Evaluation of Formulations
Clarity (pH)
All the prepared sets of formulations were found to be clear. pH of all the formulations was found to be in the range of 4.5–6.5 which is considered as nasal physiological pH range (Table V).
Table V.
Evaluation of Gels
| Formulation batch no. | Clarity | pH | Gelation temperature (°C) | Mucoadhesive strength (dyn/cm2) | Gel strength | Drug content (%) |
|---|---|---|---|---|---|---|
| T2 | ++ | 4.8 | 31°C | 1,160 ± 5.207 | 44 | 98.24 ± 0.072 |
| T5 | +++ | 5.2 | 33°C | 981 ± 4.333 | 48 | 97.65 ± 0.070 |
| T14 | ++ | 5.5 | 30°C | 904 ± 6.96 | 45 | 97.79 ± 0.043 |
| T17 | +++ | 5.7 | 30°C | 1,017 ± 5.548 | 46 | 98.01 ± 0.061 |
Values are expressed as mean ± SD; n = 3, p value < 0.0045. p < 0.05 considered statistically significant
Lysozyme is formed in the nasal secretions, which is responsible for destroying certain microbes at acidic pH. Under alkaline pH, lysozyme is inactive and nasal tissue is susceptible to microbial infection. It is therefore advisable to keep the pH of formulation in the range of 4.5 to 6.5.
Gelation Temperature
The gelation temperature of in situ gelling formulations T2, T5, T14, and T17 was determined by using Anton Paar Rheometer (model: Gmbh, 3ITT) and probe (PP25-SN 17002) using 1 ml of the sample. The point at which storage modulus crosses over the loss modulus is considered as a gelation point because it is indicative of phase transition from solution to gel. Graphs are given in Figs. 1, 2, 3, and 4; result of gelation temperature is given in Table V.
Fig. 2.
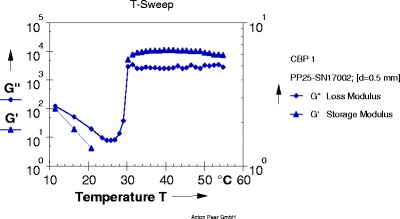
Graph of gelation temperature of formulation T2
Fig. 3.
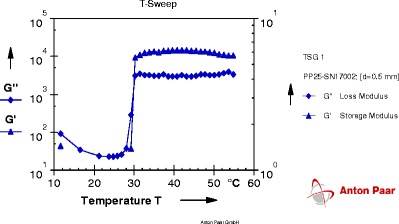
Graph of gelation temperature of formulation T14
Fig. 4.
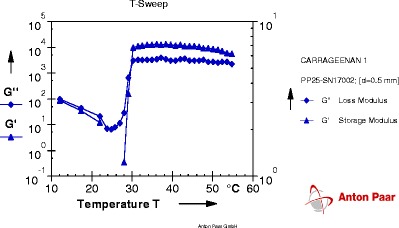
Graph of gelation temperature of formulation T17
Each graph obtained from Rheoplus software has two lines of two different parameters. One is of storage modulus G' and other is of loss modulus G". At particular temperature, when the line of loss modulus has higher value than storage modulus; the system is said to be in solution state. As the temperature goes on increasing, values of both parameters change. The temperature at which storage modulus attains higher value than loss modulus is called as a phase transition temperature because at this temperature solution system gets converted into gel system. So the crossover point of two lines is the gelation temperature.
From the results obtained from gelation study it was seen that all four formulation batches showed gel formation at nasal physiological temperature, i.e., 29°C to 34°C.
Mucoadhesive Strength
As concentrations of mucoadhesive polymer increased, there was significant decrease in gelation temperature and increase in mucoadhesive strength of formulations. From the results (Table IV), it was found that 0.2% carbopol 934 (T2), 0.3% PVP K30 (T5), 0.3% tamarind seed gum (T14), and 0.3% carrageenan (T17) showed mucoadhesive strength in the range of 900 to 1,200 dyn/cm2.
