Summary
Reasons for performing study
The 2 sites of bone marrow harvest for isolation of mesenchymal stromal cells (MSC) in the horse are the sternum and ilium. The technical procedure is based on practitioner preference but no studies have compared MSC concentrations and growth rates between each site in horses aged 2–5 years.
Objectives
The objective of this study was to compare nucleated cell counts and growth rates between the sternum and ilium and between consecutive 5 ml bone marrow aspirates. We hypothesised that there would be a higher concentration of MSCs in the sternum than the ilium, and that the first sequential aspirate from either site would yield the greatest concentration of MSCs. We hypothesised that growth rates of cells from each site would not differ.
Methods
Seven horses, aged 2–5 years old, had 2 sequential 5 ml marrow aspirates taken from the sternum and ilium. Nucleated cell counts (NCCs) were obtained pre- and post- marrow processing. Cells were expanded in culture for 3 passages and growth rate characteristics compared for all aspirates.
Results
The NCCs of the first 5 ml aspirate were higher than the second 5 ml aspirate for both sites (p<0.05). There was no difference between growth rates for any of the groups (p>0.05).
Conclusions
The NCCs and growth rates of progenitor cells in the ilium and sternum are similar for horses in the 2–5 year age category. The first 5 ml bone marrow aspirate has a higher concentration of NCCs and resulting bone marrow-derived MSC population than subsequent aspirates.
Potential Relevance
The first 5 ml aspirates from the sternum and ilium offer a rich supply of bone marrow-derived MSCs with similar growth rate characteristics. The harvesting procedure of only a 5 ml draw from either the sternum or ilium should result in adequate numbers of MSCs.
Keywords: horse, bone marrow, stem cells, mesenchymal
Introduction
In vivo studies using stromal cells in the horse emphasise the need for millions of cells injected at specific time points post-injury to optimize repair [1,2,3,4]. Thus, the goal of stromal cell harvesting is to obtain large numbers of cells in a short time frame. The wing of the ilium and the sternum are the 2 most common sites for the technical procedure of bone marrow-derived mesenchymal stromal cell (BMDMSC) collection in the horse [5]. Currently, clinician preference guides the choice of harvest site. There are practical advantages and disadvantages to both [5, 6, 7, 8], however it is unknown whether one site offers superior numbers or growth rates of cells. McDuffee et al. [9] demonstrated variability in bone-derived progenitor cells present at different bone graft harvest sites in the horse. Alternatively, Mclain et al. [10, 11] showed that human vertebral marrow had comparable concentrations of progenitor cells to matched controls of the iliac crest.
There is limited published information in the horse as to how collection site, volume of aspirate, and sequence of collection (first or second aspirate) affect MSC numbers, expansion characteristics, and final MSC counts [6, 12, 13]. Recently, it was shown in one study that sternal aspirates yielded significantly higher numbers of MSCs/ml bone marrow than ilial aspirates in middle-aged (13 year old) geldings [12]. The volumes of bone marrow used for comparisons in this study varied from 5 to 33 mls. Another study showed that the first 5 ml of sternal bone marrow had greater numbers of colony forming units than the second 5 ml aspirate in sequential aspirates from the equine sternum but the ilium was not compared to sternal samples [6]. A third study compared cell counts and growth rates of bone marrow aspirates from the equine sternum and ilium in yearlings and found no significant differences between the 2 sites. This study did find a significantly higher concentration of stromal cells in the first 60 ml bone marrow aspirate compared to the second 60 ml bone marrow aspirate [13]. Determining differences in stromal cell concentration and growth rates between harvest sites and sequential aspirates will help optimise harvest techniques for improved regenerative effects on the targeted tissue. Furthermore, the procedure of only harvesting 5 ml may decrease harvest time and morbidity of patients. Our objectives were to compare nucleated cell counts and growth rates between the sternum and ilium and between sequential aspirates. Our hypotheses were: 1) There would be significantly higher nucleated cell counts in the sternum than in the ilium. 2) The growth rates of BMDMSCs from the ilium would be higher than those of the sternum and; 3) There would be significantly higher nucleated cell counts in the primary aspirate than in the secondary aspirate from either site. The ultimate goal of this study was to guide practitioners in selection of collection site and reveal what fractions of aspirate harbor the majority of progenitor cells.
