Abstract
This paper was designed to assess the value of quality by design (QbD) to improve the manufacturing process understanding of botanical drug products. Ethanol precipitation, a widely used unit operation in the manufacture of botanical drug products was employed to illustrate the use of QbD, taking the process of danshen (the dry root of Salvia miltiorrhiza Bunge) as an example. The recovery of four active pharmaceutical ingredients (APIs) and the removal of saccharides were used to represent the performance of ethanol precipitation. Potentially critical variables, including density of concentrate, ethanol consumption, and settling temperature were identified through risk assessment methods. Design of experiments (DOE) was used to evaluate the effects of the potentially critical factors on the performance of ethanol precipitation. It was observed that higher density of concentrate leads to higher removal of saccharides, but results in lower recovery of APIs. With the rise of ethanol consumption, the recovery of different APIs behaves in different ways. A potential design space of ethanol precipitation operation was established through DOE studies. The results in this work facilitate the enhanced understanding of the relationships between multiple factors (material attributes and process parameters) and the performance of ethanol precipitation. This case study demonstrated that QbD is a powerful tool to develop manufacturing process of botanical drug products.
KEY WORDS: botanical drug products, design of experiments (DOE), design space, ethanol precipitation, quality by design (QbD)
INTRODUCTION
Botanical drugs have been widely used to treat diseases for thousands of years and have made a great contribution to human health. Botanical drugs are used not only in the way of single herbal medicine, but also usually practiced in the form of formulated preparations, which consist of several ingredient herbs in a special ratio (1). Therefore, botanical drugs are fairly complex mixtures containing hundreds of chemical compounds, many of which are considered as bioactive components.
The manufacturing process for the botanical drug products is complex and involves many unit operations. These operations are usually multivariate and the relationships between input material attributes, process parameters, and critical quality attributes (CQAs) of final products are difficult to establish due to the complex chemical constitution in botanical drugs. Traditionally, the manufacturing process of botanical drug products is performed mainly based on experience. In this mode, the effects of process variables on the product CQAs are not well understood scientifically, and the process variables usually run without proper control strategies. Consequently, the quality of final products often suffers from high variability. Therefore, enhanced understanding of the relationships between material attributes, process variables, and product CQAs is crucial to improve the manufacturing performance of botanical drug and produce desirable products with acceptable quality.
Recently, the concept of quality by design (QbD) has become a topic of high importance in pharmaceutical industry to develop robust processes and products. As defined in International Conference on Harmonization (ICH) Q8 guideline (2), QbD is a “systematic approach to development that begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management”. It is concerned with desired and predetermined specifications through relating the critical material attributes and critical process parameters to the CQAs of drug product (3). This approach involves all aspects of a pharmaceutical product from the properties of active components to the quality system. Under the QbD paradigm, pharmaceutical quality is assured by understanding and controlling formulation and manufacturing variables (4). Therefore, implementing drug development in the framework of QbD will benefit the pharmaceutical industry, the regulation agency, and most importantly the target patient population (5).
Considering the fact that experience-based methods are dominantly used during the current manufacture of botanical drug products, which fail to improve process understanding due to the complex chemical constitution and multiple process variables, it is meaningful to develop manufacturing process under the framework of QbD, which aims to move the formulation process away from empirical trial-and-error approaches and to orient the processes into predictable and precisely controlled environments to ensure product quality within the life cycle (6). This work illustrated the application of QbD principles to the process development of botanical drug products, using ethanol precipitation, which is one of the most commonly used purification methods in manufacturing botanical drug products. Based on prior knowledge, ethanol precipitation is usually identified as a critical unit operation, which may significantly impact on downstream processes and the CQAs of final products. During this operation, a large amount of impurities with poor solubilities in ethanol, such as saccharides, proteins, and salts are removed. On the other hand, it also causes the losses of some bioactive components (7,8). The contents of impurities and active pharmaceutical ingredients (APIs) in resulted supernatant are regarded as the intermediate CQAs in ethanol precipitation, and are directly related to product CQAs, including the content of hazardous substances and the purity of APIs, which are directly linked to the safety and efficacy of final products. These intermediate CQAs are highly influenced by different factors, such as input material attributes, ethanol content, and precipitation temperature (8–10). A poorly controlled operation may lead to the change of intermediate CQAs, which likely results in a high variability in the quality of final products. Therefore, enhancing process understanding and developing control strategies of ethanol precipitation are of great importance to improve batch-to-batch consistency and final product quality for the industry of botanical drug products.
MATERIALS AND CHEMICALS
Materials
Danshen, the dry root of Salvia miltiorrhiza Bunge, is one of the most popular botanical drugs to treat hyperlipoidemia, cerebrovascular, and cardiovascular diseases in China. Many chemical and pharmacological studies have demonstrated that phenolic compounds are the major active components in danshen (11–13). In this work, the ethanol precipitation process in the manufacture of a danshen preparation was exemplified to illustrate the use of QbD. The presence of multiple bioactive substances is typical of botanical drugs, and the efficacy of botanical drug products depends not only on individual active component, but also usually on the synergistic effects of several components (14–16). Therefore, four main active phenolic compounds existent in danshen, including danshensu (DSS), rosmarinic acid (RA), protocatechuic aldehyde (PA), and salvianolic acid B (SAB) (11–13) were selected here as the examples to compose the APIs.
Chemicals
Standard substances of DSS, RA, PA, SAB, and glucose were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). The acetonitrile (HPLC grade) was purchased from Merck (Darmstadt, Germany). Deionized water was produced by a Milli-Q academic water purification system (Milford, MA, USA).
