Abstract
Difficulties in visual attention are often implicated in autism spectrum disorders (ASD) but it remains unclear which aspects of attention are affected. Here, we used a multiple object tracking (MOT) task to quantitatively characterize dynamic attentional function in children with ASD aged 5–12. While the ASD group performed significantly worse overall, the group difference did not increase with increased object speed. This finding suggests that decreased MOT performance is not due to deficits in dynamic attention but instead to a diminished capacity to select and maintain attention on multiple targets. Further, MOT performance improved from 5–10 years in both typical and ASD groups with similar developmental trajectories. These results argue against a specific deficit in dynamic attention in ASD.
Keywords: Autism Spectrum Disorder, Multiple Object Tracking, Dynamic Attention, Spatial Attention, Cognitive Development
Introduction
Visual attention serves as a fundamental filter of experience, selecting a small subset of the incoming sensory information for further processing. Attention thus determines what we perceive, remember, and act upon. Previous research has suggested that people with autism spectrum disorder (ASD) have intact or even enhanced function in some visual attention abilities (Joseph et al. 2009; Kaldy et al. 2011; Mullane and Klein 2008; O’Riordan et al. 2001; Plaisted et al. 1998) but have deficits in other attentional functions (e.g. Ciesielski et al. 1990; Townsend et al. 1996; Burack 1994; for review see Allen and Courchesne 2001). In particular, several lines of work have implicated temporal aspects of attention in ASD by showing that people with ASD have difficulty adjusting the size of attentional focus dynamically (Mann and Walker 2003; Rinehart et al. 2001) and difficulty switching the locus of their attention (Elsabbagh et al. 2009; Facoetti et al. 2008; Ibanez et al. 2008; Kikuchi et al. 2011; Landry and Bryson 2004; van der Geest et al. 2001), especially in paradigms that include either dynamic, repetitive stimuli or social stimuli. Further, people with ASD may show reductions in the temporal resolution of attention, as evidenced by a greater attentional blink effect (Amirault et al. 2009) though see (Rinehart et al. 2010) and reduced abilities in temporal integration in visual (Nakano et al. 2010; Brock et al. 2002), auditory (Groen et al. 2009) and multimodal (Foss-Feig et al. 2010; Kwakye et al. 2011) tasks. These findings suggest that dynamic or spatiotemporal attention may be impaired in ASD.
Dynamic attention has also been implicated in ASD through a heterogeneous set of deficits reported in motion perception tasks. First, people with ASD appear to have a selective impairment in the detection of second-order (i.e., texture defined) motion, but not in either flicker or first-order motion detection (Bertone et al. 2005; Pellicano et al. 2005; Pellicano and Gibson 2008). Because second-order motion (unlike first-order motion or flicker detection) depends on attentive tracking mechanisms (Ashida et al. 2001) this specific pattern of impairment suggests a deficit in dynamic attention in ASD. Second, discriminating the direction of global motion, which is sometimes reported to be impaired (Milne et al. 2002; Pellicano et al. 2005; Spencer et al. 2000; Spencer and O’Brien 2006; Tsermentseli et al. 2008) and sometimes unimpaired (Brieber et al. 2010; Jones et al. 2011; Koldewyn et al. 2010; Koldewyn et al. 2011; Milne et al. 2006; Vandenbroucke et al. 2008) in ASD depends partially on the spatial scale of attention (Bulakowski et al. 2007), especially in noisy or attentionally demanding contexts. Third, biological motion perception, often reported to be impaired in ASD (Atkinson 2009; Annaz et al. 2010; Blake et al. 2003; Cook et al. 2009; Freitag et al. 2008; Klin et al. 2009; Koldewyn et al. 2010) though see (Jones et al. 2011; Murphy et al. 2009; Rutherford and Troje 2012; Saygin et al. 2010) is an attentionally demanding task (Cavanagh et al. 2001; Thornton et al. 2002). This complex pattern of preservation and loss in motion perception tasks (for review, see Kaiser and Shiffrar 2009) could be explained if people with ASD have a specific deficit in spatiotemporal or dynamic attention.
In the current study, we examined attentional function in ASD using the multiple object tracking (MOT) task (Pylyshyn and Storm 1988), in which subjects must keep track of a subset of identical objects moving on different trajectories. MOT is a powerful tool for testing attentional function in ASD because performance on MOT tasks has been well characterized in typical adults, and because the task allows several aspects of attention to be separately measured in one paradigm. In MOT, targets have to be selected from among identical distractors, attention must be divided between multiple targets and attention to targets must be maintained dynamically across time. Performance during MOT can be measured as a function of the number of targets to be tracked, the speed at which objects move and the distance maintained between tracked objects and distractors (Bettencourt and Somers 2009; Cavanagh and Alvarez 2005; Tombu and Seiffert 2008). If dynamic attention is impaired in ASD, then participants with ASD should show performance deficits primarily at faster speeds but should be just as good as typical participants at slower speeds. If, on the other hand, children with ASD show a reduction in tracking capacity at all speeds, that would instead suggest a deficit in other attention processes.
In the current study, we tested typical and ASD participants between the ages of 5 and 12 on an MOT task in which we manipulated both the number of items to be tracked (2 or 3) and the speed at which tracking was performed (5 separate speeds). We sought to answer two questions: i) do individuals with ASD have a specific deficit in dynamic attention. If so, they should perform worse in MOT than typical children, and the deficit should become greater as object speed increases. ii) What is the developmental trajectory of MOT ability between the ages of 5 and 12 and does this developmental trajectory differ between ASD and typical children? Finally, to determine if any differences between typical participants and participants with ASD in MOT ability were related to motion perception ability, we also administered a coherent motion perception task.
Methods
Participants
Participants were 34 typically developing children and 34 children with ASD aged 5–12 years (8 girls and 26 boys in each group). We matched the groups on age and non-verbal IQ, measured by the Kaufman Abbreviated Intelligence Test (see Table 1). Participants with ASD had an ASD diagnosis and met criteria for ASD or autism on the Autism Diagnostic Observation Schedule (ADOS). An additional 45 typically developing children aged 5–10 years (16 girls) and 20 typical adults (10 women) also participated in the main experiment to provide a more precise quantification of the developmental trajectory of performance in typical children. All participants had normal or corrected-to-normal visual acuity. All participants received modest monetary compensation for their participation and children additionally received small motivating prizes.
Table 1.
Participant Information
| Measure | Autism (n = 34) | Control (n=34) | t | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | |||
| Performance IQ (K-bit) | 109.62 | 16.57 | 80–139 | 111.38 | 10.821 | 83–133 | .520 | 0.605 |
| Age | 8.74 | 1.81 | 5.78–12.08 | 8.31 | 1.82 | 5.16–12.18 | −.961 | 0.340 |
| SNAP-IV* (combined score) | 1.69 | .38 | .944–2.67 | 1.89 | .48 | 0–1.17 | −9.3 | <.001 |
| ADOS (severity score) | 6.91 | 1.88 | 3–10 | - | - | - | - | - |
| ADOS (social) | 8.88 | 3.95 | 4–19 | - | - | - | - | - |
| ADOS (repetitive/restricted) | 3.5 | 1.75 | 2–7 | - | - | - | - | - |
| ADOS (total) | 12.38 | 4.74 | 6–26 | - | - | - | - | - |
Missing data on two participants with ASD and five typically developing participants.
