Abstract
The objective of the present study was to formulate stable silver sulfadiazine (SSD) nanosuspensions and nanogels suitable for topical delivery with a view to increase bactericidal activity in burn therapy. SSD nanosuspensions were formulated using the microprecipitation–high-pressure homogenization technique. An optimized microsuspension of 0.5% SSD formulated with 6% Cremophor EL and 4% Lauroglycol 90 was subjected to 30 cycles of 1,000-bar pressure to give a nanosuspension with an average particle size of 367.85 nm. Transmission electron microscopy studies revealed that ovoid- to rectangular-shaped SSD particles were present as clusters. It was evident through X-ray diffraction studies that SSD was present in amorphous state both in microprecipitate and in nanosuspension. SSD (0.5%) nanogels were prepared using 1% Carbopol 974 P for topical delivery of nanosized SSD. In vitro release studies demonstrated that SSD release was faster from solutions and nanosuspensions compared to gel formulation owing to the influence of the gel matrix on SSD release. The bacterial inhibitory efficiency of SSD nanosuspension was as good as that of SSD solution against Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa. In vivo studies revealed that a nanogel containing 0.5% SSD was more effective in wound healing compared to 0.5% and 1% marketed cream.
KEY WORDS: antibacterial study, burn, high-pressure homogenization, nanosuspension, silver sulfadiazine
INTRODUCTION
Burn injury is the most severe and traumatic injury. Burn wounds are infected with gram-positive bacteria like Staphylococcus aureus, including methicillin-resistant S. aureus (MRSA); Enterococcus species, including vancomycin-resistant species; and gram-negative bacteria like Escherichia coli, Pseudomonas aeruginosa, Klebsiella species, Enterobacter species, and Proteus species (1). Infection of burnt surfaces with microorganisms causes delay or nonhealing of the wound which results in mortality. Infection is responsible for 75% of all deaths in patients with burns exceeding 40% of the total body surface area (1). Appropriate antibacterial therapy needs to be initiated in time to avoid serious damage.
Oral and parenteral antibiotic drugs prescribed in burn wound therapy are not effective to treat the microbial infection. Topical antibiotic therapy is essential for treatment of burns. Silver sulfadiazine (SSD), a drug approved by the Food and Drug Administration, has received widespread acceptance as a topical agent to control bacterial infections in second-degree burn wounds. SSD binds to cell components including DNA and causes membrane damage (2). It achieves bacterial inhibition by binding to the base pairs in the DNA helix and thus inhibits transcription. In a similar way, it also binds to phage DNA (3–6).
SSD is a combination of silver and sulfadiazine. SSD is a polymer wherein each silver ion is tetracoordinated and surrounded by three different deprotonated sulfa molecules; each sulfa molecule in turn binds three different silver ions (7,8). Fox and Modak have reported that silver dissociated from SSD was bound by bacteria, and minute amounts of sulfadiazine appeared to be active (3). When susceptible bacteria are exposed to SSD, structural changes and weakening of the bacterial cell wall and cell membrane result, leading to distortion and enlargement of the cell (9).
Silver nanoparticles are reported to have improved antimicrobial activity due to the extremely large surface area (6). Based on the reports, we assume that nanosized SSD can closely interact with microbial colonies due to enhanced surface area. This may promote rapid and complete healing of burn wounds, reducing trauma of the patient. The polymeric nature of SSD attributes to its insolubility in aqueous and organic media. SSD is freely soluble in 30% ammonia solution. Low solubility can result in minimum toxic potential to microorganisms as well as difficulty in incorporating SSD in synthetic/natural polymeric materials to give nanoparticles (8). Strydom et al. have reported the use of poly (amidoamine) dendrimer complexes with sulfadiazine and silver for bottom–up approach to synthesize SSD nanoparticles (10). It is well established that nanosuspensions are suitable for drugs with poor solubility in aqueous media. Nanosuspensions can provide biological opportunities for site-specific dermal drug delivery owing to small size (11). Taking into account the solubility of a drug, nanosuspensions are a suitable approach for formulating nanosized SSD. Nanosuspensions can be produced either by controlled nanoprecipitation or by a high-energy particle size reduction method like milling, high-pressure homogenization. The company Baxter introduced a combination technology called Nanoedge™. The patented process uses a combination of precipitation and a second high-energy homogenization step to produce nanosuspensions (12). Pu et al. present a preparation of 10-hydroxycamptothecin nanosuspension using microprecipitation and a high-pressure homogenization technique (13). The current study utilizes a combination approach to formulate a stable, uniform SSD nanosuspension. Nanosized particles have increased surface area compared to coarse particles; hence, there is a need for surfactants to stabilize and reduce the surface free energy. It is documented that in order to sufficiently stabilize drug nanosuspension, the surfactant should have sufficient affinity for the particle surface and possess an adequately high diffusion rate to cover the generated surface rapidly (14,15). Nanosuspensions can have drug particulates in crystalline or amorphous state. The nanoprecipitation process is reported to yield amorphous suspensions (16,17). Amorphous drug nanosuspensions are prone to particle growth due to Ostwald ripening which is a process where the difference in local solubility with particle size leads to a transport of material from small to larger particles, with an accompanying increase in the mean particle size with time. It is reported that it is possible to inhibit the Ostwald ripening process in oil in water emulsions by incorporating a small amount of a second component with a very low aqueous solubility. The incorporation of a second component with low aqueous solubility leads to a difference in composition between large and small particles during the Ostwald ripening process. This difference may counterbalance the driving force for Ostwald ripening and eventually result in a termination (16). There are reports stating that hydrophobic substances with a low hydrophilic–lipophilic balance (HLB) value act as Ostwald ripening inhibitors (17). Nonionic stabilizers can be hydrophilic with higher HLB values (8~18) or lipophilic with HLB values in the range 1~8. In the present study, appropriate HLB required to avoid the Ostwald ripening effect and to stabilize nanosized SSD was achieved using a suitable combination of hydrophilic and lipophilic stabilizers.
