Abstract
Rationale
Cannabinoids have been shown to alter time perception, but existing literature has several limitations. Few studies have included both time estimation and production tasks, few control for subvocal counting, most had small sample sizes, some did not record subjects’ cannabis use, many tested only one dose, and used either oral or inhaled administration of Δ9-tetrahydrocannabinol (THC), leading to variable pharmacokinetics, and some used whole-plant cannabis containing cannabinoids other than THC. Our study attempted to address these limitations.
Objectives
To characterize the acute effects of THC and frequent cannabis use on seconds-range time perception. THC was hypothesized to produce transient, dose-related time overestimation and underproduction. Frequent cannabis smokers were hypothesized to show blunted responses to these alterations.
Methods
IV THC was administered at doses from 0.015 mg/kg to 0.05 mg/kg to 44 subjects who participated in several double-blind, randomized, counterbalanced, crossover, placebo-controlled studies. Visual time estimation and production tasks in the seconds range were presented to subjects three times on each test day.
Results
All doses induced time overestimation and underproduction. Chronic cannabis use had no effect on baseline time perception. While infrequent/non-smokers showed temporal overestimation at medium and high doses and temporal underproduction at all doses, frequent cannabis users showed no differences. THC effects on time perception were not dose-related.
Conclusions
A psychoactive dose of THC increases internal clock speed as indicated by time overestimation and underproduction. This effect is not dose-related, and is blunted in chronic cannabis smokers, who did not otherwise have altered baseline time perception.
Keywords: Cannabinoids, Δ9-tetrahydrocannabinol, time perception, temporal processing
Introduction
Cannabis, the most widely used illicit substance worldwide (Office of Applied Studies 2006; Office of National Drug Control Policy 2008), contains more than 70 different cannabinoids, and produces an array of central effects through its principal active constituent Δ9-tetrahydrocannabinol (THC), which activates brain cannabinoid receptors (CB1 receptors). One such effect, consistently reported, is alteration in seconds-to-minutes-range time perception (interval timing, or the capacity to monitor and store durations)(Tart 1971).
The capacity to time and plan the temporal order of behavioral events is an essential cognitive precursor to successful behavioral adaptation (Meck and Benson 2002), and the disruption of temporal processing can affect many cognitive and motor processes. Distinct networks and processes appear to handle temporal processing over different time scales (milliseconds, seconds, minutes, and longer)(Buonomano 2007). Alterations in time perception can be caused by drugs, disease, perceptual modality, and context reviewed in(Coull et al. 2011b). Many human behaviors rely on temporal judgments in the seconds to minutes range—for example, deciding when to cross the street based on perceptions of approaching traffic, following a beat in a musical composition, or returning to the stove just prior to the tea kettle whistling. In fact, most everyday decisions we make are based on anticipated duration and the experienced passage of time (Wittmann and Paulus 2008). Disruptions in time perception could therefore have far-reaching functional implications.
Cannabis users frequently report a subjective slowing of time in the minutes range when acutely intoxicated (Tinklenberg et al. 1972; Hicks et al. 1984; Mathew et al. 1998; McDonald et al. 2003). These subjective effects have been investigated using performance-based measures such as time estimation tasks (in which subjects estimate time between two cues) and time production tasks (in which subjects generate predetermined time intervals). Congruent with the reported subjective slowing of time under the influence of cannabis, individuals given cannabinoids consistently overestimate duration in time estimation tasks (Weil et al. 1968; Clark et al. 1970; Jones and Stone 1970; Perez-Reyes et al. 1991; Lieving et al. 2006) and underproduce durations in time production tasks (Meyer et al. 1971; Cappell et al. 1972; Tinklenberg et al. 1976, 1972; Carlini et al. 1974; Vachon et al. 1974; Hicks et al. 1984; Perez-Reyes et al. 1991; Dougherty et al. 1994; McDonald et al. 2003; O’Leary et al. 2003; Stone et al. 2010) over a range of two seconds to three minutes. Consequently, cannabis smokers’ perception that external time is passing more slowly is consistent with internal, subjective time passing more quickly.
Several preclinical studies have investigated the effects of cannabinoids on time perception. In rats, the brain CB1 receptor agonists THC and WIN-55,212 cause time underproduction (McClure and McMillan 1997; Han and Robinson 2001); in primates, THC given both orally and IV causes time overestimation and underproduction (Conrad et al. 1972; Schulze et al. 1988). Conversely, the CB1 receptor antagonist rimonabant causes time overproduction in rats (Han and Robinson 2001). Preclinical findings are thus consistent with clinical studies, and support an interpretation of increased clock speed from THC.
The clinical literature on cannabinoid effects on temporal discrimination has a number of limitations. First, few studies have included both time estimation and production tasks (Jones and Stone 1970; Perez-Reyes et al. 1991). Second, sample sizes of most studies are small. Third, some studies included subjects who smoked cannabis regularly (Morrow 1944; Weil et al. 1968; Jones and Stone 1970; Meyer et al. 1971; Dornbush and Kokkevi 1976; Heishman et al. 1997; O’Leary et al. 2003) potentially confounding results because of CB1 receptor downregulation and tolerance (Villares 2007; Hirvonen et al. 2011), while others did not record or report subjects’ cannabis exposure (Cappell et al. 1972; Dornbush et al. 1972; Karniol et al. 1975; Hicks et al. 1984; Perez-Reyes et al. 1991; McDonald et al. 2003; Stone et al. 2010). Fourth, despite well-known nonlinear and biphasic effects of cannabinoids, many studies tested only one dose (Clark et al. 1970; Jones and Stone 1970; Tinklenberg et al. 1972, 1976; Vachon et al. 1974; Hicks et al. 1984; Perez-Reyes et al. 1991; Chait and Burke 1994; Heishman et al. 1997; O’Leary et al. 2003; Stone et al. 2010). Fifth, both oral and inhaled administration of THC have wide inter- and intra-individual variability in pharmacokinetics (Azorlosa et al. 1992, 1995; Grotenhermen 2003). Some studies used the oral route of administration (Clark et al. 1970; Jones and Stone 1970; Tinklenberg et al. 1972, 1976; de Souza et al. 1974; McDonald et al. 2003); unfortunately oral administration is associated with a slow onset of effects, produces lower peak plasma levels, and prolongs the action of the THC compared to the inhaled or IV route (Lemberger et al. 1971; Ohlsson et al. 1980). Others used smoked THC (Weil et al. 1968; Jones and Stone 1970; Meyer et al. 1971; Cappell et al. 1972; Carlini et al. 1974; Vachon et al. 1974; Dornbush and Kokkevi 1976; Hicks et al. 1984; Perez-Reyes et al. 1991; Chait and Burke 1994; Dougherty et al. 1994; Heishman et al. 1997; O’Leary et al. 2003; Lieving et al. 2006), which is also associated with significant inter- and intra-individual variability in plasma levels of THC, which confounds within-subject studies (Azorlosa et al. 1992, 1995). Sixth, some studies used whole-plant cannabis (Weil et al. 1968; Clark et al. 1970; Jones and Stone 1970; Meyer et al. 1971; Cappell et al. 1972; Carlini et al. 1974; Perez-Reyes et al. 1991; Chait and Burke 1994; Dougherty et al. 1994; Heishman et al. 1997; O’Leary et al. 2003), others used just THC (Tinklenberg et al. 1972, 1976; Vachon et al. 1974; Hicks et al. 1984; McDonald et al. 2003; Lieving et al. 2006; Stone et al. 2010), and two used another cannabinoid altogether, Δ8-THC (Karniol and Carlini 1973; de Souza et al. 1974). Finally, only a minority of studies employ techniques to prevent subvocal counting (Weil et al. 1968; Clark et al. 1970; McDonald et al. 2003), which can increase accuracy and decrease coefficient of variation in time tasks (Wearden and Lejeune 2008), and some investigators even encouraged it (Tinklenberg et al. 1972; Hicks et al. 1984).
The current study attempted to address some of these limitations by administering THC intravenously in a range of doses and then measuring both time estimation and time production over the seconds range. We hypothesized that THC administration would produce transient, dose-related time overestimation and underproduction, but because repeated exposure to cannabis is associated with the development of tolerance to cannabis’ effects, that frequent cannabis smokers would show the opposite—baseline underestimation and overproduction when sober, with reduced or eliminated THC-induced effects.