METHODS
Process and the Performance Criteria for Ethanol Precipitation Operation
The drug product involved in this work is a dripping pill preparation, which is made by blending the herbal extract with excipients under thermal condition followed by dripping the mixture into an insoluble cooling liquid in which the droplets are solidified to form the “dripping pill” (13). The manufacturing process of this product involves extracting bioactive components from raw materials, concentration, ethanol precipitation, blending, dripping pelletization, heat drying, and coating, as shown in Fig. 1. The extraction process is performed using a boiling water decoction at the temperature of about 100°C. For reducing ethanol consumption in ethanol precipitation process, the water extract is then treated by a concentration operation conducted under reduced pressure at the temperature of about 80°C to obtain the concentrate, which is used as the input material for ethanol precipitation. The process of ethanol precipitation consists of two steps. The first one is the addition of ethanol to concentrate and the second one is a refrigeration procedure to further precipitate impurities. After this operation, supernatant is collected, and the ethanol contained in the supernatant is removed by another concentration process.
Fig. 1.
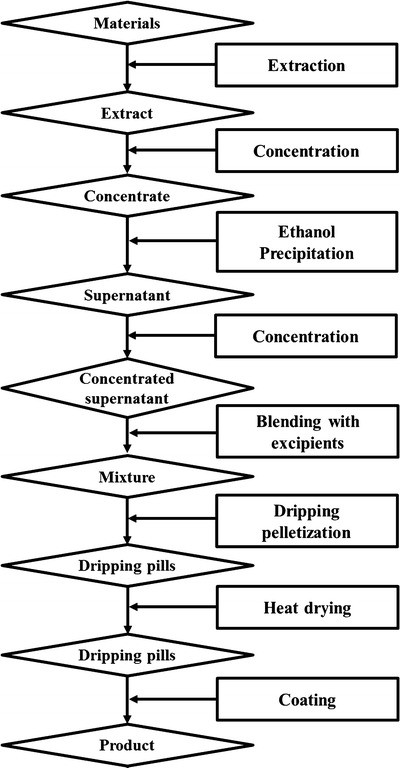
Flowchart for the manufacturing process of the botanical drug product involved in this work
The recovery of APIs can be applied to describe the impact of ethanol precipitation on the content change of active components, which is defined as following equation:
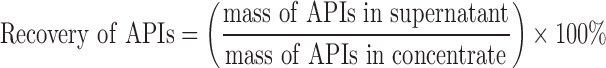 |
1 |
There are several saccharides contained in danshen, such as glucose, fructose, and sucrose (17), which can be heavily extracted by hot water. These saccharides are required to be removed during ethanol precipitation process for two reasons. Firstly, saccharides are not regarded as bioactive components. Therefore, removing saccharides can improve the purity of APIs and reduce the drug dosage of final product. Secondly, in high-temperature operations, saccharides could slowly degrade to form 5-(hydroxymethyl)-2-furancarboxaldehyde (5-HMF) (18). It has been reported that 5-HMF appeared to be a weak cytotoxic compound and would act genotoxic effects at rather high concentrations (19). Therefore, from a safety perspective, the content of this substance in final product is suggested to be controlled within a limit (8). It is inevitable that a small amount of 5-HMF could be formed during the preceding processes, such as extraction. In order to reduce the amount of 5-HMF generated in downstream processes, the saccharides need to be removed. The capability of ethanol precipitation to remove saccharides is described using following equation:
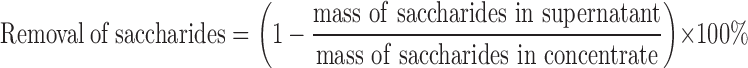 |
2 |
The recovery of APIs and the removal of saccharides are directly related to the intermediate CQAs, which are the contents of APIs and impurities, respectively. Therefore, the scientific understanding of the effects of various operating factors on the recovery of APIs and the removal of saccharides is crucial to improve the process knowledge of ethanol precipitation.
Identification of Potentially Critical Factors
A number of factors may impact the performance of ethanol precipitation. The first step toward process characterization studies is to identify factors and to assess the risk of them to the process. Quality risk assessment tools, such as fishbone diagram or failure mode and effects analysis (FMEA) can be used to quantify the degree of risk related to various variables (20). An Ishikawa diagram analysis here was performed, in accordance with ICH Q9 guideline (21), to identify an initial list of potential factors that influence the recovery of APIs and the removal of saccharides (Fig. 2). It can be seen that five main causes, including environment, attributes of material (concentrate), equipment, ethanol addition process, as well as refrigeration procedure and the associated subcauses were identified.
Fig. 2.

Ishikawa diagram analysis for the unit operation of ethanol precipitation
Then, a FMEA was conducted to quantify the risk of each potential factor and to estimate the impact of each cause on the process, producing high-risk factors for further characterization studies. The quantification of risk was performed through ranking the severity (S), probability (P), and detectability (D) of each parameter, as listed in Table I. In our method, severity is the measure of how strongly the specific parameter could impact on the process. The probability of occurrence is defined as the likelihood that the parameter could fail. Detectability is described as the ability to timely detect a failure in that parameter. For each factor, scores for S, P, and D are multiplied, yielding a risk priority number (RPN) on a scale from 1 to 36. Any factor with the value of RPN above 10 was selected for subsequent process characterization studies. Table II shows the results of our risk analysis, which achieved based on prior knowledge and historical data analysis. It can be seen that three potentially high-risk factors were identified, which were the density of concentrate, ethanol consumption, and settling temperature in the refrigeration procedure. The effects of these factors on the performance of ethanol precipitation were further investigated in this work.
Table I.
Rank for Risk Quantification of S, P, and D of Parameter
| Severity (S) | |
| 4 | High—a minor change in the parameter could cause significant impact on product quality |
| 3 | Moderate—parameter only if fluctuates in a wide range may cause significant impact on product quality |
| 2 | Low impact—always not significant |
| 1 | No impact |
| Probability (P) | |
| 3 | Highest probability that the parameter may fail |
| 2 | Failure may occur sometimes |
| 1 | Almost not fails |
| Detectability (D) | |
| 3 | Failure may be probably not detected |
| 2 | Occasionally not detected |
| 1 | Detected every time |
Table II.