MATERIALS AND METHOD
Materials
SSD was kindly gifted by Raptakos, Bret and Co. Ltd. and J P N Pharma Pvt. Ltd., Mumbai, India. Stabilizers Cremophor EL (polyoxyl 35 castor oil), Lauroglycol 90 (propylene glycol monolaurate), and Tween 80 (polyoxyethylene sorbitan monooleate) were provided by BASF India Ltd., Mumbai, India; Gattefosse India Ltd., Mumbai, India; and S.D. Fine Chemicals Ltd., Mumbai, India, respectively. Ammonia and acetone, AR grade, were purchased from S.D. Fine Chemicals Ltd., Mumbai, India. Aerosil® R 974 (colloidal silicon dioxide) and Pearlitol ®SD (spray-dried mannitol) were provided by Degussa, Mumbai, and Signet Chemical Corporation Pvt. Ltd., respectively.
Preparation of SSD Nanosuspension by Microprecipitation–High-Pressure Homogenization Method
SSD nanosuspensions were prepared by the microprecipitation—high-pressure homogenization method using a combination of hydrophilic and hydrophobic stabilizers. A batch size of 100 mL was prepared. The formulation prepared contained 6% w/v and 4% w/v of Cremophor EL and Lauroglycol 90, respectively, and 0.5% w/v SSD. Briefly, the solution of Cremophor EL in distilled water, passed through a 0.45-μm filter, was added to 0.5% w/v SSD ammoniacal solution under magnetic stirring at 100 rpm. The solution of 4% Lauroglycol 90 in acetone was quickly added to the aqueous phase. Immediately after addition, ammonia and acetone were removed by heating at 50°C under magnetic stirring at 100 rpm for 4 h. The SSD nanosuspension was obtained by high-pressure homogenization of the microsuspension employing 30 cycles of 1,000-bar pressure (Formula S2; Table I). The SSD microsuspension prepared with 0.25% w/v SSD was subjected to 15 cycles of 1,000-bar pressure (Formula S1; Table I). Figure 1 shows a schematic representation of the SSD nanosuspension preparation. The average particle size and polydispersity index of SSD were determined by photon correlation spectroscopy (PCS: Beckman coulter, N5 submicron particle size analyzer). Measurements were carried out at 90° at 25°C. Dispersions were diluted with distilled water to ensure that the light scattering intensity was within the instrument's sensitive range. Distilled water was filtered through a 0.45-μm filter (Pall Life Sciences, Mumbai). SSD suspensions were lyophilized by a freeze-drying machine (Biocryos, Korea) using Pearlitol ®SD as cryoprotectant. SSD suspensions were adsorbed on colloidal silicon dioxide in order to have a sample in dry powder form for X-ray diffraction (XRD) studies.
Table I.
List of Formulations Prepared by Varying Formulation and Process Parameters
| Formula | S1 | S2 | S3 | S4 | S5 |
|---|---|---|---|---|---|
| SSD concentration | 0.25% | 0.5% | 0.5% | 0.5% | 0.5% |
| Hydrophilic stabilizer concentration | Cremophor EL, 6% | Cremophor EL, 6% | Cremophor EL, 10% | Tween 80, 6% | Cremophor EL, 6% |
| Hydrophobic stabilizer concentration | Lauroglycol 90, 4% | Lauroglycol 90, 4% | Lauroglycol 90, 6% | Lauroglycol 90, 4% | Lauroglycol 90, 4% |
| Homogenization pressure cycles | 1,000 bars | 1,000 bars | 1,000 bars | 1,000 bars | 1,200 bars |
| Homogenization cycle numbers | 15 cycles | 30 cycles | 30 cycles | 30 cycles | 30 cycles |
SSD silver sulfadiazine
Fig. 1.
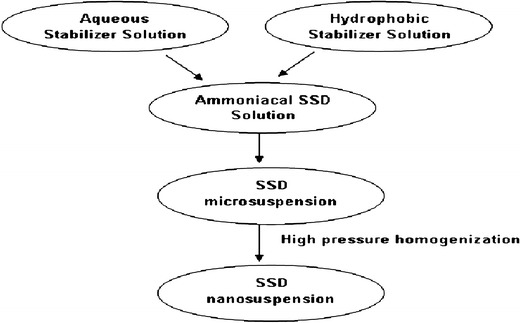
Schematic representation of SSD nanosuspension preparation
Optimization of SSD Nanosuspension
The nanosuspension was optimized with regard to formulation and process parameters presented in Table I. Two formulations were prepared with combinations of hydrophilic surfactants, 10% w/v of Cremophor EL, and 6% w/v Tween 80 with hydrophobic surfactants, 6% w/v Lauroglycol 90, and 4% w/v Lauroglycol 90, respectively (Formulae S3, S4; Table I). In order to study the effect of pressure cycles, the microsuspension prepared with 6% Cremophor EL and 4% Lauroglycol 90 was subjected to 30 cycles of 1,200-bar pressure (Formula S5; Table I).
Analysis of Drug Content
SSD nanosuspensions theoretically equivalent to 250 and 500 μg were measured by volume from 0.25% and 0.5% nanosuspension, respectively, and appropriately diluted with ammonia and methanol. The absorbance values were read in a UV/visible spectrophotometer (JascoV-530 Japan).
Transmission Electron Microscopy
Transmission electron microscopy (TEM) analysis was carried out to evaluate the external morphology of optimized 0.5% SSD nanosuspension. Briefly, a drop of nanosuspension was placed on a 300-mesh-size copper grid, and some of the SSD particles were allowed to stick to the grid. The film was allowed to dry, and the grid was observed under an electron microscope (PHILIPS Model: CM200).
X-ray Diffraction Studies
X-ray diffraction (XRD) patterns were recorded to evaluate the physical state of SSD in optimized 0.5% SSD nanosuspensions. X-ray diffraction patterns of SSD suspensions prior to and after subjecting to high-pressure homogenization were recorded. X-ray powder diffraction patterns of freeze-dried and Aerosil R 974 adsorbed SSD suspensions were recorded on a Philips PW 17291-powder X-ray diffractometer using Ni-filtered, Cu K radiation, a voltage of 40 kV, and a 25-mA current. The scanning rate employed was 1° min−1 over the 5–50° 2θ range.
Antibacterial Studies
Zone of inhibition, minimum inhibitory concentration (MIC), and minimum bactericidal concentration (MBC) studies were performed on the formulations.
Zone of Inhibition Studies
Zone of inhibition studies of the SSD formulation were carried out on Mueller Hinton agar plates against S. aureus ATCC number 6538 using the cup plate method. S. aureus suspension with 108 colony forming units was added to Mueller Hinton agar, mixed, and allowed to solidify. After solidification of agar, wells were scooped in each plate using a sterile cork borer. The nanosuspension and ammoniacal standard solution containing 0.5% SSD were evaluated. The positive control plate was prepared with only the media and organism without SSD formulation/standard SSD solution. Three percent ammonia was used as vehicle control. After incubation of the plates at 37°C for 48 h, the zone of inhibition was measured, and the diameter was recorded to the nearest millimeter with the help of a measuring scale.
