Abstract
The purpose of the research was to prepare and evaluate a topical nanolipidgel (NLH) of terbinafine hydrochloride (TRB), an antimycotic agent, for enhanced skin deposition and improved antifungal activity. Topical solid lipid nanoparticles (SLN) based nanolipidgel was formulated and evaluated. TRB-loaded SLNs were formulated by high-pressure homogenization technique. The stable TRB SLN dispersion was incorporated into a gel using 1% Carbopol 980 NF. Rheological evaluation and texture analysis of the TRB NLH was carried out. Skin permeation, skin deposition, antifungal activity, and occlusivity studies of the nanolipidgel formulation were carried out. The safety of the TRB NLH gel was evaluated using acute skin irritation test on New Zealand White rabbits. The SLN dispersion containing 10% of glyceryl monostearate, 3% of Tween 80, and 1% Plurol Oleique was the most stable. The optimized TRB SLN had a particle size and zeta potential value of 148.6 ± 0.305 nm and −20.4 ± 1.2 mV, respectively. TRB NLH had excellent rheological and texture properties to facilitate its topical application. TRB NLH showed increased skin deposition of the drug over plain (3-fold) and marketed TRB formulation (2-fold). TRB NLH had significantly enhanced antifungal activity against Candida albicans. TRB NLH showed efficient occlusivity and was non-irritant to the rabbit skin with no signs of erythema or edema. Solid lipid nanoparticles-based topical nanolipidgel of terbinafine can be an efficient, industrially scalable, and cost-effective alternative to the existing conventional formulations.
KEY WORDS: in vitro antifungal activity, rheological analysis of gel, solid lipid nanoparticles, terbinafine, texture analysis of gel
INTRODUCTION
Lipid-based colloidal drug delivery systems are well-accepted, proven, and industrially scalable platform technologies to fabricate and deliver pharmaceuticals for topical, oral, pulmonary, or parenteral delivery. Lipid-based nanoparticles are explored widely in the form of liposomes, micelles, microemulsions, nanoemulsions, self-microemulsifying/nanoemulsifying drug delivery systems (SMEDDS and SNEDDS), niosomes, solid lipid nanoparticles (SLN), and nanostructured lipid carriers (NLC). The pharmaceutical dosage forms developed using templates of lipid nanoparticles can be tailored to meet a wide range of product prerequisites dictated by disease indication, route of administration, cost, product stability, toxicity, and efficacy (1).
Nanolipidgel is a term assigned to a system comprised of solid lipid nanoparticles incorporated into a gel base. Dispersion containing solid lipid nanoparticles is formulated into a semisolid dosage form, a gel using a gelling agent. The SLN structure is composed of a solid lipid core of triglycerides, glyceride mixtures, or waxes that are solid at room temperature and human body temperature. They have particle size in the range of 50–1,000 nm (2).
Terbinafine hydrochloride belongs to a synthetic allylamine antifungal class which is prescribed for superficial fungal infections affecting the skin and nails. It is available as granules, tablets, topical cream, topical gel, topical solution, and spray formulations. It is slightly soluble in water. The stratum corneum is the primary site of action for TRB in fungal infections residing superficially into the skin layers (3–5).
For antifungal activity, the drug should be present in a concentration above its minimum inhibitory concentration (MIC) at the site of action during the period of time required for effective treatment. This can be achieved by administering TRB for a long period of time ranging from 2 to 6 weeks for skin infection and 6–12 weeks for nail infection. The long-term administration of the drug does not cause a proportional rise in the concentration of the active agent in the skin (5). Oral administration of TRB is associated with drug interactions and adverse effects like gastrointestinal effects, lactose intolerance, and hepatotoxicity. The topical delivery can be an efficient alternative to avoid systemic side effects of the drug by targeting it to the skin layers. However, poor bioavailability of the drug limits its topical delivery (6,7).
A wide range of nanotechnology-based topical formulations is available to target drug to the skin. Among them, nanolipidgel represents an attractive choice because of the numerous advantages associated with this carrier system when delivered topically (7–9). This system is composed of lipid nanoparticles present into a matrix of gel. Solid lipid nanoparticles as a template offer unique advantages. The matrix of the nanoparticles is composed of lipids and other excipients which are of GRAS status, enabling its application even on inflamed or damaged skin surfaces (10). Entrapment of actives into the lipid matrix inhibits its degradation and improves its stability (11). SLNs provide controlled release of the drugs and thus minimize the concentration required for effective therapy (12–14). They have potential to target actives to the skin layers, inhibiting its systemic uptake and associated side effects (15–17). Being a lipid-based system having colloidal particles with an extensive surface area, SLNs provide better occlusivity over the conventional oil-based semisolid preparations which help to improve the hydration status of skin. The formulation process can be performed at a lower cost and are easily scaled up (17,18).
Thus, it seemed worthy of interest to prepare a SLN-based gel, a nanolipidgel of terbinafine, for topical application for enhanced skin deposition.
MATERIALS AND METHODS
Materials
Terbinafine hydrochloride was obtained from FDC Ltd., India. Glyceryl behenate, cetyl palmitate, glyceryl distearate, stearic acid, and Plurol Oleique were obtained from Gattefosse India Pvt. Ltd. Glyceryl trimyristate, glyceryl tripalmitate, glyceryl tristearate, and glyceryl monostearate were obtained from Mohini Organics Pvt. Ltd., India. Poloxamer 188 and Cremophor RH 40 were obtained from BASF India Ltd. Sodium lauryl sulfate and Tween 80 were obtained from Merck India Pvt. Ltd. Trehalose was obtained Hayashibara Co. Ltd., Japan. Carbopol 980 NF was obtained from Lubrizol India Pvt. Ltd. Potato dextrose agar was purchased from Himedia Laboratories Pvt. Ltd., India. Culture of Candida albicans was obtained from United States Department of Agriculture, USA.
Methods
Preparation of TRB SLN
Screening of lipids
Solubility of the drug in a lipid is a key factor to achieve high entrapment of the drug into the lipid matrix. Therefore, solubility of drug in various lipids was determined in order to determine the lipid having maximum potential to solubilize the drug. Briefly, weighed amount of TRB (10 mg) was added to a glass vial. Lipid was added to the vial in gradually increasing amount. The above mixture was heated to a temperature above 5–10°C of the lipid’s melting point. A transparent solution of the drug into the melted lipid indicates solubilization of the drug into the lipid melt. This serves as an end point. The amount of each lipid added was calculated.
