Abstract
Early life stress (ELS) is a significant risk factor for psychopathology, although there are few functional imaging studies investigating its effects. Previous literature suggests that ELS is associated with changes in structure and function in the medial prefrontal cortex (MPFC), which forms the main anterior node of the default network (DN). This study investigated the impact of ELS history on resting state DN connectivity, using seed-based correlation analyses (SCA) involving the posterior cingulate cortex (PCC). Data were analyzed from 22 adult subjects without psychiatric or medical illness (13 with and 9 without ELS); none were taking psychotropic medication. Relative to controls, the ELS group had significant decreases in DN connectivity, observed between the PCC seed and the MPFC and inferior temporal cortex. Further analyses revealed a trend-level increase in connectivity between the amygdala and MPFC associated with ELS history. In conclusion, this study found that subjects with ELS, in the absence of psychiatric illness and medication exposure, demonstrated decreased DN connectivity, and trend-level increases in connectivity between the amygdala and MPFC. These findings suggest that altered resting state connectivity is a correlate of stress exposure, rather than a product of medication or psychiatric morbidity.
Keywords: Default Network, Early Life Stress, Resilience, Functional Connectivity, Post Traumatic Stress Disorder, Medial Prefrontal Cortex
INTRODUCTION
There is extensive evidence that exposure to early life stress (ELS) confers a significant risk for psychiatric illness. ELS, often defined as childhood maltreatment, abuse, neglect or parental loss, is strongly linked to post-traumatic stress disorder (PTSD), major depressive disorder (MDD), bipolar disorder, panic disorder, social phobias and substance abuse (Heim and Nemeroff, 2001; Heim et al, 2010; Kendler et al, 2000; Kendler et al, 2004). ELS is associated with poorer response to treatment, increased chronicity of symptoms, and suicide risk (Brown and Moran, 1994; Dube et al, 2001; Zlotnick et al, 2001; Zlotnick et al, 1997). ELS is also highly prevalent: reports indicate that over 6 million children in the United States are abused or neglected every year (U.S. Department of Health and Human Services), and actual numbers are likely to be higher due to under-reporting. For example, Briere et al. (Briere and Elliott, 2003) found as many as 14.2% of men and 32.3% of women reported a history of childhood sexual abuse, and 22.2% of men and 19.5% of women met criteria for physical abuse. Different kinds of abuse are often comorbid, with nearly 30% of sexually abused girls between the ages of 6 and 16 years also reporting physical abuse (Horowitz et al, 1997).
Magnetic resonance imaging (MRI) studies of samples characterized by a history of significant ELS are one way to evaluate and measure neurobiological correlates of adverse early-life experiences. Structural MRI studies have consistently demonstrated that ELS is associated with changes in specific brain regions, especially the prefrontal cortex (PFC). The PFC is relatively slower to mature than other brain regions, and as such may be more susceptible to impairment incurred during the developing years, possibly mediated by excessive glucocorticoid exposure secondary to severe environmental stressors (Conrad et al, 2007; Patel et al, 2002). ELS is associated with decreased PFC gray matter and volume (De Bellis et al, 1999; De Bellis et al, 2002), and studies of children with ELS but without PTSD have shown reduced volume in the dorsolateral PFC (DLPFC), medial PFC (MPFC), and orbitofrontal cortex (Hanson et al, 2010). In adults with a history of ELS, reduced volumes in the MPFC and DLPFC have been reported (Andersen et al, 2008). Structural changes in other brain regions have been mixed, but alterations in the hippocampus (Bremner et al, 1997; Carrion et al, 2007; Driessen et al, 2000; Vythilingam et al, 2002), amygdala (Carrion et al, 2001; Mehta et al, 2009), corpus callosum (Andersen et al, 2008; Kitayama et al, 2007), anterior cingulate cortex (Cohen et al, 2006; Hanson et al, 2010; Kitayama et al, 2006; Tomoda et al, 2009; Treadway et al, 2009) and cerebellum (Carrion et al, 2009; De Bellis and Kuchibhatla, 2006) have been described (for a review, see Hart et al. (Hart and Rubia, 2012)).
In contrast to the number of structural MRI studies, few functional MRI studies have investigated effects of ELS. One area of functional imaging research relevant to ELS is the study of the default network (DN). The MPFC, the main anterior node of the DN, is located within the regions where structural changes are highly associated with ELS. First described in 2001 (Raichle et al, 2001), the DN additionally includes the posterior cingulate cortex/precuneus (PCC), lateral parietal cortices and medial temporal regions. The DN exhibits a high degree of activity during periods of rest (Gusnard and Raichle, 2001), and thus resting state activity of DN was coined the “default mode” of brain function, to describe a period when subjects are awake and alert but not involved in a specific task (Raichle et al, 2001). Since DN regions demonstrate high levels of functional connectivity with each other (Greicius et al, 2003), resting state analyses may provide a robust baseline for comparison between samples and for use in clinical applications (Fox and Greicius, 2010).
