Abstract
Acetate supplementation in rats increases plasma acetate and brain acetyl-CoA levels. Although acetate is used as a marker to study glial energy metabolism, the effect that acetate supplementation has on normal brain energy stores has not been quantified. To determine the effect(s) that an increase in acetyl-CoA levels has on brain energy metabolism, we measured brain nucleotide, phosphagen and glycogen levels, and quantified cardiolipin content and mitochondrial number in rats subjected to acetate supplementation. Acetate supplementation was induced with glyceryl triacetate (GTA) by oral gavage (6 g/Kg body weight). Rats used for biochemical analysis were euthanized using head-focused microwave irradiation at 2, and 4 hr following treatment to immediately stop metabolism. We found that acetate did not alter brain ATP, ADP, NAD, GTP levels, or the energy charge ratio [ECR, (ATP + ½ ADP) / (ATP + ADP + AMP)] when compared to controls. However, after 4 hr of treatment brain phosphocreatine levels were significantly elevated with a concomitant reduction in AMP levels with no change in glycogen levels. In parallel studies where rats were treated with GTA for 28 days, we found that acetate did not alter brain glycogen and mitochondrial biogenesis as determined by measuring brain cardiolipin content, the fatty acid composition of cardiolipin and using quantitative ultra-structural analysis to determine mitochondrial density/unit area of cytoplasm in hippocampal CA3 neurons. Collectively, these data suggest that an increase in brain acetyl-CoA levels by acetate supplementation does increase brain energy stores however it has no effect on brain glycogen and neuronal mitochondrial biogenesis.
Keywords: acetate supplementation, brain, energy, glycogen, mitochondria, nucleotides, phosphocreatine
1. Introduction
Glyceryl triacetate (GTA), increases rat plasma acetate and brain acetyl-CoA levels within 30 min of oral administration and brain acetyl-CoA levels remain elevated 2.2-fold above controls for up to 4 hr following treatment (Reisenauer et al., 2011). Acetyl-CoA, the metabolically active form of acetate, is a substrate used for various biochemical pathways involved in carbohydrate, lipid, and protein metabolism. With regard to protein metabolism, we have shown that GTA-derived brain acetyl-CoA increases brain histone acetylation and reverses the bacterial lipopolysaccharide-induced hypoacetylation of histone 3 at lysine 9 (Soliman and Rosenberger, 2011; Soliman et al., 2012c). Nonetheless, acetyl-CoA can also be utilized in the tricarboxylic acid (TCA) cycle, where it functions to provide carbon atoms for oxidative phosphorylation involved in ATP synthesis. Stable- and radio-labeled acetate in combination with neuro-imaging techniques is used to study glial energy metabolism (Wyss et al., 2011) however the direct effect that a significant increase in circulating levels of acetate has on brain energy stores is not known.
The central nervous system depends on mitochondrial energy production to maintain normal physiologic function (Morais and De Strooper, 2010). Disruption of mitochondrial function leads to partial plasma membrane depolarization, activation of NMDA receptors, excitotoxicity, and neuronal death (Novelli et al., 1988). As early as 1 min following ischemia, brain ATP and phosphocreatine (PCr) levels fall (Lowry et al., 1964), and reduced PCr levels are observed in Alzheimer’s disease patients presenting with mild dementia (Pettegrew et al., 1994). Neurons use PCr to store energy as a reserve which when required is readily converted to ATP (Meyer et al., 1984). Increasing neuronal PCr stores protects neurons from hypoxic damage, glutamate toxicity, and Aβ-induced toxicity (Balestrino et al., 2002; Balestrino et al., 1999; Brewer and Wallimann, 2000) and is also neuroprotective in animal models of Huntington’s disease (Matthews et al., 1998), Parkinson’s disease (Matthews et al., 1999), and amyotrophic lateral sclerosis (Klivenyi et al., 1999). Interestingly, acetate supplementation prevents ATP loss, and attenuates neuroglia activation in rat models of traumatic brain injury (Arun et al., 2010), neuroinflammation (Reisenauer et al., 2011), and Lyme neuroborreliosis (Brissette et al., 2012). In brain, acetate is preferentially utilized by astrocytes (Waniewski and Martin, 1998) and is considered as a glial-specific substrate since labeled acetate is mainly incorporated into the synthetic astrocytic-glutamine pool (Minchin and Beart, 1975). During increased metabolic demand neurons depend on astrocytes for rapid removal of potassium ions and neurotransmitters from the extracellular space to maintain neuronal activity (Brown, 2004). This results in an overall increase in energy demand by both neurons and astrocytes where astrocytic glycogenolysis may play an important role. Astrocytic glycogen can serve as an important energy reserve to support neuronal energy metabolism by sparing extracellular glucose for neuronal utilization (Dinuzzo et al., 2012). However, the influence that utilization of acetate through the TCA cycle has on normal brain energetics has not been clearly demonstrated.