Screening of surfactants
TRB-loaded SLNs were produced using melt emulsification method by high-pressure homogenization technique. Different concentration of several surfactants (stabilizers) was screened for formulating TRB SLN dispersion. Briefly, glyceryl monostearate (GMS) (10% w/w) was heated 5–10°C above its melting point. TRB (1% w/w) was added to the melted lipid. Aqueous phase containing surfactant was prepared separately in double distilled water. The aqueous phase was heated to the same temperature as that of oil phase. The oil phase was then emulsified using an aqueous phase under high stirring (Remi, India). The coarse emulsion so formed was processed using a high-pressure homogenizer (Gea Nero Soavi, Italy) at a constant temperature (75°C). The nanoemulsion thus formed was then cooled to room temperature to solidify the lipid matrix and fabricate solid lipid nanoparticles.
Several surfactants like sodium lauryl sulfate, Cremophor RH 40, Lutrol F 68, and Tween 80 were screened for preparation of TRB SLN. SLN dispersion was prepared using different concentrations of these surfactants (1% to 4% w/w). The effect of these surfactants on stability of the SLNs was determined by the particle size, polydispersibility index (PI), and colloidal stability.
Screening of process parameters
Homogenization pressure and number of cycles have a direct influence on the particle size of the SLNs. The effect of two essential process variables during homogenization being homogenization pressure and number of cycles was investigated. The coarse emulsion (2.2.1.2) was processed at different homogenization pressures of 500, 800, and 1,000 bar and the samples were collected at 5, 10, and 15 cycles. The nanoemulsion so formed was cooled to room temperature to convert into SLN. The particle size of the SLN dispersion was analyzed.
Characterization of TRB SLN Dispersion
Particle size
Average particle size of the SLN dispersion was determined by photon correlation spectroscopy. The nanoparticulate dispersion was diluted with double distilled water before measurement. Particle size was analyzed at 20 ± 2°C and an angle of 90° to the incident beam. The data obtained was analyzed by Contin program on N4 Plus Submicron Particle Size Analyzer (Beckman Coulter, USA).
Entrapment efficiency
The drug entrapped into the lipid nanoparticles was determined. TRB-loaded SLNs were separated by ultracentrifugation at 50,000 rpm (Thermoscience, USA). The supernatant was separated carefully. The drug content of the supernatant was analyzed by UV spectroscopy at wavelength maxima of 283 nm. Entrapment efficiency (% EE) was calculated by the following formula:
 |
Zeta potential
The zeta potential of nanoparticles was measured by Photon Correlation Spectroscopy (PCS) using Zetasizer 3000 HSA (Malvern Instruments, Malvern, UK). Zeta potential of the SLN dispersion was measured in folded capillary cells. Measurements were performed in double distilled water adjusted with a solution of 0.1 mmol/l sodium chloride to a conductivity of 50 μS/cm at a temperature of 25°C.
Differential scanning calorimetry (DSC)
SC thermograms of lipid bulk (GMS), TRB, and TRB SLNs were recorded. TRB SLNs were freeze dried using trehalose as a cryoprotectant to obtain powder. Samples were sealed in aluminum cells and placed in a Perkin-Elmer DSC6 apparatus (Uberlingen, Germany). The empty aluminum cell served as a reference. Thermal analysis was performed at controlled heating rate of 10°C per minute in a nitrogen atmosphere.
X-ray diffractometry
XRD studies of the lipid bulk (GMS), TRB, and freeze-dried SLN were performed using an automatic X-ray diffractometer (Rigaku Dmax 2500; Rigaku, Tokyo, Japan) equipped with an X-ray generator. Nickel-filtered Cu kα1 radiation having a wavelength of 1.5106 Å, operating at 35 kW and 20 mA in the range (2θ) of 5° to 50°, was used. X-ray diffractograms were obtained at a scanning rate of 1° (2θ) per minute.
Preparation and Evaluation of TRB Gel
The optimized SLN dispersion was incorporated into the topical gel using Carbopol 980 NF. Weighed quantity of Carbopol 980 NF was dispersed in purified water (1% w/w). It was stirred for 4–5 h. TRB-SLN dispersion was centrifuged to collect the lipid nanoparticles in the form of pellets. The nanoparticles corresponding to the final concentration of TRB as 1% w/w were added to the Carbopol dispersion and stirred for an hour. Triethylamine was added to adjust the pH at 6 (19–22).
TRB nanolipidgel was assayed using HPLC. A weighed amount of gel was dissolved in methanol. The drug was extracted by sonicating for 30 min and analyzed using HPLC method. The HPLC unit consisted of a Jasco Intelligent pump (Japan) with a Lichrosphere® RP C18 (250 × 4.6 mm 5 μm) column. The mobile phase was composed of methanol: phosphate buffer (0.025 M):: 90: 10 pH 5.5 mixture with a flow rate 1.0 ml/min. Detector used was Jasco MD 2015 plus (photodiode array detector) and the eluate was monitored at 283 nm.
Rheological evaluation of TRB NLH
Non-Newtonian viscosity
The rheological behavior of plain Carbopol gel and TRB NLH was evaluated using an Anton Paar MCR 101 rheometer (Anton Paar GmbH, Graz, Austria) having a cone and plate measuring system. The sample was placed between the gap of the cone and plate, and the gap was closed gradually. The sample was subjected to dynamic shear strain from 0.1 to 100 S−1 and the shear stress and the viscosity expended by the sample by imposed shear strain was measured. All the measurements were performed at isothermal condition of 37°C (23–25).
Oscillatory rheometry
Oscillatory measurements were performed by using cone and plate measuring system (Anton Paar). A constant shear rate was maintained throughout the sample. A parallel plate measuring system was used for the testing. The material was exposed to sinusoidal varying strain and the transmitted stress was measured as a function of time. Depending upon the viscosity of the material, the amplitude of the stress wave may be smaller or higher than the amplitude of the strain wave. Therefore, the stress wave and the strain wave will be shifted out of phase. This phase angle shift which is the time shift between the strain and stress amplitudes (∆t) was measured as the damping factor which is tangent of the phase angle shift. Various parameters like loss modulus (G″), storage modulus (G′), and complex viscosity (ŋ*) were measured in order to measure the viscous and elastic behavior of the gels (26,27).