Materials and methods
Marrow collection
Samples were collected from the sternum and ilium of 7 research horses 30-60 minutes prior to their euthanasia for another study. Horses were geldings (4) and mares (3), between 430 and 500 kg, healthy, and without history of previous attempts to aspirate bone marrow. All protocols were approved by the Animal Care and Use Committee at Colorado State University. Each horse was sedated with 5 mg (0.01 mg/kg) detomidine hydrochloride (Dormosedan1) and 5 mg (0.01 mg/kg) butorphanol tartrate (Torbugesic2) intravenously prior to marrow collection. Each collection site was aseptically prepared and locally anaesthetised with 5 ml of 2% lidocaine hydrochloride3 and then a stab incision was made into the skin and subcutaneous tissue with a #15 blade. An 11-gauge jamshidi needle was used for all collections. At the sternum, the puncture site was located on midline just caudal to the olecranon, at the level of the 5th or 6th sternebrae, and the depth of insertion was approximately 2 cm. At the ilium, the puncture site was located directly in the centre of the bone where the ilium is most easily palpated and the jamshidi was inserted to a depth of approximately 5–8 cm at a slightly caudoventral angle relative to a line perpendicular with the skin [6]. Samples were collected into sterile 60 ml syringes containing 1 ml of 1000 U/ml heparin sodium4. Two consecutive 5 ml aspirates were collected from each site without redirection of the jamshidi, for a total of 4 different marrow aspirates from each horse (1st and 2nd ilium aspirate and 1st and 2nd sternal aspirate).
Marrow processing
Samples were transferred from 60 ml syringes to 15 ml conical tubes5, and a nucleated cell count of each marrow sample was taken. Nucleated cell counts were performed using a hemocytometer after diluting a 100 microliter aliquot of marrow 1:10 with ammonium chloride6 to lyse red blood cells. This initial count is the “pre-processing” nucleated cell count. Samples were refrigerated at 4°C for 22–24 h to allow separation of the plasma layer. The plasma layer was removed and transferred to a new conical tube. The remaining cell-rich layer was then centrifuged at 200 g for 5 min to separate any remaining plasma. Finally, the remaining cell-rich layer was mixed with a pre-warmed mixture of 5 ml high glucose D-MEM (Dulbecco's Modified Eagle Medium)7 and 20 μL of 10,000 U/ml heparin sodium4 and centrifuged for 5 min at 200 g. The supernatant above the red cell pellet was saved and processed with the separated plasma. All extracted fractions were pelleted by centrifugation at 1000 g for 10 min. Cells were resuspended in D-MEM7, counted again (post-processing nucleated cell count) and plated in D-MEM7 supplemented with 10 % Fetal Bovine Serum7, 1000 units/ml Penicillin-Streptomycin6, 1 ml HEPES7 at a density of 250,000 cells/cm2. T-150 or T-75 flasks (Corning® rectangular canted neck cell culture flask with vent cap)8 were used. Pre- and postprocessing counts were made to ensure that the processing methods did not affect the trends of the data. Flasks were incubated at 37°C and media was changed 24 h after seeding. The flasks were then incubated for 7–10 days without media changes to allow formation of stromal cell colonies, which were trypsinised when the majority of formed colonies were confluent. To lift colonies, flasks were washed with 5–10 ml Phosphate-buffered saline7 and then bathed in 0.25% Trypsin6 for 2–3 min or until colonies lifted. Trypsinised cells were counted with a hemocytometer and pelleted with centrifugation at 200 g for 5 min. Pelleted cells were plated in alpha-minimum essential media (α-mem media)7 supplemented with 2 ng/ml FGF-26 at a density of 800 cells/cm2. Media was changed every 2 days, and cells split when 60–80% confluent (approximately 4 days). Cells were counted at each split and passaged 3 times before freezing. Subjective observations on colony and cell morphology were noted, including colony size, shape, cell heterogeneity, cell shape and cell size.