Abnormalities of DN functional connectivity, and especially the MPFC, have been associated with ELS and PTSD. In the first study of ELS and the DN, Bluhm et al. (Bluhm et al, 2009) measured resting state DN connectivity in 17 women with PTSD from childhood abuse, and compared them with 15 healthy controls. Patients in this study had PTSD defined by the Clinician Administered PTSD Scale (CAPS)(Blake et al, 1995), in addition to a history of childhood trauma and dissociative symptoms, and were on medications. Using seed-region functional connectivity analysis (SCA) from the PCC, the authors found decreased DN connectivity in PTSD subjects, including between the PCC and MPFC. This important initial study indicated that childhood ELS exposure might be associated with decreased DN connectivity among people who developed PTSD.
Interestingly, resting state DN connectivity has been suggested as a predictor of PTSD symptoms after trauma exposure. Lanius et al. (Lanius et al, 2010) evaluated 11 subjects (6 women) who had experienced a significant motor vehicle or workplace accident, and followed them for up to 12 weeks. Using SCA, the authors found that the strength of the connectivity between the PCC and MPFC was directly related to PTSD severity immediately after the trauma. Furthermore, when assessing connectivity between the PCC and amygdala, the authors found that the strength of this connectivity at week 6 could predict the severity of PTSD symptoms at week 12. This important study indicated that resting state DN connectivity could be used as a potential prognostic indicator of PTSD. The results suggested that acute effects of trauma may be associated with decreased PCC to MPFC connectivity, but to our knowledge no study to date has investigated longer-term neural network correlates of early life traumatic events.
Other, more traditional task-based functional neuroimaging studies consistently have underscored the importance of the MPFC within the context of the DN and trauma exposure. The MPFC, along with its subcomponent the ventromedial PFC, are implicated in PTSD pathophysiology. The MPFC is a major part of the fear circuitry loop that encompasses the MPFC, hippocampus and amygdala (Shin and Handwerger, 2009) (Bryant et al, 2008). Healthy MPFC activity is required to modulate ascending amygdala activity; activation of the MPFC is required for extinction of fear conditioning in healthy individuals (Milad et al, 2007). This is in contrast to PTSD subjects, who exhibit a relative hypo-activation of MPFC during fear extinction (Rougemont-Bucking et al, 2011). These findings are supported by studies comparing emotional processing in PTSD subjects and controls: when watching fearful faces, those with PTSD demonstrate decreased MPFC activity in the context of increased amygdala activity (Shin et al, 2005). This process may not be permanent, as demonstrated by one study in which recently traumatized police officers received exposure psychotherapy, resulting in increased MPFC activity associated with decreased amygdala activity during traumatic memory retrieval (Peres et al, 2011).
Taken together, these findings indicate an association between trauma exposure and changes in DN resting state connectivity, which may be driven by abnormal MFPC function. However, to our knowledge there are no studies investigating the functional neuroimaging correlates of ELS exposure in the absence of psychiatric illness. In order to isolate potentially independent DN correlates of ELS, we focused on participants with ELS who were otherwise healthy and without psychiatric illness. This approach was designed to minimize the impact of comorbid psychiatric conditions, and to eliminate the confounding influences of psychotropic medications on connectivity (McCabe and Mishor, 2011a; McCabe et al, 2011b).
The goal of the present study was to identify and compare patterns of resting state DN connectivity between ELS and matched healthy controls, and to evaluate connectivity relationships between the amygdala and the DN. Based on the previous literature, we hypothesized that ELS participants would exhibit decreased resting state DN functional connectivity between the PCC and MPFC, and that further exploratory analyses would reveal group differences between the PCC and other DN regions.
EXPERIMENTAL PROCEDURES
Participants
Participants with a reported history of ELS exposure (n=13) and healthy controls (n=9) were recruited for this pilot study from an ongoing longitudinal study examining potential endophenotypes for mood/anxiety disorders. This study was conducted under the approval of the Brown University and Butler Hospital Institutional Review Boards, and participants were reimbursed $50 for their participation.
Study inclusion criteria were 1) a history of physical, emotional or sexual abuse as a child, defined as a Childhood Trauma Questionnaire (CTQ) (Bernstein et al, 1994) subscale classification score (Bernstein D. and L., 1998) of “moderate/severe” or “severe/extreme” (ELS group), or absence of such history confirmed with the same instrument (controls), and 2) absence of a current Axis I psychiatric disorder, assessed by the Structured Clinical Interview for DSM-IV-TR (SCID)(First, 1994). ELS and control participants were matched on the basis of age and gender. Exclusion criteria were 1) an absolute contraindication to MRI scanning (such as bodily inclusion of metallic objects), 2) current treatment with psychotropic medications, or 3) active medical illness, assessed by medical history, physical and neurological examination, electrocardiogram and standard laboratory studies. To avoid confounding influences from recent life stress, which may impact performance within the scanner (Hsu et al, 2010), any participants who reported significant life stress in the previous month, reported on the Perceived Stress Scale (PSS)(Cohen et al, 1983), were also excluded. A negative pregnancy test for women of childbearing age was required before MRI exposure.
Image Acquisition
All magnetic resonance imaging data was acquired at the Brown University MRI Research facility (www.brainscience.brown.edu/MRF) using a Siemens TIM TRIO 3 Tesla scanner (Siemens, Erlangen, Germany) equipped with a 32-channel head coil. During the scan session participants were instructed to remain awake and watch a white fixation cross against a black background. Images were acquired during two separate 5-minute epochs. Acquisition parameters for echoplanar images were TR = 2500 ms, TE = 28 ms, FOV = 192 mm2, and matrix size 642 in 3-mm axial slices. This sequence yielded 147 whole brain volumes for each functional imaging run, with spatial resolution of 3 mm3 per voxel. Whole-brain high-resolution (1 mm3) T1 images were acquired prior to resting state scans for anatomic reference.