Texture Analysis of the TRB NLH
Spreadability
The efficacy of the topical semisolid formulation depends on the even spreading of the formulation on to the skin. Thus spreadability is a critical parameter affecting the delivery of the drug to the targeted site (23). The spreadability of the blank gel and the nanolipidgel was determined by Texture Analyzer (Brookfield Engineering Laboratories, USA). The specifications prescribed by Brookfield Engineering Laboratories, USA were used for the spreadability determination. The spreadability assembly was fixed. The female cone was fixed on the base holder. The sample was added to the female cone. The male cone was attached to the load cell. The male cone and the female cone were aligned such that the male cone coincides with the female cone. The compression type of test was performed. The male cone was lowered at a pretest speed of 1 mm/s. The test speed and the post-test speed were set to 2 mm/s. The spreadability parameters of the plain Carbopol gel and the nanolipidgel were compared in terms of work done to deform the samples at specified distance.
Adhesiveness
The adhesiveness of the nanolipidgel is the work required to overcome the attractive forces between the sample (gel) and the probe of the texture analyzer which comes in contact with it gradually.
A 10-mm-diameter cylindrical probe was used for analysis. A force of 5×g was applied to the sample at a pre-test speed of 0.5 mm/s with holding time of 10 s. The probe was withdrawn at 1 mm/s speed. The maximum force required to separate the probe from the sample was recorded (28).
In Vitro Skin Permeation and Deposition Studies
In vitro skin permeation study was performed using modified Keshary Chien type of diffusion cell. Male Wistar Rat skin was carefully removed and cleaned. The upper epidermis was separated and mounted on the diffusion cell. Permeation medium consisted of phosphate buffer: 1-propanol:: 1:1. TRB NLH was applied on the donor area. A constant temperature (37 ± 0.5°C) was maintained throughout the experiment using circulating water bath. Samples (2 ml) were withdrawn at different time intervals over a period of 24 h. The sink conditions were maintained by replacing the aliquots with the fresh medium. The drug concentration was then determined using a validated HPLC method as described earlier. At the end of 24 h, applied skin area was washed with distilled water and separated. It was cut into fine pieces and extracted into methanol by sonication. The extract was subsequently filtered through a 0.45-μm filter and was analyzed for drug content.
Primary Skin Irritation Test
The primary skin irritation of the SLN-based TRB gel was evaluated by acute skin irritation test. The overall procedure was carried out as per OECD guideline #404. The protocol for the study was approved by the Institutional Animal Ethical Committee (ICT/IAEC/0909/06). Animals used for the study were healthy male New Zealand white rabbits weighing between 2.5 and 3.0 kg. Animals were categorized into four groups (n = 3) on the basis of formulations applied. The groups were named as positive control (formalin), plain TRB gel, marketed formulation, and TRB NLH.
Rabbit’s back was shaved carefully. Then 0.5 g of the test samples was applied to the hair-free area of 0.4 cm2 of skin. The rabbit skin was observed carefully after 24 h for any signs of erythema and edema which are indicators of irritation potential (29).
In Vitro Antifungal Activity
The developed formulation was studied for its in vitro antifungal activity against Candida albicans using agar diffusion method. Modified agar diffusion method, i.e., ditch plate technique, was used. Potato dextrose agar (PDA) was used for the preparation of cultures and incubation of fungal species.
Cultivation/incubation media was prepared and sterilized (by autoclaving at 15 psig pressure, 121°C for 15 min). Fresh cultures of C. albicans were prepared and incubated at 37 ± 2°C for 48 h in dark condition. Sterilized PDA plates were prepared and a spherical ditch was made with a sterile borer in an aseptic area (30–34).
Each of the formulation (blank gel, TRB NLH, plain TRB gel, and marketed formulation) was mixed thoroughly with the medium and was poured in the ditch made on agar plate under sterile conditions. The plates were dried and incubated at 37 ± 2°C for 48 h. Zone of inhibition was measured at the end of incubation.
In Vitro Occlusion Test
The method adopted by Vringer et al. was used for determining the occlusivity of the formulations (13). Plain TRB formulation, marketed formulation, and TRB NLH were evaluated for its occlusivity by measuring the percent water loss. Distilled water (25 g) was placed in different beakers. Each beaker was covered with a Whatman glass microfiber filter (9.0 cm). Test formulations were then applied on its surface. The beaker covered with filter in which no test formulation was applied served as a control for water loss. These beakers were then placed at 30 ± 2°C/60 ± 5% RH for a period of 48 h. All the formulations were tested in triplicate keeping all the condition constant. Percent water loss was calculated in reference to the control (2).
Statistical Analysis
All the results were statistically evaluated using one-way ANNOVA. The significance of the result was analyzed at p <0.05.
RESULTS
Preparation of Solid Lipid Nanoparticles
Screening of Lipids
TRB is slightly soluble in water. The solubility of the drug in lipid determines the entrapment of the drug into the lipid nanoparticles. Lipid in which drug has maximum solubility will have a higher entrapment efficiency. TRB showed highest solubility in glyceryl monostearate (10 ± 2.4 mg/mg); therefore, it was selected for SLN preparation (Fig. 1a).
Fig. 1.
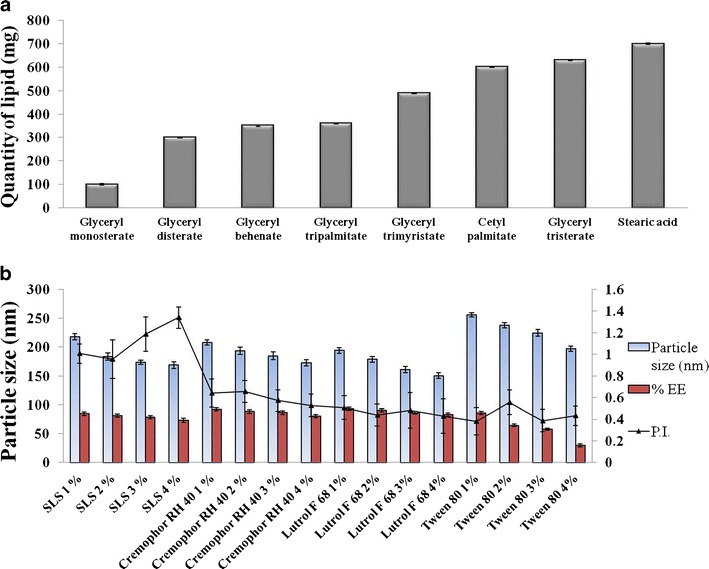
Optimization of formulation components. a Solubility of TRB in various lipids. b Screening of surfactants
Screening of Surfactants
SLNs were prepared by high-pressure homogenization technique using different surfactants. Surfactant type and concentration plays a key role in the stabilization of the SLN dispersion (32). The SLN dispersion prepared with sodium lauryl sulfate (SLS) had an initial particle size of around 200 nm. The nanoparticles in the dispersion were showing aggregation in a day, producing particles in the micron-size range. SLNs fabricated using Cremophor RH 40 and Lutrol F 68 had a particle size below 200 nm. However, they were showing a gelation tendency (phenomena characterized by a time-dependent transformation of low-viscosity SLN dispersion into viscous gel). Tween 80 at a concentration of 3% was able to stabilize aggregation- and gelation-prone SLNs. It had a particle size of 224.7 ± 0.388 nm. However, there was decreased entrapment of the drug into the nanoparticles (58.1 ± 1.8%) which is attributed to Tween-80-induced micellar solubilization of TRB in water (Fig. 1b). Incorporation of a lipophilic cosurfactant would enhance the entrapment of the drug into lipid nanoparticles and will further aid in stabilization of the system.