Risk Assessment using FMEA for the Operational Factors of Ethanol Precipitation
| Cause | Factors | Severity | Probability | Detectability | RPN |
|---|---|---|---|---|---|
| Environment | Temperature | 1 | 2 | 2 | 4 |
| Concentrate attributes | Density | 4 | 3 | 1 | 12 |
| Temperature | 3 | 1 | 1 | 3 | |
| pH value | 4 | 2 | 1 | 8 | |
| Equipment | Style of addition | 1 | 1 | 1 | 1 |
| Position of stir | 1 | 1 | 1 | 1 | |
| Ethanol addition | Ethanol content | 3 | 1 | 1 | 3 |
| Ethanol consumption | 4 | 2 | 2 | 16 | |
| Flow rate | 3 | 1 | 2 | 6 | |
| Stir rate | 3 | 1 | 2 | 6 | |
| Refrigeration | Settling temperature | 4 | 3 | 1 | 12 |
| Settling time | 3 | 1 | 1 | 3 |
Experimental Design
The design of experiments (DOE) was used to evaluate the effects of the three potentially high-risk factors, including density of concentrate (in grams per milliliter), ethanol consumption (in milliliters of ethanol per milliliter of concentrate), and the settling temperature (degree Celcius) on the recovery of APIs and the removal of saccharides. The ultimate goal of DOE was to achieve enhanced process understanding and establish the design space of ethanol precipitation. A response surface methodology of Box–Behnken design with four center points (total 16 runs) was chosen to perform the study, as shown in Table III. All DOE runs were conducted in a random order. The true values of these three variables in experiments were firstly coded in the range of −1 to 1, which are shown in Table IV. Then, the coded values were used to develop models and analyze the effects of these variables. The statistical analysis was based on the confidence level of 0.95. The DOE and the data analysis were conducted in Design Expert 7.13 (State-Ease Inc., MN, USA) and JMP 9 (SAS Institute Inc., NC, USA).
Table III.
Box–Behnken Design with three Factors and four Center Points for Ethanol Precipitation
| Run | Concentrate density (g/ml, at 20°C) | Ethanol consumption (ml/ml) | Settling temperature (°C) |
|---|---|---|---|
| 1 | 1.25 | 2.8 | 15 |
| 2 | 1.25 | 2.3 | 5.0 |
| 3 | 1.19 | 2.3 | 5.0 |
| 4 | 1.22 | 2.3 | 15 |
| 5 | 1.19 | 2.3 | 25 |
| 6 | 1.22 | 2.8 | 5.0 |
| 7 | 1.22 | 2.3 | 15 |
| 8 | 1.19 | 2.8 | 15 |
| 9 | 1.22 | 1.8 | 25 |
| 10 | 1.22 | 2.3 | 15 |
| 11 | 1.25 | 2.3 | 25 |
| 12 | 1.25 | 1.8 | 15 |
| 13 | 1.22 | 1.8 | 5.0 |
| 14 | 1.22 | 2.3 | 15 |
| 15 | 1.22 | 2.8 | 25 |
| 16 | 1.19 | 1.8 | 15 |
Table IV.
True Values and Coded Values for the Design Factors
| Variables | Symbols | Coded values | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Density of concentrate | A | 1.19 | 1.22 | 1.25 |
| Ethanol consumption | B | 1.8 | 2.3 | 2.8 |
| Settling temperature | C | 5 | 15 | 25 |
Ethanol Precipitation
In this study, the DOE runs were performed in lab scale. The concentrates with different density were prepared at 100 ml scale for each run. During the process of ethanol precipitation, 95% ethanol solution (v/v) was pumped into the concentrate at the flow rate of 10 ml/min under a continuous stirring (400 rpm). As soon as the consumption of ethanol reached the designed amount, the ethanol addition process was stopped. The mixtures then was sealed and refrigerated at planned temperature for 24 h. Finally, supernatant was collected and analyzed to evaluate the recovery of APIs and the removal of saccharides.
Sample Analysis
The quantitative analysis of API was performed by UPLC method (22). A Waters Acquity UPLC system (Waters, Milford, MA, USA) coupled with an Acquity UPLC HSS T3 column (100 mm × 2.1 mm, 1.8 μm) was used. The mobile phase consisted of 0.02% (v/v) phosphoric acid in water (A) and 0.02% (v/v) phosphoric acid-80% (v/v) acetonitrile in water (B). The separation of samples was achieved using the gradient elution program as follow: 9–22% B at 0–1.6 min, 22–26% B at 1.6–1.8 min, 26–39% B at 1.8–8.0 min, 39–9% B at 8.0–8.4 min and 9% B at 8.4–10 min. The solvent flow rate was 0.4 ml/min and the column temperature was kept at 40°C. Peaks were recorded at 280 nm.
The determination of saccharides was performed using the phenol–sulfuric colorimetric method established by Dubois et al. (23), and glucose was used as a standard. Of the sample or standard solution, 1.0 ml was transferred to a conical flask, and then 2.0 ml of 3% phenol solution was added followed by 10.0 ml of 98% sulfuric acid. The operation was performed under magnetic stirring to make the system mix well. The mixture was then kept in a water bath of 30°C for 1 h and the absorbance of the solution was measured at 490 nm using a T6 UV–vis spectrophotometer (Beijing Purkinje General Equipment Co., Ltd., China). The saccharide content was expressed as glucose-equivalent saccharides in weight.