Method
Time perception data were collected over two years during the course of five THC administration studies that had identical methodologies, but posed different questions. The studies were double-blind, randomized, counterbalanced, and placebo-controlled, and included 44 subjects with a range of cannabis exposure (Supplemental Table 1). The studies were pooled for analysis using methods described previously (Ranganathan et al. 2009); none of these data have yet been published. Within each study, subjects completed a placebo test day and either one or two active THC test days. A small number of subjects (less than 10%) participated in more than one study. All studies were conducted at the Neurobiological Studies Unit (NSU, VA Connecticut Healthcare System, West Haven, CT) under IND 51,671 with the approval of the IRBs of both the Yale University School of Medicine (Human Investigation Committee) and the VA Connecticut Healthcare System (Human Subjects Subcommittee) and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Subjects
Subjects were recruited by advertisements and by word of mouth, and were paid for their participation. Screening procedures are described in detail elsewhere (D’Souza et al. 2004a). After obtaining written informed consent, subjects (18 to 35 years old) underwent a structured psychiatric interview (First et al. 2007) and were excluded if they had any DSM Axis I lifetime psychiatric or substance use disorder (excluding cannabis use) or family history of psychosis. Cigarette smokers were included only if they could abstain from smoking for the entire test session (~8 hours). Also excluded were subjects with a history of counseling (other than for a life circumstance disorder), any recent significant psychosocial stressor, or no previous use of cannabis. All subjects were asked to estimate their lifetime cannabis exposure, heaviest use, and last exposure to cannabis. Past-month cannabis use was quantified using a time-line-follow-back approach. Subjects who were cannabis naïve were excluded for ethical reasons. Collateral information was gathered from a telephone interview with a spouse or family member. Subjects received a physical and neurological examination, EKG and laboratory tests (serum electrolytes, liver function tests, complete blood count with differential and urine toxicology). Subjects were instructed to refrain from alcohol, illicit drugs (other than cannabis) or prescription drugs not approved by the research team for at least one week before the study and throughout study participation; those who had not used cannabis in the month prior to screening and who self-identified as infrequent/nonsmokers of cannabis were required to have a negative urine toxicology at screening. Unfortunately, there is no conclusive way to objectively verify abstinence from cannabis for the previous 24 hours, because THC has metabolites that can last for a week or more beyond last use, so twenty-four-hour abstinence from cannabis was determined by self-report. A number of reports suggest a strong concordance between urine toxicology and self-reported drug use (Martin et al. 1988; Ball et al. 1994; Carpenter et al. 2009), suggesting that self-report of drug use is accurate when elicited in a non-judgmental fashion in a research setting.
Drugs
THC doses in the five studies ranged from 0.015 mg/kg to 0.05 mg/kg, and for the purpose of analysis were categorized into four groups: placebo (0 mg/kg), low (0.01 to 0.02 mg/kg), medium (0.03 to 0.04 mg/kg), and high dose (0.05 mg/kg). THC was administered IV via automated infusion pump in order to reduce inter- and intra-individual variability in plasma THC levels and to mimic the time course of plasma THC levels associated with subjective “high” (Ohlsson et al. 1980; Lindgren et al. 1981; Agurell et al. 1986). All doses were given in counterbalanced order between test days.
Test Days
Test days were spaced at least three days apart in order to minimize carryover effects (Wall et al. 1976). Subjects fasted overnight and reported to the test facility around 8 am. Safety procedures are described elsewhere (Carbuto et al. 2012). During the study, subjects were monitored by the research nurse, research assistant, and psychiatrist in order to assess changes in physical or mental state and to provide support in case of distress. Exogenous cues to the passage of time were removed from the environment.
Timing tasks
Two outcome measures were utilized; a time estimation task and a time production task, both over the range of seconds (Time Estimation 2.0, ©2001 Brainmetric Software). The time estimation task (TET) consisted of three sessions of five trials, each ranging from 5 to 30 seconds. Prior to study initiation, fifteen trial durations were generated randomly and grouped into three fixed-order sessions: session 1 (9, 30, 19, 14, and 7 seconds); session 2 (5, 21, 26, 20, and 17 seconds), and session 3 (23, 24, 13, 13, 21 seconds). Each subject then received one session at baseline, one at peak drug effect, and (in some studies) one after the drug had worn off, in a randomized and counterbalanced way, but—in order to minimize practice effects—never the same session twice on a given day. Each trial began with the computer screen flashing once. The subject then had to count the number of “B”s appearing amongst random letters appearing sequentially at random places on the computer screen, which served as a distractor and prevented subjects from counting to themselves. The screen then flashed a second time, at which point the subject had to indicate both how many “B”s had appeared and how much time had elapsed. The time production task (TPT) used the same software and a similar methodology, except that the subject was instructed to hold down a mouse button for a defined period of seconds, intervals again predetermined, counterbalanced, and randomly ranging from 5 to 30 seconds. On each test day, timing tasks were presented before drug administration, at peak drug effect (~25 minutes after the completion of drug administration), and a third time after most effects of THC were expected to have worn off (~240 minutes).
Data analysis
Data were analyzed using SAS, version 9.2 (SAS Institute, Cary, NC). The primary outcome measure was time perception: the ratio of estimated time to actual time for the TET and the ratio of produced time to actual time for the TPT. Each outcome was tested for normality using Kolmogorov–Smirnov test statistics and normal probability plots. Time perception was then analyzed using linear mixed models that included dose (placebo, low, medium, high) and study-day time (baseline, peak, post) as within-subjects effects and random subject effects. The best-fitting variance-covariance structure was determined using information criteria. Significant interactions between dose and study-day time were interpreted using appropriate post-hoc tests and graphical displays. The subjects were then categorized into “frequent” or “infrequent/non-smokers” based on current use of cannabis. Those who had used cannabis eight or more times in the past month (i.e., more than twice weekly) were categorized as “frequent smokers”, and those who used cannabis less than twice per week or not at all in the previous month were categorized as “infrequent/nonsmokers”. This dichotomy was chosen based on data from our laboratory that have indicated that detectable tolerance to cognitive and physiological effects of THC starts to appear when subjects use cannabis more than twice a week but not when they use less. Our presumption therefore was that tolerance to the time-perception altering effects of cannabis would manifest at the same level (D’Souza et al. 2008b; Ranganathan et al. 2009). Background characteristics were compared between frequent smokers and infrequent/non-smokers using t-tests or chi-square/Fisher’s tests as appropriate, and the above analyses repeated for each group. Finally, effect sizes for repeated measures ANOVA were calculated as Cohen’s d, for which effect sizes are classified as small (0.2 to 0.5), medium (0.5 to 0.7) or large (>0.7)(Cohen 1973).
Results
Information on demographics and cannabis use are presented in Table 1. Most subjects were men in their early 20s. There were no statistically significant differences between groups in sex, education, weight, height, cigarette smoking, intelligence, race, or handedness; however, infrequent/nonsmokers were slightly older than frequent smokers. THC produced physiological, cognitive and subjective effects consistent with previous reports (D’Souza et al. 2004b, 2008a; b, 2012; Ranganathan et al. 2009, 2012; Carbuto et al. 2012). Physiological and subjective data are not presented here; nor are cognitive tasks other than time perception and reaction time; the latter was negligibly affected by THC dose.
Table 1.
Demographics
| Cannabis frequent smokers (n=10) | Cannabis infrequent/nonsmokers (n=34) | p | Total (n=44) mean±SD | |
|---|---|---|---|---|
| Male (%) | 6 (60%) | 27 (79%) | 0.24 | 33 (75%) |
| Age | 20.7±1.4 | 23.1±3.6 | 0.05 | 23.2±3.6 |
| Education | 14.6±1.3 | 15.2±1.8 | 0.33 | 15.4±2.1 |
| Race | 0.17 | |||
| White | 10 (100%) | 23 (70%) | 33 (76%) | |
| Black | 0 | 6 (15%) | 6 (12%) | |
| Other | 0 | 5 (15%) | 5 (15%) | |
| BMI | 24.5±2.8 | 25.5±4.9 | 0.54 | 25.5±4.4 |
| IQ (NART) | 115±7 | 117±6 | 0.38 | 117±6 |
| Cigarette smoker? | 3 (30%) | 1 (3%) | 0.06 | 4 (10%) |
| Right-handed (%) | 10 (100%) | 32 (94%) | 1.00 | 42 (95%) |
BMI=body mass index; IQ=Intelligence Quotient; NART=National Adult Reading Test
Time Estimation Task
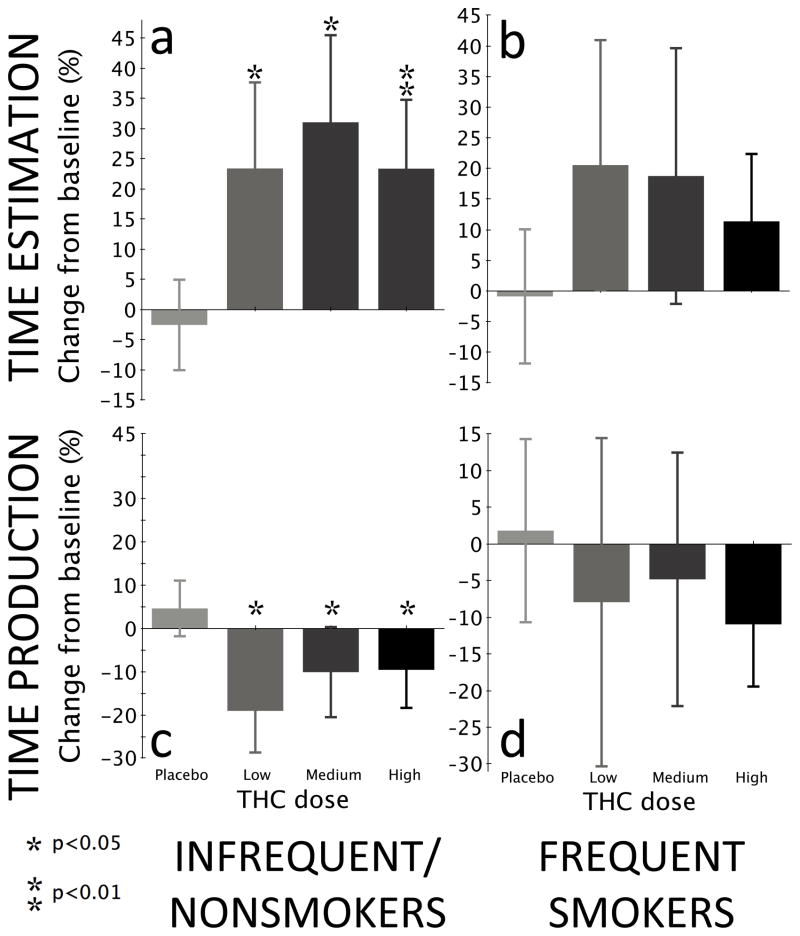
On the TET, there was a significant main effect of time (F2,197=12.37, p<0.0001) and a dose-by-time interaction (F6,197=2.92, p=0.009) but no main effect of dose (F3,197=1.07, p=0.36). There was an overall time effect at low (F2,197=4.02, p<0.02), medium (F2,197=4.78, p<0.01), and high (F2,197=7.05, p=0.001) doses but not for placebo (F2,197=0.21, p=0.81). There was significantly increased overestimation relative to placebo from high-dose THC at peak effects (F1,197=8.56, p=0.004, Figures 1a–1b and Table 2). Effect sizes were medium-to-large for the comparison of placebo to low (d=0.31), medium (d=0.63) and high dose (d=0.59). In an analysis restricted to infrequent/non-smokers, a similar main effect of time (F2,149=10.05, p<0.0001) and interaction between dose × time (F6,149=2.35, p=0.03) was observed, but again no main effect of dose (F3,149=0.85, p=0.47). There was an overall time effect at low (F2,149=3.49, p=0.03), medium (F2,149=3.73, p<0.03), and high (F2,149=5.77, p=0.004) doses but not for placebo (F2,149=0.16, p=0.86). Overestimation was increased from both medium-dose (F1,149=3.8, p=0.05) and high-dose (F1,149=7.85, p=0.006) THC administration compared to placebo. An analysis restricted to frequent cannabis users revealed no main effects of dose (F3,25=2.47, p=0.09) or time (F2,12=1.72, p=0.22), nor any interaction between dose and time (F6,25=0.67, p=0.68).