Image Preprocessing
After image acquisition, anatomic data were transformed to standard Talairach stereotaxic space (Talairach, 1988). Echoplanar data were reconstructed into 3D + time datasets, which were concatenated and registered to the sixth volume of the first series to minimize movement artifact and generate motion correction parameters for use as covariates in subsequent analysis. Similar to Greicius et al. (Greicius et al, 2003), bandpass filtering was performed at .0083 sec < f < 0.15 sec to reduce the effect of high frequency noise and low frequency drift. Preprocessing steps utilized the Analysis of Functional Images (AFNI) (Cox, 1996) software.
Seed Generation
One known limitation of the SCA approach is misplacement of seed region, leading to spurious results (Cole et al, 2012). To mitigate this potential issue, we generated individual PCC seeds to isolate grey matter and to eliminate white matter or cerebrospinal fluid signals. The PCC was selected as the principal seed region because it correlates significantly with other DN regions in healthy adults (Fox et al, 2005; Fransson and Marrelec, 2008; Greicius et al, 2003), and is most commonly used in DN studies. High-resolution T1 images were segmented using statistical parametric mapping (SPM9; Functional Imaging Laboratory, University College London, London, United Kingdom), aligned and registered to stereotaxic space. PCC seeds were created in T1 volume as two 5-mm radius spheres surrounding Talairach coordinates ± 5, 55, 25, and re-sampled to accommodate echoplanar resolution. The segmented cortical ribbon and ROI sphere then were combined using a 75% probability map. This conjunction methodology generated unique PCC seed regions valid for each participant. PCC time series data were extracted and combined from these seed regions of interest (ROI) and used for subsequent data analysis, described below.
Seed-Based Correlation Analysis and Statistical Analysis of the DN
We used SCA to identify and evaluate the DN. This method requires the selection of a priori seed regions, used to generate time-course models of functional connectivity. Resultant time series data are used as regressors in a general linear model (GLM) analysis to generate whole-brain voxel-wise functional connectivity maps with shared covariance with the original seed region. This methodology has been demonstrated to be a robust analytic approach to identify resting state networks (Biswal et al, 2010; Cole et al, 2012; Smith et al, 2010), and has been utilized in multiple studies of the DN (Bluhm et al, 2009; Fox et al, 2005; Greicius et al, 2003; Lanius et al, 2010).
Voxel-based GLM was used to quantify functional relationships between the observed mean blood-oxygen-level-dependent (BOLD) signal over time in the PCC seed region and the BOLD time series data in the rest of the gray matter ribbon. Independent variables in the GLM were PCC time series data and covariates of non-interest, such as linear drift and movement parameters. GLM results yielded individual R2 values for time series data, which were normalized into Z values using Fisher’s R-to-Z transformation. Initial one-sample t-tests of these Z values were used to describe the spatial extent of the DN. Individual maps of Z values that represented the strength of the PCC BOLD time-series relationship to each voxel in the cortical ribbon served as the basic measure to generate SCA functional connectivity maps.
Planned group-level contrasts used a priori MPFC coordinates adapted from Greicius et al. (Greicius et al, 2003) to evaluate our primary hypothesis of diminished resting state connectivity between the PCC and MPFC. The statistical threshold for this comparison was set at a two-tailed p < .05, since the MPFC was an a priori selected brain region. The convention of using a priori ROIs as primary outcome measures has proven to be a useful method to ensure accurate identification of involved regions, ensure the independence of statistical testing (Poldrack and Mumford, 2009) and decrease false positive results associated with multiple comparisons (Smith et al, 2010).
Because of the close relationship between the MPFC and amygdala in the neurobiology of stress exposure, we compared resting state connectivity between the amygdala and MPFC between groups. We created a 5-mm radius seed region within bilateral amygdala (Talairach coordinates −22 −5 −15 and 23 −5 −16, for left and right amygdala, respectively), and performed a similar data analysis as described above to directly compare mean BOLD time-series data from the amygdala with the mean MPFC time series BOLD signal. As previous literature has indicated that increased connectivity between the PCC and amygdala may significantly correlate with the clinical severity of PTSD, connectivity between these regions was also evaluated. ANOVA was used to contrast results from ELS and healthy controls, with statistical significance set at two-tailed p < .05.
In addition, we performed exploratory analyses as follows. First, using other DN ROIs from Greicius et al., we investigated group differences in resting state DN connectivity between the PCC and medial frontal gyrus, inferior temporal cortex (ITC), parahippocampal regions, and angular gyrus. Correlations between ELS severity and DN changes were also investigated using Pearson r. Overall ELS severity was calculated as the sum of the 5 severity classification scores (0 to 3 for each of the CTQ subscales representing different types of maltreatment i.e., emotional abuse, physical abuse, sexual abuse, emotional neglect and physical neglect)(Bernstein D. et al, 1998), with a range of 0–15. SPSS Statistics 19 (IBM Corporation, Armonk, NY) was used for statistical analyses.