RESULTS
Effects of the Potentially High-Risk Factors on the Recovery of APIs
The experimental results of API recovery at each run are displayed in Table V. Response surface models were developed to evaluate the effects of the design factors on the recovery of each API and the parameter estimates for the terms of each model are listed in Table VI. It can be seen that concentrate density and the second-order concentrate density have the significant impacts on the recovery of all the APIs (p values < 0.05). The main effect of ethanol consumption shows significance on the recovery of DSS, SAB, and PA, while is insignificant on the recovery of RA. The term of quadratic ethanol consumption has apparent effect on the recovery of PA. Settling temperature has the significant impact on the recovery of DSS, SAB, and PA. The second-order settling temperature is significant on influencing the recovery of RA and PA. All the interaction terms are not significant with their high p values.
Table V.
Experimental Data of Responses for Each Run
| Run | Recovery of DSS (%) | Recovery of RA (%) | Recovery of SAB (%) | Recovery of PA (%) | Removal of saccharides (%) |
|---|---|---|---|---|---|
| 1 | 41.1 | 69.9 | 48.3 | 83.7 | 77.4 |
| 2 | 42.3 | 71.9 | 50.8 | 82.5 | 80.9 |
| 3 | 56.6 | 81.7 | 66.0 | 90.1 | 56.2 |
| 4 | 47.4 | 74.3 | 57.0 | 84.4 | 68.8 |
| 5 | 62.0 | 85.0 | 70.6 | 93.3 | 42.8 |
| 6 | 46.1 | 76.2 | 53.5 | 87.9 | 74.4 |
| 7 | 46.2 | 73.2 | 55.9 | 83.4 | 68.2 |
| 8 | 55.6 | 81.0 | 65.1 | 91.5 | 65.9 |
| 9 | 52.0 | 76.8 | 62.0 | 85.6 | 53.9 |
| 10 | 46.0 | 74.6 | 54.4 | 83.4 | 70.5 |
| 11 | 45.2 | 72.7 | 54.6 | 84.4 | 67.4 |
| 12 | 46.2 | 70.9 | 56.7 | 80.3 | 59.5 |
| 13 | 48.7 | 76.0 | 58.7 | 84.7 | 60.2 |
| 14 | 45.4 | 72.8 | 54.6 | 83.3 | 70.2 |
| 15 | 47.3 | 77.0 | 62.1 | 90.6 | 79.7 |
| 16 | 63.0 | 83.1 | 72.6 | 90.7 | 38.1 |
Table VI.
The Parameter Estimates for all Terms in the Model of the Recovery of each API
| Model terms | DSS | RA | SAB | PA | ||||
|---|---|---|---|---|---|---|---|---|
| Estimate | Prob > |t| | Estimate | Prob > |t| | Estimate | Prob > |t| | Estimate | Prob > |t| | |
| A | −7.7862 | <0.0001* | −5.6706 | <0.0001* | −8.0001 | <0.0001* | −4.3131 | <0.0001* |
| B | −2.4799 | 0.0008* | −0.3288 | 0.3420 | −2.6393 | 0.0070* | 1.5412 | 0.0013* |
| C | 1.5982 | 0.0071* | 0.7137 | 0.0664 | 2.5384 | 0.0084* | 1.0756 | 0.0073* |
| A 2 | 4.1134 | 0.0003* | 1.9067 | 0.0055* | 3.2993 | 0.0121* | 1.6532 | 0.0050* |
| B 2 | 1.1363 | 0.0909 | 0.6012 | 0.2306 | 1.9129 | 0.0855 | 1.2803 | 0.0155* |
| C 2 | 1.1803 | 0.0815 | 2.2017 | 0.0028* | 1.7020 | 0.1170 | 2.3078 | 0.0009* |
| A × B | 0.5669 | 0.3541 | 0.2725 | 0.5676 | −0.2345 | 0.8094 | 0.6472 | 0.1416 |
| B × C | −0.5371 | 0.3782 | 0.0163 | 0.9724 | 1.3317 | 0.2022 | 0.4670 | 0.2679 |
| A × C | −0.6362 | 0.3029 | −0.6306 | 0.2113 | −0.2072 | 0.8311 | −0.3388 | 0.4098 |
*p < 0.05
Figure 3 shows how the design factors impact the recovery of APIs based on the established models. It can be observed that increasing density of concentrate leads to rapid decrease in the recovery of each API, indicating that the input material with higher density will cause more losses of APIs during the process of ethanol precipitation. Higher settling temperature results in higher recovery of DSS and SAB (Fig. 3a and c). On the other hand, as the increase of ethanol consumption, the recovery of DSS and SAB decreases. The effect of settling temperature on the recovery of SAB is smaller with the lower ethanol consumption, while the effect of ethanol consumption on the recovery of SAB is larger with the lower settling temperature, as shown in Fig. 3c. With the rise of ethanol consumption, the recovery of PA increases gradually, which is contrary to that on the recovery of DSS and SAB as illustrated in Fig. 3d. Increasing settling temperature makes the recovery of RA and PA first decrease and then increase (Fig. 3b and d).
Fig. 3.
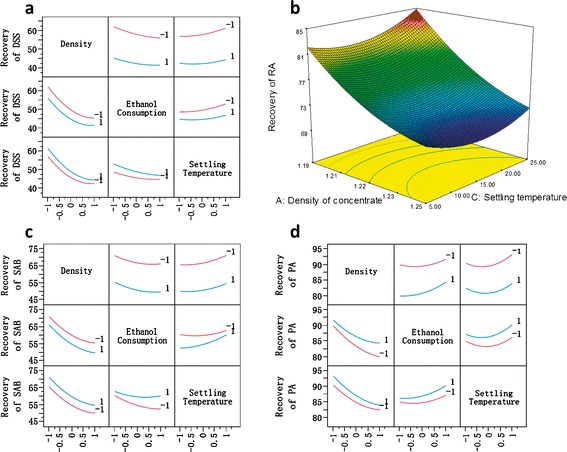
Effects of design factors on the recovery of each API: a interaction profile for each factor on the recovery of DSS; b surface plot for the effects of settling temperature and concentrate density on the recovery of RA with ethanol consumption at center level; c interaction profile for each factor on the recovery of SAB; d interaction profile for each factor on the recovery of PA
Final models fitted for the recovery of each API were established after the insignificant terms were removed. Analysis of variance (ANOVA) was performed and model-fit statistics are shown in Fig. 4. The large value of R2 for each model indicates a very good fit. Models are significant with small p values.