Fig. 1.
Time estimation and production by THC dose for all subjects, frequent cannabis smokers, and infrequent/nonsmokers. Bars represent the change in the ratio of reported time between baseline (before drug or placebo administration) and peak drug effect, in other words (truebaseline × estimatedpeak)/(truepeak × estimatedbaseline). If the same trial duration had been given at both peak and baseline (which it was not), this would simplify to estimatedpeak/estimatedbaseline. The same calculation holds for time production. This is a simple within-group comparison, as opposed to the linear mixed model depicted in Tables 2 and 3.
Table 2.
Time Estimation Ratio of estimated time to true time
| Group | Dose | n | Baseline | Peak | Post | p |
|---|---|---|---|---|---|---|
| Infrequent/nonsmokers | Placebo | 32 | 0.83±0.23 | 0.81±0.27 | 0.79±0.13 | N/A |
| Low | 12 | 0.77±0.23 | 0.95±0.26 | 0.88±0.21 | 0.17 | |
| Medium | 9 | 0.78±0.23 | 1.03±0.14 | 0.89±0.12 | 0.05 | |
| High | 20 | 0.80±0.22 | 0.99±0.29 | 0.89±0.30 | 0.006 | |
| smokers Frequent | Placebo | 10 | 0.91±0.25 | 0.90±0.18 | 0.88±0.22 | No main effects of dose or time or dose × time interaction |
| Low | 4 | 0.65±0.14 | 0.78±0.17 | 0.75±0.23 | ||
| Medium | 3 | 0.73±0.11 | 0.87±0.23 | 0.76±0.21 | ||
| High | 5 | 0.84±0.14 | 0.94±0.12 | 0.98±0.17 |
Ratio of estimated time to true time derived from a linear mixed model that included and random subject effects as well as dose and time as within-subjects effects. P-values are for comparison between dose and placebo.
When data were reanalyzed separately for each trial length, linear regression of estimated time to true time versus trial length was nonsignificant for both placebo (p=0.41) and THC (p=0.15). Linear regression of produced time to true time versus trial length was also nonsignificant for both placebo (p=0.30) and THC (p=0.32) (one-tailed significance calculations).
Time Production Task
The combined analysis revealed main effects of dose (F3,198=3.93, p<0.01) and time (F2,198=6.94, p=0.001) as well as a significant dose-by-time interaction (F6,198=3.91, p=0.001). There was an overall time effect for low-dose (F2,198=5.43, p=0.005) and high-dose (F2,198=6.17, p<0.003) THC, but not placebo (F2,198=1.36, p=0.26) or medium (F2,198=1.54, p=0.22) doses. Compared to placebo, significantly increased underproduction resulted from both medium (F1,198=4.86, p=0.03) and high (F1,198=10.2, p=0.002) THC doses at peak effects (Figures 1c–1d and Table 3). Effect sizes were small-to-medium for the comparison of placebo to low (d=0.29), medium (d=0.36) and high dose (d=0.45). An analysis restricted to infrequent/non-smokers revealed a main effect of time (F2,150=7.53, p<0.001) but not dose (F3,150=2.31, p=0.08), and a significant dose-by-time interaction (F6,150=4.22, p=0.0006) that resulted from underproduction at low (F1,150=4.09, p<0.05), medium (F1,150=4.43, p=0.03), and high (F1,150=5.47, p=0.02) THC doses relative to placebo at peak effects. There was an overall time effect at both low (F2,150=7.27, p=0.001) and high (F2,150=5.65, p=0.004) doses but not in placebo (F2,150=1.33, p=0.27) or medium (F2,150=1.53, p=0.22) doses. The analysis restricted to frequent cannabis users showed no main effects of dose (F3,25=2.06, p=0.13) or time (F2,12=0.27, p=0.77) nor any interaction between dose and time (F6,25=0.37, p=0.89).
Table 3.
Time Production Ratio of produced time to true time
| Group | Dose | n | Baseline | Peak | Post | p |
|---|---|---|---|---|---|---|
| Infrequent/nonsmokers | Placebo | 32 | 1.06±0.27 | 1.11±0.27 | 1.19±0.26 | N/A |
| Low | 12 | 1.17±0.37 | 0.95±0.25 | 1.10±0.28 | 0.05 | |
| Medium | 9 | 1.04±0.25 | 0.93±0.22 | 1.03±0.22 | 0.04 | |
| High | 20 | 1.12±0.29 | 1.01±0.32 | 0.98±0.32 | 0.02 | |
| Frequentsmokers | Placebo | 10 | 1.15±0.29 | 1.18±0.33 | 1.17±0.50 | No main effects of dose or time or dose × time interaction |
| Low | 4 | 1.41±0.41 | 1.30±0.46 | 1.32±0.43 | ||
| Medium | 3 | 1.33±0.15 | 1.26±0.37 | 1.31±0.35 | ||
| High | 5 | 1.02±0.17 | 0.91±0.10 | 0.93±0.13 |
Ratio of produced time to true time derived from a linear mixed model that included and random subject effects as well as dose and time as within-subjects effects. P-values are for comparison between dose and placebo.
Discussion
At baseline, before any drug was administered, subjects tended to overestimate time (by 16%) and underproduce (by 9%), a phenomenon that did not differ between frequent smokers and infrequent/non-smokers. In most previous reports, investigators have not calculated or reported baseline inaccuracy in time perception, but those who have report results of similar magnitude—baseline overestimation of 7% (Clark et al. 1970), 33% (Bech et al. 1973), 35% (Menhiratta et al. 1978), and 15% (Menhiratta et al. 1978) with baseline underproduction of 49% (Stone et al. 2010). Other investigators report the opposite—baseline underestimation of 15% (Menhiratta et al. 1978) and overproduction of 17% (Tinklenberg et al. 1972), 10% (Vachon et al. 1974), and 10% to 30% (Hicks et al. 1984). The reason for this disparity is unclear, and clearly because of factors not controlled for in these experiments.
All doses of THC induced time overestimation and underproduction compared with placebo, consistent with a speeded internal clock (Gibbon et al. 1984). Infrequent/non-smokers showed temporal overestimation compared with placebo at medium and high doses and temporal underproduction at all doses; frequent users, however, showed no significant difference in temporal estimation between THC and placebo at any dose. Our results are therefore consistent with previous studies reporting that THC causes temporal overestimation and underproduction, with the additional finding that this effect appears to be blunted or eliminated in subjects who use cannabis two to three times a week or more.
Dose Response
The absence of a dose-response relationship was surprising, since several studies from our laboratory using similar methodologies have observed that the physiological, subjective, cognitive, and endocrine effects that THC produces in humans are dose-related. The clinical literature on the effects of cannabinoids on time perception is contradictory, with some studies reporting dose-related effects, others failing to show such effects, some suggesting an inverted-U dose response, and others suggesting a loss of a dose-related effects at longer duration (Weil et al. 1968; Cappell et al. 1972; Bech et al. 1973; Borg et al. 1975; Dougherty et al. 1994; McDonald et al. 2003; Lieving et al. 2006). Some preclinical studies also suggest lack of dose response with THC (Schulze et al. 1988; Han and Robinson 2001). Thus, while cannabinoid agonist WIN-55,212 caused dose-related time underproduction in rats, and the cannabinoid antagonist rimonabant caused dose-related time overproduction, THC caused time underproduction that was not dose-related (Han and Robinson 2001). That a full CB1 receptor agonist (WIN-55,212) and full CB1 receptor antagonist (rimonabant) both show a linear but opposite dose-response on time perception suggests that time perception is at least partly mediated via CB1 receptors. The intermediate physiological effects on time perception seen with THC might thus be a reflection of its partial agonism at CB1.