MIC and MBC Studies
The broth dilution method using Luria Bertani medium was employed to determine the minimal concentration of antimicrobial agent to inhibit or kill the microorganism. Three 96-well microtiter plates were used in the study, one for each bacteria to be tested, namely S. aureus, E. coli, P. aeruginosa. A standard 0.5% SSD ammoniacal solution and two SSD nanosuspensions with 0.25% and 0.5% SSD were evaluated. Based on the references available, samples were appropriately diluted and tested in concentration ranges 15–150, 5–45, and 1–40 ppm against S. aureus, E. coli, and P. aeruginosa, respectively (18–20). The MIC of the ammonia solution in the range 0.015–0.75% v/v and stabilizers 4% w/v Cremophor EL and 6% w/v Lauroglycol 90 used in nanosuspensions were tested against all three organisms. The stabilizer solutions were treated in the same way as formulations tested in concentration ranges 110–150, 25–45, and 1–40 ppm against S. aureus, E. coli, and P. aeruginosa, respectively. The plates were incubated for 48 h at 37°C. The optical densities of the contents in the wells were measured in a microplate reader before and after incubation.
For MBC measurement, 25 μl of colorless triphenyl tetrazolium chloride (TTC) dye was added to each well. The plate was incubated at 37°C for 24 h. MBC was determined by visual observation of red color.
In Vitro Release Studies
Formulation of SSD Nanogel
A topical gel containing 0.5% SSD was formulated using Carbomer 974P as gelling agent. Briefly, 1% Carbomer 974P and 0.1% sodium salt of methyl paraben were added to the nanosuspension containing 0.5% w/v SSD. The mixture was kept aside for 1 h for swelling and was intermittently stirred with a glass rod. Later, a drop of 0.3% ammonia solution was added to neutralize the pH.
Release Study
Diffusion through a dialysis membrane is a widely used technique to evaluate the release from colloidal dispersions and topical formulations (21,22). An SSD marketed formulation contains 1% w/w SSD. The marketed formulation was mixed with equal weight of no drug carbopol gel to give a gel with 0.5% SSD.
In the present study, nanogel (1 g), modified marketed cream (1 g), nanosuspension (1 mL), and ammoniacal solution (1 mL) were evaluated. All the samples evaluated contained 0.5% SSD. Samples were placed in a dialysis bag (molecular weight cutoff, 12,000 Da, Himedia, India) immersed in a beaker with 25 mL phosphate-buffered saline (PBS: pH 7.4). The bags were tied with threads at both the ends and suspended in a beaker. The medium was stirred with the help of a magnetic stirrer (Remi equipments, India) at 50 rpm. An aliquot of 1 mL dissolution medium was removed at a series of various points (0.5, 1, 2, 3, 4, 6, 8, 10, 12, 24, 36, and 48 h), and the same volume of fresh dissolution medium was added accordingly. The aliquots were analyzed at 263 nm by UV spectrophotometer.
In Vivo Study
The main purpose of in vivo studies was to evaluate the effectiveness of 0.5% SSD nanogel in treating hot water-induced burn wounds. In vivo efficacy was evaluated on female Sprague Dawley rats (n = 6). The protocol for the study was approved by the Institutional Animal Ethics Committee of the Bombay College of Pharmacy, Mumbai, India, and the animal handling throughout the experiment was performed in accordance to the Committee for the Purpose of Control and Supervision on Experiments on Animals guidelines. Female Sprague Dawley rats were provided by Glenmark Pharmaceuticals Ltd., Navi Mumbai.
Female Sprague Dawley rats (200–220 g) were divided into five groups of six animals each wherein group 1, 2, 3, and 4 were treated with 0.5% nanogel (quantity applied—500 mg), 0.25% nanogel (quantity applied—1 g), 1% marketed cream (quantity applied—250 mg), and 0.5% modified marketed formulation (quantity applied—500 mg), respectively. Group 5 animals were control animals with no treatment.
Procedure
The dorsum of each Sprague Dawley rat was shaved. The hair on the back of the rats was clipped with a clipper, and the remaining hair was removed using a depilatory. The shaved rats were allowed a rest period of 24 h to recover from any skin injury that might have occurred during shaving of the skin. Burn wounds were inflicted on animals under ketamine HCl (22 mg/kg body weight, i.m.) anesthesia. The trauma was performed by exposing the shaved backskin of anesthetized animals to hot water. For this procedure, a cylindrical-shaped bar with a radius of 1 cm was placed on the backs of the rats, and then, hot water (95°C) was poured into this bar and held for 120 s. After the formation of standard, second-degree burn wounds, the formulations were repeatedly applied (one application every day) to the burned areas for 14 days post wounding (23). The wound contraction was calculated as the percentage of the original wound size for each animal of a group. Wound contraction was noted by following the progressive changes in wound area planimetrically, excluding the day of wounding. The size of the wounds was traced on transparent paper throughout the monitoring period. The tracing was then transferred to graph paper from which wound surface area was evaluated (24). The evaluated surface area was then employed to calculate the percentage of wound contraction, taking the initial size of the wound as 100% by using the following equation:
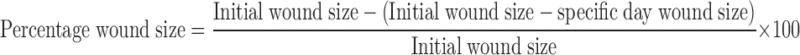 |
Photographs of the wound surfaces were taken for visual comparison. One-way ANOVA followed by Newman–Keuls multiple comparison posttest was applied to the data.