RESULTS
Participants
22 participants (12 female) meeting entry criteria were scanned between November 2010 and March 2012. All participants were right-handed. Demographic and clinical characteristics of the two participant groups are shown on Table 1. Groups did not significantly differ in terms of age, gender or education. Within the healthy control group, no participants met criteria for lifetime depressive or anxiety disorders. Within the ELS group, four participants endorsed diagnostic criteria for “probable” past (lifetime) psychiatric disorders, though all considered themselves free of psychiatric illness and none had ever sought or received past psychotropic drug treatment for their symptoms. Lifetime diagnoses included PTSD (n = 1), MDD (n = 1), depressive disorder not otherwise specified (n = 2), and social phobia (n = 1); one participant endorsed past symptoms meeting criteria consistent with both PTSD and MDD. In addition to physical, sexual, and emotional abuse, the ELS population scored significantly higher than controls on two other CTQ subscales (physical neglect and emotional neglect), and 76% of ELS participants endorsed threshold severity for more than one type of ELS.
Table 1.
Demographic and Clinical Characteristics of ELS and Control Groups
| Characteristic | ELS (n = 13) | Control (n = 9) | p |
|---|---|---|---|
| Age (Mean ± SD years) | 36 ± 10 | 34 ± 9 | ns |
| Gender (n, % Female) | 8 (62) | 4 (44) | ns |
| College Education (%) | 62 | 52 | ns |
| CTQ | |||
| Category (n, %)a | |||
| Emotional Abuse | 4 (30) | - | |
| Physical Abuse | 8 (62) | - | |
| Sexual Abuse | 8 (62) | - | |
| Emotional Neglect | 7 (54) | - | |
| Physical Neglect | 6 (46) | - | |
| Summary Score (Mean ± SD)b | 7 ± 4 | ||
SD, standard deviation, CTQ, childhood trauma questionnaire
Participants endorsing at least moderate scores in CTQ categories.
Derived from CTQ categories, where ELS severity is indicated by “none/minimal”= 0, “low to moderate”= 1, “moderate to severe”= 2, or “severe to extreme”= 3, with a total range of 0–15
Connectivity Analyses
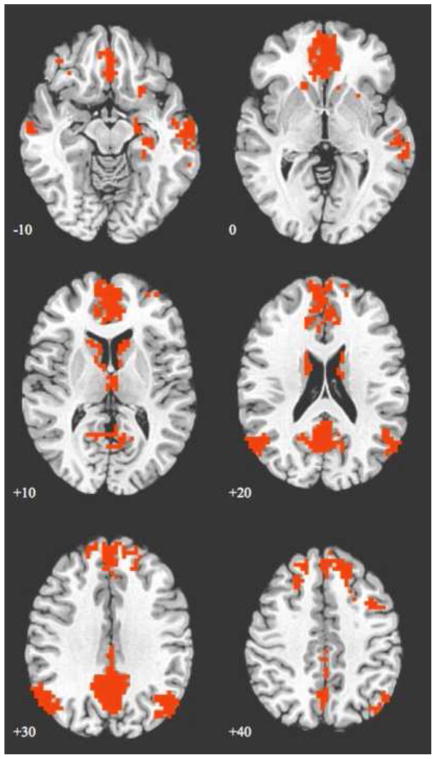
Data from one ELS participant was removed due to movement greater than 3 mm during image acquisition, resulting in an evaluable sample of 21 participants (12 ELS, 9 controls). Whole-brain, one sample t-tests (p < .001, false discovery rate [FDR](Benjamini, 1995)-corrected) of connectivity revealed the expected spatial pattern of DN regions in both ELS and healthy control participants, with a composite map of both ELS and controls shown in Figure 1. Significant peaks of connectivity occurred in the MPFC, PCC/precuneus, bilateral angular gyrus, medial temporal cortex and ITC. The a priori ROI analysis demonstrated statistically significant decreased connectivity between the PCC and MPFC in the ELS group, compared to controls (p < .04).
Figure 1.
Axial images showing a map of default network connectivity derived from a seed-based connectivity analysis. The map illustrated is a combination of early life stress (ELS) and healthy individuals, with whole–brain results of one sample t-tests of connectivity, thresholded at p < .001 (FDR-corrected) and cluster size >20 adjacent voxels. Images are shown using radiologic convention. Z coordinates of each slice are shown on the bottom left of the corresponding image.
When the amygdala was utilized as a seed region, there was a trend-level increase in resting state connectivity between the amygdala and MPFC in the ELS group (p < .07) relative to controls. There were no statistically significant group differences in connectivity between the amygdala and PCC (two-tailed p > .10).