For all nucleated cell count comparisons, the median nucleated cell count was used due to the small sample size (n = 7). Comparisons were made between the nucleated cell counts of sternal aspirate 1 and sternal aspirate 2, ilial aspirate 1 and ilial aspirate 2, sternal aspirate 1 and ilial aspirate 1, and sternal aspirate 2 and ilial aspirate 2, both pre- and post- processing of marrow. For each 5 ml marrow aspirate, the following assessments were made:
Nucleated cell number and concentration (number of nucleated cells per ml marrow aspirate) pre-processing of marrow
Nucleated cell number and concentration post-processing of marrow
Growth rate of cells
Data Analysis
Statistical calculations were made using SAS v. 9.2 (SAS Institute Incorporated, Cary, NC). The data were analysed for normality of spread using the Shapiro-Wilk test. The data were not distributed normally so they were log-transformed prior to analysis. The nucleated cell count and concentration (nucleated cells per ml marrow) pre- and post- marrow processing were statistically compared between each aspirate using the signed rank test (non-parametric paired t-test to compare median values). The values of growth rates of cells from each aspirate were log transformed and then compared using a multivariable linear regression analysis to adjust for the effect of the ‘day’. A p-value less than 0.05 was determined to represent statistical significance.
Results
For both collection sites, the first 5 ml aspirate had a significantly higher nucleated cell count per ml marrow than the second 5 ml aspirate, both pre- and post- marrow processing (p<0.05) (Table 1, 2). For the sternum, the pre-processing median nucleated cell count per ml marrow of the first 5 ml aspirate was 46 million compared to 16 million for the second aspirate. For the ilium, the pre-processing median nucleated cell count per ml marrow of the first 5 ml aspirate was 22 million compared to 7 million for the second aspirate. Post-processing values followed the same trends.
Table 1.
Pre-processing nucleated cell count analysis for each aspirate.
| Aspirate | Median | Minimum | Maximum | Mean | Std Dev | 95% confidence limit of mean | |
|---|---|---|---|---|---|---|---|
| minimum | maximum | ||||||
| ST1 | 46.2 a | 10.4 | 198.0 | 74.8 | 70.1 | 10.0 | 140.0 |
| ST2 | 16.3 b | 9.9 | 28.4 | 17.2 | 6.6 | 11.1 | 23.3 |
| IL1 | 21.9 a | 9.4 | 181.0 | 50.3 | 62.7 | 0.0 | 108.0 |
| IL2 | 7.4 b | 4.1 | 15.4 | 7.8 | 3.9 | 4.2 | 11.5 |
Numbers are in millions of cells. N = 7 horses for each aspirate. ST = sternum; IL = Ilium. 1 and 2 represent first or second 5 ml aspirates at each site. “a” and “b” are used to denote statistical significance.
Table 2.
Post-processing nucleated cell count analysis for each aspirate.
| Aspirate | Median | Minimum | Maximum | Mean | Std Dev | 95% confidence limit of mean | |
|---|---|---|---|---|---|---|---|
| minimum | maximum | ||||||
| ST 1 | 29.4 a | 4.7 | 47.1 | 27.7 | 14.2 | 14.5 | 40.8 |
| ST 2 | 12.2 b | 3.3 | 29.3 | 12.7 | 8.5 | 4.8 | 20.5 |
| IL 1 | 10.9 a | 8.7 | 51.6 | 20.0 | 15.8 | 5.5 | 34.6 |
| IL 2 | 4.0 b | 1.8 | 10.0 | 5.0 | 3.5 | 1.8 | 8.2 |
Numbers are in millions of cells. N = 7 horses for each aspirate. ST = sternum; IL = Ilium. 1 and 2 represent first or second 5 ml aspirates at each site. “a” and “b” are used to denote statistical significance.