Plurol Oleique is reported as a cosurfactant in microemulsion-based formulations (23). It is a lipophilic cosurfactant having Hydrophilic Lipophilic Balance (HLB) value of 6. Incorporating Plurol Oleique into SLN formulation increases the loading of the drug into the lipid nanoparticles (78 ± 2.4%) by increasing its solubilization into the lipid phase (Fig. 2a).
Fig. 2.
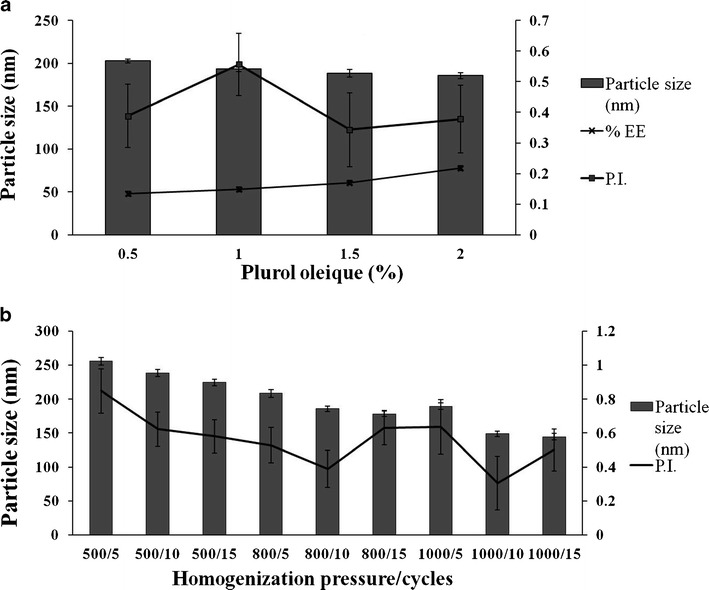
a Effect of Plurol Oleique on particle size. b Optimization of process parameters
Combination of Tween 80 (3%) and Plurol Oleique (1%) was optimized for preparation of stable TRB SLN.
Screening of Process Parameters
The effect of process variables on the properties of SLN dispersion was investigated. TRB SLNs were prepared at homogenization pressure from 500 bar to 1,000 bar. Samples were withdrawn at 5, 10, and 15 cycles (Fig. 2b).
SLN’s particle size was decreased with the increase in homogenization pressure (500–1,000 bar) and cycles (5–15) from 255 ± 5.8 nm (PI 0.848 ± 0.13) to 144.6 ± 4.7 nm (PI 0.501 ± 0.126). Homogenization pressure of 1,000 bar with 10 homogenization cycles was optimized to prepare TRB-loaded SLN which gave a particle size of 148.8 ± 3.8 nm with PI of 0.305 ± 0.158.
Characterization of Optimized SLN Dispersion
Particle Size and Zeta Potential
Blank SLN dispersion had a particle size of 136.4 nm with PI of 0.365. The zeta potential value was observed to be −26.94 ± 1.25 mV. The drug loaded dispersion had a particle size of 148.6 nm with a PI of 0.305. The zeta potential of the TRB dispersion was −20.4 ± 1.18 mV.
Differential Scanning Calorimetry
DSC thermographs of TRB, GMS, TRB SLNs, and trehalose are shown in Fig. 3a. Bulk glyceryl monostearate melts at 68.28°C with a sharp peak. When incorporated into SLN, it showed a broad melting peak at 53°C. TRB showed a sharp melting endotherm at 213.63°C, which was absent in the TRB SLN indicating amorphous nature of the drug in the form of SLN (Fig. 3a).
Fig. 3.
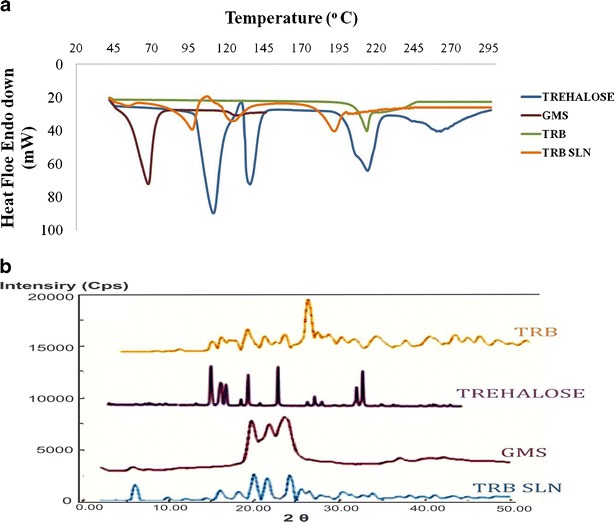
Characterization of TRB SLNs. a DSC study of bulk drug glyceryl monostearate, TRB, TRB SLNs, and trehalose. b XRD pattern of bulk TRB, trehalose, glyceryl monostearate, and TRB SLNs
XRD Study
XRD studies of the powdered drug-loaded SLNs was carried out. XRD pattern showed a decrease in the intensity of the peaks of the drug and the lipid when incorporated into SLN (Fig. 3b).
Preparation and Evaluation of TRB NLH
TRB SLNs were incorporated into a semisolid gel using 1% Carbopol 980 NF. TRB NLH was opaque in nature with a pH of 6 ± 0.2. Assay confirmed the drug content of 100.23 ± 1.23%.
Rheological Testing of the Nanolipidgel
This study was performed to evaluate the rheological behavior of plain Carbopol gel and the TRB NLH prepared using Carbopol 980 NF as a gelling agent. The concave nature of the rheogram in Fig. 4a, inclined towards the shear rate axis, shows both the gels had a pseudoplastic flow (shear thinning system).
Fig. 4.