Gel Strength
All formulations had gel strength in the range of 25–50 s (Table V). From the results, it was found that all formulations showed suitable gel strength, did not have postnasal drip and were easily administered as drops.
The gel strength values between 25 and 50 s are considered sufficient, as gel strength of less than 25 s may not preserve its integrity and may erode rapidly while gels with strength greater than 50 s are too stiff and may cause discomfort.
Drug Content
Formulations should have drug content within the limit of 97.5–102.5% for nasal drops. Drug content was determined by using micropipette (20 μl). All the formulations were found to have drug content in between 97.65% and 98.24% (w/w) (Table V).
Viscosity Studies
The plot of viscosity vs. temperature for in situ gelling formulations T2, T5, T14, and T17 is shown in Fig. 5. There was no considerable change in viscosity up to the point of gelation temperature. Sharp rise in viscosity was observed at the point of Tsol–gel transition.
Fig. 5.
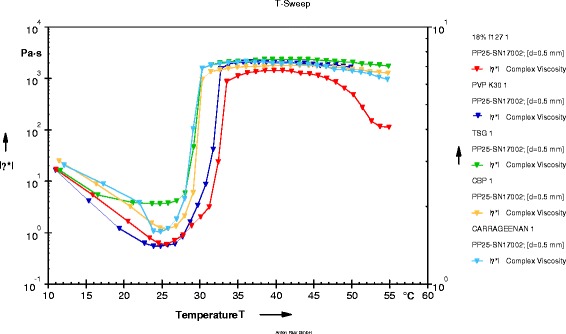
Plot of viscosity vs. temperature
From the plot, it was clear that viscosity of each formulation decreased initially with increase in temperature. At about 25°C, all formulations were having lowest viscosity and were in solution form. Sharp rise in viscosity was observed at gelation temperature and maintained a constant value of up to 50°C.
From viscosity studies, it was concluded that all formulations were in liquid state at room temperature and were converted into gel at nasal physiological temperature.
Ex vivo Permeation Studies
The permeation study of T2, T5, T14, and T17 was carried out by using Franz diffusion cell apparatus in which sheep’s nasal mucosa was used as a diffusion membrane and PBS at pH 7.4 was used as a diffusion medium. Drug release profile was obtained by plotting percent drug release against time (Fig. 6), and results of permeation study are given in Table VI.
Fig. 6.
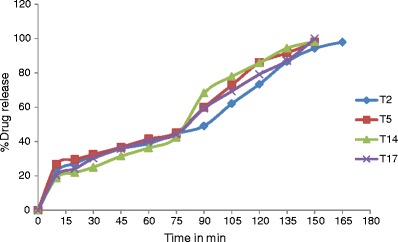
Compiled release profile of T2, T5, T14, and T17 formulation
Table VI.
Result of Permeation Study
| Formulation batch No. | Time (min) | % drug release | Permeation coefficient (mg cm−2 min−1) | Flux (mg cm−2 min−1) |
|---|---|---|---|---|
| T2 | 165 | 97.85 ± 0.052 | 0.01415 | 0.1415 |
| T5 | 150 | 97.86 ± 0.073 | 0.01545 | 0.1545 |
| T14 | 150 | 98.09 ± 0.1202 | 0.01721 | 0.1721 |
| T17 | 150 | 99.15 ± 0.3623 | 0.01561 | 0.1561 |
Values are expressed as mean ± SD; n = 3, p value < 0.0045. p < 0.05 considered statistically significant
From the drug release profiles (Fig. 6), it was concluded that the initial release rate from each formulation was very rapid, this may be due to incomplete gel formation in the earlier time period, but the release became slow in latter period after complete gel formation. The results showed that the formed gels had the ability to retain VENH for the duration of 150 min. In vitro release study indicated that the release of drug from each formulation was almost same. All four formulations exhibited almost the same release profile for 150 min (97.85% to 99.15%). T17 formulation showed comparatively higher release of 99.15% than others.