A close metabolic correlate to acetate supplementation with regard to brain bioenergetics is the ketogenic diet which increases cellular acetyl-CoA levels (Yudkoff et al., 2005). The ketogenic diet is a high-fat low-carbohydrate diet used to treat refractory cases of epilepsy and is considered neuroprotective (Bough et al., 2006). While the mechanisms underlying the therapeutic effect of the diet remain unknown, a variety of studies suggest mitochondrial bioenergetics to be key in this regard (Maalouf et al., 2009). Ingestion of the diet produces ketone bodies that cross the blood brain barrier and are metabolized to acetyl-CoA, which increases mitochondrial biogenesis in hippocampal neurons and improves brain ATP production (Bough et al., 2006; Nylen et al., 2009). Mitochondrial biogenesis is a process that stimulates the synthesis of mitochondria, import of nuclear-encoded proteins, and fusion of organelles to form a network that increases metabolic function (Nisoli and Carruba, 2006). Cardiolipin, a phospholipid class, mainly located in the inner mitochondrial membrane (Daum, 1985) is involved in the regulation of mitochondrial bioenergetics (Hoch, 1992), is important for the activity of mitochondrial proteins, and can be used as a marker for mitochondrial mass (Houtkooper and Vaz, 2008; Schlame, 2007).
Because both the ketogenic diet and acetate supplementation increase brain acetyl-CoA levels, we propose that acetate supplementation will increase brain bioenergetics similar to that found with the ketogenic diet. To address this hypothesis, we measured brain nucleotide, phosphagen, glycogen, and cardiolipin levels and neuronal mitochondrial number in hippocampal CA3 neurons of rats subjected to acetate supplementation. We observed a significant increase in brain PCr following 4 hr of treatment which is indirect evidence that an increase in acetyl-CoA metabolism in response to acetate supplementation stimulates brain energy metabolism. We did not observe significant changes in other brain nucleotides and glycogen with the exception of a decrease in AMP consistent with the normal state of rats used in this study. Long-term acetate supplementation did not alter brain glycogen and mitochondrial number in CA3 neurons nor did it alter whole brain cardiolipin mass. Thus, the increase in PCr levels and reduced AMP levels coupled with no changes in brain glycogen and mitochondrial biogenesis suggest that acetate supplementation increases brain energy stores however unlike ketogenic diet does not alter neuronal mitochondrial biogenesis.
2. Materials and methods
2.1. Reagents
Nucleotide (ATP, ADP, AMP, GTP, NAD) and phosphagen (PCr, Cr) standards, glyceryl triacetate (99%), Freon (trichlorofluromethane), trioctylamine, 6-(p-Toluidino)-2-naphthalenesulfonic acid sodium salt (TNS), glyceryl triacetate (GTA), and gelatin were purchased from Sigma-Aldrich (St. Louis, MO). Creatinine standard was from MP Biomedicals, LLC (Solon, OH). 14C-labeled ATP, ADP, and AMP were obtained from American Radiolabeled Chemicals, Inc. (St. Louis, MO). Tetrabutylammonium phosphate (TBAP) was purchased from Mallinckrodt Baker, Inc. (Phillipsburg, NJ) and HPLC grade acetonitrile, mono-basic potassium phosphate (KH2PO4), perchloric acid, acetone, sodium hydroxide, and bovine serum albumin were obtained from EMD Chemicals Inc. (Gibbstown, NJ). Bradford reagent was from Bio-Rad Laboratories, Inc. (Hercules, CA). Glutaraldehyde, paraformaldehyde, osmium tetraoxide, uranyl acetate, lead citrate, embed-812 (Epon-812 substitute) WPE #154.3; araldite-502 (modified bisphenol A epoxy); BEEM embedding capsule size#3; cresyl fast violet C.I.#51180, pre-cleaned Gold Seal micro-slides and formvar carbon support film specimen 2 × 1mm slot grids were purchased from Electron Microscopy Science (Hatfield, PA).