Previous Exposure to Cannabis
The lack of significant baseline differences between frequent smokers and infrequent/non-smokers suggests that chronic cannabis exposure does not alter time perception over the seconds range, even though chronic exposure blunts the acute effects of THC on time perception. Three previous studies have examined time perception in chronic cannabis smokers over the minutes (rather than seconds) range and are contradictory: one found that daily bhang drinkers/ganja smokers in India overestimated a two-minute interval (Menhiratta et al. 1978), another found that daily cannabis smokers underestimated a three-minute interval (Dornbush and Kokkevi 1976), and the last found that daily cannabis smokers underproduced a two-minute interval, although this was significant only in trials where subjects were given performance feedback (Webb et al. 1993). This discrepancy may have been because the minutes length of the tasks used in these studies placed heavier demands on working memory than our seconds-length tasks, and thus were more apt to show impairment from chronic use; alternatively, the comparatively heavier cannabis use by subjects in these studies compared with ours (daily use compared with twice weekly in our subjects) may have led to greater sensitivity for chronic effects on time perception.
Frequent cannabis smokers experienced less temporal distortion from THC than infrequent/non-smokers. Although frequent smokers do not develop tolerance to the euphoria derived from smoking THC, they show blunted responses to THC’s psychotomimetic, perceptual altering, amnestic, and endocrine effects, which has been interpreted as evidence of tolerance (Lichtman and Martin 2005; D’Souza et al. 2008a; b, 2012; Ranganathan et al. 2009), consistent with recent in vivo studies that have demonstrated CB1 receptor downregulation associated with chronic cannabis use (Hirvonen et al. 2011). Therefore, it is not unreasonable to suppose that tolerance also develops to the temporal effects of THC. Four previous studies enrolled both chronic and infrequent smokers. Two did not compare the groups (Morrow 1944; Weil et al. 1968). One noted that casual smokers showed more pronounced effects on a time production task after smoking 0.9% THC than heavy smokers, but the difference did not reach statistical significance, likely because of small sample size (Meyer et al. 1971). The last study reported a greater effect on a time production task in chronic users than occasional users, but the difference did not reach statistical significance, and the time production tasks used were millisecond-range, a different timescale than that tested here (O’Leary et al. 2003).
“State-dependent learning” is another possible explanation for blunted THC effects, as frequent smokers may have recalibrated their sense of duration based on frequent experiencing of cannabis’ altered state of consciousness. This effect has been noted in rats, which ceased to show effects of altered clock speed from cocaine when it was given continuously for two weeks, presumably because the memories of durations that served as comparators to study durations became progressively updated to the new clock speed (Matell et al. 2004). However, this is unlikely to be the explanation in our subjects. For one thing, the frequent smokers were not chronic daily smokers but rather smoked twice a week or more, and thus spent far more time sober than they did in an altered state of consciousness such that they would relearn subjective duration lengths. For another, such state-dependent learning should cause a rebound in the opposite direction when not under the influence of the drug, yet baseline time estimation and production did not differ between groups according to frequency of smoking.
Implications
THC transiently impaired time estimation and production in the seconds range. Even the lowest dose range of THC, much less than that seen with recreational cannabis use, produced changes in time perception. The effects disappeared after a few hours. These temporal processing changes may or may not have functional consequences, which were not measured in this study. However, seconds-range temporal processing is relevant to many everyday tasks; for example, driving, playing music, and using power tools. Processes underlying time perception from milliseconds to minutes may be particularly relevant to cognition, as measures of temporal precision in humans correlate well with measures of general intelligence and working memory (Troche and Rammsayer 2009). Furthermore, timing deficits are characteristic of disorders such as schizophrenia, autism, and attention-deficit hyperactivity disorder (Meck 2005; Allman and Meck 2012), suggesting that timing alterations may be an endophenotype of psychiatric disease.
Possible Mechanism
A number of studies suggest two parallel timing systems to account for the full range of durations resolved by the theoretical “internal clock” (Madison 2001; Ivry and Schlerf 2008). The first is a sensory, “bottom-up” mechanism for durations in the milliseconds range, which is considered important for motor coordination and computed by the cerebellum. The second is a more cognitively mediated “top-down” mechanism for durations in the seconds-to-minutes range, which is considered important for temporal estimation, computed by frontal–striatal circuits, and able to concatenate smaller intervals generated locally or by the cerebellum (Kagerer et al. 2002; Lewis and Miall 2003; Wittmann et al. 2007; Gutyrchik et al. 2010; Zélanti and Droit-Volet 2012) . This hypothesis is supported by neuroimaging studies that reveal cerebellar involvement for durations shorter than 1 to 2 seconds (Ivry and Spencer 2004) and thalamo-cortico-striatal and prefrontal involvement for longer durations reviewed by (Lewis and Miall 2006; Coull et al. 2011b).
Neuroanatomically, the time scales measured in this study are most likely striatally mediated. Ninety five % of the neurons in the striatum are medium spiny projection neurons (Kelland et al. 1991). An estimated 10 to 30 thousand separate afferents project to each striatal spiny neuron from the cortex and thalamus (Matell and Meck 2004). Three quarters are cortical and glutamatergic (Cowan and Wilson 1994), and almost always synapse onto the head of the spines of the striatal spiny neurons(Kemp and Powell 2012). An additional 9% are dopaminergic, originate almost entirely in the substantia nigra, commonly co-localize with glutamatergic afferents, and synapse on either the spine head or neck (Freund et al. 1984). A lateral inhibitory network of GABAergic and cholinergic inputs comes from neighboring striatal neurons (Kitai et al. 1976; Surmeier et al. 2007).
One neuroanatomical model of interval timing is the striatal beat frequency (SBF) model, which relies on cortical oscillatory neurons firing with different frequencies and converging onto striatal spiny neurons (Matell and Meck 2004). At the beginning of an interval, phasic dopaminergic input from the ventral tegmental area synchronizes these oscillating neurons and dopaminergic input from the substantia nigra resets the spiny neurons. A pulse of dopamine marking the end of the interval strengthens (through long-term potentiation and long-term depression) the striatal synapses in the striatum that are activated at that moment as a result of the beat frequency pattern of these cortical neurons. These strengthened and weakened synaptic weights record the target duration. Later, neostriatal GABAergic spiny neurons compare the cortical neuron activation pattern stored in memory with the activation pattern in the moment, and fire if the patterns match, signaling that the target duration has been reached. In the SBF model, tonic dopamine–glutamate activity in the ventral tegmental area–to-cortex pathway modulates the frequency of cortical oscillations and thus clock speed.
Short- and long-term synaptic efficacy changes are also mediated by the cannabinoid system. Endocannabinoids act as retrograde messengers (Freund et al. 2003; Chevaleyre et al. 2006; Kano et al. 2009) at presynaptic CB1 receptors in the corticostriatal pathway (Herkenham et al. 1991; Katona et al. 2006). CB1 receptor activation reduces both the release and uptake of glutamate in striatal slices, possibly by decreasing glutamate transporter activity, increasing synaptic cleft glutamate concentration, and activating presynaptic mGluRs, which then decrease glutamate release (Brown 2003). Unlike many excitatory synapses in the brain that are potentiated by high-frequency afferent stimulation, striatal medium spiny neuron synapses undergo long term depression (LTD) from repeated activation of glutamatergic afferents in the presence of tonic levels of dopamine. This process requires dopamine D2 receptor activation, high levels of intracellular calcium, and activation of presynaptic CB1 receptors through retrograde release of endocannabinoids (Kheirbek 2007), or in this case exogenous cannabinoids.
In this way, the “top-down” corticostriatal interval timing is optimized by phasic dopaminergic activity in corticostriatal circuits, modulated by glutamate activity (Cheng et al. 2006, 2007; LL et al. 2008; Coull et al. 2011b), which is in turn mediated by the cannabinoid system. A speeded “internal clock” from exogenous cannabinoids could therefore result from increased glutamate in the synaptic cleft of striatal spiny neurons, which increases the frequency of cortical oscillations and thus clock speed. Alternately, cannabinoids may have differential effects on medium spiny neurons in the direct pathway (which have an excitatory effect on the cortex) and the indirect pathway (which have inhibitory effects), the balance tending toward increased clock speed.