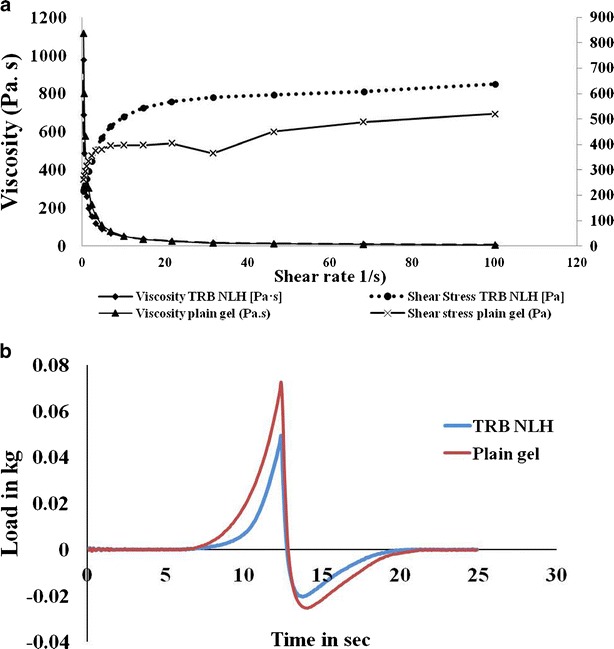
Characterization of TRB NLH. a Rheological behavior of plain Carbopol gel and TRB NLH. b Spreadability of plain Carbopol gel and TRB NLH by texture analyzer
Oscillatory Rheometry
The rotational experiments provides the rheological behavior of the system; however, the dynamic oscillation testing provides the detailed structure of viscoelastic material. Strain sweep test is used to determine the viscous and elastic region of the material. The storage modulus (stored energy per unit volume) G′ as a measure of elasticity of the material and the loss modulus (dissipated energy per unit volume) G″ as a measure of viscosity of the material were determined. Damping factor being the ratio of loss modulus and storage modulus shows the interaction of internal structures. The rheogram indicates that, even at low angular frequency, the rheological properties of the sample are more elastic than viscous. The storage modulus was always higher than loss modulus. Damping factor of plain Carbopol gel was greater than 1 throughout the frequency range (Fig. 5a). However, when TRB SLNs were incorporated into a gel, a nanolipidgel, it showed high storage modulus, with a damping factor of less than 1, showing the elastic nature of the TRB NLH at 37°C, ensuring uniform spreadability of the formulation with less force. Storage modulus of TRB NLH is less dependent on the frequency sweep indicating uniform particles dispersed in the gel, which ensures high stability of the TRB NLH (Fig. 5b) (26,27).
Fig. 5.
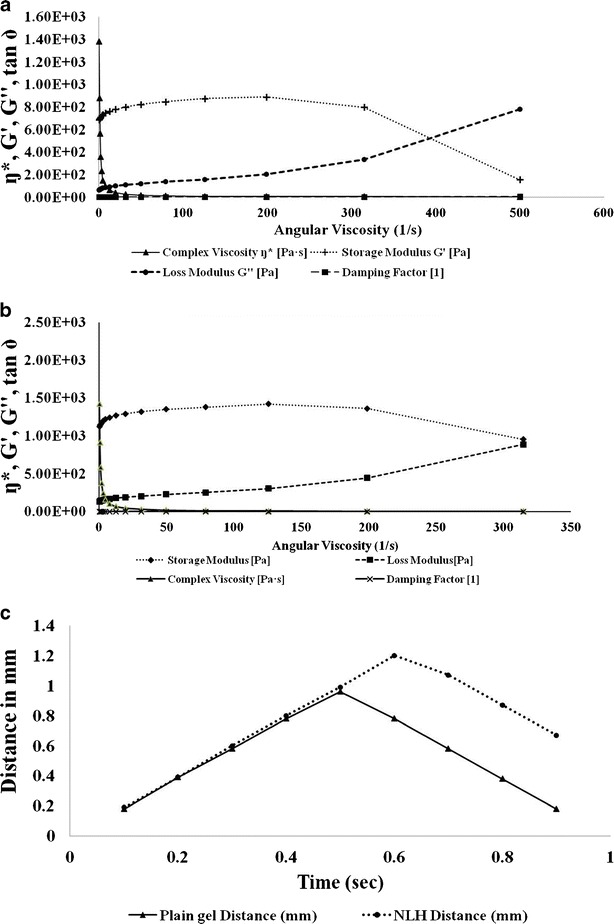
Characterization of TRB NLH. a Oscillatory sweep test plain Carbopol gel. b Oscillatory sweep test TRB NLH. c Adhesion test by texture analyzer
Texture Analysis
Spreadability
The maximum value on the spreadability curve measures the firmness of the sample at the specified depth. The area under the positive curve is the force required to deform the sample to the defined distance. Lower peak load with lower hardness work value indicates a good spreadability of the sample. Thus, TRB NLH had better spreadability over the plain Carbopol gel (Fig. 4b).
Adhesiveness
The adhesiveness of the TRB NLH was less than the plain Carbopol gel, which indicates that the lipid nanoparticles which are uniformly dispersed in water do not interfere with the swelling mechanism of the Carbopol (Fig. 5c) (28).
In Vitro Skin Permeation and Deposition Study
In vitro skin permeation of TRB through TRB NLH was determined on Wistar Rat skin using a modified Keshary Chien cell. The cumulative percent of the drug permeated was calculated at each time point (Fig. 6a). All the formulations showed less than 10% drug permeation in 24 h. There was no significant difference in the release profile of the drug from all the tested formulations (p < 0.05). The aim of developing the nanolipidgel was to enhance the deposition of drug into the skin. Skin deposition of the drug into the skin was analyzed using HPLC. The study showed almost a 3-fold and 2-fold rise in the deposition of the drug from TRB NLH (16.48 ± 1.35%) over plain gel (31.34 ± 0.99%) and marketed formulation (Mkt formulation) (54.50 ± 2.35%) (Fig. 6b). There was a significant increase in the deposition of the drug in the form of nanolipidgel over plain and marketed formulation (p < 0.05).
Fig. 6.
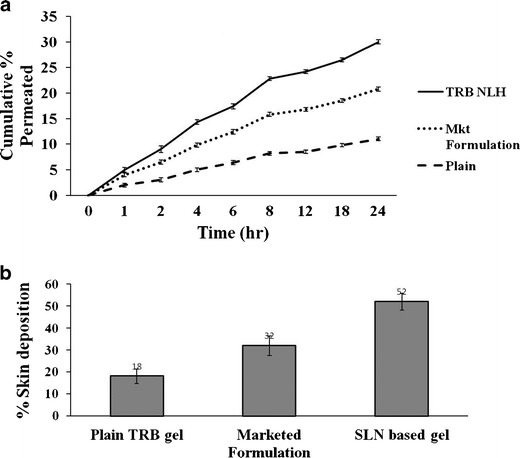
Skin permeation studies. a Skin permeation study on Wistar Rat skin. b Skin deposition study on Wistar Rat skin
Skin Irritation Test
TRB NLH of TRB was free of any irritation when applied to the skin. There was no sign of erythema and edema (Fig. 7).