RESULTS AND DISCUSSION
Preparation of SSD Nanosuspension by Microprecipitation–High-Pressure Homogenization Method
Nanosuspensions of SSD were prepared by the combination of microprecipitation and high-pressure homogenization method. A combination of hydrophilic and hydrophobic stabilizers was employed in formulating stable nanosuspensions. Based on preliminary experiments, we concluded that Cremophor EL, when used with Lauroglycol 90, gave a stable SSD microsuspension. Lindfors et al. address the requirement of the miscibility of the Ostwald ripening inhibitor with the dispersion medium and have also demonstrated that when these two components do not mix, inhibition of the ripening is not achieved (16). With this view, acetone was utilized as solvent and distributing agent for Lauroglycol 90. The SSD microsuspension prepared with 0.25% drug load was subjected to 15 cycles of 1,000-bar pressure. The results are shown in Fig. 2. There was particle size reduction up to the eighth cycle after which there was insignificant change. The polydispersity index was reduced from an initial value of 1.95 to 0.87 at the end of the 15th high-pressure cycle. The formulation prepared with 0.5% SSD was subjected to 30 cycles of 1,000-bar pressure. With the increase in the SSD load to 0.5% w/v, 25 pressure cycles were required to get the polydispersity index below 1 as seen in Fig. 3. After the tenth cycle, the particle size did not change significantly. However, the polydispersity index decreased with the increase in homogenization pressure cycles. There was gradual decrease in polydispersity index from an initial value of 1.8 to 0.75 at the end of the 30th cycle. It has been reported that in order to achieve a product of similar size as low concentrated nanosuspension, more cycles (disintegration energy) need to be applied to the highly concentrated suspension (25). Keck and Muller explain the two-step diminution process in a homogenizer. In the first step, the majority of the particles (bulk population) reach smaller size relatively fast. Further homogenization cycles have little effect on the mean diameter of the bulk but reduce the width of the distribution by eliminating the remaining few large crystals in the second step. Therefore, even when the mean diameter of the bulk population has reached its minimum and stays constant, additional homogenization cycles improve the homogeneity of the population which further reduces the potential effects of Ostwald ripening (26). The results obtained after high-pressure homogenization are in agreement to both the observations reported.
Fig. 2.
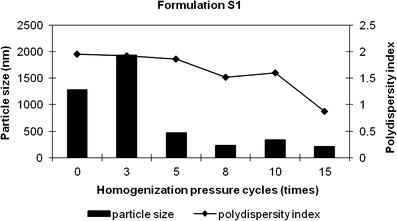
Particle size distribution of formulation S1. Bars represent particle size data, and lines represent polydispersity index data
Fig. 3.
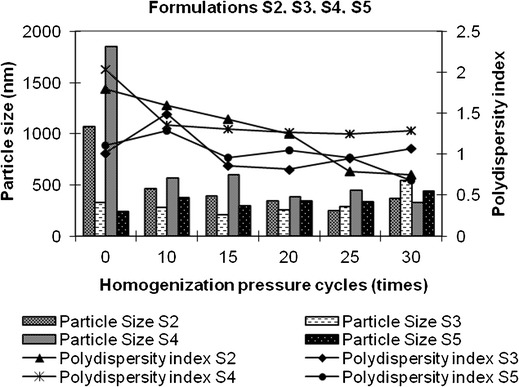
Particle size distribution of S2, S3, S4, and S5 formulations. Bars represent particle size data, and lines represent polydispersity index data
Optimization of SSD Nanosuspension
The SSD formulation (0.5%) was optimized with regard to formulation and process parameters. With the process parameters controlled, an increase in the concentration of Cremophor EL as well as Lauroglycol 90 to 10% and 6%, respectively, as in Formula S3 (Table I) did not give nanosuspensions with a polydispersity index less than 1 as shown in Fig. 3. As 1,000-bar pressure cycles increased from 0 to 30, there was a decrease and then apparent increase in particle size and polydispersity index. At the 20th cycle, the particle size and polydispersity index had reduced to 251.6 nm and 0.81 from 325 to 1.01, respectively. Similar particle size distribution was obtained after subjecting formula S2 to 30 cycles of 1,000-bar pressure. It can be inferred that when other parameters are kept constant, an increase in surfactant concentrations requires a lesser number of pressure cycles to obtain a similar particle size and polydispersity index. However, a further increase in pressure cycles to 30 increases particle size and polydispersity index. Figure 3 shows that after 30 cycles of 1,000-bar pressure, a combination of 6% w/v Tween 80 and 4% w/v Lauroglycol 90 yielded 327.5-nm-sized SSD particles, but the polydispersity index was more than 1. The hydrophilic stabilizer, Tween 80, selected to stabilize the suspension, was not effective enough and exhibited polydispersity index 1.29 even after 30 pressure cycles of 1,000 bars. In the case of the high-pressure homogenization method, pressure cycles play an important role in determining the particle size characteristics. In order to study the effect, the SSD microsuspension prepared using 6% w/v Cremophor EL and 4% w/v Lauroglycol 90 was subjected to 30 cycles of 1,200-bar pressure (Formula S5, Table I). The results are shown in Fig. 3. A comparison was made with nanosuspension formula S2. It was observed that the increase in homogenization pressure to 1,200 bars did not show any significant difference in particle size and polydispersity index seen in nanosuspension formula S2. It was concluded that 30 cycles of 1,000-bar pressure is the optimum pressure cycle required to produce 0.5% SSD nanosuspension with 6% Cremaphor EL and 4% Lauroglycol 90.
Analysis of Drug Content
The assay of 0.25% and 0.5% nanosuspensions showed that the calculated contents of SSD correlated with the values of theoretical content in the system. All the SSD present in the system was accounted for in the assay.
Transmission Electron Microscopy
TEM studies were done on 0.5% SSD nanosuspension to get more insight of the system. The SSD nanoparticles were found to be ovoid- to rectangular-shaped and seen as clusters as shown in Fig. 4. The particle size obtained by TEM was 50 nm. However, PCS studies showed an average particle size of 367 nm. A polydispersity index of 0.75 indicates broad particle size distribution. PCS is based on the dynamic light scattering technique which could explain the larger size in PCS studies.
Fig. 4.

Morphology of SSD nanosuspension using transmission electron microscopy
X-ray Diffraction Studies
SSD is a highly crystalline molecule. A strong intense peak of SSD crystal has been reported at 10.20° (27). A characteristic sharp peak at around 10.18° was observed in the XRD spectrum of the SSD sample. XRD patterns recorded for freeze-dried and Aerosil R 974 adsorbed powder did not show sharp peaks of crystalline SSD indicating the presence of the amorphous state of SSD as shown in Fig. 5. A less intense peak at 10.18° was observed in all the spectra which indicates significant loss of crystallinity in the SSD nanosuspension both prior to and after subjecting to high-pressure homogenization. SSD was in an amorphous state after microprecipitation prior to high-pressure homogenization. Based on the results obtained, we can conclude that the high-pressure homogenization process did not change the physical nature of SSD in the suspension. However, there are reports stating that when a precipitated particle suspension is subsequently homogenized, the “annealing” process converts all precipitated particles to crystalline material. Keck and Muller report that in such a case, drug nanocrystals possess a definite crystalline state (26). In another report of the research work by Pu et al., XRD spectra of lyophilized 10-hydroxy camptothecin nanosuspension, prepared by the combination of microprecipitation and high-pressure homogenization methods, showed the absence of crystalline peaks of the drug indicating its amorphous nature after freeze-drying (13). Results obtained in the current study are in agreement with this report.