Converging preclinical evidence suggests interactions between cannabinoid and dopamine systems reviewed in(Gardner 2005; Laviolette and Grace 2006). CB1 and D2 receptors show coexpression (Hermann et al. 2002) and convergence of signal transduction in several brain regions (Meschler and Howlett 2001). THC activates dopaminergic mesolimbic neurons (French 1997; French et al. 1997; Diana et al. 1998; Gardner 2005) and induces dopamine release in the striatum (Chen et al. 1990a; b; Tanda et al. 1997, 2000). In vivo microdialysis studies show markedly increased dopamine release from the nucleus accumbens from cannabinoid receptor activation by systemic exogenous cannabinoids (Tanda et al. 1997; Fadda et al. 2006) or endocannabinoids (Solinas et al. 2006). Similarly, systemically administered cannabinoids can modulate the activity of dopaminergic pathways in the prefrontal cortex either directly or indirectly, by influencing the activity of dopaminergic neurons through either pre- or postsynaptic mechanisms (Pistis et al. 2002; Egerton et al. 2006; Laviolette and Grace 2006). If the effects of cannabinoids on timing are mediated by their capacity to release dopamine in brain regions relevant to timing such as the striatum and prefrontal cortex, then one would expect a clock speed to increase, which is what we observed. In a previous study we showed that the D2 antagonist haloperidol did not block the psychotomimetic effects of THC in healthy subjects (D’Souza et al. 2008a) unfortunately, time tasks were not administered in that study.
Comparison to pharmacological and pathophysiological states
A number of other drugs with disparate mechanisms of action share in common the capacity to disrupt time perception in the suprasecond range. For example, benzodiazepines produce a pronounced impairment in temporal processing of seconds-range intervals while sparing millisecond-range interval processing (Rammsayer and Vogel 1992; Rammsayer 1999). Time perception is also affected by the dopaminergic system. Reduced dopaminergic activity (as from antipsychotic drugs) results in temporal underestimation while increased dopaminergic activity (as from cocaine or methamphetamine) results in temporal overestimation (Meck and Church 1983; MacDonald and Meck 2005; Matell et al. 2006; Coull et al. 2011b). The glutamate (NMDA) receptor antagonist ketamine also affects time perception in subtle ways (Pomarol-Clotet et al. 2006; Stone and Pilowsky 2006), possibly related to disruption of temporal information manipulation in working memory (Coull et al. 2011a). Ketamine facilitates the ability of cocaine to increase clock speed in rats despite their having received extended counter-training (Cheng et al. 2007). Thus, time perception is a complicated phenomenon affected by GABAergic, dopaminergic, and glutamatergic agents as well as cannabinoids.
Schizophrenia is a disorder characterized by core cognitive deficits including problems with the temporal coordination of information processing (Andreasen et al. 1999). Several studies show that schizophrenia patients have reduced accuracy in temporal estimation over a range of milliseconds to seconds (Tysk 1983; Davalos et al. 2003; Carroll et al. 2009) and minutes to hours (Rabin 1957; Johnson and Petzel 1971). However, since many of the patients were taking D2 receptor antagonists known to alter time perception, interpretation of these results is difficult. The THC-induced alterations in time perception observed in this study adds to a list of psychotropic, cognitive, and psychophysiological effects induced by THC in healthy volunteers that resemble those seen in schizophrenia (D’Souza et al. 2004b, 2012; Sewell et al. 2010).
Strengths and limitations
Strengths include IV administration, which permitted precise control over THC dose and blood levels; a sample size larger than any previous study; a wide range of administered doses; and control for subvocal counting. Participants with a range of exposure to cannabis were included, permitting assessment of the effects of recent exposure to cannabis on time perception. Engagement of subjects in a distractor task prevented them from counting internally (which increases precision and decreases variance in a non-scalar way) or standardizing the attentional resources allocated to the task.
Limitations include the inability to distinguish between THC’s effects on clock, memory, and decision stages of temporal discrimination, as well as THC’s effects on memory and attention. In animals, three distinct stages in processing the duration of a signal in a temporal discrimination task have been described: the clock stage, in which objective time is transformed into subjective time; the memory stage, in which the clock reading is stored, and the decision stage, in which the clock reading is compared with memory of similar intervals and a decision is made whether the durations are similar enough (Meck 1983, 1996). These stages can be independently affected. Although we hypothesize that THC has increased clock speed, THC is also known to affect both episodic and working memory (Ranganathan and Souza 2006). As the methodology of this study was not sufficient to disentangle the effects of THC on the clock stage from its effects on the decision or memory stages, future studies are warranted to dissociate THC effects on timing from its effects on memory and attention. Marijuana produces inconsistent effects on simple reaction time tests, with some studies reporting modest impairment and others showing no effect (Chait and Zacny 1992); reaction time did not appear to be a confound in our study.
The trial durations used (5 to 30 s) were not “trained” by the experimenter, but instead were inferred to have been learned by the subject based on real-life experience, potentially leading to increased between-subject variability. Use of a symbolic representation for duration (verbal labels) added further variability. Both could be avoided in future studies by training the subject to learn a set of specific stimulus durations against which other stimulus durations are compared (Paule et al. 1999). The use of an active control such as ketamine or amphetamine would have strengthened the study, as would have the inclusion of everyday tasks in order to characterize the functional consequences of the time perception abnormalities. It is possible that the relative lack of effect in frequent users could be due to the small subject numbers in this group (n=10) compared to the infrequent group (n=34), which may have obscured otherwise significant results. Future studies to specifically examine this question should consider using daily smokers rather than merely frequent smokers in order to maximize tolerance, and be powered appropriately.
Conclusions and future directions
THC at a psychoactive dose causes time overestimation and underproduction. Chronic exposure to cannabis does not change time perception significantly. However, the effect of THC on time overestimation and underproduction was blunted in chronic cannabis smokers, who also show tolerance to other cognitive, physiological, and endocrine effects of cannabinoids. Future studies are necessary to examine whether this effect, seen over the seconds range, also holds true over the millisecond and minute timescales, and to determine the contributions of attention and working memory on the timing effects of THC. Future studies using neuroimaging could help characterize the neural circuitry of the temporal processing abnormalities produced by cannabinoids.
Supplementary Material
Acknowledgments
Funding
Mohini Ranganathan has in the past three years and currently receives research grant support administered through Yale University School of Medicine from Eli Lilly Inc. Deepak Cyril D’Souza has in the past three years and currently receives research grant support administered through Yale University School of Medicine from Astra Zeneca, Abbott Laboratories, Eli Lilly Inc., Organon, Pfizer Inc., and Sanofi; he is also a consultant for Bristol Meyers Squibb.
The authors wish to acknowledge support from the (1) Department of Veterans Affairs, (2) National Institute of Mental Health, (3) National Institute of Drug Abuse, (4) National Institute of Alcoholism and Alcohol Abuse (NIAAA) and (5) the Yale Center for Clinical Investigation (YCCI). This research project was funded in part by grants from NIH (R01 DA012382, R21 DA020750, R21 MH086769, R21 AA016311 to DCD). The authors also thank Angelina Genovese, R.N.C., M.B.A.; Michelle San Pedro, R.N.; Elizabeth O’Donnell, R.N.; Brenda Breault, R.N., B.S.N.; Sonah Yoo, R.Ph.; Rachel Galvan, R.Ph.; and Willie Ford of the Neurobiological Studies Unit at the VA Connecticut Healthcare System, West Haven Campus for their central contributions to the success of this project. The experiments comply with the current laws of the USA.
Footnotes
Conflict of Interest
Patrick Skosnik, Ashley Williams, Ashley Schnakenberg, Rajiv Radhakrishnan, Brian Pittman, and R. Andrew Sewell report no financial relationships with commercial interests.