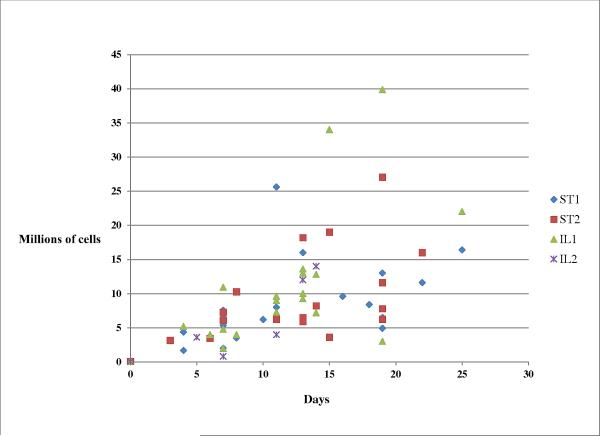
The cell growth rates were not significantly different for any comparisons. Fig 1 shows the growth of all aspirates over time. This figure reveals that, despite having adequate initial cell counts, only 2 out of 7 second 5 ml aspirates from the ilium proliferated. In contrast, all of the first 5 ml aspirates from the ilium proliferated well. Fig 1 also shows that 2 out of 7 samples from the first ilium aspirates achieved 3rd passage cell counts over 30 million cells, which were the greatest values obtained from all sites. In contrast, the greatest value achieved by either sternal site was 27 million cells from a second sternal aspirate.
Fig 1.
Growth of all stromal cells in culture over time. Day 0 represents first passage of cells out of colony at 800 cells/cm2 (60,000 cells/T-75 flask). ST represents sternum, IL represents ilium, and 1 and 2 represent the first or second sample from each site.
We noted differences in colony morphology between cells from the sternum and the ilium. Subjectively, stromal cell colonies from the ilium were more organised than those of the sternum (Fig 2 a, b). Sternal cells formed less discreet colonies which were often surrounded by individual adherent cells. There were no appreciable morphological differences between the cells from the sternum and ilium at later passages (Fig 2 c, d).
Fig 2.
Phase contrast microscopic images of stromal cells from the 1st 5 ml sample from both sternum and ilium. Fig 2a and 2b show cells in colony from the ilium and sternum, respectively. Stromal cell colonies from the ilium appeared more organised and discreet than those of the sternum. Fig 2c and 2d show 2nd passage cells from the ilium and sternum, respectively. Expanding cells from the sternum and ilium are virtually indistinguishable from each other.
Discussion
This study shows a significant difference between the nucleated cell counts obtained from the first 5 ml bone marrow aspirate compared to the second 5 ml aspirate at both the sternum and the ilium. Our results corroborate the findings of Muschler et al. and McLain et al. [11, 14] in which there were significant differences in the concentration of connective tissue progenitors in different aspirate volumes of human vertebral and ilial bone marrow. Kasashima et al. (2011) demonstrated the same phenomenon occurring with sequential aspirates of marrow from the equine sternum [6] and Toupadakis et al. (2010) noted significantly greater yields in the first 60 ml of marrow from either sternum or ilium [13]. With increasing aspiration volumes, connective tissue progenitor concentration decreases due to dilution of the aspirate with peripheral blood [1]. A similar phenomenon likely occurs in both humans and horses.
We demonstrated that the sternum and ilium share similar nucleated cell counts and cell growth rates. Our findings are similar to those of Muschler and colleagues in which human vertebral and ilial aspirates had comparable concentrations of stromal cells [1] and Toupadakis et al. in which sternal and ilial aspirates had comparable concentrations of stromal cells in yearling horses [13]. However, our findings contrast with those of Delling et al. (2012) in which ilial harvests yielded significantly fewer stromal cells than sternal harvests in middle-aged horses [12]. One possible explanation is that ilial marrow quality declines with age, such that ilial marrow is suboptimal in older horses. In the study by Delling et al. (2012), ilial harvest volumes varied from 5 to 33 ml, making it difficult to make direct comparisons between different sites [12]. It is possible that the low concentrations of stromal cells in ilial harvests from that study are a result of dilution of ilial marrow with peripheral blood due to excessively large harvest volumes from the ilium.