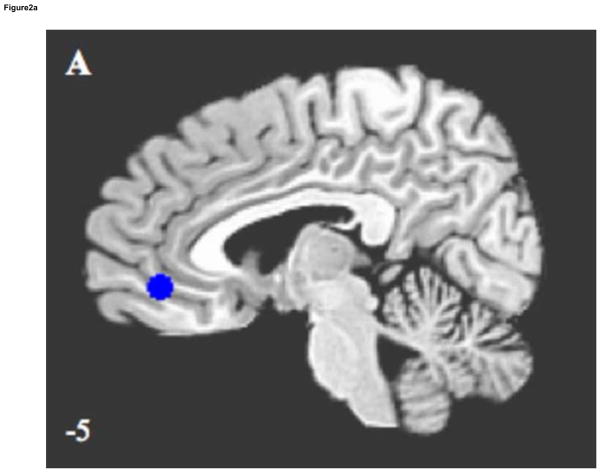
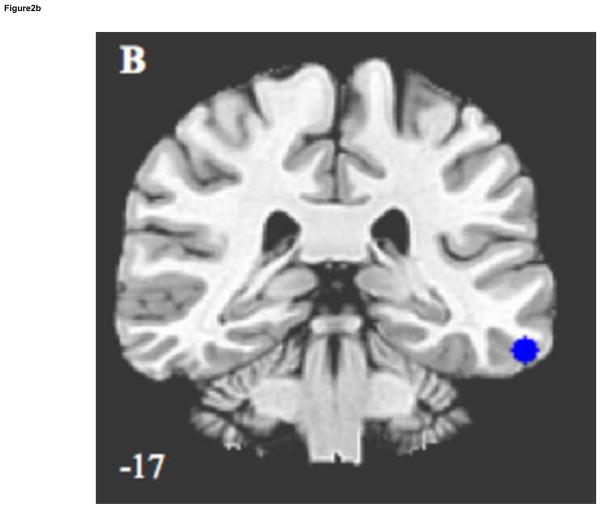
Exploratory evaluations of other DN regions found ELS associated with decreased connectivity between the PCC and left ITC (p < .04), shown in Figure 2A and B, and in Table 2. Negative correlations between ELS severity and connectivity strength were found in the left ITC (r = −.46, p < .03), with trends towards significance in the right ITC (r = −.39, p < .07) and MPFC (r = −.38, p < .08).
Figure 2.
Sagittal and coronal images illustrating significantly decreased default network connectivity in ELS participants, compared to controls, using region of interest analysis. Results are (A) medial prefrontal cortex/ventral anterior cingulate cortex, and (B) left inferior temporal cortex. Z coordinates of each slice are shown on bottom left.
Table 2.
Group Differences in SCA Functional Connectivity between PCC and DN ROIs
| Brain Regions | x, y, za | Mean Z Score ± SD
|
p | |
|---|---|---|---|---|
| ELS | Control | |||
| MPFC/vACC (BA 32/10/11) | 5 41 −4 | .30 ± .11 | .40 ± .10 | .04 |
| Left SFG (BA 8/9) | −10 35 50 | .18 ± .08 | .23 ± .09 | ns |
| Right SFG (BA 8/9) | 14 35 50 | .16 ± .09 | .22 ± .13 | ns |
| Left AG (BA 39) | −43 −64 35 | .40 ± .13 | .40 ± .15 | ns |
| Right AG (BA 39) | 50 −64 53 | .27 ± .13 | .27 ± .10 | ns |
| Left PHG (BA 35) | −22 −25 −16 | .09 ± .07 | .09 ± .06 | ns |
| Left ITC (BA 20/21) | −58 −31, −16 | .02 ± .06 | 0.11 ± .10 | .04 |
SCA, seed connectivity analysis; PCC, posterior cingulate cortex/precuneus; DN, default network; ROI, region of interest; MPFC, medial prefrontal cortex; vACC, ventral anterior cingulate cortex; SFG; superior frontal gyrus; AG angular gyrus, PHG, parahippocampal gyrus; ITC, inferior temporal cortex; SD, standard deviation
LPI coordinates indicating center of mass, 19 voxel ROIs
DISCUSSION
Using a seed-based connectivity approach (SCA), our study demonstrated decreased resting state DN connectivity associated with early-life stress (ELS) in medication-free adults without current psychiatric or medical illness. In comparison to non-ELS controls, our ELS participants showed decreased DN connectivity, while exhibiting trend-level increased connectivity between the amygdala and MPFC. These results suggest that diminished resting state DN connectivity may be a correlate of ELS exposure, and provide preliminary evidence in support of the notion that the DN patterns observed to be associated with ELS do not reflect confounds from current psychiatric morbidity or medication use.
Our finding of decreased PCC-to-MPFC connectivity among healthy adults reporting histories of significant ELS is generally consistent with the previously published literature, which shows decreased connectivity in the DN associated with multiple psychiatric disorders whose etiopathologies include links to stress during early brain development, including PTSD (Bluhm et al, 2009), MDD (Greicius et al, 2007; Zhu et al, 2012), and schizophrenia (Bluhm et al, 2007; Calhoun et al, 2008). Our study builds on the existing literature, and suggests that decreased DN connectivity may be an independent correlate of ELS exposure measurable in the absence of, or perhaps prior to development of, psychiatric disorders. Previous reviewers of the work in this area have hypothesized that ELS disrupts the posterior-to-anterior connectivity established as part of the normal developmental process (Fransson et al, 2011; Smyser et al, 2011), in part based on observations by researchers regarding similarity in the patterns of DN connectivity measured in adults with PTSD with those found in healthy 7- to 9-year-old children (Daniels et al, 2011). Results from our study in a healthy and unmedicated adult population suggest that ELS could be a determinant of diminished DN connectivity that endures beyond childhood. Future, longitudinal imaging studies of individuals with ELS are needed to investigate this possibility.