Children with ASD were recruited through the Simons Foundation and the Boston Autism Consortium. Typically developing children were recruited from the local community. Potential participants were excluded if they had any history of birth or brain trauma, non-corrected visual impairments or a non-verbal IQ of less than 80. Typically developing participants were further excluded if they scored higher than 11 on the Social Communication Questionnaire (see description below), had a diagnosis of any developmental disorder or any history of ASD in their immediate family. Every participant signed an assent form and a parent or guardian signed an informed consent approved by the Committee on the Use of Humans as Experimental Subjects.
Standardized Measures
All children were tested on a number of standardized tests. Data from these standardized measures are presented in Table 1.
Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 2000)
A structured observational assessment that provides opportunities for interaction and play while measuring social, communicative and repetitive behaviors that are diagnostic of ASD.
Social Communication Questionnaire (SCQ)
A parent-report screening questionnaire to evaluate communication and social skills in people aged 4 years and above (Rutter et al., 2003). The SCQ was obtained from all participants. Although a score of 15 is typically used in clinical settings to indicate concern that a child may have ASD, we used a more conservative cutoff score of 11 for the typically developing group.
The Kaufman Brief Intelligence Test (k-bit) (Kaufman & Kaufman, 2004)
The K-bit provides a short and reliable means of assessing intelligence in individuals aged 4–90. Only the nonverbal subtest was used, testing skills such as pattern recognition, analogy completion and mental rotation.
Swanson, Nolan and Pelham Questionnaire, revised (SNAP-IV) (Bussing et al., 2008)
A parent report where parents assess their child on symptoms of ADHD as defined by the DSM-IV (American, 1994).
General Procedure
Experiments were programmed using psychtoolbox (Brainard 1997; Pelli 1997) implemented in MATLAB. Responses in the MOT task were made on a touch screen monitor (resolution: 1280 × 1024 pixels, refresh rate: 60Hz). Responses for the coherent motion perception task were made using two large buttons connected to a MacBook Pro (resolution: 1920 × 1200 pixels, refresh rate: 60Hz).
Multiple Object Tracking Task Procedure
This (main) experiment was designed to test children’s ability to track multiple objects (either 2 or 3) among distracters at five different speeds. Each child performed five practice trials followed by 40 experimental trials.
Practice/Warm-up trials
The first five trials, presented separately, served to introduce children to the experiment and demonstrate the different speeds used during the experiment. These practice trials were identical to the subsequent test trials except that participants were only asked to track one target object among seven distractors during these trials. All participants were 100% accurate in tracking one object at all speeds.
Experimental trials
For each trial, participants were presented with 8 squares (1.3° × 1.3°) on a black background, either two or three of which were first presented as pictures of a kitten while the rest were simple red squares (see figure 1). When participants pressed the picture of one of the kittens, all of the kittens would change to red squares and all squares then moved around the screen on independent trajectories at one of five speeds (6.4°/sec, 9.3°/sec, 13.7°/sec, 19.9°/sec, 28.8°/sec). Participants were asked to keep track of “which squares the kittens were hiding in” as the squares moved for 3 seconds. Once movement stopped, subjects indicated all squares in which a kitten was hidden by touching each one. After the correct number of squares (2 or 3) had been chosen, participants received feedback: if they were correct, the square would change back to the picture of the kitten, if they were not correct, the correct square would be highlighted by changing to bright green. If participants got at least one square correct, they were presented with the sound of a kitten meowing. If they missed all of the squares, a pre-recorded voice stated “Oops, let’s try again”. Four trials were presented at each speed for each number of tracked objects (2 or 3) for a total of 40 trials.
Figure 1.
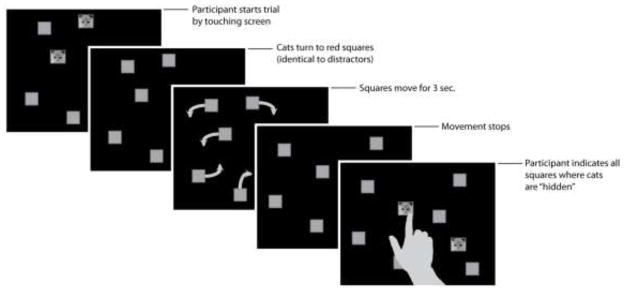
Schematic of the multiple object tracking task. Note that squares depicted as grey here were originally red and the cat picture was in full color (orange tabby kitten on a red background)
Coherent Motion Perception Procedure
This experiment tested children’s ability to perceive coherent motion in a cloud of moving dots. We utilized this task primarily to look at the relationship between multiple object tracking and a simpler motion perception task within our autism group. Data from this experiment was collected on as many of the participants as possible (time allowing), 28 participants with autism and 26 typically developing participants.
Stimulus
Coherent motion perception was assessed through a Global Dot Motion task (Newsome and Pare 1988) in which 100 white dots (.2° visual angle in diameter) were presented in a black square (12.5° × 12.5°), centered on a black screen. Dots moved across the screen at 11°/sec. We manipulated the coherence of the display by changing the percentage of dots that were moving in the same direction, here either directly left (50% of trials) or directly right while all other dots moved in randomly chosen directions. Dots had a “lifetime” of 50 msec, so that coherent motion direction could not be determined by monitoring individual dot motion (Newsome and Pare 1988). Each stimulus was shown for 1 second. Participants entered their response (left or right motion) after stimulus offset.
Practice/Warm-up trials
Participants were first presented with several trials as a demonstration of what the stimuli would look like (presented at 100% coherence). They were then asked to complete 10 trials at 80% coherence and given feedback to be sure that they understood the task and could discriminate leftward from rightward motion in the displays when the motion information was very strong.
Experimental trials
Participants were first presented with additional practice trials, at 80% coherence, and were required to get 4 of these practice trials correct in a row for the program to move on to the experimental trials. If subjects could not do this over the course of 12 practice trials, the experiment would end and the experimenter would re-explain the task and re-present the demonstration trials. Once experimental trials began, a QUEST staircase procedure (Watson and Pelli 1983) was used to adjust the difficulty of the task by changing the coherence of the global motion (percentage of the dots moving in the same direction). This procedure produced an estimate of the motion coherence at which participants could discriminate between leftward and rightward global motion with an accuracy of 75% correct, providing a threshold for coherent motion perception for each individual. QUEST parameters were: number of trials = 30; Beta = 3.5; Delta = .01; Grain = 1. In addition to the 30 experimental trials, 6 lapse trials were included where the stimulus was presented at 100% coherence to ensure that participants were attending to the task across the entire session. On each trial, participants were asked to indicate the direction of global motion and encouraged to guess when they did not know. No feedback was provided.
Results
Multiple Object Tracking
Accuracy was calculated for each trial by averaging the responses for all targets (either 2 or 3). For instance, if a participant correctly identified 2 of 3 targets, accuracy would be 67% on that trial. We then converted average accuracy on each trial type into a a k-score that reflects the average number of items that a participant was capable of tracking for that particular trial type. To calculate k, we used the high threshold guessing model (Hulleman 2005): k = (oc − t2)/(o + c − 2t). Here, o = total objects including targets and distracters (8 for the current study), c = average correctly identified targets, and t = the number of targets to be tracked (either 2 or 3). This measure was used for all of the following statistical analyses.