References
- Agurell S, Halldin M, Lindgren JE, Ohlsson A, Widman M, Gillespie H, Hollister L. Pharmacokinetics and metabolism of delta 1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacological Reviews. 1986;38:21–43. [PubMed] [Google Scholar]
- Allman MJ, Meck WH. Pathophysiological distortions in time perception and timed performance. Brain. 2012;135:656–77. doi: 10.1093/brain/awr210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Nopoulos P, O’Leary DS, Miller DD, Wassink T, Flaum M. Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biological Psychiatry. 1999;46:908–20. doi: 10.1016/s0006-3223(99)00152-3. [DOI] [PubMed] [Google Scholar]
- Azorlosa JL, Greenwald MK, Stitzer ML. Marijuana smoking: effects of varying puff volume and breathhold duration. J Pharmacol Exp Ther. 1995;272:560–9. [PubMed] [Google Scholar]
- Azorlosa JL, Heishman SJ, Stitzer ML, Mahaffey JM. Marijuana smoking: effect of varying delta 9-tetrahydrocannabinol content and number of puffs. J Pharmacol Exp Ther. 1992;261:114–22. [PubMed] [Google Scholar]
- Ball SA, Carroll KM, Rounsaville BJ. Sensation seeking, substance abuse, and psychopathology in treatment-seeking and community cocaine abusers. Journal of Consulting and Clinical Psychology. 1994;62:1053–7. doi: 10.1037//0022-006x.62.5.1053. [DOI] [PubMed] [Google Scholar]
- Bech P, Rafaelsen L, Rafaelsen OJ. Cannabis and alcohol: effects on estimation of time and distance. Psychopharmacologia. 1973;32:373–81. doi: 10.1007/BF00429474. [DOI] [PubMed] [Google Scholar]
- Borg J, Gershon S, Alpert M. Dose effects of smoked marihuana on human cognitive and motor functions. Psychopharmacologia. 1975;42:211–8. doi: 10.1007/BF00421258. [DOI] [PubMed] [Google Scholar]
- Brown TM, Brotchie JM, Fitzjohn SM. Cannabinoids decrease corticostriatal synaptic transmission via an effect on glutamate uptake. J Neurosci. 2003;23:11073–7. doi: 10.1523/JNEUROSCI.23-35-11073.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomano DV. The biology of time across different scales. Nat Chem Biol. 2007;3:594–7. doi: 10.1038/nchembio1007-594. [DOI] [PubMed] [Google Scholar]
- Cappell H, Webster CD, Herring BS, Ginsberg R. Alcohol and marihuana: a comparison of effects on a temporally controlled operant in humans. J Pharmacol Exp Ther. 1972;182:195–203. [PubMed] [Google Scholar]
- Carbuto M, Sewell RA, Williams A, Forselius-Bielen K, Braley G, Elander J, Pittman B, Schnakenberg A, Bhakta S, Perry E, Ranganathan M, D’Souza DC. The safety of studies with intravenous delta(9)-tetrahydrocannabinol in humans, with case histories. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2417-y. [DOI] [PubMed] [Google Scholar]
- Carlini EA, Karniol IG, Renault PF, Schuster CR. Effects of marihuana in laboratory animals and in man. Br J Pharmacol. 1974;50:299–309. doi: 10.1111/j.1476-5381.1974.tb08576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter KM, McDowell D, Brooks DJ, Cheng WY, Levin FR. A preliminary trial: double-blind comparison of nefazodone, bupropion-SR, and placebo in the treatment of cannabis dependence. Am J Addict. 2009;18:53–64. doi: 10.1080/10550490802408936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll CA, O’Donnell BF, Shekhar A, Hetrick WP. Timing dysfunctions in schizophrenia span from millisecond to several-second durations. Brain and Cognition. 2009;70:181–90. doi: 10.1016/j.bandc.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Chait LD, Burke KA. Preference for high- versus low-potency marijuana. Pharmacol Biochem Behav. 1994;49:643–7. doi: 10.1016/0091-3057(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Chait LD, Zacny JP. Reinforcing and subjective effects of oral delta 9-THC and smoked marijuana in humans. Psychopharmacology (Berl) 1992;107:255–62. doi: 10.1007/BF02245145. [DOI] [PubMed] [Google Scholar]
- Chen J, Paredes W, Lowinson JH, Gardner EL. Delta 9-tetrahydrocannabinol enhances presynaptic dopamine efflux in medial prefrontal cortex. Eur J Pharmacol. 1990a;190:259–62. doi: 10.1016/0014-2999(90)94136-l. [DOI] [PubMed] [Google Scholar]
- Chen JP, Paredes W, Li J, Smith D, Lowinson J, Gardner EL. Delta 9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology. 1990b;102:156–62. doi: 10.1007/BF02245916. [DOI] [PubMed] [Google Scholar]
- Cheng RK, Ali YM, Meck WH. Ketamine “unlocks” the reduced clock-speed effects of cocaine following extended training: evidence for dopamine--glutamate interactions in timing and time perception. Neurobiol Learn Mem. 2007;88:149–59. doi: 10.1016/j.nlm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Cheng RK, MacDonald CJ, Meck WH. Differential effects of cocaine and ketamine on time estimation: implications for neurobiological models of interval timing. Pharmacol Biochem Behav. 2006;85:114–22. doi: 10.1016/j.pbb.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Clark LD, Hughes R, Nakashima EN. Behavioral effects of marihuana. Experimental studies. Arch Gen Psychiatry. 1970;23:193–8. doi: 10.1001/archpsyc.1970.01750030001001. [DOI] [PubMed] [Google Scholar]
- Cohen J. Eta-Squared and Partial Eta-Squared in Communication Science. Human Communication Research. 1973;28:473–490. [Google Scholar]
- Conrad DG, Elsmore TF, Sodetz FJ. 9 -tetrahydrocannabinol: dose-related effects on timing behavior in chimpanzee. Science. 1972;175:547–50. doi: 10.1126/science.175.4021.547. [DOI] [PubMed] [Google Scholar]
- Coull JT, Cheng RK, Meck WH. Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology. 2011a;36:3–25. doi: 10.1038/npp.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Morgan H, Cambridge VC, Moore JW, Giorlando F, Adapa R, Corlett PR, Fletcher PC. Ketamine perturbs perception of the flow of time in healthy volunteers. Psychopharmacology (Berl) 2011b;218:543–56. doi: 10.1007/s00213-011-2346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan RL, Wilson CJ. Spontaneous firing patterns and axonal projections of single corticostriatal neurons in the rat medial agranular cortex. J Neurophysiol. 1994;71:17–32. doi: 10.1152/jn.1994.71.1.17. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Braley G, Blaise R, Vendetti M, Oliver S, Pittman B, Ranganathan M, Bhakta S, Zimolo Z, Cooper T, Perry E. Effects of haloperidol on the behavioral, subjective, cognitive, motor, and neuroendocrine effects of Delta-9-tetrahydrocannabinol in humans. Psychopharmacology (Berl) 2008a;198:587–603. doi: 10.1007/s00213-007-1042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza DC, Fridberg DJ, Skosnik PD, Williams A, Roach B, Singh N, Carbuto M, Elander J, Schnakenberg A, Pittman B, Sewell RA, Ranganathan M, Mathalon D. Dose-Related Modulation of Event-Related Potentials to Novel and Target Stimuli by Intravenous Delta(9)-THC in Humans. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, Braley G, Gueorguieva R, Krystal JH. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–72. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Ranganathan M, Braley G, Gueorguieva R, Zimolo Z, Cooper T, Perry E, Krystal J. Blunted psychotomimetic and amnestic effects of delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology. 2008b;33:2505–16. doi: 10.1038/sj.npp.1301643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos DB, Kisley MA, Ross RG. Effects of interval duration on temporal processing in schizophrenia. Brain and Cognition. 2003;52:295–301. doi: 10.1016/s0278-2626(03)00157-x. [DOI] [PubMed] [Google Scholar]
- de Souza MR, Karniol IG, Ventura DF. Human tonal preferences as a function of frequency under delta8-tetrahydrocannabinol. Pharmacol Biochem Behav. 1974;2:607–11. doi: 10.1016/0091-3057(74)90028-8. [DOI] [PubMed] [Google Scholar]
- Dornbush R, Clare G, Zaks A, Crown P, Volavka J, Fink M. 21-Day administration of marijuana in male volunteers. In: Lewis M, editor. Current Research in Marijuana. Academic Press; New York: 1971. pp. 115–128. [Google Scholar]
- Dornbush RL, Kokkevi A. Acute effects of cannabis on cognitive, perceptual, and motor performance in chronic hashish users. Ann N Y Acad Sci. 1976;282:313–22. doi: 10.1111/j.1749-6632.1976.tb49906.x. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Cherek DR, Roache JD. The effects of smoked marijuana on progressive-interval schedule performance in humans. J Exp Anal Behav. 1994;62:73–87. doi: 10.1901/jeab.1994.62-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton A, Allison C, Brett RR, Pratt JA. Cannabinoids and prefrontal cortical function: insights from preclinical studies. Neurosci Biobehav Rev. 2006;30:680–95. doi: 10.1016/j.neubiorev.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Fadda P, Scherma M, Spano MS, Salis P, Melis V, Fattore L, Fratta W. Cannabinoid self-administration increases dopamine release in the nucleus accumbens. Neuroreport. 2006;17:1629–32. doi: 10.1097/01.wnr.0000236853.40221.8e. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for Axis I DSM-IV Disorders, Research Version. B.R. Department, New York State Psychiatric Institute; New York: 2007. [Google Scholar]
- French ED. delta9-Tetrahydrocannabinol excites rat VTA dopamine neurons through activation of cannabinoid CB1 but not opioid receptors. Neuroscience Letters. 1997;226:159–62. doi: 10.1016/s0304-3940(97)00278-4. [DOI] [PubMed] [Google Scholar]