2.2. Animal Procedures
Male Sprague-Dawley rats were purchased from Harlan Laboratories (Indianapolis, IN) and experiments were performed following the Guide for the Care and Use of Laboratory Animals (NIH publication number 80–23) as approved by the University of North Dakota Animal Care and Use Committee. All rats were allowed to acclimate for seven days prior to inclusion in the study, were maintained on standard laboratory chow diet, and provided water ad libitum. For nucleotide and phosphagen analysis rats with weighing 150–250 g were given a single dose of GTA (6 g/kg body weight) by oral gavage while control rats received 6 g/kg body weight water. Rats were anaesthetized with sodium pentobarbital (50 mg/kg, i.v.) and subjected to head-focused microwave (6 kW, 2s) irradiation at 2, and 4 hr post-treatment to immediately stop metabolism. Microwave-fixed brains were flash frozen, pulverized in liquid nitrogen, and then stored at −80° C until use. Parallel sets of rats (100–250 g) used to measure brain cardiolipin content and to quantify neuronal mitochondria received a daily dose of GTA or water (6 g/kg body weight) for 28 days and were euthanized 1 hr after the last treatment. The 28 day treatment regimen for the mitochondrial number, and cardiolipin content experiments is based on the 24.4 day half-life of brain mitochondria (Menzies and Gold, 1971) and previous results demonstrating a therapeutic effect in our rat model of neuroinflammation (Reisenauer et al., 2011). Rats used for cardiolipin measurement were euthanized and subjected to head-focused microwave irradiation as described above. Rats used for measuring mitochondrial number were anaesthetized using isoflurane (Butler Animal Health Supply, Dublin, OH) and euthanized by cardiac perfusion with heparinized saline followed by a mixture of 4 % paraformaldehyde and 2 % glutaraldehyde in 0.1 M phosphate buffer. Whole brain was removed and post-fixed in the same mixture for 48 hr at 4° C after which they were transferred to 0.1 M phosphate buffered saline (PBS, pH 7.4) and stored at 4° C until use.
2.3. Nucleotide and Phosphagen Extraction
Nucleotides and phosphagens were extracted using a modified tissue extraction protocol as described (Hammer et al., 1988). In brief, a sample of tissue was homogenized in ice cold 0.6 N perchloric acid in a pre-cooled Tenbroeck glass homogenizer then transferred to a micro-centrifuge tube. The homogenizer was then washed twice with 0.6 N perchloric acid and the combined homogenates were centrifuged at 13,000 × g at 25° C for 2 min. The supernatant was neutralized with ice cold Freon/trioctylamine (4:1, by Vol.) by vortex mixing for 30 sec and the pellet was saved for protein determination. The neutralized extract was centrifuged at 13,000 × g for 2 min to induce phase separation and the upper aqueous layer was collected and stored at 4° C until analysis. Phosphagens were analyzed within 3 hr of extraction and nucleotides were analyzed within 24 hr using high performance liquid chromatography (HPLC) as outlined below.
2.4. Nucleotide Analysis
Nucleotide analysis was performed using a gradient elution as described (Hammer et al., 1988) with slight modifications. The separation was performed on an ISCO HPLC system with an in-line spectrophotometer (model V4) and a Waters Sunfire™ ODS column (5µm, 250 × 4.6 mm, Milford, MA) equipped with a C18 SecurityGuard cartridge (Torrance, CA). Column temperature was maintained at 40° C. Pump control and peak integration was performed using Clarity advanced chromatography software (Ver. 2.6, DataApex, Prague, Czech Republic). The mobile phase consisted of buffer A, 30 mM KH2PO4 + 7.5 mM TBAP, pH 5.45; and buffer B, acetonitrile/30 mM KH2PO4 (1:1, by Vol.) + 7.5 mM TBAP, pH 7.0 (adjusted before addition of the organic phase). Buffers were filtered using a 0.45-µm Supor-450 membrane filter (Pall Corporation, Ann Arbor, MI) prior to addition of the ion-pairing reagent (TBAP). The 40 min elution program consisted of 10% buffer B initially maintained for 0.5 min and then increased to 25% over a period of 8.5 min and held constant for 2 min. The gradient was then increased to 50% buffer B over 4 min followed by a gradient to 60% over 19 min. At 34 min the proportion of buffer B was decreased to 10% over a 4 min period, held constant for 2 min, and the column was allowed to equilibrate for 20 min before injection of the next sample. The flow rate was held constant at 1 ml/min throughout the separation and the nucleotide detection was performed at 260 nm. The peaks were identified and quantified by comparison of sample retention times and peak area to known standards. The results obtained are expressed as nmoles of nucleotide per mg of protein.