K-score increased with the number of items to be tracked (F(1, 66) = 97.483, p < 0.001) and decreased with their speed (F(4, 264) = 274.256, p < .001). Participants with ASD had a lower tracking capacity than typically developing participants (F(1, 66) = 7.69, p = 0.007). The deficit in the ASD group was more obvious when three targets were tracked than two, reflected in a trend of an interaction between group and items to be tracked (F(1, 66) = 3.626, p = 0.061). Importantly, we found no interaction between group and speed (F(4, 264) = .727, p = .574) and no three-way-interaction between speed, items tracked and group (F(4, 264) = .181, p = .592). All of these results remained the same when PIQ and age were entered as covariates, either separately or together. These results indicate that although participants with ASD have a lower tracking capacity overall, they were not disproportionately worse at higher speeds.
To test whether floor or ceiling effects might be obscuring a possible interaction between group and speed in our capacity measure, our next analysis included only conditions that were far from floor or ceiling for all children: speeds 3 and 4 while participants were tracking 3 objects. Despite focusing on the data with the most dynamic range to detect such a relationship, we still saw no interaction between group and speed (F(1, 66) = .072, p = .79) while both a main effect of speed (F(1, 66) = 63.568 p < .001) and a main effect of group (F(1, 66) = 4.413, p = .039) remained. Again, although individuals with ASD performed worse overall, they did not suffer disproportionally at faster speeds.
Multiple Object Tracking Across Development
MOT skill development in typically developing children
To measure with greater resolution any changes in tracking capacity across age, we used data collected from all 76 typically developing children between the ages of 5–10, placing them in age groups by year (see Table 2). We focused on this age range because only a few children (three in each group) in the age and IQ matched groups were older than 10 years. As some older children reached ceiling across all speeds in the track-2-item condition, we focused our analyses on the track-3-item condition. ANOVA including all child age groups (5y, 6y, 7y, 8y, 9y, 10y) showed a robust main effect of age-group (F(5, 70) = 25.924, p < .001), indicating that tracking capacity increased with age. Interestingly, it also showed not only the expected main effect of speed (F(4, 280) = 340.236, p < 0.001) but also a speed by age-group interaction (F(20, 280) = 3.597, p < .001) indicating that both attentional capacity and dynamic attention develop significantly during this age range. Figure 3 illustrates these age-related changes.
Table 2.
Typical participant information by age-group
| Age-Group | Ages for typical children (n = 76) | |||
|---|---|---|---|---|
| N | Mean | SD | Range | |
| 5-year-olds | 14 | 5.43 | .34 | 5.03–5.97 |
| 6-year-olds | 11 | 6.38 | .25 | 6.02–6.83 |
| 7-year-olds | 15 | 7.60 | .28 | 7.01–7.95 |
| 8-year-olds | 11 | 8.66 | .31 | 8.03–8.98 |
| 9-year-olds | 14 | 9.56 | .32 | 9.07–9.98 |
| 10-year-olds | 11 | 10.69 | .28 | 10.13–10.94 |
Figure 3.
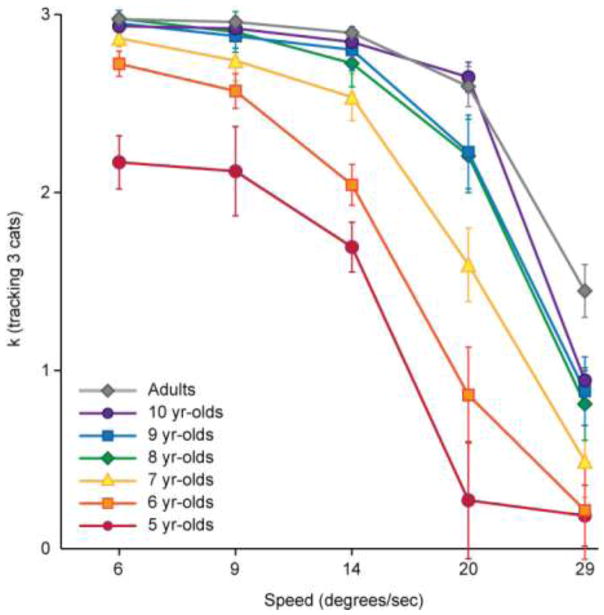
K-values for the entire group of typical children (N = 76), binned by age, across the five speeds while children tracked three objects. Adults (N = 20) are included in the graph for illustrative purposes but were not included in the main ANOVA investigating developmental change. Developmental change between the ages of 5 and 10 is evident, with further development after the age of 10 only evident at the highest speed
Further analysis showed that between the age spans of 5–7 years, the overall improvement in capacity was the same for all speeds (main effect of age F(2, 37) = 14.008, p < .001; interaction between age and speed, F(8, 148) = 1.088, p = .211). Seven-year-olds reached near adult performance levels when the objects moved at the slowest speed (t = 1.79, p = .09), but not at higher speeds. However, from 7–10 years of age, increasing age was associated with greater ability to track at higher speeds (main effect of age F(3, 47) = 3.779, p = .016, interaction between age and speed, F(12, 188) = 2.055, p = .022). Ten-year-old performed at adult levels except for the fastest speed (ps > .1 for speeds 1 – 4; t = 3.031, p = .005 for speed 5). These results suggest that development of MOT skills is primarily driven by improvements in attentional capacity before the age of seven, while dynamic attention continues to improve until at least the age of ten, if not into adolescence.
MOT skill development in ASD
Next, to test whether the development of tracking capacity might differ between typically developing children and those with ASD, we performed an ANOVA similar to that done in the entire typically developing group, but looking just at our age and IQ matched groups. Because of both the smaller group size and the fact that children were not evenly distributed across our age range, we binned participants by 2-year intervals (5–6y, 7–8y, 9–10y and 11–12y). This ANOVA showed the expected main effects of speed (F(4, 240) = 117.94, p < .001) and group (F(1, 60) = 4.725, p = .034). This analysis also showed the same developmental trends revealed in the larger typically developing group: a main effect of age-group (F(3, 60) = 4.799, p = .005) and an age-group by speed interaction (F(12, 240) = 2.042, p = .02). Crucially, however, there was no interaction between group and age-group (F(3, 60) = 1.425, p = .22), nor was there a speed by group by age-group interaction (F(12, 240) = .515, p = .90). These results indicate that developmental change did not differ between groups, despite the overall between-group difference.
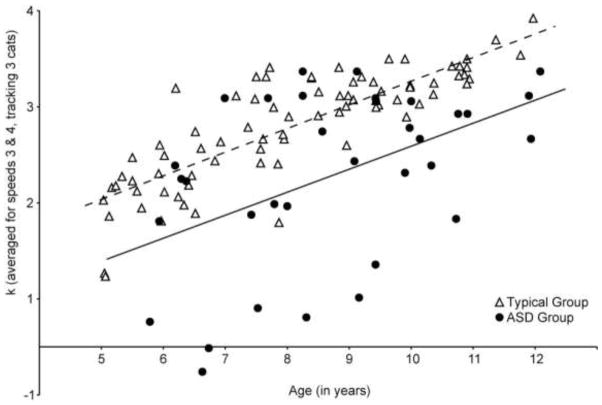
To make sure that we were not missing possible between-group differences in development, we once again focused on our most dynamic range, averaging k-scores across speeds 3 and 4 for trials where participants tracked 3 targets. We then ran a regression with this k-score average as the dependent variable and performance IQ, age (as a continuous variable), group and an age by group interaction term as predictors. Both performance IQ (t(62) = 3.310, p = .002) and age (t(62) = 3.563, p = .001) were significant predictors of tracking capacity. As shown in figure 4, however, developmental change did not significantly differ between groups as assessed by either slope (t(62) = .097, p = .92) or intercept (t(62) = −.714, p = .48).
Figure 4.