Fig. 5.
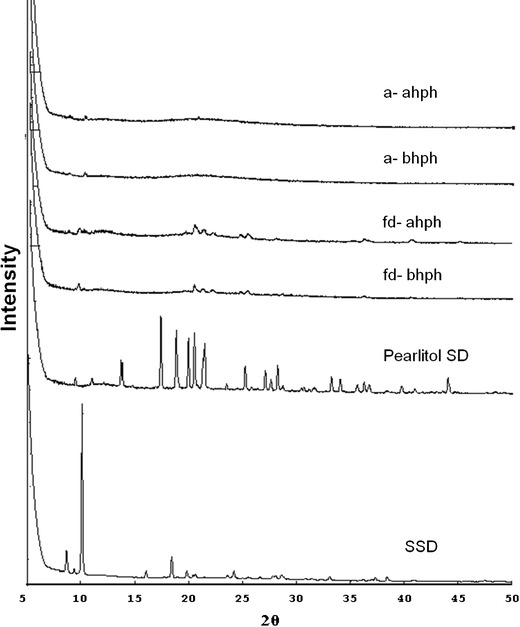
X-ray diffraction patterns of SSD in microsuspension and nanosuspension. SSD silver sulfadiazine; Pearlitol SD, fd-bhph freeze-dried sample of SSD suspension before subjecting to high-pressure homogenization; fd-ahph freeze-dried sample of SSD suspension after subjecting to high-pressure homogenization; a-bhph Aerosil R 974 adsorbed sample of SSD suspension before subjecting to high-pressure homogenization; a-ahph Aerosil R 974 adsorbed sample of SSD suspension after subjecting to high-pressure homogenization
Antibacterial Studies
Zone of Inhibition Studies
The inhibitory activity of SSD was determined against S. aureus. After incubation for 48 h, matted growth was obtained on the positive control plate which confirms the growth of microorganisms and the suitability of the medium and experimental conditions in the study. Distinct zones of inhibition were obtained for the SSD nanosuspension and SSD standard solution. The SSD formulation showed a 15-mm zone whereas the SSD ammoniacal solution showed 33.5 mm zone size (Table II). However, 3% ammonia solution, the vehicle in the SSD standard solution, showed a 30-mm zone. On accounting for vehicle control, i.e., subtracting the values obtained by SSD ammoniacal solution and 3% ammonia solution, 3.5 mm is the zone size for SSD standard drug solution. Since the zone of inhibition of the SSD nanosuspension is greater than that of the standard solution, we can infer that the antibacterial activity of the formulation is superior to the drug solution probably owing to close interaction of nanosized silver with S. aureus.
Table II.
Zone of Inhibition Values of SSD in Nanosuspension and Solution
| Formulation | Zone of inhibition (mm) |
|---|---|
| 0.5% SSD nanosuspension | 15 |
| 0.5% SSD ammoniacal standard solution | 33.5 |
| 3% ammonia solution (SSD vehicle) | 30 |
SSD silver sulfadiazine
MIC and MBC Studies
The broth dilution method used in the present study gives an advantage of using the same wells for MIC and MBC determinations. MIC, the lowest concentration of nanoparticles that inhibits growth of bacteria, was determined. Luria Bertani broth was used in the present study as it supports growth of S. aureus, E. coli, and P. aeruginosa (28). Cremophor EL (6% w/v) and Lauroglycol 90 (4% w/v) were treated similar to the formulations tested for MIC. The MIC value of SSD formulations and solution was found to be less than 15 ppm which was the lowest concentration used in the study against S. aureus. Cremophor EL (6% w/v) did not show any inhibitory activity against S. aureus. However, ammonia and 4% w/v Lauroglycol 90 solution showed a MIC value 0.75% and concentration equivalent to <110 ppm formulation, respectively (Table III). Therefore, there could be some contribution of Lauroglycol 90 and ammonia in the MIC value of SSD formulations and solution, respectively. MIC values obtained against E. coli were less than 5 ppm for the SSD solution and formulations which was the lowest concentration of SSD used in the present study against E. coli. Ammonia displayed inhibitory activity at 0.15% concentration. The inhibitory activity of ammonia may contribute to the MIC of 0.5% w/v SSD ammoniacal solution. The MIC value of the formulation and solutions was found to be same for SSD against S. aureus and E. coli. Thirumurugan et al. have compared the MIC values of silver nanoparticles synthesized from Phytophthora infestans and silver nitrate solution against E. coli and S. aureus. Silver nanoparticles gave lower MIC readings compared to solubilized silver in silver nitrate solution (29). SSD nanosuspensions and solution showed the same MIC value of 10 ppm against P. aeruginosa. Surfactants and ammonia did not display any inhibitory activity. However, with P. aeruginosa, in vitro sensitivity does not consistently predict therapeutic efficacy in experimentally infected burned rats (9). MIC studies by broth dilution method indicate the prevention of growth of microorganisms in the presence of an antibacterial agent. There is no indication that microorganisms have been killed by an antimicrobial agent. The presence of viable microorganisms was tested using colorless TTC dye. MBC value was considered as the lowest concentration of the formulation giving colorless well after incubation. The SSD (0.5%) nanosuspension and solution showed 30 and 20 ppm MBC value, respectively against S. aureus. The minimum bactericidal concentrations of 0.25% and 0.5% SSD nanosuspension against E. coli were found to be 30 and 25 ppm, respectively, as seen in Table III, whereas the 0.5% SSD solution showed higher activity with MBC of 15 ppm against E. coli. Interaction of solubilized SSD was more than nanosized SSD against E. coli. SSD (0.25%) nanosuspension and 0.5% SSD solution showed an equivalent bactericidal activity with MBC of 10 ppm whereas the 0.5% SSD nanosuspension showed lower activity with a higher MBC value of 20 ppm against P. aeruginosa. It is expected that both nanosized SSD formulations would show equivalent activity. Ammonia and surfactant solutions did not exhibit any bactericidal activity against all organisms. SSD serves as a reservoir of silver and slowly liberates silver ions. It has been reported that the bactericidal effect of silver nanoparticles has been attributed to their small size and high surface-to-volume ratio, which allows them to interact closely with microbial membranes and is not merely due to the release of metal ions in solution. It is also published that the bactericidal effect of silver nanoparticles decreases as the size increases (30). SSD nanosuspensions are expected to exhibit bactericidal activity at least equivalent to that of the SSD solution. Surprisingly, the results obtained were not in agreement with the report.