Fig. 7.
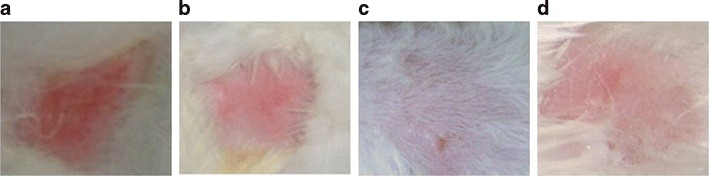
Skin irritation studies on rabbits. a Positive control. b Plain TRB gel. c Marketed formulation. d TRB NLH
In Vitro Antifungal Activity
C. albicans is primarily responsible for various skin and nail infections, and hence is used as a reference for evaluation of antifungal activity of actives. Zone of inhibition serves as an important in vitro parameter for its measurement. In vitro antifungal activity of the formulations was compared by modified agar diffusion technique by measuring the zone of inhibition over 48 h. The zone of inhibition for the TRB-loaded formulations was in the order of plain TRB gel (5.2 ± 0.20 mm) < Mkt formulation (7.8 ± 0.23 mm) < SLN-based terbinafine HCL gel (11.74 ± 0.12 mm) (Fig. 8a). TRB NLH had a significantly increased antifungal activity over plain TRB gel and Mkt formulation (p < 0.05).
Fig. 8.
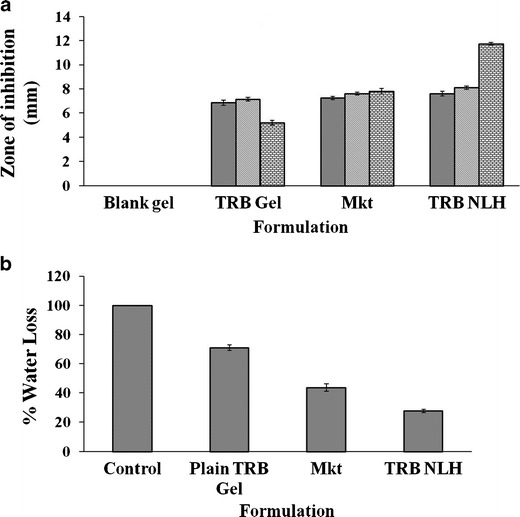
Evaluation of TRB NLH. a In vitro antifungal activity of various formulations of TRB against C. albicans. b In vitro occlusion study of TRB SLN gel
In Vitro Occlusion Study
Occlusivity of TRB NLH was determined by measuring percent epidermal water loss over 24 h. The formulations with minimum water loss will provide better occlusivity. This would further enhance the hydration status of the skin, augmenting the deposition of the drug into the skin. Water-containing beaker covered with filter paper without formulation served as a control. The water loss from the various formulations was compared to the control whose value was considered as 100%. TRB NLH showed a significant decrease in the percent epidermal water loss (27.7 ± 1.27%) compared to plain TRB gel (70.82 ± 1.35%) and Mkt formulation (43.59 ± 2.50%) at p <0.05 (Fig. 8b).
Discussion
Solid lipid nanoparticles-based nanolipidgel system was formulated and evaluated for topical delivery of terbinafine. TRB SLNs were fabricated by melt emulsification method using high-pressure homogenization technique. Surfactant type and concentration plays a crucial role in the stabilization of SLNs in terms of particle size, aggregation, and gelation phenomena (35). SLS being too hydrophilic in nature (HLB 40) have less affinity for lipid nanoparticles and hence is not able to stabilize the surface of lipid nanoparticles efficiently. This further causes their coalescence to produce aggregates in the micron-size range. SLNs formulated using Cremophor RH 40 and Lutrol F 68 were showing time-dependent gelation. The phenomenon of gelation is attributed to polymorphic changes taking place into the lipid structure when subjected to heating and cooling cycles. This leads to nanoparticles aggregation with increased viscosity of the dispersion to form gel-like structures. Cremophor RH 40 and Lutrol F 68 may not be able to inhibit the polymorphic changes in the lipids causing gelation. Tween 80, at 3% concentration, produced stable SLNs with a particle size of 224.7 ± 0.388. However, there was decreased entrapment of the drug in the lipid nanoparticles, attributed to its micellar solubilization in water. Incorporation of Plurol Oleique, a lipophilic cosurfactant (HLB 6) which has affinity towards the lipid nanoparticles, enhances the loading of the drug into nanoparticles and also provides improved stability in terms of aggregation and gelation. Process parameters like homogenization pressure and cycles further influence the particle size and PI of the SLN dispersion. There was a decrease in the particle size from 255.8 ± 5.8 nm to 144.6 ± 4.7 nm with increase in homogenization pressure from 500 to 1,000 bar. Increasing the homogenization pressure induces cavitation forces and causes a highly localized rise in the temperature and pressure within the fluid, resulting in decrease of particle size and inhibition of agglomeration. Homogenization pressure of 1,000 bar with 10 homogenization cycles provides optimum particle size and PI (148.8 ± 3.8 nm and 0.305 ± 0.158). Optimized TRB SLNs were then characterized using DSC and XRD studies. The DSC thermographs of the plain drug, crude lipid, freeze-dried TRB SLNs, and trehalose (cryoprotectant) were plotted. The shift in the sharp melting endotherm of lipid (from 68.28°C to 53°C) and the absence of the characteristic drug melting endotherm at 213.63°C were attributed to reduced crystallinity of the lipid and amorphization of the drug when incorporated in SLN. XRD studies of the powdered TRB SLNs showed decreased crystallinity of both drug and lipid in the form of SLNs.
TRB-loaded SLNs were formulated into a nanolipidgel using 1% Carbopol 980 NF. Semisolid properties of the TRB NLH were evaluated by rheological and texture analysis studies. It was compared with the plain TRB gel. TRB NLH showed a pseudoplastic flow, a shear thinning behavior which facilitates its topical application. Rheograms of all tested semisolid formulations showed a non-Newtonian plastic behavior. These systems do not flow till the yield value is achieved and shows a decrease in the viscosity of the formulations with increasing shear rate (shear thinning system). TRB NLH had increased yield values than plain TRB gel, which favors its topical application and improves stability of semisolid formulations during storage. Incorporation of the lipid nanoparticles in the nanolipidgel causes the formation of colloidal network that aligns itself in the direction of applied shear, which results in a slight increase in viscosity compared to plain Carbopol gel (24,25). Texture analysis showed better spreadability and adhesiveness of TRB NLH over plain Carbopol gel. The colloidal nature of the lipid nanoparticles does not interfere with the hydration properties of Carbopol.