Individual k-values for the entire group of typical children (N = 79) and the group of children with ASD (N = 34) plotted against age to show developmental change in both groups. The dotted line is the line of best fit for the typical group while the solid black line shows the line of best fit for the ASD group
Correlation analyses
To assess potential relationships between tracking capacity and other possible factors, we used our most sensitive measure: the average tracking capacity at speeds 3 and 4 while participants were tracking 3 objects.
Correlations with Standardized Measures
Performance IQ
Not unexpectedly, tracking capacity was positively correlated with performance IQ for both groups (Typical Group: r = .404, p = .018; ASD group: r = .480, p = .004).
SNAP-IV
No child in the typical group scored high enough on the SNAP-IV to be considered at risk for ADHD. In the ASD group, 8 children fall within the clinical range for ADHD. However, tracking capacity did not correlate with SNAP-IV scores, (r = .047, p = .85) and this relationship remained similar when both age and IQ were controlled.
ADOS
No correlations were found between autism severity as assessed by the ADOS calibrated severity score and tracking capacity in the ASD group (r = .2, p = .308) even when both IQ and age were controlled (r = .104, p = .613).
Correlations with Motion Coherence Perception ability
The two groups did not differ on motion coherence ability as assessed by 75% thresholds (ASD mean: 42.56 Typical Mean: 41.03; t(44) = −.281, p = .78). Additionally, neither group showed a significant relationship between motion coherence perception ability and tracking capacity (Typical Group: r = −.243, p = .498; ASD group: r = −.267, p = .23) even when both age and performance IQ were controlled for: (Typical Group: r = .335, p = .417; ASD group: r = −.089, p = .71). Both of these results suggest that possible between-group differences in motion perception cannot explain the between-group differences in tracking capacity during MOT.
Discussion
Children with ASD tracked fewer objects successfully than did typically developing children across all conditions in the MOT task. Contrary to our predictions, however, children with ASD did not perform disproportionately worse at higher speeds, but instead showed a similar performance deficit at all speeds. These results argue against the selective deficit in dynamic attention in ASD that we hypothesized, which predicts greater performance disparities at higher speeds. In addition, the lack of a deficit in coherent motion perception in ASD, as well as the lack of a correlation between performance on MOT and direction discrimination ability in the coherent motion task indicates that neither basic motion perception ability nor the dynamic nature of MOT can explain the lower performance we observed in children with ASD in the MOT task.
How can the apparent lack of a deficit in dynamic attention found here be squared with the evidence for such a deficit reviewed in the introduction? Perhaps deficits in the perception of biological motion are more related to the social nature of the stimuli than to their dynamic nature (Pelphrey et al. 2011), a possibility that could be tested with perceptual discrimination tasks on static body stimuli. More generally, our data suggest that it may be worthwhile to revisit some of the prior evidence that temporal/dynamic stimuli pose special processing challenges for people with ASD.
How can we account for the deficits we do find in the number of items that can be tracked in MOT tasks? MOT involves at least three partially distinct aspects of attentional processing: dynamic attention (tracking targets over time), selective attention (distinguishing targets from nontargets), and divided attention (spreading attention over multiple targets). As our results are not consistent with a deficit in dynamic attention, could a deficit in either selective attention or divided attention explain our between-group differences?
Selective attention deficits have been reported in autism primarily on tasks where irrelevant information is presented in the same stimulus as task-relevant information (e.g., local features in a global shape) (Burack 1994; Ciesielski et al. 1990; Plaisted et al. 1999; Remington et al. 2009). On tasks where irrelevant stimuli are separate object distractors (e.g., a visual search task), people with ASD have generally performed as well or even better (Baldassi et al. 2009; Joseph et al. 2009; Kaldy et al. 2011; O’riordan 2004; O’Riordan and Plaisted 2001; O’Riordan et al. 2001; Plaisted et al. 1998) than a typical comparison group. MOT requires attentional selection among objects (not features): one must attend to some objects (targets) while filtering out others (distracters), relying purely on attention (as opposed to, say, a color difference). Thus, it might be expected that people with ASD would not experience difficulty with the selective attention demands of the MOT task. Indeed, evidence from our study suggests that differences in selective attention are not driving our group effect in MOT. In particular, as speed increases during MOT, selection becomes more difficult because at higher speeds there are more events when the targets and distracters are near each other spatially, each resulting in a chance of selection failure (Franconeri et al. 2008; Franconeri et al. 2010; Shim et al. 2008). If difficulties in selective attention were driving between-group differences in MOT capacity, the differences between groups should, again, be greater at higher speeds. Because our results show consistent between-group performance differences across speed, it is unlikely that selective attention deficits in the ASD group are driving our results. This could be tested more explicitly in future research by directly manipulating the distance between distracters and targets to determine if doing so would affect performance similarly in both typical and ASD populations.
Might deficits in divided attention then account for the lower MOT performance we observed in children with ASD? The literature on divided attention in ASD is mixed, with some studies suggesting that people with ASD have typical divided attention abilities (Bogte et al. 2009; Rutherford et al. 2007) while others conclude that people with ASD show a deficit in divided attention abilities (Althaus et al. 1996; Ciesielski et al. 1995; Pierce et al. 1997). MOT relies on divided attention because it requires attending to multiple, independently moving objects. In order to continue to track objects as they move among identical distracters, it is theorized that an attentional index is established for each object, and this index must be maintained as objects move (Cavanagh and Alvarez 2005; Pylyshyn and Storm 1988). A reduction in the ability to establish these attentional indices and then divide attention over them would lead to a reduction in tracking capacity, regardless of object speed. This is what our data show: children with ASD have reduced capacity at all speeds relative to the typical comparison group suggesting that those with ASD may have a selective deficit in spatial indexing rather than temporal updating. Thus, a deficit in divided attention is consistent with our results.
Another possibility is that differences in spatial working memory ability could underlie the deficits we observe in individuals with ASD in the MOT task. Previous research has shown that tracking capacity during MOT is directly related to spatial working memory capacity in typical adults (Allen et al. 2006; Cavanagh and Alvarez 2005), predicting much of the individual variability in MOT capacity (Trick et al. 2012). As working memory deficits have been reported in autism for static displays (Bennetto et al. 1996; Lopez et al. 2005; Luna et al. 2007; Poirier et al. 2011; Russo et al. 2007), though see (Griffith et al. 1999; Ozonoff and Strayer 2001; Russell et al. 1996) it certainly seems possible that deficits in working memory capacity in ASD could at least partially explain the current results. This possibility could be tested in future work in which MOT capacity and working memory ability are examined in the same individuals with ASD.
The current results also provide a possible target for future brain-imaging studies of attentional function in ASD. A region in posterior parietal cortex has been shown in several studies to be engaged during MOT tasks (Culham et al. 1998; Culham et al. 2001; Jovicich et al. 2001). This region also appears to increase its response only when the number of items to be tracked is increased (Howe et al. 2009; Shim et al. 2010) but, importantly, not when the speed of targets is increased (Shim et al. 2010). Thus, this region may be specifically involved in the indexing of items to be attended while other regions, such as motion sensitive cortex in the middle temporal area (MT+), the frontal eye fields (FEF) and the anterior portion of the inferior parietal sulcus (AIPS), may be more involved in tracking targets dynamically, increasing their response when the speed of targets increases (Shim et al. 2010). The current results would predict that participants with autism would show reduced response in posterior parietal cortex during MOT, a difference that could be especially noticeable when the number of items to be tracked is manipulated. The current results would also predict that regions more involved in the dynamic aspects of the task (MT+, FEF, AIPS) would all be expected to respond at similar levels in typical and ASD groups.