We noted subjective differences in morphology between cells from the 2 sites. The stromal cell colonies from the ilium were more organised than those of the sternum. Sternal colonies were less discreet and often surrounded by individual adherent cells. The significance of this finding is unclear. One possibility is that the adherent cells in sternal cultures that were not in discreet colonies represent contaminant cell populations. If so, they did not appreciably affect cell growth, since cell growth rates at both sites were similar.
In our study, sample size was the greatest limitation. There is significant individual variation in nucleated cell concentration, even among similarly-aged, healthy individuals [1, 15]. Also, not every sample resulted in a growing cell population, especially the second ilial samples. We restricted the age of our study population to between 2 and 5 years to avoid the influence of age on cell concentration and growth. It has been our experience that horses older than 5–7 years have fewer nucleated cells in the ilial cavity; more “failures” of bone marrow expansions have occurred from ilial aspirates than from sternal aspirates. This observation has been noted by Delling et al. as well [12].
The objectives of this study were met in that we were able to compare the effects of bone marrow aspirate sites and fractions on nucleated cell counts and growth rates. Our first hypothesis that there would be significantly higher nucleated cells counts in the sternum than the ilium was disproven as we were not able to detect a significant difference either pre- or post-processing of the cells when sternum and ilium were compared. Our second hypothesis that the growth rates of BMDMSCs from the ilium would be higher than the sternum was also disproven as similar trends in growth rates were seen. Finally, our third hypothesis was proven as we were able to show significantly higher concentrations of nucleated cells in the first 5 ml aspirates compared to the second 5 ml aspirates at both sites. There are 2 major practical points to be taken from this study. One is that a statistically significant difference in stromal cell quantity or rate of growth between the sternum and the ilium was not detected in this study population. The decision between sternal or ilial harvest in young horses aged 2–5 years old should be based on clinician preference, not on the presumption that one site is optimal in terms of numbers of cells or growth rates. The second important point is that the 1st 5 ml aspirate contains significantly more nucleated cells than subsequent aspirates. Harvest procedure times may be shortened and patient morbidity decreased by reducing total harvest volume to the first 5–10 ml from either collection site.
Acknowledgments
Source of funding
This study was funded in part by a PVM Student Grant from the Center for Companion Animal Studies at Colorado State University and NIH K08 AR054903-01A2.
Footnotes
Author contributions
Dr Adams – primary author; worked with other authors on study design; collected data and executed study; helped with data analysis and interpretation; wrote manuscript. Dr Goodrich – assisted in all aspects of the study including design, data collection, study execution, data analysis and interpretation. Huge role in editing and helping prepare the manuscript. Drs Rao and Olea-Popelka – statisticians. Nikki Philliips – assisted with data collection, data analysis and the preparation of figures. Dr Kisiday – assisted with study design, data collection and editing manuscript. Dr McIlwraith – assisted with study design, data collection and study execution.
Authors’ declaration of interests
The authors have declared the following competing interests: Drs Goodrich, Kisiday and McIlwraith have financially invested in Advanced Regenerative Therapies, Inc. a company that processes bone marrow for stem cell therapies.
Pfizer Inc., New York, New York, 10017, USA.
Fort Dodge, Fort Dodge, Iowa, 50501, USA.
VEDCO Inc., St. Joseph, Missouri, 64507, USA.
APP Pharmaceuticals LLC, Schaumburg, Illinois, 60173, USA.
BD Falcon Tubes, BD Biosciences, Maryland, 21152, USA.
Sigma-Aldrich, St. Louis, Missouri, 63103, USA.
GIBCO/Invitrogen, Grand Island, New York, 14072, USA.
Corning Incorporated Life Sciences, Lowell, Massachusetts, 01851, USA.