Table III.
MIC and MBC Values of SSD in Nanosuspension and Solution
| Formulation | Concentration in ppm | |||||
|---|---|---|---|---|---|---|
| S. aureus | E. coli | P. aeruginosa | ||||
| MIC | MBC | MIC | MBC | MIC | MBC | |
| 0.25% SSD nanosuspension | <15 | <15 | <5 | 30 | 10 | 10 |
| 0.5% SSD nanosuspension | <15 | 30 | <5 | 25 | 10 | 20 |
| 0.5% SSD solution | <15 | 20 | <5 | 15 | 10 | 10 |
| Ammoniaa | 0.75 | – | 0.15 | – | – | – |
| Surfactantsb | Lauroglycol 90 < 110 ppm | – | – | – | – | – |
ppm part per million, MIC minimum inhibitory concentration, MBC minimum bactericidal concentration, SSD silver sulfadiazine
aConcentration in percentage
bConcentration equivalent to formulation
In Vitro Release Studies
Homogenous, smooth SSD nanogels were prepared using Carbopol 974 P as gelling agent. In order to compare release profiles of 0.5% SSD nanogels with marketed formulation, 1% marketed cream was diluted with Carbopol gel to 0.5%. Nascimento et al. have reported the use of a dialysis bag to determine SSD release from chitosan gels and marketed cream (22). PBS was used as a medium to analyze SSD release from SSD chitosan wound dressings (31). Based on the references available on in vitro release studies of SSD formulations, a diffusion technique using PBS pH 7.4 medium was employed in the present study. SSD release from 0.5% SSD nanogel and 0.5% SSD modified marketed formulation was compared. SSD nanosuspension and ammoniacal solution were used as control. The results are shown in Fig. 6. Initially, up to 3 h, SSD release from SSD nanogel was slow compared to the release from modified marketed formulation. SSD release from nanogel and marketed formulation were 11.89% and 15.57%, respectively, at 3 h. However, as time progressed, the release from nanogel was fast compared to marketed preparation. At the end of 48 h, 42.79% SSD was released from SSD modified marketed formulation whereas an equivalent amount (45.5%) was released from SSD nanogel at the end of 24 h. Faster release may be due to large surface area of nanosized SSD present in nanogel, increasing its dissolution velocity. We can infer that in comparison to marketed formulation, there was an increase in the release of SSD from nanogel which would result in improved antimicrobial activity. An unpaired t test was applied to the release data. Statistically significant difference was observed on comparing the release data of SSD from nanogel and marketed formulation at 12-, 24-, 36-, and 48-h time points (P < 0.05). At the end of the study, SSD released from nanosuspension and ammoniacal solution was 73.65% and 90.53%, respectively. It is reported that the release of an active substance from formulation and its penetration through the diffusion barriers is inversely related to viscosity of the formulation (32). Therefore, SSD release from nanosuspension was significantly high compared to SSD nanogel (P < 0.05). Since SSD is in solubilized state in ammoniacal solution, the diffusion through the dialysis membrane is faster compared to the nanosuspension and gel formulations (P < 0.05).
Fig. 6.
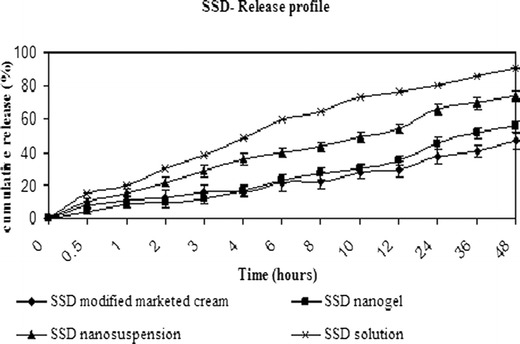
In vitro release data of SSD from nanogel, modified marketed formulation, nanosuspension, and solution. Data expressed as mean ± SD (standard deviation)
In Vivo Study
Second-degree burn wounds were induced by adding hot water into the metal mold placed on the dorsal surface of rats. The quantity of preparation applied was such that the amount of drug delivered to the burn wound site was constant. Animals were treated with 500 mg of 0.5% SSD nanogel, 0.5% SSD modified marketed formulation. One gram of 0.25% SSD nanogel and 250 mg of 1% SSD marketed cream were applied. Burn wounds were traced every day for 14 days. Comparison among groups was done on the basis of wound size on the particular day. The initial wound size before application of formulations was taken as 100%. The results of percent wound size on days 3, 6, 9, 12, and 14 are shown in Fig. 7. At the end of the study, the wound contraction observed by treatment with 0.5% SSD nanogels (P < 0.05) and 1% SSD marketed cream (P < 0.05) was significantly higher than that of the control group. The wound size of animals treated with 0.25% SSD nanogels and 0.5% SSD modified marketed formulation was less compared to the control group though not statistically different. Therefore, we can conclude that 0.5% SSD nanogel and 1% SSD marketed cream were effective in healing burn wounds. On day 3, the mean wound size of animals treated with 0.5% SSD nanogel and 1% marketed SSD cream was 70.41% and 78.66%, respectively, whereas 0.25% SSD nanogels reduced the wound size to only 94.21%. There was no wound contraction observed in animals treated with 0.5% modified marketed preparation till day 3. On applying statistics, a significant difference was observed in wound size of animals treated with 0.5% SSD nanogel and 0.5% marketed formulation (P < 0.05). Similarly, on day 9, 0.5% nanogel exhibited better activity compared to marketed formulations (P < 0.05). The wound size observed by treatment with 0.5% nanogel was 32% whereas with 1% marketed and 0.5% modified marketed formulations, the wound size was 66.5% and 67.6%, respectively. The wound size observed on treatment with 0.25% SSD was 52.5%. On day 6 and 12, wound contraction was observed in all groups; however, there was no statistically significant difference in the wound size. Treatment with 0.5% SSD nanogel and 0.5% SSD modified marketed formulation reduced the wound size to 8.18% and 31.01%, respectively, on the 14th day, the last day of the study. There was significant difference in the wound contraction (P < 0.05). The wound size observed on treatment with 1% marketed cream and 0.25% SSD nanogels was 15.16% and 27.68%, respectively. This establishes the fact that 0.5% SSD nanogel is more efficient than the marketed formulation prepared with same concentration of SSD. Wound healing rate of 0.5% SSD nanogel is higher than conventional marketed cream. It was reported in an article by Fox and Modak that the efficacy of silver sulfadiazine results from its slow and steady reactions with serum and other sodium chloride-containing body fluids, which permits the slow and sustained delivery of silver ions into the wound environs (3). Small size and large surface area of released nano silver aids in closer interaction with bacteria. Therefore, 0.5% SSD nanogel shows higher activity than marketed formulation with micron-sized drug particles. However, 0.25% SSD nanogels where the quantity of application was large showed lesser wound contraction compared to 0.5% SSD nanogels and 1% SSD marketed cream. This could be due to the slow and less diffusion through the thick layer of 1 g applied onto the burnt surface. Based on the results, it can be concluded that 0.5% SSD nanogels shows enhanced wound healing activity compared to marketed preparations. The results obtained by in vivo studies were satisfactory. Representative photographs of wounds of different groups 1–5 on day 1 and 14 are shown in Fig. 8.