The current study also highlights the importance of examining cognitive skills in ASD within a developmental framework. While caution must be taken in interpreting the developmental data because of the cross-sectional design, our results suggest that both tracking capacity and dynamic attention develop significantly between the ages of five and 10. Although the dynamic aspects of the MOT task have not been manipulated in earlier developmental studies, the current results are consistent with previous studies of MOT performance in children in which the number of objects to be tracked was manipulated to examine tracking capacity (O’Hearn et al. 2010; Trick et al. 2005). A decrease in tracking capacity during MOT has been reported in other developmental disorders, including both William’s syndrome (O’Hearn et al. 2005; O’Hearn et al. 2010) and Turner Syndrome (Beaton et al. 2010). Given that both disorders are associated with general reductions in spatial skills (Landau et al. 2006; Lepage et al. 2011; Mazzocco et al. 2006) while people with autism are generally considered to have typical or even superior spatial skills (for review see: Edgin and Pennington 2005), capacity decreases in other developmental disorders may not reflect the same underlying cause as in ASD. Further, in contrast to people with William’s syndrome, where development of MOT skills appears to plateau at the developmental level of 4-year-old typical children while the ability to remember multiple static locations develops further, (O’Hearn et al. 2010), our data show similar developmental trajectories of MOT performance for typical children and children with ASD. In the larger typical group in our study, the performance of the 10-year-old group appears to be virtually identical to adult performance in all conditions except at the highest speed when 3 objects were being tracked. This finding suggests that MOT capacity typically approaches adult levels by the age of ten, while some development continues to occur in dynamic attention. Future studies will be necessary to determine whether individuals with ASD eventually reach the same performance levels on this task as typical adults, or whether they plateau at a lower level.
The data in Figure 4 appear to show a group of participants with ASD that lag behind both typical individuals and other children with ASD of similar ages. Indeed, while 80% of participants with ASD performed at more than 1 standard deviation below the mean of typical individuals in their age group, only 35% of ASD participants (12 of 34) performed at more than 2 standard deviations below that mean. This group of 12 participants did not have significantly different IQs, ADOS scores or scores on the SNAP-IV. They also did not perform differently on the coherent motion task. Nonetheless, it is possible that these children may constitute a subgroup in whom attention function is particularly delayed developmentally – a possibility that could be further investigated by retesting these individuals on this task as well as on other measures of attentional function.
What impact might the deficits reported here have on real world cognitive skills? Difficulty dividing attention between multiple targets could potentially impact other visual cognitive skills in ASD, especially in understanding complex social situations and interpersonal interactions. The ability to track multiple targets at the same time is also important for skills like sports and monitoring traffic when crossing streets. Differences in the ability to divide attention between multiple targets may be important in understanding other aspects of the cognitive profile in children with ASD, especially the interactions between attention, executive function and visual perception skills during development. The suggestion that dynamic attention does not play a major role in ASD is particularly important, although this finding will need to be replicated not only in MOT but also in other paradigms where dynamic attention can be manipulated in different ways. That MOT skills both develop significantly during middle to late childhood and appear to develop in similar ways in both ASD and typical groups is encouraging as it suggests that divided attention skills could be a target for clinical intervention.
Figure 2.
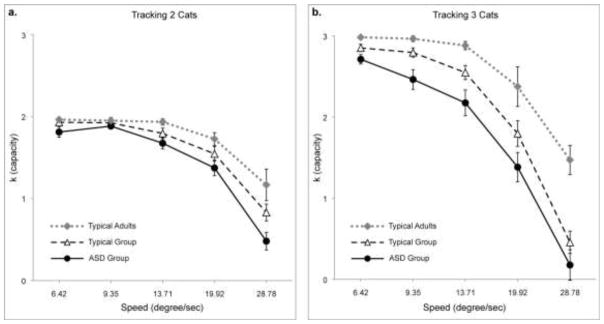
Group performance (k-score) across all speeds a) while tracking 2 objects and b) while tracking 3 objects. Error bars represent the standard error of the mean. Adults are included here for illustrative purposes only
Acknowledgments
The authors would especially like to thank all the participants and their families for their time and contribution to our research. We are grateful to all of the families at the participating SFARI Simplex Collection (SSC) sites, as well as the principal investigators (A. Beaudet, R. Bernier, J. Constantino, E. Cook, E. Fombonne, D. Geschwind, E. Hanson, D. Grice, A. Klin, R. Kochel, D. Ledbetter, C. Lord, C. Martin, D. Martin, R. Maxim, J. Miles, O. Ousley, K. Pelphrey, B. Peterson, J. Piggot, C. Saulnier, M. State, W. Stone, J. Sutcliffe, C. Walsh, Z. Warren, E. Wijsman). We are also grateful to all of the families participating in the Autism Consortium collection, as well as the principal investigators. This study was supported by funds from the Ellison Medical Foundation and the Simons Foundation.
Footnotes
Kami Koldewyn is at Massachusetts Institute of Technology in the McGovern Center for Brain Research and the Department of Brain and Cognitive Sciences in Cambridge Massachusetts, USA. Sarah Weigelt was also at Massachusetts Institute of Technology in the McGovern Center for Brain Research and the Department of Brain and Cognitive Sciences in Cambridge Massachusetts, USA. Nancy Kanwisher is the Walter A. Rosenblith Professor of Cognitive Neuroscience in the Department of Brain and Cognitive Sciences and at the McGovern Center for Brain Research at Massachusetts Institute of Technology in Cambridge Massachusetts, USA. Yuhong Jiang is a professor in the Psychology department at the University of Minnesota in Minneapolis Minnesota, USA.