The decreased connectivity between the PCC and inferior temporal cortex (ITC) has not previously been reported in relation to ELS, and may represent a novel finding if replicated. The ITC is associated with semantic knowledge (Warburton et al, 1996), although little research has evaluated its role in psychiatric conditions or related symptoms, likely due to the considerable apparent heterogeneity of this brain region (Blaizot et al, 2010). In one study of pain sensitivity in comorbid borderline personality disorder and PTSD, decreased ITC BOLD signal was observed while participants watched a script designed to induce a dissociative state (Ludascher et al, 2010).
We found trend-level increases in functional connectivity between the amygdala and MPFC associated with ELS, and no differences between groups in amygdala-to-PCC connectivity. Results from other studies of amygdala connectivity have been mixed, with one study finding no differences in amygdala-to-prefrontal connectivity associated with PTSD (Rabinak et al, 2011), another finding reduced connectivity between the amygdala and rostral anterior cingulate cortex in PTSD (Sripada et al, 2012), and a third that demonstrated decreased amygdala-to-MPFC connectivity in healthy individuals with anxiety (Kim et al, 2011). While trend-level results need to be interpreted with caution, it is possible that changes in resting state connectivity reflects impaired modulation of amygdala activity, and that inadequate top-down DN modulation of this signal may be an alternate component of PTSD neurobiology.
Another interpretation of our results incorporates the concept of heightened resilience to development of psychopathology in the face of ELS exposure. Of our ELS subjects, over three quarters endorsed at least moderate severity of trauma involving multiple different types of childhood maltreatment, yet they had not developed significant psychiatric disorders by the time of participation in this study. While there are few neuroimaging studies of resilience, in a study by New et al. (New et al, 2009) women who had been sexually assaulted but did not develop PTSD demonstrated increased MPFC activity, compared to matched women who developed PTSD and healthy controls. An important caveat is that New et al reported changes in brain activity, rather than connectivity, which complicates direct comparison with our results. However, our preliminary observation of decreased DN connectivity and increased MPFC-to-amygdala connectivity in a sample defined by history of ELS and no significant adult psychopathology provides a potential signal for further investigation and clarification within larger, prospective neuroimaging studies of resilient populations.
There were several important limitations to our study. Our sample size was modest, although comparable in size with other similar studies, and unique in terms of absence of psychiatric illness and psychotropic exposure. We sought to mitigate the influence of sample size by employing an a priori ROI analysis with limited regions to minimize type I error. However, the ROI analysis itself may be a limitation, as other brain regions that may be involved in ELS were not measured. While our participants did not have psychiatric illness at the time of this study, it is possible they may develop psychiatric illness in the future. As with other studies, categories of ELS exposure were often comorbid, so it is not possible to assess which type(s) of ELS significantly impacted DN connectivity in our sample. Lastly, a one-time scan session does not provide information on the stability of our findings; future studies would benefit from a longitudinal and prospective design.
In summary, we found decreased resting state DN connectivity in a group of participants with ELS in the absence of psychiatric illness and psychotropic drug exposure, and a suggestion of increased connectivity between the amygdala and the MPFC. Our findings suggest that neuroimaging correlates of ELS and resilience could be an important area of future research.
Acknowledgments
Role of Funding Source
This study was supported by NIH grant 5R01MH068767-08 (LLC), and grants from the Brown MRI Research Facility (NSP) and Rhode Island Foundation (NSP). These funding sources had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. We thank all of the participants.
Footnotes
This study was presented in part at the 68th Annual Meeting of the Society of Biological Psychiatry, Philadelphia, PA, 2012
Contributors
Dr. Philip was involved at all levels of the study, including design, data acquisition and analyses and manuscript drafting. Dr. Sweet wrote the imaging protocol, Drs. Tyrka and Price contributed to data analyses, Ms. Bloom assisted with data acquisition and analysis, and Dr. Carpenter contributed to participant acquisition and data analysis. All authors contributed to and approved the final manuscript.