Fig. 4.
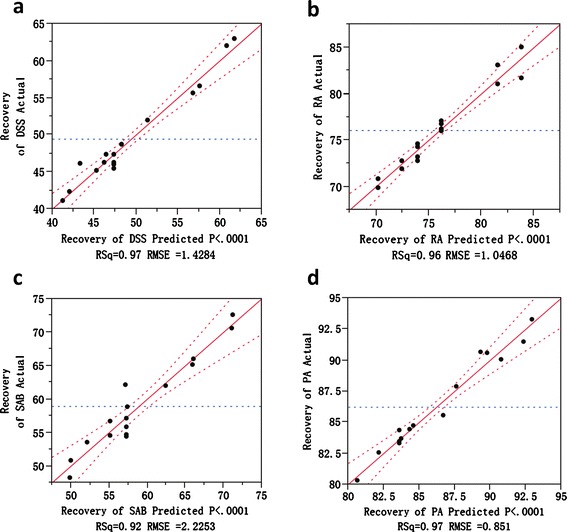
Model fit statistics for the recovery of each API: a the actual by predicted plot for the recovery of DSS; b the actual by predicted plot for the recovery of RA; c the actual by predicted plot for the recovery of SAB; d the actual by predicted plot for the recovery of PA
Effects of the Potentially High-Risk Factors on the Removal of Saccharides
The primary aim of ethanol precipitation is to get rid of saccharides. The content of total saccharides in concentrate and the supernatant yielded from ethanol precipitation operation was measured and the experimental data for the removal of saccharides was listed in Table V. Response surface model was established, and the estimated parameter of model terms is shown in Table VII. It can be seen that ethanol consumption most significantly impacts on the recovery of saccharides, followed by the density of concentrate and the second-order concentrate density.
Table VII.
The Parameter Estimates for all Terms in the Model of the Removal of Saccharides
| Terms | Estimate | Prob > |t| |
|---|---|---|
| A | 10.2848 | 0.0007* |
| B | 10.7107 | 0.0006* |
| C | −3.4822 | 0.0737 |
| A 2 | −7.1834 | 0.0197* |
| B 2 | −1.9657 | 0.4210 |
| C 2 | −0.3769 | 0.8739 |
| A × B | −2.4563 | 0.3220 |
| B × C | 2.8887 | 0.2514 |
| A × C | −0.0343 | 0.9885 |
*p < 0.05
Figure 5 displays surface plots for the effects of the variables on the removal of saccharides. Ethanol consumption is the most significant term that influences the removal of saccharides (p value in Table VII). It can be observed that higher ethanol consumption leads to higher removal of saccharides, while lower ethanol consumption results in lower removal of saccharides, indicating that more ethanol could precipitate more saccharides. As shown in Fig. 5a, increasing density of concentrate can also rapidly increase the removal of saccharides and the effect of density on the removal is larger at the lower level of ethanol consumption than that at the higher level of ethanol consumption. From Fig. 5b, it can be seen that decreasing settling temperature at lower ethanol consumption leads to a gradual increase in the removal of saccharides. While at the higher level of ethanol consumption, the change of settling temperature does not appear to impact on the removal of saccharides significantly.
Fig. 5.
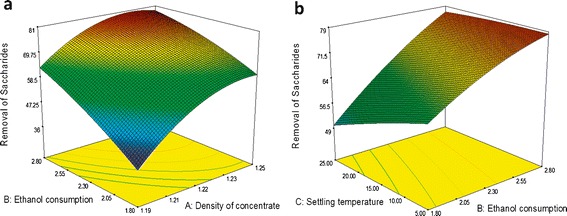
Surface plots for the effects of variables on the removal of saccharides: a the effects of concentrate density and ethanol consumption on the removal of saccharides; b the effects of ethanol consumption and settling temperature on the removal of saccharides
The final model was established after the insignificant terms were removed. ANOVA results were summarized in Fig. 6. R2 of 0.87 indicate a good model fit, and the model is significant with p value <0.0001.
Fig. 6.

ANOVA for the removal of saccharides
Design Space
Essentially, QbD involves identifying critical input material attributes and process variables and determining the relationships linking these factors to product CQAs to establish the design space of process. According to ICH Q8 guideline, design space is “the multidimensional combination and interaction of input variables (e.g., material attributes) and process parameters that have been demonstrated to provide assurance of quality”. Regardless of how a design space is developed, it is expected that operation within the design space will result in a product meeting the defined quality (2). There have been many reports on the development of design space. Lepore and Spavins (24) outlined the steps and elements for determining a design space for pharmaceutical products. Boukouvala et al. (25) proposed the approaches for defining design space based on different data-driven modeling techniques. Huang et al. (26) used a combination of DOE and multivariate analysis to establish design space for ensuring desired CQAs. In our work, response surface methodology was applied to develop design space for the unit operation of ethanol precipitation.
The potentially high-risk factors, including the density of concentrate, ethanol consumption, and settling temperature were identified by the risk assessment, and their relationships to the performance of ethanol precipitation were established by DOE. A satisfactory design space for a unit operation should meet the specifications of all CQAs to ensure that when the process is run within the proposed design space, product of desired quality can be produced. In this study, as the criteria to evaluate the performance of ethanol precipitation, the recovery of each API and the removal of saccharides were taken into consideration to develop the design space. Specification of each response was set up according to the knowledge obtained from industry, as listed in Table VIII. Each specification can be used to achieve an independent design space for its respective response. These separate design spaces were then overlaid, and the resultant space within which all specifications are met would represent the proposed design space. In this study, an orthogonal design space for ethanol precipitation was established, as illustrated in Fig. 7. The ranges of these factors for the orthogonal design space were: density of concentrate to be 1.20–1.21 g/ml (at 20°C), ethanol consumption to be 2.6–2.8 ml/ml, and settling temperature to be 12–15°C. This design space can provide new knowledge about the unit operation of ethanol precipitation.