Sarah Weigelt is now at Westfälische Wilhelms-Universität Münster in the Institut für Psychologie in Münster, Germany.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Allen G, Courchesne E. Attention function and dysfunction in autism. Frontiers in Bioscience: A Journal and Virtual Library. 2001;6:D105–D119. doi: 10.2741/allen. [DOI] [PubMed] [Google Scholar]
- Allen R, McGeorge P, Pearson DG, Milne A. Multiple-target tracking: A role for working memory? Quarterly Journal of Experimental Psychology (2006) 2006;59(6):1101–1116. doi: 10.1080/02724980543000097. [DOI] [PubMed] [Google Scholar]
- Althaus M, deSonneville LMJ, Minderaa RB, Hensen LGN, Til RB. Information processing and aspects of visual attention in children with the DSM-III-R diagnosis “pervasive developmental disorder not otherwise specified” (PDDNOS) .1. Focused and divided attention. Child Neuropsychology. 1996;2(1):17–29. [Google Scholar]
- Amirault M, Etchegoyhen K, Delord S, Mendizabal S, Kraushaar C, Hesling I, et al. Alteration of attentional blink in high functioning autism: a pilot study. Journal of Autism and Developmental Disorders. 2009;39(11):1522–1528. doi: 10.1007/s10803-009-0821-5. [DOI] [PubMed] [Google Scholar]
- Annaz D, Remington A, Milne E, Coleman M, Campbell R, Thomas MS, et al. Development of motion processing in children with autism. Developmental Science. 2010;13(6):826–838. doi: 10.1111/j.1467-7687.2009.00939.x. [DOI] [PubMed] [Google Scholar]
- Ashida H, Seiffert AE, Osaka N. Inefficient visual search for second-order motion. Journal of the Optical Society of America. 2001;18(9):2255–2266. doi: 10.1364/josaa.18.002255. [DOI] [PubMed] [Google Scholar]
- Atkinson AP. Impaired recognition of emotions from body movements is associated with elevated motion coherence thresholds in autism spectrum disorders. Neuropsychologia. 2009;47(13):3023–3029. doi: 10.1016/j.neuropsychologia.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Baldassi S, Pei F, Megna N, Recupero G, Viespoli M, Igliozzi R, et al. Search superiority in autism within, but not outside the crowding regime. Vision Research. 2009;49(16):2151–2156. doi: 10.1016/j.visres.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Beaton EA, Stoddard J, Lai S, Lackey J, Shi J, Ross JL, et al. Atypical functional brain activation during a multiple object tracking task in girls with Turner syndrome: neurocorrelates of reduced spatiotemporal resolution. American Journal on Intellectual and Developmental Disabilities. 2010;115(2):140–156. doi: 10.1352/1944-7558-115.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetto L, Pennington BF, Rogers SJ. Intact and impaired memory functions in autism. Child Development. 1996;67(4):1816–1835. [PubMed] [Google Scholar]
- Bertone A, Mottron L, Jelenic P, Faubert J. Enhanced and diminished visuo-spatial information processing in autism depends on stimulus complexity. Brain. 2005;128(Pt 10):2430–2441. doi: 10.1093/brain/awh561. [DOI] [PubMed] [Google Scholar]
- Bettencourt KC, Somers DC. Effects of target enhancement and distractor suppression on multiple object tracking capacity. Journal of Vision. 2009;9(7):9. doi: 10.1167/9.7.9. [DOI] [PubMed] [Google Scholar]
- Blake R, Turner LM, Smoski MJ, Pozdol SL, Stone WL. Visual recognition of biological motion is impaired in children with autism. Psychological Science. 2003;14(2):151–157. doi: 10.1111/1467-9280.01434. [DOI] [PubMed] [Google Scholar]
- Bogte H, Flamma B, Van Der Meere J, Van Engeland H. Divided attention capacity in adults with autism spectrum disorders and without intellectual disability. Autism. 2009;13(3):229–243. doi: 10.1177/1362361309103793. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10(4):433–436. [PubMed] [Google Scholar]
- Brieber S, Herpertz-Dahlmann B, Fink GR, Kamp-Becker I, Remschmidt H, Konrad K. Coherent motion processing in autism spectrum disorder (ASD): An fMRI study. Neuropsychologia. 2010;48(6):1644–1651. doi: 10.1016/j.neuropsychologia.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Brock J, Brown CC, Boucher J, Rippon G. The temporal binding deficit hypothesis of autism. Development and Psychopathology. 2002;14(2):209–224. doi: 10.1017/s0954579402002018. [DOI] [PubMed] [Google Scholar]
- Bulakowski PF, Bressler DW, Whitney D. Shared attentional resources for global and local motion processing. J Vis. 2007;7(10):1–10. doi: 10.1167/7.10.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burack JA. Selective attention deficits in persons with autism: preliminary evidence of an inefficient attentional lens. Journal of Abnormal Psychology. 1994;103(3):535–543. doi: 10.1037//0021-843x.103.3.535. [DOI] [PubMed] [Google Scholar]
- Cavanagh P, Alvarez GA. Tracking multiple targets with multifocal attention. Trends in Cognitive Sciences. 2005;9(7):349–354. doi: 10.1016/j.tics.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Cavanagh P, Labianca AT, Thornton IM. Attention-based visual routines: sprites. Cognition. 2001;80(1–2):47–60. doi: 10.1016/s0010-0277(00)00153-0. [DOI] [PubMed] [Google Scholar]
- Ciesielski KT, Courchesne E, Elmasian R. Effects of focused selective attention tasks on event-related potentials in autistic and normal individuals. Electroencephalography and Clinical Neurophysiology. 1990;75(3):207–220. doi: 10.1016/0013-4694(90)90174-i. [DOI] [PubMed] [Google Scholar]
- Ciesielski KT, Knight JE, Prince RJ, Harris RJ, Handmaker SD. Event-related potentials in cross-modal divided attention in autism. Neuropsychologia. 1995;33(2):225–246. doi: 10.1016/0028-3932(94)00094-6. [DOI] [PubMed] [Google Scholar]
- Cook J, Saygin AP, Swain R, Blakemore SJ. Reduced sensitivity to minimum-jerk biological motion in autism spectrum conditions. Neuropsychologia. 2009;47(14):3275–3278. doi: 10.1016/j.neuropsychologia.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culham JC, Brandt SA, Cavanagh P, Kanwisher NG, Dale AM, Tootell RB. Cortical fMRI activation produced by attentive tracking of moving targets. Journal of Neurophysiology. 1998;80(5):2657–2670. doi: 10.1152/jn.1998.80.5.2657. [DOI] [PubMed] [Google Scholar]
- Culham JC, Cavanagh P, Kanwisher NG. Attention response functions: characterizing brain areas using fMRI activation during parametric variations of attentional load. Neuron. 2001;32(4):737–745. doi: 10.1016/s0896-6273(01)00499-8. [DOI] [PubMed] [Google Scholar]
- Edgin JO, Pennington BF. Spatial cognition in autism spectrum disorders: superior, impaired, or just intact? Journal of Autism and Developmental Disorders. 2005;35(6):729–745. doi: 10.1007/s10803-005-0020-y. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Volein A, Holmboe K, Tucker L, Csibra G, Baron-Cohen S, et al. Visual orienting in the early broader autism phenotype: disengagement and facilitation. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2009;50(5):637–642. doi: 10.1111/j.1469-7610.2008.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facoetti A, Ruffino M, Peru A, Paganoni P, Chelazzi L. Sluggish engagement and disengagement of non-spatial attention in dyslexic children. Cortex. 2008;44(9):1221–1233. doi: 10.1016/j.cortex.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Foss-Feig JH, Kwakye LD, Cascio CJ, Burnette CP, Kadivar H, Stone WL, et al. An extended multisensory temporal binding window in autism spectrum disorders. Experimental Brain Research. 2010;203(2):381–389. doi: 10.1007/s00221-010-2240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franconeri SL, Jonathan SV, Scimeca JM. Tracking multiple objects is limited only by object spacing, not by speed, time, or capacity. Psychological Science. 2010;21(7):920–925. doi: 10.1177/0956797610373935. [DOI] [PubMed] [Google Scholar]