- French ED, Dillon K, Wu X. Cannabinoids excite dopamine neurons in the ventral tegmentum and substantia nigra. Neuroreport. 1997;8:649–52. doi: 10.1097/00001756-199702100-00014. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–66. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Freund TF, Powell JF, Smith AD. Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience. 1984;13:1189–215. doi: 10.1016/0306-4522(84)90294-x. [DOI] [PubMed] [Google Scholar]
- Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol Biochem Behav. 2005;81:263–84. doi: 10.1016/j.pbb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Melis M, Muntoni AL, Diana M. Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. European Journal of Pharmacology. 1998;341:39–44. doi: 10.1016/s0014-2999(97)01442-8. [DOI] [PubMed] [Google Scholar]
- Gibbon J, Church RM, Meck WH. Scalar timing in memory. Ann N Y Acad Sci. 1984;423:52–77. doi: 10.1111/j.1749-6632.1984.tb23417.x. [DOI] [PubMed] [Google Scholar]
- Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42:327–60. doi: 10.2165/00003088-200342040-00003. [DOI] [PubMed] [Google Scholar]
- Gutyrchik E, Churan J, Meindl T, Bokde AL, von Bernewitz H, Born C, Reiser M, Poppel E, Wittmann M. Functional neuroimaging of duration discrimination on two different time scales. Neurosci Lett. 469:411–5. doi: 10.1016/j.neulet.2009.12.040. [DOI] [PubMed] [Google Scholar]
- Han CJ, Robinson JK. Cannabinoid modulation of time estimation in the rat. Behav Neurosci. 2001;115:243–6. doi: 10.1037/0735-7044.115.1.243. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Arasteh K, Stitzer ML. Comparative effects of alcohol and marijuana on mood, memory, and performance. Pharmacol Biochem Behav. 1997;58:93–101. doi: 10.1016/s0091-3057(96)00456-x. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, de Costa BR, Richfield EK. Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Res. 1991;547:267–74. doi: 10.1016/0006-8993(91)90970-7. [DOI] [PubMed] [Google Scholar]
- Hermann H, Marsicano G, Lutz B. Coexpression of the cannabinoid receptor type 1 with dopamine and serotonin receptors in distinct neuronal subpopulations of the adult mouse forebrain. Neuroscience. 2002;109:451–60. doi: 10.1016/s0306-4522(01)00509-7. [DOI] [PubMed] [Google Scholar]
- Hicks RE, Gualtieri CT, Mayo JP, Jr, Perez-Reyes M. Cannabis, atropine, and temporal information processing. Neuropsychobiology. 1984;12:229–37. doi: 10.1159/000118144. [DOI] [PubMed] [Google Scholar]
- Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, Pike VW, Volkow ND, Huestis MA, Innis RB. Reversible and regionally selective downregulation of brain cannabinoid CB(1) receptors in chronic daily cannabis smokers. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivry RB, Schlerf JE. Dedicated and intrinsic models of time perception. Trends Cogn Sci. 2008;12:273–80. doi: 10.1016/j.tics.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivry RB, Spencer RM. The neural representation of time. Curr Opin Neurobiol. 2004;14:225–32. doi: 10.1016/j.conb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Petzel TP. Temporal orientation and time estimation in chronic schizophrenics. Journal of Clinical Psychology. 1971;27:194–6. doi: 10.1002/1097-4679(197104)27:2<194::aid-jclp2270270210>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Jones RT, Stone GC. Psychological studies of marijuana and alcohol in man. Psychopharmacologia. 1970;18:108–17. doi: 10.1007/BF00402390. [DOI] [PubMed] [Google Scholar]
- Kagerer FA, Wittmann M, Szelag E, Steinbuchel N. Cortical involvement in temporal reproduction: evidence for differential roles of the hemispheres. Neuropsychologia. 2002;40:357–66. doi: 10.1016/s0028-3932(01)00111-7. [DOI] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–80. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Karniol IG, Carlini EA. Comparative studies in man and in laboratory animals on 8 - and 9 -trans-tetrahydrocannabinol. Pharmacology. 1973;9:115–26. doi: 10.1159/000136375. [DOI] [PubMed] [Google Scholar]
- Karniol IG, Shirakawa I, Takahashi RN, Knobel E, Musty RE. Effects of delta9-tetrahydrocannabinol and cannabinol in man. Pharmacology. 1975;13:502–12. doi: 10.1159/000136944. [DOI] [PubMed] [Google Scholar]
- Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–37. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelland MD, Chiodo LA, Freeman AS. Dissociative anesthesia and striatal neuronal electrophysiology. Synapse. 1991;9:75–8. doi: 10.1002/syn.890090111. [DOI] [PubMed] [Google Scholar]
- Kemp JM, Powell TP. The site of termination of afferent fibres in the caudate nucleus. Philos Trans R Soc Lond B Biol Sci. 1971;262:413–27. doi: 10.1098/rstb.1971.0104. [DOI] [PubMed] [Google Scholar]
- Kheirbek MA. A molecular switch for induction of long-term depression of corticostriatal transmission. J Neurosci. 2007;27:9824–5. doi: 10.1523/JNEUROSCI.2938-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitai ST, Kocsis JD, Preston RJ, Sugimori M. Monosynaptic inputs to caudate neurons identified by intracellular injection of horseradish peroxidase. Brain Res. 1976;109:601–6. doi: 10.1016/0006-8993(76)90039-1. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA. Cannabinoids Potentiate Emotional Learning Plasticity in Neurons of the Medial Prefrontal Cortex through Basolateral Amygdala Inputs. J Neurosci. 2006a;26:6458–68. doi: 10.1523/JNEUROSCI.0707-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA. The roles of cannabinoid and dopamine receptor systems in neural emotional learning circuits: implications for schizophrenia and addiction. Cell Mol Life Sci. 2006b doi: 10.1007/s00018-006-6027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemberger L, Axelrod J, Kopin IJ. Metabolism and disposition of delta-9-tetrahydrocannabinol in man. Pharmacol Rev. 1971;23:371–80. [PubMed] [Google Scholar]
- Lewis PA, Miall RC. Distinct systems for automatic and cognitively controlled time measurement: evidence from neuroimaging. Curr Opin Neurobiol. 2003;13:250–5. doi: 10.1016/s0959-4388(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Miall RC. A right hemispheric prefrontal system for cognitive time measurement. Behav Processes. 2006;71:226–34. doi: 10.1016/j.beproc.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. Cannabinoid tolerance and dependence. Handbook of Experimental Pharmacology. 2005:691–717. doi: 10.1007/3-540-26573-2_24. [DOI] [PubMed] [Google Scholar]
- Lieving LM, Lane SD, Cherek DR, Tcheremissine OV. Effects of marijuana on temporal discriminations in humans. Behav Pharmacol. 2006;17:173–83. doi: 10.1097/01.fbp.0000197458.08892.fc. [DOI] [PubMed] [Google Scholar]
- Lindgren JE, Ohlsson A, Agurell S, Hollister L, Gillespie H. Clinical effects and plasma levels of delta 9-tetrahydrocannabinol (delta 9-THC) in heavy and light users of cannabis. Psychopharmacology (Berl) 1981;74:208–12. doi: 10.1007/BF00427095. [DOI] [PubMed] [Google Scholar]
- MacDonald CJ, Meck WH. Differential effects of clozapine and haloperidol on interval timing in the supraseconds range. Psychopharmacology (Berl) 2005;182:232–44. doi: 10.1007/s00213-005-0074-8. [DOI] [PubMed] [Google Scholar]
- Madison G. Variability in isochronous tapping: higher order dependencies as a function of intertap interval. J Exp Psychol Hum Percept Perform. 2001;27:411–22. doi: 10.1037//0096-1523.27.2.411. [DOI] [PubMed] [Google Scholar]
- Martin GW, Wilkinson DA, Kapur BM. Validation of self-reported cannabis use by urine analysis. Addict Behav. 1988;13:147–50. doi: 10.1016/0306-4603(88)90004-4. [DOI] [PubMed] [Google Scholar]
- Matell MS, Bateson M, Meck WH. Single-trials analyses demonstrate that increases in clock speed contribute to the methamphetamine-induced horizontal shifts in peak-interval timing functions. Psychopharmacology (Berl) 2006;188:201–12. doi: 10.1007/s00213-006-0489-x. [DOI] [PubMed] [Google Scholar]
- Matell MS, King GR, Meck WH. Differential modulation of clock speed by the administration of intermittent versus continuous cocaine. Behav Neurosci. 2004;118:150–6. doi: 10.1037/0735-7044.118.1.150. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH. Cortico-striatal circuits and interval timing: coincidence detection of oscillatory processes. Cognitive Brain Research. 2004;21:139–70. doi: 10.1016/j.cogbrainres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH, Turkington TG, Coleman RE. Cerebellar activity and disturbed time sense after THC. Brain Res. 1998;797:183–9. doi: 10.1016/s0006-8993(98)00375-8. [DOI] [PubMed] [Google Scholar]
- McClure GY, McMillan DE. Effects of drugs on response duration differentiation. VI: differential effects under differential reinforcement of low rates of responding schedules. J Pharmacol Exp Ther. 1997;281:1368–80. [PubMed] [Google Scholar]
- McDonald J, Schleifer L, Richards JB, de Wit H. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology. 2003;28:1356–65. doi: 10.1038/sj.npp.1300176. [DOI] [PubMed] [Google Scholar]
- Meck WH. Selective adjustment of the speed of internal clock and memory processes. J Exp Psychol Anim Behav Process. 1983;9:171–201. [PubMed] [Google Scholar]
- Meck WH. Neuropharmacology of timing and time perception. Brain Res Cogn Brain Res. 1996;3:227–42. doi: 10.1016/0926-6410(96)00009-2. [DOI] [PubMed] [Google Scholar]