In vitro skin permeation and deposition studies of plain TRB gel, marketed formulation, and TRB NLH were carried out on Wistar Rat skin. There was a 3-fold and 2-fold increase in TRB deposition into the skin from TRB NLH. Plain TRB gel was prepared by simple dispersion of the drug into a Carbopol base. The drug deposition was hence primarily dependent on the deposition characteristics of the drug per se. The marketed formulation, however, contained TRB in the cream base. Cream formulations usually contain an oil phase, emulsified by aqueous phase following addition of surfactants. This composition of the formulation can be responsible for the higher deposition compared to the plain gel. SLN-based formulations present lipid particles in the colloidal size range with an extensive surface area and thus increase the deposition of the drug into the skin. Increased occlusivity of the SLN further improves the hydration status of the skin and enhances deposition of the drug in deeper tissues (9).
In vitro antifungal activity showed a significant increase in zone of inhibition of TRB in TRB NLH. The blank SLN gel (without TRB) did not have antifungal activity of its own. Blank gel formulation was a mere Carbopol 980 NF-based gel formulation without the addition of any antifungal active. The formulation thus showed no inhibition of fungal colonies and did not have antifungal activity. It was used as a negative control during the study. Marketed formulation comprised of a cream-based formulation of TRB. Semisolid formulations like creams consist of an oil phase emulsified by the use of a blend of surfactants. Surfactants are known to have antifungal activity which is attributed to inhibition of the steroidal component of the fungi which forms an integral part of its cell wall. TRB NLH presents a lipid nanoparticle-based gel system, which increases the water solubility of TRB facilitating its diffusion in the SDA medium, enhancing its antifungal activity. The colloidal nature of the particles also enhances the permeability of the drug into the fungal cell providing improved antifungal activity. Nanoparticles offer an extensive surface area which enhances the interaction of the drug with the binding sites of the yeast wall, further enhancing the drug diffusion through the yeast cell membrane. Surfactants are incorporated into the lipid nanoparticles as stabilizers for colloidal system. Surfactants also have antifungal activity by inhibition of steroidal component of fungal cell wall by their surface-tension-lowering activity (31–34). There was a gradual increase in the zone of inhibition of TRB in the form of SLN which can be due to the slow release of the drug from the matrix of the lipid nanoparticles (Fig. 8a).
In vitro occlusivity study of TRB NLH was performed. Solid lipid nanoparticles present the semisolid lipid-based system, having particles in a colloidal size range. Colloidal lipid particles offer an extensive surface area over the conventional semisolid formulations. This minimizes the epidermal water loss resulting in improved hydration status of the skin which further augments the drug penetration into deeper skin tissues (13).
Conclusion
Surfactant concentration and type plays an important role in determining stability of the SLN dispersion in terms of particle size, entrapment, and colloidal stability. A blend of surfactants serves as a good stabilizer over a single surfactant. Process parameters like homogenization pressure and cycles have a direct influence on particle size. There was decreased crystallinity of the drug and the lipid when incorporated in SLN which was confirmed by DSC and XRD studies. Nanolipidgel presents an attractive delivery system to deliver TRB in the form of solid lipid nanoparticles. Rheological and texture analysis showed TRB NLH had the required rheological and texture properties to facilitate its topical application. TRB NLH showed an enhanced skin deposition, improved in vitro antifungal activity, and efficient occlusion properties. TRB NLH was free of any skin irritation. Thus, development of topical terbinafine nanolipidgel can be a novel, cost-effective, industrially scalable, and effective alternative to the conventional dosage forms available in the market.
Acknowledgments
The authors want to acknowledge UGC for fellowship and AICTE/NAFETIC for proving laboratory facilities.
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Martins S, Sarmento B, Ferreira DC, Souto EB. Lipid-based colloidal carriers for peptide and protein delivery—liposomes versus lipid nanoparticles. Int J Nanomedicine. 2007;2:595–607. [PMC free article] [PubMed] [Google Scholar]
- 2.Jannin V, Musakhanian J, Marchaud D. Approaches for the development of solid and semisolid lipid-based formulations. Adv Drug Deliv Rev. 2008;60:734–6. doi: 10.1016/j.addr.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Alberti I, Kalia YN, Naik A, Bonny J, Guy RH. Effect of ethanol and isopropyl myristate on the availability of topical terbinafine in human stratum corneum, in vivo. Int J Pharm. 2001;219:11–9. doi: 10.1016/S0378-5173(01)00616-0. [DOI] [PubMed] [Google Scholar]
- 4.Ryder NS. Terbinafine: mode of action and properties of the squalene epoxidase inhibition. Br J Dermatol. 1992;126:2–7. doi: 10.1111/j.1365-2133.1992.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 5.Novartis. Prescribing information for lamisil (terbinafine hydrochloride), antifungal agent. Dorval, 1993; control no. 107515.