Conflict of Interest
Drs. Philip, Sweet, Tyrka, Price and Carpenter have received research support from the National Institutes of Health. Dr. Philip has received research support from Neuronetics and has served as a paid consultant to Gerson Lehrman. Drs. Tyrka, Carpenter, and Price have received research support from Cyberonics, the Department of Defense, Medtronic, and Neuronetics. Dr. Tyrka received an honorarium for continuing medical education from Lundbeck. Dr. Price has served as a paid consultant to Gerson Lehrman, and Dr. Price has served as a paid consultant to Wiley, Springer, Qatar National Research Fund, Abbott, and AstraZeneca. Dr. Carpenter has served as a paid consultant to Abbott, and also acted in a consultant/speaker role without compensation for Neuronetics. Dr. Sweet and Ms. Bloom report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20(3):292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57:289–300. [Google Scholar]
- Bernstein DLF. Childhood Trauma Questionnaire: A Retrospective Self-report. Pearson Education, Inc; San Antonio, Texas: 1998. [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151(8):1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, et al. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107(10):4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaizot X, Mansilla F, Insausti AM, Constans JM, Salinas-Alaman A, Pro-Sistiaga P, et al. The human parahippocampal region: I. Temporal pole cytoarchitectonic and MRI correlation. Cereb Cortex. 2010;20(9):2198–2212. doi: 10.1093/cercor/bhp289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RW, et al. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33(4):1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm RL, Williamson PC, Osuch EA, Frewen PA, Stevens TK, Boksman K, et al. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J Psychiatry Neurosci. 2009;34(3):187–194. [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, et al. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse--a preliminary report. Biol Psychiatry. 1997;41(1):23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briere J, Elliott DM. Prevalence and psychological sequelae of self-reported childhood physical and sexual abuse in a general population sample of men and women. Child Abuse Negl. 2003;27(10):1205–1222. doi: 10.1016/j.chiabu.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Brown GW, Moran P. Clinical and psychosocial origins of chronic depressive episodes. I: A community survey. Br J Psychiatry. 1994;165(4):447–456. doi: 10.1192/bjp.165.4.447. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Kemp AH, Felmingham KL, Liddell B, Olivieri G, Peduto A, et al. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: an fMRI study. Hum Brain Mapp. 2008;29(5):517–523. doi: 10.1002/hbm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Kiehl KA, Pearlson GD. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum Brain Mapp. 2008;29(7):828–838. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, et al. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry. 2001;50(12):943–951. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Reiss AL. Stress predicts brain changes in children: a pilot longitudinal study on youth stress, posttraumatic stress disorder, and the hippocampus. Pediatrics. 2007;119(3):509–516. doi: 10.1542/peds.2006-2028. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Watson C, Eliez S, Menon V, Reiss AL. Converging evidence for abnormalities of the prefrontal cortex and evaluation of midsagittal structures in pediatric posttraumatic stress disorder: an MRI study. Psychiatry Res. 2009;172(3):226–234. doi: 10.1016/j.pscychresns.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, et al. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry. 2006;59(10):975–982. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci. 2012;4:8. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, McLaughlin KJ, Harman JS, Foltz C, Wieczorek L, Lightner E, et al. Chronic glucocorticoids increase hippocampal vulnerability to neurotoxicity under conditions that produce CA3 dendritic retraction but fail to impair spatial recognition memory. J Neurosci. 2007;27(31):8278–8285. doi: 10.1523/JNEUROSCI.2121-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. AFNI: software for analysis and visualization of functioanl magnetic resonance images. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Daniels JK, Frewen P, McKinnon MC, Lanius RA. Default mode alterations in posttraumatic stress disorder related to early-life trauma: a developmental perspective. J Psychiatry Neurosci. 2011;36(1):56–59. doi: 10.1503/jpn.100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, et al. A.E. Bennett Research Award. Developmental traumatology. Part I: Biological stress systems. Biol Psychiatry. 1999;45(10):1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J, et al. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry. 2002;52(11):1066–1078. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Kuchibhatla M. Cerebellar volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry. 2006;60(7):697–703. doi: 10.1016/j.biopsych.2006.04.035. [DOI] [PubMed] [Google Scholar]
- Driessen M, Herrmann J, Stahl K, Zwaan M, Meier S, Hill A, et al. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch Gen Psychiatry. 2000;57(12):1115–1122. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- Dube SR, Anda RF, Felitti VJ, Chapman DP, Williamson DF, Giles WH. Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: findings from the Adverse Childhood Experiences Study. JAMA. 2001;286(24):3089–3096. doi: 10.1001/jama.286.24.3089. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM-IV Disorders. Biometrics Research Department. New York State Psychiatric Institute; New York: 1994. [Google Scholar]
- Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Aden U, Blennow M, Lagercrantz H. The functional architecture of the infant brain as revealed by resting-state fMRI. Cereb Cortex. 2011;21(1):145–154. doi: 10.1093/cercor/bhq071. [DOI] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage. 2008;42(3):1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62(5):429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2(10):685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, et al. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. J Neurosci. 2010;30(22):7466–7472. doi: 10.1523/JNEUROSCI.0859-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H, Rubia K. Neuroimaging of child abuse: a critical review. Front Hum Neurosci. 2012;6:52. doi: 10.3389/fnhum.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49(12):1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol. 2010;52(7):671–690. doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