Table VIII.
Specification of Each Response
| Responses | Specification (%) |
|---|---|
| Recovery of DSS | 47.0–52.0 |
| Recovery of PA | 86.0–90.0 |
| Recovery of RA | 75.0–79.0 |
| Recovery of SAB | 56.0–61.0 |
| Removal of saccharides | 68.0–76.0 |
Fig. 7.
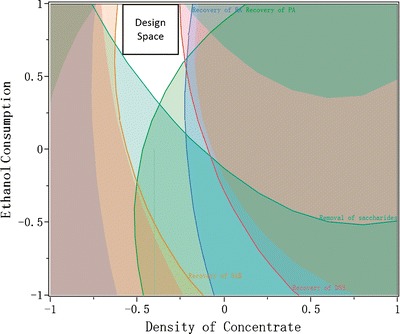
The illustration of the design space for ethanol precipitation (settling temperature at 15°C): white area the desirable parameter ranges that meet the specification of each response, rectangle area the established design space of ethanol precipitation
DISCUSSION
The losses of bioactive components in the ethanol precipitation of danshen have been observed in the previous work (8). It is important to understand the effects of the studied factors on the losses of APIs under the framework of QbD. The density of concentrate has the significant impact on the recovery of each API in that with the rise of concentrate density, more bioactive components are lost (Fig. 3). A possible reason is that during ethanol precipitation, the depositing particles will absorb the active components due to electric effects and their complicated interactions (7). As shown in Fig. 5a, higher density of concentrate makes more saccharides precipitate, leading to a mass of depositing particles. These particles can exert significant absorbance on the active ingredients. Therefore, more APIs are precipitated at the higher level of concentrate density. With the rise of ethanol consumption, the recovery of DSS and SAB decreases (Fig. 3a and c). One potential cause for this phenomenon is that more particles resulted from higher ethanol consumption could absorb more active components, as discussed above. Another potential explanation proposed by Gong et al. (8) is that DSS and SAB are phenolic acids, and these phenolic acids are extracted from materials in the form of ions, which are less soluble in the solutions of ethanol–water than in pure water. Therefore, higher ethanol content makes more of these compounds precipitate. On the other hand, PA has the highest recovery among all the active compounds (more than 80%), and with the increase of ethanol consumption, the recovery of PA increases (Fig. 3d), which is different from DSS and SAB. It may be due to the fact that PA is a kind of aldehyde, which exists in the form of molecular instead of ion naturally, and the solubility of PA molecular is higher in ethanol–water solutions than in pure water (27). Therefore, the supernatant with higher ethanol content could dissolve PA from precipitate over again. These results indicate that the mechanisms of the losses of bioactive components are complicated and different compounds behave in different ways during ethanol precipitation.
With the rise of ethanol consumption, the removal of saccharides increases (Fig. 5). This can be explained by the fact that many kinds of saccharides are less soluble in ethanol solutions than in water, and the solubilities of saccharides decrease with the increase of ethanol content (28–31). Therefore, more ethanol consumption could precipitate more saccharides in our experiments. Lower settling temperature leads to higher removal of saccharides. It is because the solubilities of a number of sugars decrease as the decrease of equilibrium temperatures (30,31). Settling temperature has the significant impact on the removal of saccharides at the lower ethanol consumption, but the impact seems insignificant at the higher ethanol consumption (Fig. 5b). The aim of refrigeration procedure is to further remove the impurities that are not precipitated by the addition of ethanol. The more residual impurities are left in supernatant, the more they can be removed by refrigeration procedure, which indicates a significant impact on the removal of impurities. At the higher level of ethanol consumption, most of saccharides are already precipitated by the addition of ethanol (more that 75%, as in Fig. 5b), leaving a small amount of saccharides in the supernatant prior to refrigeration procedure. As the result, the impact of settling temperature on the removal of saccharides is not obvious. It indicates that increasing ethanol consumption can compensate for the impact of settling temperature on the removal of saccharides.
As discussed above, the density of concentrate had the significant impact on every response, in that with the rise of the density, the removal of saccharides increases, but the recovery of APIs decreases. On the other hand, higher level of settling temperature leads to higher recovery of APIs, while results in lower removal of saccharides. Increasing ethanol consumption increases the removal of saccharides, but decreases the recovery of DSS and SAB. These results show that the operating conditions in favor of removing saccharides may cause more losses of bioactive components. Therefore, the recovery of APIs and the removal of saccharides cannot reach high levels simultaneously.
According to ICH Q8 guideline (2), design space is a combination and interaction of input variables and process parameters that provide assurance of product quality. Process is able to deliver desired products when operated in design space. The quality of botanical drug products usually suffers from high variability, which is profoundly related to the variation of chemical constitution. To improve the consistency of product quality, it is necessary to setup the specification of each response and map the design space of ethanol precipitation. It has to be noted that response specifications definitively affect the establishment of design space. As pointed out before, the combination of parameters leading to high recovery of APIs could result in low removal of saccharides and vice versa. To produce the products with required safety and efficacy, the process design space should situate both API recovery and saccharide removal in moderate levels. The specification of each response with approved scale was proposed using the knowledge from industry (Table VIII) and an orthogonal design space was generated based on the specifications. According to Fig. 7, it can be seen that the desirable parameter ranges which meet the specifications are wider than the defined orthogonal design space. The orthogonal design space for a unit operation is more feasible than the one that is mathematically expressed, and makes it simple for manufacturing process control. Actually, if creating an orthogonal space dose not significantly narrow the design space, then an orthogonal design space can be proposed (32). As a large part of the desirable parameter ranges has been covered (Fig. 7), the orthogonal design space generated is regarded as the design space for the ethanol precipitation unit operation. The proposed design space will promote the process understanding and is crucial for pharmaceutical industry to develop a robust operation of ethanol precipitation. The design space can be further refined based on additional experience obtained during commercial manufacture.