- Franconeri SL, Lin JY, Pylyshyn ZW, Fisher B, Enns JT. Evidence against a speed limit in multiple-object tracking. Psychonomic Bulletin & Review. 2008;15(4):802–808. doi: 10.3758/pbr.15.4.802. [DOI] [PubMed] [Google Scholar]
- Freitag CM, Konrad C, Häberlen M, Kleser C, von Gontard A, Reith W, et al. Perception of biological motion in autism spectrum disorders. Neuropsychologia. 2008;46(5):1480–1494. doi: 10.1016/j.neuropsychologia.2007.12.025. [DOI] [PubMed] [Google Scholar]
- Griffith EM, Pennington BF, Wehner EA, Rogers SJ. Executive functions in young children with autism. Child Development. 1999;70(4):817–832. doi: 10.1111/1467-8624.00059. [DOI] [PubMed] [Google Scholar]
- Groen WB, van Orsouw L, Huurne N, Swinkels S, van der Gaag RJ, Buitelaar JK, et al. Intact spectral but abnormal temporal processing of auditory stimuli in autism. Journal of Autism and Developmental Disorders. 2009;39(5):742–750. doi: 10.1007/s10803-008-0682-3. [DOI] [PubMed] [Google Scholar]
- Howe PD, Horowitz TS, Morocz IA, Wolfe J, Livingstone MS. Using fMRI to distinguish components of the multiple object tracking task. Journal of Vision. 2009;9(4):1–10. doi: 10.1167/9.4.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulleman J. The mathematics of multiple object tracking: from proportions correct to number of objects tracked. Vision Research. 2005;45(17):2298–2309. doi: 10.1016/j.visres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Ibanez LV, Messinger DS, Newell L, Lambert B, Sheskin M. Visual disengagement in the infant siblings of children with an autism spectrum disorder (ASD) Autism. 2008;12(5):473–485. doi: 10.1177/1362361308094504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CR, Swettenham J, Charman T, Marsden AJ, Tregay J, Baird G, et al. No evidence for a fundamental visual motion processing deficit in adolescents with autism spectrum disorders. Autism Research. 2011;4(5):347–357. doi: 10.1002/aur.209. [DOI] [PubMed] [Google Scholar]
- Joseph RM, Keehn B, Connolly C, Wolfe JM, Horowitz TS. Why is visual search superior in autism spectrum disorder? Developmental Science. 2009;12(6):1083–1096. doi: 10.1111/j.1467-7687.2009.00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J, Peters RJ, Koch C, Braun J, Chang L, Ernst T. Brain areas specific for attentional load in a motion-tracking task. Journal of Cognitive Neuroscience. 2001;13(8):1048–1058. doi: 10.1162/089892901753294347. [DOI] [PubMed] [Google Scholar]
- Kaiser MD, Shiffrar M. The visual perception of motion by observers with autism spectrum disorders: a review and synthesis. Psychonomic Bulletin & Review. 2009;16(5):761–777. doi: 10.3758/PBR.16.5.761. [DOI] [PubMed] [Google Scholar]
- Kaldy Z, Kraper C, Carter AS, Blaser E. Toddlers with Autism Spectrum Disorder are more successful at visual search than typically developing toddlers. Developmental Science. 2011;14(5):980–988. doi: 10.1111/j.1467-7687.2011.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y, Senju A, Akechi H, Tojo Y, Osanai H, Hasegawa T. Atypical disengagement from faces and its modulation by the control of eye fixation in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2011;41(5):629–645. doi: 10.1007/s10803-010-1082-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Lin DJ, Gorrindo P, Ramsay G, Jones W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459(7244):257–261. doi: 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koldewyn K, Whitney D, Rivera SM. The psychophysics of visual motion and global form processing in autism. Brain. 2010;133(Pt2):599–610. doi: 10.1093/brain/awp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koldewyn K, Whitney D, Rivera SM. Neural correlates of coherent and biological motion perception in autism. Developmental Science. 2011;14(5):1075–1088. doi: 10.1111/j.1467-7687.2011.01058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwakye LD, Foss-Feig JH, Cascio CJ, Stone WL, Wallace MT. Altered auditory and multisensory temporal processing in autism spectrum disorders. Frontiers in Integrative Neuroscience. 2011;4:129. doi: 10.3389/fnint.2010.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau B, Hoffman J, Reiss J, Dilks D, Lakusta L, Chunyo G. Specialization, Breakdown, and Sparing in Spatial Cognition. Williams-Beuren Syndrome: Research, Evaluation, and Treatment. 2006:207–236. [Google Scholar]
- Landry R, Bryson SE. Impaired disengagement of attention in young children with autism. Journal of Child Psychology and Psychiatry. 2004;45(6):1115–1122. doi: 10.1111/j.1469-7610.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- Lepage JF, Dunkin B, Hong DS, Reiss AL. Contribution of executive functions to visuospatial difficulties in prepubertal girls with Turner syndrome. Developmental Neuropsychology. 2011;36(8):988–1002. doi: 10.1080/87565641.2011.584356. [DOI] [PubMed] [Google Scholar]
- Lopez BR, Lincoln AJ, Ozonoff S, Lai Z. Examining the relationship between executive functions and restricted, repetitive symptoms of Autistic Disorder. Journal of Autism and Developmental Disorders. 2005;35(4):445–460. doi: 10.1007/s10803-005-5035-x. [DOI] [PubMed] [Google Scholar]
- Luna B, Doll SK, Hegedus SJ, Minshew NJ, Sweeney JA. Maturation of executive function in autism. Biological Psychiatry. 2007;61(4):474–481. doi: 10.1016/j.biopsych.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Mann TA, Walker P. Autism and a deficit in broadening the spread of visual attention. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2003;44(2):274–284. doi: 10.1111/1469-7610.00120. [DOI] [PubMed] [Google Scholar]
- Mazzocco MM, Singh Bhatia N, Lesniak-Karpiak K. Visuospatial skills and their association with math performance in girls with fragile X or Turner syndrome. Child Neuropsychology. 2006;12(2):87–110. doi: 10.1080/09297040500266951. [DOI] [PubMed] [Google Scholar]
- Milne E, Swettenham J, Hansen P, Campbell R, Jeffries H, Plaisted K. High motion coherence thresholds in children with autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2002;43(2):255–263. doi: 10.1111/1469-7610.00018. [DOI] [PubMed] [Google Scholar]
- Milne E, White S, Campbell R, Swettenham J, Hansen P, Ramus F. Motion and form coherence detection in autistic spectrum disorder: Relationship to motor control and 2:4 digit ratio. Journal of Autism and Developmental Disorders. 2006;36(2):225–237. doi: 10.1007/s10803-005-0052-3. [DOI] [PubMed] [Google Scholar]
- Mullane JC, Klein RM. Literature review: visual search by children with and without ADHD. Journal of Attention Disorders. 2008;12(1):44–53. doi: 10.1177/1087054707305116. [DOI] [PubMed] [Google Scholar]
- Murphy P, Brady N, Fitzgerald M, Troje NF. No evidence for impaired perception of biological motion in adults with autistic spectrum disorders. Neuropsychologia. 2009;47(14):3225–3235. doi: 10.1016/j.neuropsychologia.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Nakano T, Ota H, Kato N, Kitazawa S. Deficit in visual temporal integration in autism spectrum disorders. Proceedings of the Royal Society: Biological Sciences. 2010;277(1684):1027–1030. doi: 10.1098/rspb.2009.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome WT, Pare EB. A selective impairment of motion perception following lesions of the middle temporal visual area (MT) Journal of Neuroscience. 1988;8(6):2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hearn K, Hoffman JE, Landau B. Developmental profiles for multiple object tracking and spatial memory: typically developing preschoolers and people with Williams syndrome. Developmental Science. 2010;13(3):430–440. doi: 10.1111/j.1467-7687.2009.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hearn K, Landau B, Hoffman JE. Multiple object tracking in people with Williams syndrome and in normally developing children. Psychological Science. 2005;16(11):905–912. doi: 10.1111/j.1467-9280.2005.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Riordan M, Plaisted K. Enhanced discrimination in autism. The Quarterly Journal of Experimental Psychology. 2001;54(4):961–979. doi: 10.1080/713756000. [DOI] [PubMed] [Google Scholar]