The release profiles exhibited an inflection point, which indicated gel formation on the diffusion membrane in donor compartment of diffusion cell. During gel formation, formulation got converted into the gel phase and thus drug release became slow. All formulations showed almost the same release profile though the mucoadhesive polymers are different because the main release-controlling polymer was LF127, and the concentration of which was constant in all four formulations.
Histopathological Evaluation of Mucosa
The compiled result of all four formulations related to histopathological evaluation of mucosa is given in Table VII, and findings of this study are given in Figs. 7 and 8. Histopathological study revealed that formulation T2 and T5 were not causing necrosis to nasal mucosa. In case of T2 formulation, detachment of epithelial cells was observed but formulation was not damaging the columnar structure of epithelial cell as well as it was not causing necrosis. Formulation T14 and T17 were found to be causing necrosis. While formulation T5 was found to be the safest of all and can be considered as an optimized formulation as it was harmless to nasal mucosa and so was selected as optimized batch.
Table VII.
The Compiled Result of Histopathological Evaluation of Mucosa
| Formulation batch No. | Detachment of epithelial cells | Loss of columnar structure of epithelial cells | Detachment of glandular cells from basement membrane | Loss of nucleus of glandular cells |
|---|---|---|---|---|
| T2 | √ | × | × | × |
| T5 | × | × | × | × |
| T14 | √ | √ | √ | √ |
| T17 | √ | × | √ | √ |
Fig. 7.
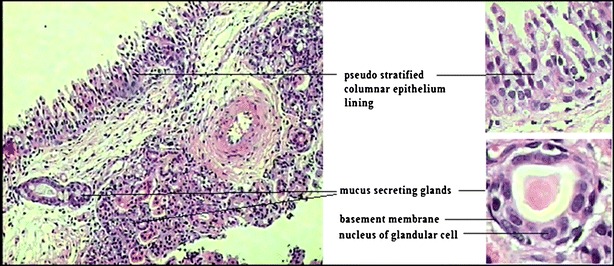
Microscopy of normal nasal mucosa
Fig. 8.
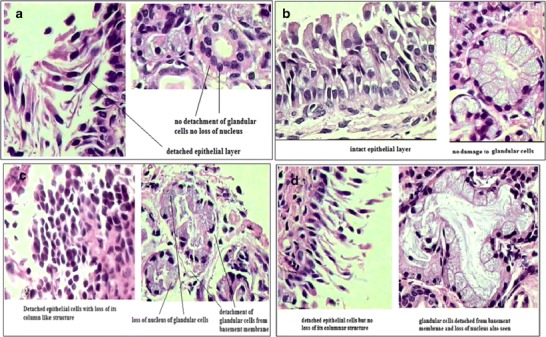
Histopathological evaluation of mucosa on exposure to formulation T2, T5, T14, and T17. a Histopathological evaluation of mucosa on exposure to formulation T2. b Histopathological evaluation of mucosa on exposure to formulation T5. c Histopathological evaluation of mucosa on exposure to formulation T14. d Histopathological evaluation of mucosa on exposure to formulation T17
Detachment of nasal epithelial layer was observed which may be due to physical injury to epithelial cells and basement membrane. Physical injury may have occurred because of sudden pH shock. Initially, nasal membrane was exposed to PBS at pH 7.4 as being a diffusion medium for 30 min to stabilize the membrane. So membrane got stabilized in pH 7.4 buffer. pH of the formulation was adjusted to 4.5. Application of formulation to stabilized membrane may have given pH shock to the highly delicate epithelial layer. This sudden change in pH may have dislocated the epithelial cell layer and also damaged the cell structure. Formulation contained mucoadhesive polymers which adhere to mucosal layer by physical entanglement. Polymers like carbopol has a tendency to swell. At the time of gel formation, these polymers adhere to mucosal layer and due to swelling of these polymers; damage to epithelial cell layer may have taken place. Damage to superficial epithelial layer as well as basement membrane allowed the excipients to penetrate to glandular cells where they caused necrosis, may be by damaging cell wall of glandular cells due to pH shock and swelling.