Fig. 7.
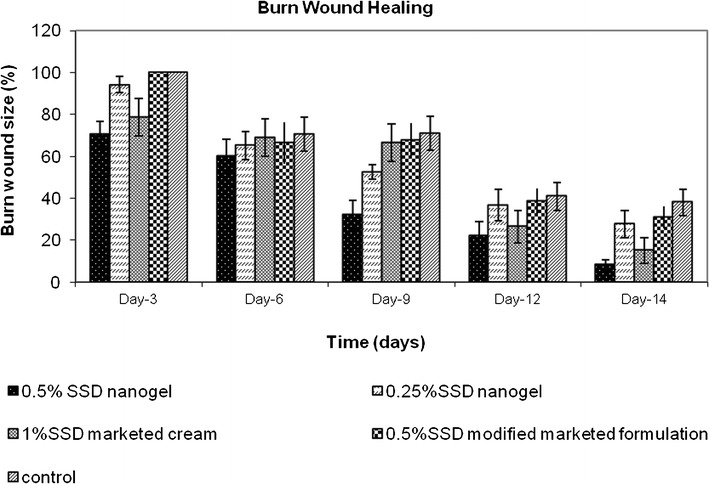
Graphical representation of burn wound healing in rats (n = 6). Data expressed as mean ± SEM (standard error of mean)
Fig. 8.
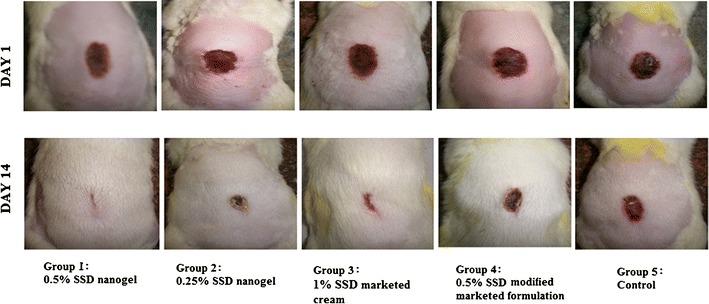
Representative photographs of burn wounds in rats
CONCLUSION
Stable SSD nanosuspensions were prepared with a combination of hydrophilic and hydrophobic stabilizers, Cremophor EL and Lauroglycol 90, using microprecipitation–high-pressure homogenization technique. SSD nanogels were prepared for topical delivery of nanosized SSD using Carbopol as gelling agent. SSD nanosystems exhibited improved antibacterial activity against pathogens commonly invading burn wounds. The wound healing activity of SSD nanogel was superior to that of SSD marketed cream in hot water-induced burn wounds in female Sprague Dawley rats. SSD nanogels show good potential for faster burn wound healing which will reduce the trauma of the patient. SSD nanosuspensions and nanogels are promising systems to provide relief to patients suffering from large-surface-area burns.
ACKNOWLEDGMENTS
The authors are thankful to Raptakos, Bret and Co. Ltd. and J P N Pharma Pvt. Ltd., Mumbai, India, for gift samples of silver sulfadiazine. The authors also thank BASF India Ltd., Mumbai, India; and Gattefosse India Ltd., Mumbai, India, for providing gift samples of excipients. The authors wish to thank Glenmark Pharmaceuticals Ltd., Navi Mumbai, for providing the animals. The authors are grateful to TIFR, SAIF IITB for help in XRD and TEM studies, and also thank MKR Laboratory and the microbiology department of C.H.M College for help with antibacterial studies.
REFERENCES
- 1.Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006;19:403–434. doi: 10.1128/CMR.19.2.403-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atiyeh BS, Costagliola M, Hayek SN, Dibo SA. Effect of silver on burn wound infection control and healing: review of the literature. Burns. 2007;33:139–148. doi: 10.1016/j.burns.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Fox CL, Modak SM. Mechanism of silver sulfadiazine action on burn wound infections. Antimicrob Agents Chemother. 1974;5:582–588. doi: 10.1128/AAC.5.6.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maple PAC, Hamilton-Miller JMT, Brranfltt W. Comparison of the in-vitro activities of the topical antimicrobials azelaic and, nitroforazone, silver sulphadiazine and mupirocin against methcillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1992;29:661–668. doi: 10.1093/jac/29.6.661. [DOI] [PubMed] [Google Scholar]
- 5.Mcdonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12:147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27:76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Bult A. Silver sulfadiazine and related antibacterial metal sulfanilamides: facts and fancy. Pharm Int. 1982;3:400–404. [Google Scholar]
- 8.Capelli CC, Wis K, inventor; Biointerface Technologies, Inc., Madison, Wis, assignee. Metal Based antimicrobial coating. United States Patent 4933178.1990 June 12.
- 9.Dollery C. Silver sulfadiazine. In: Therapeutic drugs. 2nd ed. Edinburgh: Churchill Livingstone; 1999. p. S32–5.