- Horowitz LA, Putnam FW, Noll JG, Trickett PK. Factors affecting utilization of treatment services by sexually abused girls. Child Abuse Negl. 1997;21(1):35–48. doi: 10.1016/s0145-2134(96)00129-9. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Langenecker SA, Kennedy SE, Zubieta JK, Heitzeg MM. fMRI BOLD responses to negative stimuli in the prefrontal cortex are dependent on levels of recent negative life stress in major depressive disorder. Psychiatry Res. 2010;183(3):202–208. doi: 10.1016/j.pscychresns.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Bulik CM, Silberg J, Hettema JM, Myers J, Prescott CA. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953–959. doi: 10.1001/archpsyc.57.10.953. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Prescott CA. Childhood sexual abuse, stressful life events and risk for major depression in women. Psychol Med. 2004;34(8):1475–1482. doi: 10.1017/s003329170400265x. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex. 2011;21(7):1667–1673. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama N, Brummer M, Hertz L, Quinn S, Kim Y, Bremner JD. Morphologic alterations in the corpus callosum in abuse-related posttraumatic stress disorder: a preliminary study. J Nerv Ment Dis. 2007;195(12):1027–1029. doi: 10.1097/NMD.0b013e31815c044f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama N, Quinn S, Bremner JD. Smaller volume of anterior cingulate cortex in abuse-related posttraumatic stress disorder. J Affect Disord. 2006;90(2–3):171–174. doi: 10.1016/j.jad.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Bluhm RL, Coupland NJ, Hegadoren KM, Rowe B, Theberge J, et al. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr Scand. 2010;121(1):33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- Ludascher P, Valerius G, Stiglmayr C, Mauchnik J, Lanius RA, Bohus M, et al. Pain sensitivity and neural processing during dissociative states in patients with borderline personality disorder with and without comorbid posttraumatic stress disorder: a pilot study. J Psychiatry Neurosci. 2010;35(3):177–184. doi: 10.1503/jpn.090022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C, Mishor Z. Antidepressant medications reduce subcortical-cortical resting-state functional connectivity in healthy volunteers. Neuroimage. 2011a;57(4):1317–1323. doi: 10.1016/j.neuroimage.2011.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C, Mishor Z, Filippini N, Cowen PJ, Taylor MJ, Harmer CJ. SSRI administration reduces resting state functional connectivity in dorso-medial prefrontal cortex. Mol Psychiatry. 2011b;16(6):592–594. doi: 10.1038/mp.2010.138. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SC, et al. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. J Child Psychol Psychiatry. 2009;50(8):943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62(5):446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- New AS, Fan J, Murrough JW, Liu X, Liebman RE, Guise KG, et al. A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biol Psychiatry. 2009;66(7):656–664. doi: 10.1016/j.biopsych.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Patel R, McIntosh L, McLaughlin J, Brooke S, Nimon V, Sapolsky R. Disruptive effects of glucocorticoids on glutathione peroxidase biochemistry in hippocampal cultures. J Neurochem. 2002;82(1):118–125. doi: 10.1046/j.1471-4159.2002.00948.x. [DOI] [PubMed] [Google Scholar]
- Peres JF, Foerster B, Santana LG, Fereira MD, Nasello AG, Savoia M, et al. Police officers under attack: resilience implications of an fMRI study. J Psychiatr Res. 2011;45(6):727–734. doi: 10.1016/j.jpsychires.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Mumford JA. Independence in ROI analysis: where is the voodoo? Soc Cogn Affect Neurosci. 2009;4(2):208–213. doi: 10.1093/scan/nsp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Welsh RC, Kenndy AE, Lyubkin M, Martis B, et al. Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Front Psychiatry. 2011;2:62. doi: 10.3389/fpsyt.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougemont-Bucking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez-Romaguera J, et al. Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS Neurosci Ther. 2011;17(4):227–236. doi: 10.1111/j.1755-5949.2010.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Handwerger K. Is posttraumatic stress disorder a stress-induced fear circuitry disorder? J Trauma Stress. 2009;22(5):409–415. doi: 10.1002/jts.20442. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62(3):273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Smith SM, Miller KL, Salimi-Khorshidi G, Webster M, Beckmann CF, Nichols TE, et al. Network modelling methods for FMRI. Neuroimage. 2010;54(2):875–891. doi: 10.1016/j.neuroimage.2010.08.063. [DOI] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, et al. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex. 2011;20(12):2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, et al. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci. 2012;37(2):110069. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar sterotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. Thieme Medical Publishers, Inc; Stuttgart, Germany: 1988. [Google Scholar]
- Tomoda A, Suzuki H, Rabi K, Sheu YS, Polcari A, Teicher MH. Reduced prefrontal cortical gray matter volume in young adults exposed to harsh corporal punishment. Neuroimage. 2009;47(Suppl 2):T66–71. doi: 10.1016/j.neuroimage.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Grant MM, Ding Z, Hollon SD, Gore JC, Shelton RC. Early adverse events, HPA activity and rostral anterior cingulate volume in MDD. PLoS One. 2009;4(3):e4887. doi: 10.1371/journal.pone.0004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services Aoc, youth and families. Child maltreatment 2007. U.S. Government Printing Office; Washington, DC: 2007. [Google Scholar]
- Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 2002;159(12):2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton E, Wise RJ, Price CJ, Weiller C, Hadar U, Ramsay S, et al. Noun and verb retrieval by normal subjects. Studies with PET. Brain. 1996;119 (Pt 1):159–179. doi: 10.1093/brain/119.1.159. [DOI] [PubMed] [Google Scholar]
- Zhu X, Wang X, Xiao J, Liao J, Zhong M, Wang W, et al. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol Psychiatry. 2012;71(7):611–617. doi: 10.1016/j.biopsych.2011.10.035. [DOI] [PubMed] [Google Scholar]
- Zlotnick C, Mattia J, Zimmerman M. Clinical features of survivors of sexual abuse with major depression. Child Abuse Negl. 2001;25(3):357–367. doi: 10.1016/s0145-2134(00)00251-9. [DOI] [PubMed] [Google Scholar]
- Zlotnick C, Warshaw M, Shea MT, Keller MB. Trauma and chronic depression among patients with anxiety disorders. J Consult Clin Psychol. 1997;65(2):333–336. doi: 10.1037//0022-006x.65.2.333. [DOI] [PubMed] [Google Scholar]