Pharmacodynamic Study in Rats
Formulated nasal in situ gel system T5 was evaluated for pharmacodynamic activity in rats as a nasal drop. Pharmacodynamic activity of T5 formulation was compared with orally administered dose. Pharmacodynamic activity study involved two tests. One was FST and second was locomotor activity. Immobility period of normal, orally treated and formulation-treated group was compared. Total immobility period would decrease if high concentration of venlafaxine reaches target site. So by this study, the comparison of bioavailability by different routes can be done. Locomotor activity study is a supportive test for FST. It conforms that results obtained by FST are due to antidepressant activity of drug and not due to the hyper activity of rats.
Forced Swim Test
Venlafaxine, in a dose range of 15 mg/kg produced a decrease in immobility period (in seconds) with respect to control group, while optimized thermoreversible formulation given intra nasally as nasal drops further decreased the immobility period (Table VIII).
Table VIII.
Results of Forced Swim Test
| Treatment group | Total immobility period in seconds | Mean | |||
|---|---|---|---|---|---|
| Normal control | 154 | 152 | 149 | 139 | 148.5 ± 3.32 |
| Venlafaxine oral | 97 | 102 | 107 | 113 | 104.8 ± 3.42 |
| Thermoreversible in situ gel | 92 | 93 | 89 | 84 | 89.5 ± 2.02 |
p value < 0.0001
Results of FST revealed that there was significant reduction in total immobility period in seconds by treating the animals with antidepressant drug VENH. There was significant (p = 0.0001) difference in total immobility period of normal control compared with venlafaxine-treated group (43.75 ± 4.776 s) (Table IX) Thermoreversible in situ gel reduced total immobility period by 15.25 ± 3.976 s than oral treatment (p = 0.0086).
Table IX.
Data of Comparative Study on Forced Swim Test
| Compared groups | p value | Difference between means | Are the means significantly different? |
|---|---|---|---|
| Normal control vs. venlafaxine oral | 0.0001 | 43.75 ± 4.776 | Yes |
| Venlafaxine oral vs. thermoreversible in situ gel | 0.0086 | 15.25 ± 3.976 | Yes |
Locomotor Activity
Locomotion (number of squares crossed) was defined as the rat placing its four paws into the quadrant and going to the adjacent quadrant (Table X). The number of squares crossed by the animals of normal control group (84.25 ± 1.75), orally treated group (88.75 ± 0.85), and thermoreversible nasal in situ gel-treated group (91.75 ± 1.652) were found to be statistically similar. No significant difference was observed in number of squares crossed by animal, when all four groups were compared (p = >0.05) (Table XI). From this, it can be concluded that the animals were not hyperactive; the results of FST were due to antidepressant activity of VENH and not due to the hyperactivity of the animals.
Table X.
Results of Locomotor Activity Test
| Treatment group | Number of squares crossed | Mean | |||
|---|---|---|---|---|---|
| Normal control | 80 | 83 | 86 | 88 | 84.25 ± 1.75 |
| Venlafaxine oral | 87 | 88 | 89 | 91 | 88.75 ± 0.85 |
| Thermoreversible in situ gel | 87 | 94 | 92 | 94 | 91.75 ± 1.65 |
p value < 0.0341
Table XI.
Data of Comparative Study on Locomotor Test
| Compared groups | p value | Difference between means | Are the means significantly different? |
|---|---|---|---|
| Normal control vs. venlafaxine oral | 0.602 | −4.500 ± 1.947 | No |
| Venlafaxine oral vs. thermoreversible in situ gel | 0.1578 | −3.000 ± 1.860 | No |
From the results of FST and locomotor activity, it can be concluded that VENH was more effective as an antidepressant by nasal route as in situ gel nasal drops in comparison to oral administration of equivalent dose. Drug was reaching to target site in the brain in more amount by nasal route compared with oral route therefore total immobility period was reduced.