References
- 1.Smith RK, Korda M, Blunn GW, Goodship AE. Isolation and implantation of autologous equine stem cells from bone marrow into the superficial digital flexor tendon as a potential novel treatment. Equine Vet. J. 2003;35:909–102. doi: 10.2746/042516403775467388. [DOI] [PubMed] [Google Scholar]
- 2.Smith RK. Autogenous stem cell implantation. Proceedings of ACVS Veterinary Symposium. 2004:204–206. [Google Scholar]
- 3.Fortier LA. Stem cells: classifications, controversies, and clinical applications. Vet. Surg. 2005;34:415–423. doi: 10.1111/j.1532-950X.2005.00063.x. [DOI] [PubMed] [Google Scholar]
- 4.Smith RK, Webbon PM. Harnessing the stem cell for the treatment of tendon injuries: heralding a new dawn? Br. J. Sports Med. 2005;39:582–584. doi: 10.1136/bjsm.2005.015834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodrich L, Frisbie DD, Kisiday JD. How to harvest bone marrow derived mesenchymal stem cells for expansion and injection. Proc. Am. Ass. Equine practnrs. 2008;54:253–257. [Google Scholar]
- 6.Kasashima Y, Ueno T, Tomita A, Goodship E, Smith RKW. Optimisation of bone marrow aspiration from the equine sternum for the safe recovery of mesenchymal stem cells. Equine vet. J. 2011;43:288–294. doi: 10.1111/j.2042-3306.2010.00215.x. [DOI] [PubMed] [Google Scholar]
- 7.Fortanier C, Kuentz M, Sutton L, Milpied N, Michalet M, Macquart-Moulin G, Faucher C, Le Corroller AG, Moatti JP, Blaise D. Healthy sibling donor anxiety and pain during bone marrow or peripheral blood stem cell harvesting for allogeneic transplantation: results of a randomised study. Bone Marrow Transplant. 2002;29:145–9. doi: 10.1038/sj.bmt.1703338. [DOI] [PubMed] [Google Scholar]
- 8.Durando MM, Zarucco L, Schaer TP, Ross M, Reef VB. Pneumopericardium in a horse secondary to sternal bone marrow aspiration. Equine Vet. Educ. 2006;18:75–79. [Google Scholar]
- 9.McDuffee LA, Anderson GI, Wright GM, Ryan DAJ. In vitro heterogeneity of osteogenic cell populations at various equine skeletal sites. Can. J. Vet. Res. 2006;70:277–284. [PMC free article] [PubMed] [Google Scholar]
- 10.McLain RF, Fleming JE, Boehm CA, Muschler GF. Aspiration of osteoprogenitor cells for augmenting spinal fusion: Comparison of progenitor cell concentrations from the vertebral body and iliac crest. J. Bone Joint Surg. Am. 2005;87:2655–2661. doi: 10.2106/JBJS.E.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLain RF, Boehm CA, Rufo-Smith C, Muschler G. Transpedicular aspiration of osteoprogenitor cells from the vertebral body: progenitor cell concentrations affected by serial aspiration. Spine J. 2009;9:995–1002. doi: 10.1016/j.spinee.2009.08.455. [DOI] [PubMed] [Google Scholar]
- 12.Delling U, Lindner K, Ribitsch I, Jülke H, Brehm W. Comparison of bone marrow aspiration at the sternum and the tuber coxae in middle-aged horses. Can. J. Vet.Res. 2012;76:52–56. [PMC free article] [PubMed] [Google Scholar]
- 13.Toupadakis CA, Wong A, Genetos DC, Cheung WK, Borjesson DL, Ferraro GL, Galuppo LD, Leach JK, Owens SD, Yellowley CE. Comparison of the osteogenic potential of equine mesenchymal stem cells from bone marrow, adipose tissue, umbilical cord blood, and umbilical cord tissue. Am. J. Vet. Research. 2010;71:1237–1245. doi: 10.2460/ajvr.71.10.1237. [DOI] [PubMed] [Google Scholar]
- 14.Muschler GF, Boehm C, Easley K. Aspiration to obtain osteoblast progenitor cells from human bone marrow: The influence of aspiration volume. J. Bone Joint Surg. Am. 1997;79-A:1699–1709. doi: 10.2106/00004623-199711000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Yoo JU, Barthel TS, Nishimura K, Solchaga L, Caplan AI, Goldberg VM, Johnstone B. The Chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J. Bone Joint Surg. Am. 1998;80-A:1745–1757. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]