- 6.Amichai BA, Grunwald MH. Adverse drug reactions of the new oral antifungal agents—terbinafine, fluconazole, and itraconazole. Int J Dermatol. 1998;37:410–5. doi: 10.1046/j.1365-4362.1998.00496.x. [DOI] [PubMed] [Google Scholar]
- 7.Sachdeva V, Siddoju S, Yu YY, Kim HD, Friden PM, Banga AK. Transdermal iontophoretic delivery of terbinafine hydrochloride: quantitation of drug levels in stratum corneum and underlying skin. Int J Pharm. 2010;88:24–31. doi: 10.1016/j.ijpharm.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 8.Wissing SA, Muller RH. Cosmetic applications for solid lipid nanoparticles (SLN) Int J Pharm. 2003;254:65–8. doi: 10.1016/S0378-5173(02)00684-1. [DOI] [PubMed] [Google Scholar]
- 9.Agnihotri SM, Vavia PR. Diclofenac-loaded biopolymeric nanosuspensions for ophthalmic application. Nanomedicine. 2009;5:90–5. doi: 10.1016/j.nano.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Mehnert W, Mader K. Solid lipid nanoparticles production, characterization and applications. Adv Drug Deliv Rev. 2001;47:165–96. doi: 10.1016/S0169-409X(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 11.Muller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002;54:S131–55. doi: 10.1016/S0169-409X(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 12.Muller RH, Mader K, Gohla SH. Solid lipid nanoparticles (SLN) for controlled drug delivery review of the state of the art. Eur J Pharm Biopharm. 2000;50:161–17. doi: 10.1016/S0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 13.Souto EB, Wissing SA, Barbosa CM, Muller RH. Development of controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int J Pharm. 2004;278:71–7. doi: 10.1016/j.ijpharm.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 14.ZurMuhlen A, Schwarz C, Mehnert W. Solid lipid nanoparticles (SLN) for controlled drug delivery—drug release and release mechanism. Eur J Pharm Biopharm. 1998;45:149–55. doi: 10.1016/S0939-6411(97)00150-1. [DOI] [PubMed] [Google Scholar]
- 15.Jenning V, Gysler A, Korting MS, Gohla SH. Vitamin A loaded solid lipid nanoparticles for topical use: occlusive properties and drug targeting to the upper skin. Eur J Pharm Biopharm. 2003;49:211–8. doi: 10.1016/S0939-6411(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, Chang X, Du D, Liu W, Liu J, Weng T, Yang Y, Xu H, et al. Podophyllotoxin-loaded solid lipid nanoparticles for epidermal targeting. J Control Release. 2006;110:296–306. doi: 10.1016/j.jconrel.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 17.Liua J, Hub W, Chena H, Nib Q, Xua H, Yang X. Isotretinoin-loaded solid lipid nanoparticles with skin targeting for topical delivery. Int J Pharm. 2007;328:191–5. doi: 10.1016/j.ijpharm.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Wissing SA, Muller RH. The influence of solid lipid nanoparticles on skin hydration and viscoelasticity—in vivo study. Eur J Pharm Biopharm. 2003;56:67–72. doi: 10.1016/S0939-6411(03)00040-7. [DOI] [PubMed] [Google Scholar]
- 19.Lippacher A, Muller RH, Mader K. Preparation of semisolid drug carriers for topical application based on solid lipid nanoparticles. Int J Pharm. 2001;214:9–12. doi: 10.1016/S0378-5173(00)00623-2. [DOI] [PubMed] [Google Scholar]
- 20.Jannin V, Musakhanian J, Marchaud D. Approaches for the development of solid and semi-solid lipid-based formulations. Adv Drug Deliv Rev. 2008;60:734–46. doi: 10.1016/j.addr.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Bhalekar MR, Pokharkar V, Madgulkar A, Patil N, Patil N. Preparation and evaluation of miconazole nitrate-loaded solid lipid nanoparticles for topical delivery. AAPS PharmSciTech. 2009;10:289–96. doi: 10.1208/s12249-009-9199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandawgade SD, Patravale VB. Development of SLNs from natural lipids: application to topical delivery of tretinoin. Int J Pharm. 2008;363:132–8. doi: 10.1016/j.ijpharm.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 23.Bonacucina G, Cespi M, Palmieri GF. Characterization and stability of emulsion gels based on acrylamide/sodium acryloyldimethyl taurate copolymer. AAPS PharmSciTech. 2009;10:368–75. doi: 10.1208/s12249-009-9218-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chawla V, Saraf SA. Rheological studies on solid lipid nanoparticle based carbopol gels of aceclofenac. Colloids Surf B Biointerfaces. 2012;92:293–8. doi: 10.1016/j.colsurfb.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy RS, Kennedy ML. Effect of non-ionic surfactants on the flow behaviour of aqueous veegum suspensions. AAPS PharmSciTech. 2007;8:E171–6. doi: 10.1208/pt0801025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park EK, Song PW. Rheological evaluation of petroleum jelly as a base material in ointment and cream formulations: steady shear flow behaviour. Arch Pharm Res. 2010;33:141–50. doi: 10.1007/s12272-010-2236-4. [DOI] [PubMed] [Google Scholar]
- 27.Chow KT, Chan LW, Heng PW. Formulation of hydrophilic nonaqueous gel: drug stability in different solvents and rheological behaviour of gel matrices. Pharm Res. 2008;25:207–17. doi: 10.1007/s11095-007-9457-3. [DOI] [PubMed] [Google Scholar]
- 28.Jones DS, Lawlor MS, Woolfson D. Examination of flow rheological and texture properties of polymer gel composed of poly(methyl vinylether-co-maleic anhydride) and poly (vinylpyrrolidone): rheological and mathematical interpretation of textural parameters. J Pharm Sci. 2002;91:2090–101. doi: 10.1002/jps.10195. [DOI] [PubMed] [Google Scholar]
- 29.Pople PV, Singh KK. Development and evaluation of topical formulation containing solid lipid nanoparticles of vitamin A. AAPS PharmSciTech. 2006;7:E63–9. doi: 10.1208/pt070491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viet DQ, Sarawade PB, Hilonga A, Kim J, Chai YG, Kim SH, et al. Preparation of silver nanoparticle containing silica micro beads and investigation of their antibacterial activity. Appl Surf Sci. 2011;257:6963–70. doi: 10.1016/j.apsusc.2011.03.041. [DOI] [Google Scholar]
- 31.McCarron PA, Donnelly RF, Marouf W, Calvert DE. Anti-adherent and antifungal activities of surfactant-coated poly (ethyl cyanoacrylate) nanoparticles. Pharma Nanotech. 2007;340:182–90. doi: 10.1016/j.ijpharm.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 32.Wang Q, Zhang N, Hu X, Yang J, Du Y. Alginate/polyethylene glycol blend fibers and their properties for drug controlled release. J Biomed Mater Res. 2007;82A:122–8. doi: 10.1002/jbm.a.31075. [DOI] [PubMed] [Google Scholar]
- 33.Wang Q, Hu X, Du Y, Kennedy JF. Alginate/starch blend fibers and their properties for drug controlled release. Carbohydr Polym. 2010;82:842–7. doi: 10.1016/j.carbpol.2010.06.004. [DOI] [Google Scholar]
- 34.Wang Q, Zhang N, Hu X, Yang J, Du Y. Chitosan/polyethylene glycol blend fibers and their properties for drug controlled release. J Biomed Mater Res A. 2008;85A:881–7. doi: 10.1002/jbm.a.31544. [DOI] [PubMed] [Google Scholar]
- 35.Helgason T, Awad TS, Kristbergsson K, McClements DJ, Weiss J. Effect of surfactant coverage on formation of solid lipid nanoparticles (SLN) J Colloid Interface Sci. 2009;334:75–81. doi: 10.1016/j.jcis.2009.03.012. [DOI] [PubMed] [Google Scholar]