CONCLUSIONS
For the formulation of thermoreversible gel, Lutrol F127 18% was used as thermoreversible gel-forming polymer as it formed gel at nasal physiological temperature. Carbopol 937, PVP K30, HPMC K4M, sodium alginate, tamarind seed gum, and carrageenan were tried as mucoadhesive polymers in different concentrations. These formulations were tested for mucoadhesive strength by using sheep nasal mucosa. Depending upon mucoadhesive strength, formulation T2, T5, T14, and T17 were selected for further evaluation of gel strength, viscosity, drug content, and diffusion through sheep nasal mucosa. All four selected batches were found to be having almost same release profile, for 150 min, i.e., 97.85 ± 0.05 to 99.15 ± 0.36% of drug release was achieved. Formulation T14 and T17 were found to be causing necrosis while T2 was found to be causing detachment of epithelial cell layer in histopathological evaluation. T5 was found to be safe for nasal administration. Formulation T5 was selected as the final optimized formulation and used for pharmacodynamic study in rats where FST and locomotor activity tests were performed. From the pharmacodynamic study mainly FST, it was concluded that VENH was more effective as an antidepressant by nasal route than oral route when administered as in situ gel nasal drops. Thus, in the present study nasal drug delivery of VENH was successfully formulated in the form of thermoreversible nasal in situ gel which can be a good option for administration of the drug as well as it can also be thought of a drug delivery route for such CNS drugs for brain targeting.
ACKNOWLEDGMENTS
The authors are thankful to Lupin Research Park, Pune, for providing VENH as a gift sample.
REFERENCES
- 1.Feighner JP. The role of venlafaxine in rational antidepressant therapy. J Clin Psychiatry. 1994;55:98–100. [PubMed] [Google Scholar]
- 2.Hussein A. Intranasal drug delivery. Adv Drug Deliv Rev. 1998;29:39–49. doi: 10.1016/S0169-409X(97)00060-4. [DOI] [PubMed] [Google Scholar]
- 3.Ugwoke MI, Verbeke N, Kinget R, Agu RU, Vanbilloen H, Baetens J. Scintigraphic evaluation in rabbits of nasal drug delivery system based on carbopol 971 P and carboxymethylcellulose. J Control Release. 2000;68:207–214. doi: 10.1016/S0168-3659(00)00258-3. [DOI] [PubMed] [Google Scholar]
- 4.Behl CR, Pimplaskar HK, Sileno AP, DeMeireles J, Romeo VD. Effects of physicochemical properties and other factors on systemic nasal drug delivery. Adv Drug Deliv Rev. 1998;29:89–116. doi: 10.1016/S0169-409X(97)00063-X. [DOI] [PubMed] [Google Scholar]
- 5.Shinde JS. In situ mucoadhesive nasal gels of metoclopramide hydrochloride: preformulation and formulation studies. J Pharm Res. 2008;1(1):88–96. [Google Scholar]
- 6.Edsman K, Carlfors J, Petersson R. Rheological evaluation of poloxamer as an in situ gel for ophthalmic use. Eur J Pharm Sci. 1998;6:105–112. doi: 10.1016/S0928-0987(97)00075-4. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Parson DL, Navarre C, Kompella UB. Development and in-vitro evaluation of sustained release poloxamer 407 (P407) gel formulations of ceftiofur. J Control Release. 2002;85:73–81. doi: 10.1016/S0168-3659(02)00273-0. [DOI] [PubMed] [Google Scholar]
- 8.Johnston TP, Punjabi MA, Froelich CJ. Sustained delivery of interleukin-2 from a poloxamer gel matrix following intraperitonial injection in mice. Pharm Res. 1992;9:425–434. doi: 10.1023/A:1015815624334. [DOI] [PubMed] [Google Scholar]
- 9.Schmolka IR. Artificial skin I: preparation and properties of pluronic-127 gels for treatment of burns. J Biomed Mater Res. 1972;6:571–582. doi: 10.1002/jbm.820060609. [DOI] [PubMed] [Google Scholar]