It is necessary to develop a proper control strategy for ethanol precipitation under QbD paradigm. The control strategy is defined as “a planned set of controls, derived from current product and process understanding, that assures process performance and product quality” (33). In our work, the control strategy for ethanol precipitation arises from the process characterization studies and the established design space, aiming to maintain the recovery of APIs and the removal of saccharides within the specifications (Table VIII) to ensure the consistency of product quality. To achieve this target, the density of concentrate, ethanol consumption, and settling temperature will be controlled. The acceptable ranges of these operating factors were established in the form of design space. As the input material of ethanol precipitation, concentrate is the output intermediate from upstream unit operation, which is concentration process, and the attributes of concentrate depend heavily on the performance of the upstream process. Therefore, a well understood and controlled concentration unit operation is crucial to situate the density of concentrate in the range of 1.20–1.21 g/ml (at 20°C). For ethanol consumption, a flowmeter can be selected to determine whether the end point of ethanol addition process is reached. Near-infrared spectroscopy has proven to be an efficient tool for inline monitoring of ethanol precipitation process, in that the faults occurring in initial density of starting materials and the volume of ethanol could be detected (34). During the refrigeration procedure, a thermostat can be employed to ensure that the settling temperature remains within the acceptable range (12–15°C). The development of control strategy is considered as a dynamic activity, which means that the new knowledge gained from lab or routine manufacture in the future will be utilized for the continual evolvement of the control strategy.
CONCLUSION
This case study demonstrated that QbD is a powerful tool in improving the knowledge of the relationships between potentially high-risk factors and the performance of ethanol precipitation. It was found that operating conditions which promote the removal of saccharides will likely cause more losses of bioactive components. Therefore, the recovery of APIs and the removal of saccharides cannot reach high levels simultaneously. The mechanisms for the losses of active components are complicated, partly related to the chemical and physical properties of bioactive compounds. The potential design space that met the predetermined specification of each response was defined. The process characterization studies and the design space provide opportunities for establishing control strategies of ethanol precipitation. It is envisioned that developing manufacturing process of botanical drug products under the framework of QbD not only enhances process understanding, but also facilitates the optimization and control of process to produce the products with desired quality.
Acknowledgments
This work was financially supported by the China International Science and Technology Cooperation Project (2010DFB33630) and Zhejiang Provincial Natural Science Foundation of China (LQ12H29004).
References
- 1.Yang L, Wang Y, Wang L, Xiao H, Wang Z, Hu Z. Rapid quantification of iridoid glycosides analogues in the formulated Chinese medicine Longdan Xiegan Decoction using high-performance liquid chromatograph coupled with mass spectromentry. J Chromatogr A. 2009;1216:2098–2103. doi: 10.1016/j.chroma.2008.06.043. [DOI] [PubMed] [Google Scholar]
- 2.ICH Q8 (R2). Pharmaceutical development. 2009. http://www.ich.org/products/guidelines/quality/article/quality-guidelines.html. Accessed 18 May 2012.
- 3.Basalious EB, El-Sebaie W, El-Gazayerly O. Application of pharmaceutical QbD for enhancement of the solubility and dissolution of a class II BCS drug using polymeric surfactants and crystallization inhibitors: development of controlled-release tablets. AAPS Pharm Sci Tech. 2011;12(3):799–810. doi: 10.1208/s12249-011-9646-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu LX. Pharmaceutical quality by design: product and process development, understanding, and control. Pharm Res. 2008;25(4):781–791. doi: 10.1007/s11095-007-9511-1. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Lionberger RA, Davit BM, Yu LX. Utility of physiological based absorption modeling in implementing quality by design in drug development. AAPS J. 2011;13(1):59–71. doi: 10.1208/s12248-010-9250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Hallak MHDK, Azarmi S, Xu Z, Maham Y, Löbenberg R. Isothermal microcalorimetry as a quality by design tool to determine optimal blending sequences. AAPS J. 2010;12(3):417–423. doi: 10.1208/s12248-010-9202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Jin Y, Cai S, Cheng Y, Qu H. Study Progresses: TCM ethanol precipitation techniques and associated equipment. World Sci Technol. 2007;9(5):16–19. [Google Scholar]
- 8.Gong X, Wang S, Qu H. Comparison of two separation technologies applied in the manufacture of botanical injections: second ethanol precipitation and solvent extraction. Ind Eng Chem Res. 2011;50:7542–7548. doi: 10.1021/ie2004972. [DOI] [Google Scholar]