- Meck WH. Neuropsychology of timing and time perception. Brain Cogn. 2005;58:1–8. doi: 10.1016/j.bandc.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Meck WH, Benson AM. Dissecting the brain’s internal clock: how frontal-striatal circuitry keeps time and shifts attention. Brain Cogn. 2002;48:195–211. doi: 10.1006/brcg.2001.1313. [DOI] [PubMed] [Google Scholar]
- Meck WH, Church RM. A mode control model of counting and timing processes. J Exp Psychol Anim Behav Process. 1983;9:320–34. [PubMed] [Google Scholar]
- Menhiratta SS, Wig NN, Verma SK. Some psychological correlates of long-term heavy cannabis users. Br J Psychiatry. 1978;132:482–6. doi: 10.1192/bjp.132.5.482. [DOI] [PubMed] [Google Scholar]
- Meschler JP, Howlett AC. Signal transduction interactions between CB1 cannabinoid and dopamine receptors in the rat and monkey striatum. Neuropharmacology. 2001;40:918–26. doi: 10.1016/s0028-3908(01)00012-0. [DOI] [PubMed] [Google Scholar]
- Meyer RE, Pillard RC, Shapiro LM, Mirin SM. Administration of marijuana to heavy and casual marijuana users. Am J Psychiatry. 1971;128:198–204. doi: 10.1176/ajp.128.2.198. [DOI] [PubMed] [Google Scholar]
- Morrow R Mayor’s Committee on Marihuana btNYAoM. The Marihuana Problem in the City of New York. City of New York: 1944. Psychophysical and other functions. [Google Scholar]
- O’Leary DS, Block RI, Turner BM, Koeppel J, Magnotta VA, Ponto LB, Watkins GL, Hichwa RD, Andreasen NC. Marijuana alters the human cerebellar clock. Neuroreport. 2003;14:1145–51. doi: 10.1097/00001756-200306110-00009. [DOI] [PubMed] [Google Scholar]
- Office of Applied Studies (Office of Applied Studies 2006; Office of National Drug Control Policy 2008) Results from the 2005 National Survey on Drug Use and Health: National findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2006. [Google Scholar]
- Office of National Drug Control Policy. The Marijuana Factbook. ONDCP; Washington, DC: 2008. Marijuana: The Greatest Cause of Illegal Drug Abuse. [Google Scholar]
- Ohlsson A, Lindgren JE, Wahlen A, Agurell S, Hollister LE, Gillespie HK. Plasma delta-9 tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clin Pharmacol Ther. 1980;28:409–16. doi: 10.1038/clpt.1980.181. [DOI] [PubMed] [Google Scholar]
- Paule MG, Meck WH, McMillan DE, McClure GY, Bateson M, Popke EJ, Chelonis JJ, Hinton SC. The use of timing behaviors in animals and humans to detect drug and/or toxicant effects. Neurotoxicol Teratol. 1999;21:491–502. doi: 10.1016/s0892-0362(99)00015-x. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes M, Burstein SH, White WR, McDonald SA, Hicks RE. Antagonism of marihuana effects by indomethacin in humans. Life Sci. 1991;48:507–15. doi: 10.1016/0024-3205(91)90465-n. [DOI] [PubMed] [Google Scholar]
- Pistis M, Ferraro L, Pira L, Flore G, Tanganelli S, Gessa GL, Devoto P. Delta(9)-tetrahydrocannabinol decreases extracellular GABA and increases extracellular glutamate and dopamine levels in the rat prefrontal cortex: an in vivo microdialysis study. Brain Res. 2002;948:155–8. doi: 10.1016/s0006-8993(02)03055-x. [DOI] [PubMed] [Google Scholar]
- Pomarol-Clotet E, Honey GD, Murray GK, Corlett PR, Absalom AR, Lee M, McKenna PJ, Bullmore ET, Fletcher PC. Psychological effects of ketamine in healthy volunteers. Phenomenological study. Br J Psychiatry. 2006;189:173–9. doi: 10.1192/bjp.bp.105.015263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin AI. Time estimation of schizophrenics and nonotics. Journal of Clinical Psychology. 1957;13:88–90. doi: 10.1002/1097-4679(195701)13:1<88::aid-jclp2270130125>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Rammsayer TH. Neuropharmacological evidence for different timing mechanisms in humans. Q J Exp Psychol B. 1999;52:273–86. doi: 10.1080/713932708. [DOI] [PubMed] [Google Scholar]
- Rammsayer TH, Vogel WH. Pharmacologic properties of the internal clock underlying time perception in humans. Neuropsychobiology. 1992;26:71–80. doi: 10.1159/000118899. [DOI] [PubMed] [Google Scholar]
- Ranganathan M, Braley G, Pittman B, Cooper T, Perry E, Krystal J, D’Souza DC. The effects of cannabinoids on serum cortisol and prolactin in humans. Psychopharmacology (Berl) 2009;203:737–44. doi: 10.1007/s00213-008-1422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan M, Carbuto M, Braley G, Elander J, Perry E, Pittman B, Radhakrishnan R, Sewell RA, D’Souza DC. Naltrexone does not attenuate the effects of intravenous Delta9-tetrahydrocannabinol in healthy humans. International Journal of Neuropsychopharmacology. 2012:1–14. doi: 10.1017/S1461145711001830. [DOI] [PubMed] [Google Scholar]
- Ranganathan M, D’Souza DC. The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology (Berl) 2006;188:425–44. doi: 10.1007/s00213-006-0508-y. [DOI] [PubMed] [Google Scholar]
- Schulze GE, McMillan DE, Bailey JR, Scallet A, Ali SF, Slikker W, Jr, Paule MG. Acute effects of delta-9-tetrahydrocannabinol in rhesus monkeys as measured by performance in a battery of complex operant tests. J Pharmacol Exp Ther. 1988;245:178–86. [PubMed] [Google Scholar]
- Sewell RA, Skosnik PD, Garcia-Sosa I, Ranganathan M, D’Souza DC. Behavioral, cognitive and psychophysiological effects of cannabinoids: relevance to psychosis and schizophrenia. Revista Brasileira de Psiquiatria. 2010;32(Suppl 1):S15–30. [PubMed] [Google Scholar]
- Solinas M, Justinova Z, Goldberg SR, Tanda G. Anandamide administration alone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rats. J Neurochem. 2006;98:408–19. doi: 10.1111/j.1471-4159.2006.03880.x. [DOI] [PubMed] [Google Scholar]
- Stone JM, Morrison PD, Nottage J, Bhattacharyya S, Feilding A, McGuire PK. Delta-9-tetrahydrocannabinol disruption of time perception and of self-timed actions. Pharmacopsychiatry. 2010;43:236–7. doi: 10.1055/s-0030-1255030. [DOI] [PubMed] [Google Scholar]
- Stone JM, Pilowsky LS. Antipsychotic drug action: targets for drug discovery with neurochemical imaging. Expert Review of Neurotherapeutics. 2006;6:57–64. doi: 10.1586/14737175.6.1.57. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–35. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997a;276:2048–50. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism.[comment] Science. 1997b;276:2048–50. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Tart CT. Marijuana intoxication common experiences. Nature. 1970;226:701–4. doi: 10.1038/226701a0. [DOI] [PubMed] [Google Scholar]
- Tinklenberg JR, Kopell BS, Melges FT, Hollister LE. Marihuana and alcohol, Time production and memory functions. Arch Gen Psychiatry. 1972;27:812–5. doi: 10.1001/archpsyc.1972.01750300074013. [DOI] [PubMed] [Google Scholar]
- Tinklenberg JR, Roth WT, Kopell BS. Marijuana and ethanol: differential effects on time perception, heart rate, and subjective response. Psychopharmacology (Berl) 1976;49:275–9. doi: 10.1007/BF00426830. [DOI] [PubMed] [Google Scholar]
- Troche S, Rammsayer T. Temporal and non-temporal sensory discrimination and their predictions of capacity- and speed-related aspects of psychometric intelligence. Personality and Individual Differences. 2009;47:52–57. [Google Scholar]
- Tysk L. Time estimation by healthy subjects and schizophrenic patients: a methodological study. Perceptual and Motor Skills. 1983;56:983–8. doi: 10.2466/pms.1983.56.3.983. [DOI] [PubMed] [Google Scholar]
- Vachon L, Sulkowski A, Rich E. Marihuana effects on learning, attention and time estimation. Psychopharmacologia. 1974;39:1–11. doi: 10.1007/BF00421453. [DOI] [PubMed] [Google Scholar]
- Villares J. Chronic use of marijuana decreases cannabinoid receptor binding and mRNA expression in the human brain. Neuroscience. 2007;145:323–34. doi: 10.1016/j.neuroscience.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Wall M, Brine D, Perez-Reyes M. Metabolism of cannabinoids in man. In: Braude M, Szara S, editors. Pharmacology of marihuana. Raven; New York: 1976. p. 536. [Google Scholar]
- Wearden JH, Lejeune H. Scalar properties in human timing: conformity and violations. Q J Exp Psychol (Hove) 2008;61:569–87. doi: 10.1080/17470210701282576. [DOI] [PubMed] [Google Scholar]
- Webb P, Strube F, Leavitt J, Norris G, Fitz-Gerald M, Nixon F, Straumanis J. Time distortion as a persistent sequelae of chronic THC use. In: Harris L, editor. NIDA Research Monograph 132, Problems of Drug Dependence. US Government Printing Office; Washington, DC: 1993. [Google Scholar]
- Weil AT, Zinberg NE, Nelsen JM. Clinical and psychological effects of marihuana in man. Science. 1968;162:1234–42. doi: 10.1126/science.162.3859.1234. [DOI] [PubMed] [Google Scholar]
- Williamson LL, Cheng RK, Etchegaray M, Meck WH. “Speed” warps time: methamphetamine’s interactive roles in drug abuse, habit formation, and the biological clocks of circadian and interval timing. Curr Drug Abuse Rev. 2008;1:203–12. doi: 10.2174/1874473710801020203. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Leland DS, Churan J, Paulus MP. Impaired time perception and motor timing in stimulant-dependent subjects. Drug Alcohol Depend. 2007;90:183–92. doi: 10.1016/j.drugalcdep.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Paulus MP. Decision making, impulsivity and time perception. Trends in Cognitive Science. 2008;12:7–12. doi: 10.1016/j.tics.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Zelanti PS, Droit-Volet S. Cognitive abilities explaining age-related changes in time perception of short and long durations. J Exp Child Psychol. 109:143–57. doi: 10.1016/j.jecp.2011.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.