- 10.Choi HG, Oh YK, Kim CK. In situ gelling and mucoadhesive liquid suppository containing acetaminophen: enhanced bioavailability. Int J Pharm. 1998;165:23–32. doi: 10.1016/S0378-5173(97)00385-2. [DOI] [Google Scholar]
- 11.Yong CS, Choi JS, Quan QZ, Rhee JD, Kim CK, Lim SJ. Effect of Na chloride on the gelation temperature, gel strength and bioadhesive force of poloxamer gels containing diclofenac Na. Int J Pharm. 2001;226:195–205. doi: 10.1016/S0378-5173(01)00809-2. [DOI] [PubMed] [Google Scholar]
- 12.Jones DS, Woolfson AD, Brown AF, Coulter WA, McClelland C, Irwin CR. Design characterization and preliminary clinical evaluation of a novel mucoadhesive topical formulation containing tetracycline for the treatment of periodontal disease. J Control Release. 2000;67:357–368. doi: 10.1016/S0168-3659(00)00231-5. [DOI] [PubMed] [Google Scholar]
- 13.Chng HS, Park H, Kelly P, Robinson JR. Bioadhesive polymers as platforms for oral controlled drug delivery II. Synthesis and evaluation of some swelling water-insoluble bioadhesive polymers. J Pharm Sci. 1985;74:339–405. doi: 10.1002/jps.2600740407. [DOI] [PubMed] [Google Scholar]
- 14.Keny RV, Lourenco CF. Formulation and evaluation of thermoreversible in situ gelling and mucoadhesive diltiazem hydrochloride liquid suppository. Int J Pharma Biol Sci. 2010;1(1):1–17. [Google Scholar]
- 15.Choi HG, Kim CK, Jung JH, Ryu JM, Yoon SJ. Development of in situ-gelling and mucoadhesive acetaminophen liquid suppository. Int J Pharm. 1998;165:33–44. doi: 10.1016/S0378-5173(97)00386-4. [DOI] [Google Scholar]
- 16.Pisal S, Shelke V, Mahadik K, Kadam S. Effect of organogel components on in vitro nasal delivery of propranolol hydrochloride. AAPS PharmSciTech. 2004;5:1–9. doi: 10.1208/pt050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng Y, Watts P, Hinchcliffe M, Hotchkiss R, Nankervis NF, Faraj A. Development of a novel nasal nicotine formulation comprising an optimal pulsatile and sustained plasma nicotine profile for smoking cessation. J Control Release. 2002;79:243–249. doi: 10.1016/S0168-3659(01)00553-3. [DOI] [PubMed] [Google Scholar]
- 18.Tas C, Ozkan CK, Savaser A, Ozkan Y, Tasdemir U, Altunay H. Nasal absorption of metoclopramide from different carbopol 981 based formulations: in vitro, ex vivo, and in vivo evaluation. Eur J Pharm Biopharm. 2006;64:246–254. doi: 10.1016/j.ejpb.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 19.Majithiya RJ, Ghosh PK. Thermoreversible-mucoadhesive gel for nasal delivery of sumatriptan. AAPS PharmSciTech. 2006;7(3):1–7. doi: 10.1208/pt070367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhir A, Kulkarni SK. Involvement of l arginine nitric oxide cyclic guanosine monophosphate pathway in the antidepressant-like effect of venlafaxine in mice. Prog Neuro-Psychopharmacol Biol Psychiatry. 2007;31:921–925. doi: 10.1016/j.pnpbp.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Mygind N, Dahl R. Anatomy, physiology and function of the nasal cavity in health and disease. Adv Drug Del Rev. 1998;29:3–12. doi: 10.1016/S0169-409X(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 22.Chien YW, Su KSE, Chang SF. Nasal systemic drug delivery. New York: Marcel Dekker; 1999. pp. 39–78. [Google Scholar]
- 23.Abd Elhady SS, Mortada N, Awad GAS, Zaki NM, Taha RA. Development of in situ gelling and mucoadhesive mebevarine hydrochloride solution for rectal administration. Saudi Pharm J. 2003;11:159–171. [Google Scholar]
- 24.Dumortier G, Grosslord JL, Agnely F, Chaumeil JC. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res. 2006;23:2709–2727. doi: 10.1007/s11095-006-9104-4. [DOI] [PubMed] [Google Scholar]