- 9.Koh GY, Chou G, Liu Z. Purification of a water extract of Chinese sweet tea plant (Rubus suavissimus S. Lee) by alcohol precipitation. J Agric Food Chem. 2009;57:5000–5006. doi: 10.1021/jf900269r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmourlo G, Mendonça-Filho RR, Alviano CS, Costa SS. Screening of antifungal agents using ethanol precipitation and bioautography of medicinal and food plants. J Ethnopharmacol. 2005;96:563–568. doi: 10.1016/j.jep.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Morris-Natscbke SL, Lee KH. New developments in the chemistry and biology of the bioactive constituents of Tanshen. Med Res Rev. 2007;27(1):133–148. doi: 10.1002/med.20077. [DOI] [PubMed] [Google Scholar]
- 12.Li YG, Song L, Liu M, Hu ZB, Wang ZT. Advancement in analysis of Salviae miltiorrhizae Radix et Rhizoma (Danshen) J Chromatogr A. 2009;1216:1941–1953. doi: 10.1016/j.chroma.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 13.Zhou L, Zuo Z, Chow MSS. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol. 2005;45:1345–1359. doi: 10.1177/0091270005282630. [DOI] [PubMed] [Google Scholar]
- 14.Lau KM, Lai KK, Liu CL, Tam JCW, To MH, Kwok HF, et al. Synergistic interaction between Astragali Radix and Rehmanniae Radix in a Chinese herbal formula to promote diabetic wound healing. J Ethnopharmacol. 2012;141:250–256. doi: 10.1016/j.jep.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 15.Izumi E, Ueda-Nakamura T, Veiga VF, Pinto AC, Nakamura CV. Terpenes from Copaifera demonstrated in vitro antiparasitic and synergic activity. J Med Chem. 2012;55:2994–3001. doi: 10.1021/jm201451h. [DOI] [PubMed] [Google Scholar]
- 16.Herranz-Lopez M, Fernandez-Arroyo S, Perez-Sanchez A, Barrajon-Catalan E, Beltran-Debon R, Menedez JA, et al. Synergism of plant-derived polyphenols in adipogenesis: perspectives and implications. Phytomedicine. 2012;19:253–261. doi: 10.1016/j.phymed.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Song F, Zheng Z, Liu Z, Liu S. Characterization of saccharides and phenolic acids in the Chinese herb Tanshen by ESI-FT-ICR-MS and HPLC. J Mass Spectrom. 2008;43:1545–1552. doi: 10.1002/jms.1441. [DOI] [PubMed] [Google Scholar]
- 18.Falcone PM, Tagliazucchi D, Verzelloni E, Giudici P. Sugar conversion induced by the application of heat to grape must. J Agric Food Chem. 2010;58:8680–8691. doi: 10.1021/jf101110s. [DOI] [PubMed] [Google Scholar]
- 19.Severin I, Dumont C, Jondeau-Cabaton A, Graillot V, Chagnon MC. Genotoxic activities of the food contaminant 5-hydroxymethylfurfural using different in vitro bioassays. Toxicol Lett. 2010;192:189–194. doi: 10.1016/j.toxlet.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 20.Adam S, Suzzi D, Radeke C, Khinast JG. An integrated quality by design (QbD) approach towards design space definition of a blending unit operation by discrete element method (DEM) simulation. Eur J Pharm. 2011;42:106–115. doi: 10.1016/j.ejps.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 21.ICH Q9. Quality risk management. 2005. http://www.ich.org/products/guidelines/quality/article/quality-guidelines.html. Accessed 18 May 2012.
- 22.Commission CP. Chinese Pharmacopoeia. Beijing: China Medical Science Press; 2010. p. 907. [Google Scholar]
- 23.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28(3):350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- 24.Lepore J, Spavins J. PQLI design space. J Pharm Innov. 2008;3:79–87. doi: 10.1007/s12247-008-9034-2. [DOI] [Google Scholar]
- 25.Boukouvala F, Muzzio FJ, Ierapetritou MG. Design space of pharmaceutical processes using data-driven-based methods. J Pharm Innov. 2010;5:119–137. doi: 10.1007/s12247-010-9086-y. [DOI] [Google Scholar]
- 26.Huang J, Kaul G, Cai C, Chatlapalli R, Hernandez-Abad P, Ghosh K, Nagi A. Quality by design case study: an integrated multivariate approach to drug product and process development. Int J Pharm. 2009;382:23–32. doi: 10.1016/j.ijpharm.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Gong X, Wang Y, Qu H. Solubilities of protocatechuic aldehyde, caffeic acid, d-galactose, and d-raffinose pentahydrate in ethanol-water solutions. J Chem Eng Data. 2012;57:2018–2022. doi: 10.1021/je300323g. [DOI] [Google Scholar]
- 28.Alves LA, Silva JBA, Giulietti M. Solubility of d-glucose in water and ethanol/water mixtures. J Chem Eng Data. 2007;52(6):2166–2170. doi: 10.1021/je700177n. [DOI] [Google Scholar]
- 29.Bouchard A, Hofland GW, Witkamp GJ. Properties of sugar, polyol, and polysaccharide water-ethanol solutions. J Chem Eng Data. 2007;52(5):1838–1842. doi: 10.1021/je700190m. [DOI] [Google Scholar]
- 30.Gong X, Wang S, Qu H. Solid–liquid equilibria of d-glucose, d-fructose and sucrose in the mixture of ethanol water from 273.2 K to 293.2 K. Chin J Chem Eng. 2011;19(2):1–6. doi: 10.1016/S1004-9541(11)60157-2. [DOI] [Google Scholar]
- 31.Macedo EA, Peres AM. Thermodynamics of ternary mixtures containing sugars. SLE of d-fructose in pure and mixed solvents. Comparison between modified UNIQUAC and modified UNIFAC. Ind Eng Chem Res. 2001;40(21):4633–4640. doi: 10.1021/ie0102596. [DOI] [Google Scholar]
- 32.Kapsi SG, Castro LD, Muller FX, Wrzosek TJ. Development of a design space for a unit operation: illustration using compression-mix blending process for the manufacture of a tablet dosage form. J Pharm Innov. 2012;7:19–29. doi: 10.1007/s12247-012-9122-1. [DOI] [Google Scholar]
- 33.ICH Q10. Pharmaceutical quality system. 2008. http://www.ich.org/products/guidelines/quality/article/quality-guidelines.html. Accessed 18 May 2012.
- 34.Huang H, Qu H. In-line monitoring of alcohol precipitation by near-infrared spectroscopy in conjunction with multivariate batch modeling. Anal Chem Acta. 2011;707:47–56. doi: 10.1016/j.aca.2011.09.031. [DOI] [PubMed] [Google Scholar]