- O’riordan MA. Superior visual search in adults with autism. Autism. 2004;8(3):229–248. doi: 10.1177/1362361304045219. [DOI] [PubMed] [Google Scholar]
- O’Riordan MA, Plaisted KC, Driver J, Baron-Cohen S. Superior visual search in autism. Journal of Experimental Psychology Human Perception and Performance. 2001;27(3):719–730. doi: 10.1037//0096-1523.27.3.719. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Strayer DL. Further evidence of intact working memory in autism. Journal of Autism and Developmental Disorders. 2001;31(3):257–263. doi: 10.1023/a:1010794902139. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10(4):437–442. [PubMed] [Google Scholar]
- Pellicano E, Gibson LY. Investigating the functional integrity of the dorsal visual pathway in autism and dyslexia. Neuropsychologia. 2008;46(10):2593–2596. doi: 10.1016/j.neuropsychologia.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Pellicano E, Gibson L, Maybery M, Durkin K, Badcock DR. Abnormal global processing along the dorsal visual pathway in autism: a possible mechanism for weak visuospatial coherence? Neuropsychologia. 2005;43(7):1044–1053. doi: 10.1016/j.neuropsychologia.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Shultz S, Hudac CM, Vander Wyk BC. Research review: Constraining heterogeneity: the social brain and its development in autism spectrum disorder. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2011;52(6):631–644. doi: 10.1111/j.1469-7610.2010.02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Glad KS, Schreibman L. Social perception in children with autism: an attentional deficit? Journal of Autism and Developmental Disorders. 1997;27(3):265–282. doi: 10.1023/a:1025898314332. [DOI] [PubMed] [Google Scholar]
- Plaisted K, O’Riordan M, Baron-Cohen S. Enhanced visual search for a conjunctive target in autism: a research note. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1998;39(5):777–783. [PubMed] [Google Scholar]
- Plaisted K, Swettenham J, Rees L. Children with autism show local precedence in a divided attention task and global precedence in a selective attention task. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1999;40(5):733–742. [PubMed] [Google Scholar]
- Poirier M, Martin JS, Gaigg SB, Bowler DM. Short-term memory in autism spectrum disorder. Journal of Abnormal Psychology. 2011;120(1):247–252. doi: 10.1037/a0022298. [DOI] [PubMed] [Google Scholar]
- Pylyshyn ZW, Storm RW. Tracking multiple independent targets: evidence for a parallel tracking mechanism. Spatial Vision. 1988;3(3):179–197. doi: 10.1163/156856888x00122. [DOI] [PubMed] [Google Scholar]
- Remington A, Swettenham J, Campbell R, Coleman M. Selective attention and perceptual load in autism spectrum disorder. Psychological Science. 2009;20(11):1388–1393. doi: 10.1111/j.1467-9280.2009.02454.x. [DOI] [PubMed] [Google Scholar]
- Rinehart N, Tonge B, Brereton A, Bradshaw J. Attentional blink in young people with high-functioning autism and Asperger’s disorder. Autism. 2010;14(1):47–66. doi: 10.1177/1362361309335718. [DOI] [PubMed] [Google Scholar]
- Rinehart NJ, Bradshaw JL, Moss SA, Brereton AV, Tonge BJ. A deficit in shifting attention present in high-functioning autism but not Asperger’s disorder. Autism. 2001;5(1):67–80. doi: 10.1177/1362361301005001007. [DOI] [PubMed] [Google Scholar]
- Russell J, Jarrold C, Henry L. Working memory in children with autism and with moderate learning difficulties. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1996;37(6):673–686. doi: 10.1111/j.1469-7610.1996.tb01459.x. [DOI] [PubMed] [Google Scholar]
- Russo N, Flanagan T, Iarocci G, Berringer D, Zelazo PD, Burack JA. Deconstructing executive deficits among persons with autism: implications for cognitive neuroscience. Brain and Cognition. 2007;65(1):77–86. doi: 10.1016/j.bandc.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Rutherford MD, Troje NF. IQ Predicts Biological Motion Perception in Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2012;42(4):557–565. doi: 10.1007/s10803-011-1267-0. [DOI] [PubMed] [Google Scholar]
- Rutherford MD, Richards ED, Moldes V, Sekuler AB. Evidence of a divided-attention advantage in autism. Cognitive Neuropsychology. 2007;24(5):505–515. doi: 10.1080/02643290701508224. [DOI] [PubMed] [Google Scholar]
- Saygin AP, Cook J, Blakemore SJ. Unaffected perceptual thresholds for biological and non-biological form-from-motion perception in autism spectrum conditions. PloS One. 2010;5(10):e13491. doi: 10.1371/journal.pone.0013491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim WM, Alvarez GA, Jiang YV. Spatial separation between targets constrains maintenance of attention on multiple objects. Psychonomic Bulletin & Review. 2008;15(2):390–397. doi: 10.3758/PBR.15.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim WM, Alvarez GA, Vickery TJ, Jiang YV. The number of attentional foci and their precision are dissociated in the posterior parietal cortex. Cerebral Cortex. 2010;20(6):1341–1349. doi: 10.1093/cercor/bhp197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer J, O’Brien J, Riggs K, Braddick O, Atkinson J, Wattam-Bell J. Motion processing in autism: evidence for a dorsal stream deficiency. Neuroreport. 2000;11(12):2765–2767. doi: 10.1097/00001756-200008210-00031. [DOI] [PubMed] [Google Scholar]
- Spencer JV, O’Brien JM. Visual form-processing deficits in autism. Perception. 2006;35(8):1047–1055. doi: 10.1068/p5328. [DOI] [PubMed] [Google Scholar]
- Thornton IM, Rensink RA, Shiffrar M. Active versus passive processing of biological motion. Perception. 2002;31(7):837–853. doi: 10.1068/p3072. [DOI] [PubMed] [Google Scholar]
- Tombu M, Seiffert AE. Attentional costs in multiple-object tracking. Cognition. 2008;108(1):1–25. doi: 10.1016/j.cognition.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend J, Harris NS, Courchesne E. Visual attention abnormalities in autism: delayed orienting to location. Journal of the International Neuropsychological Society. 1996;2(6):541–550. doi: 10.1017/s1355617700001715. [DOI] [PubMed] [Google Scholar]
- Trick LM, Jaspers-Fayer F, Sethi N. Multiple-object tracking in children: The Catch the Spies task. Cognitive Development. 2005;20(3):373–387. [Google Scholar]
- Trick LM, Mutreja R, Hunt K. Spatial and visuospatial working memory tests predict performance in classic multiple-object tracking in young adults, but nonspatial measures of the executive do not. Attention, Perception & Psychophysics. 2012;74(2):300–311. doi: 10.3758/s13414-011-0235-2. [DOI] [PubMed] [Google Scholar]
- Tsermentseli S, O’Brien JM, Spencer JV. Comparison of form and motion coherence processing in autistic spectrum disorders and dyslexia. Journal of Autism and Developmental Disorders. 2008;38(7):1201–1210. doi: 10.1007/s10803-007-0500-3. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke MW, Scholte HS, van Engeland H, Lamme VA, Kemner C. Coherent versus component motion perception in autism spectrum disorder. Journal of Autism and Developmental Disorders. 2008;38(5):941–949. doi: 10.1007/s10803-007-0467-0. [DOI] [PubMed] [Google Scholar]
- van der Geest JN, Kemner C, Camfferman G, Verbaten MN, van Engeland H. Eye movements, visual attention, and autism: a saccadic reaction time study using the gap and overlap paradigm. Biological Psychiatry. 2001;50(8):614–619. doi: 10.1016/s0006-3223(01)01070-8. [DOI] [PubMed] [Google Scholar]
- Watson AB, Pelli DG. QUEST: a Bayesian adaptive psychometric method. Perception & Psychophysics. 1983;33(2):113–120. doi: 10.3758/bf03202828. [DOI] [PubMed] [Google Scholar]