- 10.Strydom SJ, Rose WE, Otto DP, Liebenberg W, de Villers MM. Poly (amidoamine) dendrimer-mediated synthesis and stabilization of silver sulfonamide nanoparticles with increased antibacterial activity. Nanomedicine. 2012. doi:10.1016/j.nano.2012.03.006. [DOI] [PubMed]
- 11.Mishra PR, Shaal LA, Müller RH, Keck CM. Production and characterization of hersperetin nanosuspensions for dermal delivery. Int J Pharm. 2009;371:182–189. doi: 10.1016/j.ijpharm.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 12.Kipp JE, Wong JCT, Doty MJ, Rebbeck CL, inventor; Baxter International Inc., assignee. Microprecipitation method for preparing submicron suspensions. US Patent 6869617 B2. 2005 Mar 22.
- 13.Pu X, Sun J, Wang Y, Wang Y, Liu X, Zhang P, et al. Development of a chemically stable 10-hydroxycamptothecin nanosuspensions. Int J Pharm. 2009;379:167–173. doi: 10.1016/j.ijpharm.2009.05.062. [DOI] [PubMed] [Google Scholar]
- 14.Gao L, Zhang D, Chen M. Drug nanocrystals for the formulation of poorly soluble drugs and its application as a potential drug delivery system. J Nanoparticles Res. 2008;10:845–862. doi: 10.1007/s11051-008-9357-4. [DOI] [Google Scholar]
- 15.Xia D, Quan P, Piao H, Piao H, Sun S, Yin Y, et al. Preparation of stable nitrendipine nanosuspensions using the precipitation–ultrasonication method for enhancement of dissolution and oral bioavailability. Eur J Pharm Sci. 2010;40:325–334. doi: 10.1016/j.ejps.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Lindfors L, Skantze P, Skantze U, Rasmusson M, Zackrisson A, Olsson U. Amorphous drug nanosuspensions. 1. Inhibition of Ostwald ripening. Langmuir. 2006;22:906–910. doi: 10.1021/la0523661. [DOI] [PubMed] [Google Scholar]
- 17.Sigfridsson K, Forssen S, Hollander P, Skantze U, de Verdier J. A formulation comparison, using a solution and different nanosuspensions of a poorly soluble compound. Eur J Pharm Biopharm. 2007;67:540–547. doi: 10.1016/j.ejpb.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Carr HS, Wlodkowski TJ, Rosenkranz HS. Silver sulfadiazine: in vitro antibacterial activity. Antimicrob Agents Chemother. 1973;4:585–587. doi: 10.1128/AAC.4.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamilton-Miller JMT, Shah S. A microbiological assessment of silver fusidate, a novel topical antimicrobial agent. Int J Antimicrob Agents. 1996;7:97–99. doi: 10.1016/0924-8579(96)00302-0. [DOI] [PubMed] [Google Scholar]
- 20.Waasbergen LG, Fajdetic I, Fianchini M, Dias HVR. Antimicrobial properties of highly fluorinated tris(pyrazolyl)borates. J Inorg Biochem. 2007;101:1180–1183. doi: 10.1016/j.jinorgbio.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Verger MLL, Fluckiger L, Kim Y, Hoffman M, Maincent P. Preparation and characterization of nanoparticles containing an antihypertensive agent. Eur J Pharm Biopharm. 1998;46:137–143. doi: 10.1016/S0939-6411(98)00015-0. [DOI] [PubMed] [Google Scholar]
- 22.Nascimento EG, Sampaio TB, Medeiros AC, Azevedo EP. Evaluation of chitosan gel with 1% silver sulfadiazine as an alternative for burn wound treatment in rats. Acta Cir Bras. 2009;24:460–465. doi: 10.1590/S0102-86502009000600007. [DOI] [PubMed] [Google Scholar]
- 23.Alemdaroglu C, Degim Z, Celebi N, Zor F, Ozturk S, Erdogan D. An investigation on burn wound healing in rats with chitosan gel formulation containing epidermal growth factor. Burns. 2006;32:319–327. doi: 10.1016/j.burns.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Srivastava P, Durgaprasad S. Burn wound healing property of Cocos nucifera: an appraisal. Indian J Pharmacol. 2008;40:144–146. doi: 10.4103/0253-7613.43159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krause KP, Müller RH. Production and characterisation of highly concentrated nanosuspensions by high pressure homogenisation. Int J Pharm. 2001;214:21–24. doi: 10.1016/S0378-5173(00)00626-8. [DOI] [PubMed] [Google Scholar]
- 26.Keck CM, Müller RH. Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation. Eur J Pharm Biopharm. 2006;62:3–16. doi: 10.1016/j.ejpb.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Bult A, Plug CM. Silver sulfadiazine. In: Florey K, editor. Analytical profile of drug substances. New York: Academic; 1984. pp. 553–571. [Google Scholar]
- 28.Ginalska G, Osinska M, Uryniak A, Urbanik-Sypniewska T, Belcarz A, Rzeski W, et al. Antibacterial activity of gentamicin-bonded gelatin-sealed polyethylene terephthalate vascular prostheses. Eur J Vasc Endovasc Surg. 2005;29:419–424. doi: 10.1016/j.ejvs.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 29.Thirumurugan G, Shaheedha SM, Dhanaraju MD. In-vitro evaluation of anti-bacterial activity of silver nanoparticles synthesised by using Phytophthora infestans. Int J ChemTech Res. 2009;1:714–716. [Google Scholar]
- 30.Panáek A, Kvítek L, Prucek R, Kolář M, Veeřová R, Pizúrová N, et al. Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J Phys Chem B. 2006;110:16248–16253. doi: 10.1021/jp063826h. [DOI] [PubMed] [Google Scholar]
- 31.Mi FL, Wu YB, Shyu SS, Chao AC, Lai JY, Su CC. Asymmetric chitosan membranes prepared by dry/wet phase separation: a new type of wound dressing for controlled antibacterial release. J Membr Sci. 2003;212:237–254. doi: 10.1016/S0376-7388(02)00505-7. [DOI] [Google Scholar]
- 32.Tas C, Ozkan Y, Savaser A, Baykara T. In vitro release studies of chlorpheniramine maleate from gels prepared by different cellulose derivatives. II Farmaco. 2003;58:605–611. doi: 10.1016/S0014-827X(03)00080-6. [DOI] [PubMed] [Google Scholar]