2.5. Phosphagen Analysis
Phosphagens analysis was performed as described (Dunnett et al., 1991) using the HPLC system described above with a modified solvent and gradient system, and a Waters Spherisorb® ODS2 column (5µm, 250 × 4.6 mm Milford, MA). The mobile phase consisted of buffer A, 14.7 mM KH2PO4 + 2.3 mM TBAP, pH 3; and buffer B, acetonitrile/14.7 mM KH2PO4 (1:1, by Vol.) + 2.3 mM TBAP, pH 6.5 (prepared in the same manner as described for nucleotide analysis). The 35 min elution gradient was started at 100% buffer A for the first 5 min followed by a gradient increase in buffer B to 10% over a period of 6 min. At 11 min, the composition of buffer B was increased to 50% over 5 min and held constant for 5 min. The gradient was returned to zero over 5 min and held constant for 9 min. The column was allowed to equilibrate for 20 min prior to the next run and the flow rate was held constant at 1 ml/min throughout the separation. Phosphagen detection was performed at 210 nm and the peaks were quantified using external calibration standards. The results are expressed as nmoles of phosphagen per mg protein.
2.6. Glycogen Analysis
Whole brain glycogen was measured from microwave-fixed pulverized brain samples as per the method described by (Cruz and Dienel, 2002) with modifications. Approximately, 50 mg brain powder was homogenized in phosphate buffered saline (pH 7.0) followed by glucose solubilization and glycogen precipitation with ethanol (65% of total Vol.). The samples were vortex mixed and centrifuged at 13,000 × g at 4° C for 10 min. The supernatant containing endogenous glucose was discarded and the pellet was homogenized in 1.5 mL of 0.03 M hydrochloric acid. Samples were than heated at 90° C for 45 min followed by centrifugation at 13,000 × g at 4° C for 10 min. A 10 µL aliquot of the supernatant containing glycogen was incubated with amyloglucosidase enzyme to release glucosyl units which were quantified using a commercial fluorometric kit (Cayman Chemical Company, Ann Arbor, MI). Basal glucose levels obtained from samples not incubated with the enzyme served as background and were subtracted from total glucosyl units released from glycogen. Brain glycogen levels are expressed as µmol of glucosyl units released per gram brain.
2.7. Cardiolipin Analysis
Samples were extracted in n-hexane: 2-propanol (3:2, by Vol.) using a Tenbroeck homogenizer (Radin, 1981). Tissue extracts and cardiolipin standards, dissolved in chloroform, were isolated on 20 cm × 20 cm TLC Silica gel 60 plates (EMD Chemicals Inc., Gibbstown, NJ) using a two solvent system. Solvent A was chloroform/methanol/acetic acid/formic acid/water (70:30:12:4:2, by Vol.), and Solvent B was hexane/di-isopropyl ether/acetic acid (63:35:2, by Vol.). Two TLC chambers were equilibrated with the solvents for at least 1 hr before separation. Samples and standard were spotted on the plate, dried for 5 min at 85° C then placed into the TLC chamber containing solvent A and eluted for 10 cm. The TLC plate was air dried for 15 min then eluted to the top of the plate with solvent B. Bands were visualized using either iodine vapors (phosphorus assay) or with a TNS solution (50 mM in 25 mM Tris buffer, pH 7.4) using UV light (fatty acid analysis). Cardiolipin mass was measured using a phosphorus assay as described (Rouser et al., 1966) using KH2PO4 dissolved in deionized distilled water (0.125–4.0 mM) as standards. The fatty acid composition of cardiolipin was measured by gas liquid chromatography as described (Long et al., 2010). Fatty acid standards were used to identify and quantify the fatty acid components in the samples based on their retention times and concentration factors, respectively.
2.8. Protein Analysis
Protein pellets from nucleotide, phosphagen, and cardiolipin analysis were washed with acetone, dried using a nitrogen evaporator and then re-suspended for 24 hr in 1 N sodium hydroxide. The dissolved pellets were boiled for 5 min and protein content was measured as described (Bradford, 1976).
2.9. Electron Microscopy
Brain tissue was embedded in a plastic mold using 12% gelatin and egg yolk (1:2, by Vol.) then post-fixed in 4% paraformaldehyde/2% glutaraldehyde for 48 hr at 4° C. Transverse 40 µm sections were isolated from the CA3 region of the hippocampus using a Vibratome (Bannockburn, IL), and placed in PBS (pH 7.4) at 4° C. The sections were incubated in 2% osmium tetraoxide in 0.1M PBS at 37° C for 45 min, dehydrated with increasing concentrations of ethanol/propylene oxide, infiltrated overnight in Epon/Araldite embedding medium (Ted Pella), flat mounted between silanized glass slides, then polymerized at 60° C for 72 hr. The Vibratome sections embedded in Epon/Araldite medium were visualized under light microscope to morphologically identify the stratum pyramidale cell bodies in the CA3 region of the hippocampus and were glued to an Epon/Araldite bullet for ultrathin sectioning. Ultrathin sections (90–120 nm) were serial mounted on copper (2 × 1 mm) formvar/carbon stabilized slot grids. The slot grids were stained in a Leica EM AC20 autostainer (Richmond, IL) using 0.5% uranyl acetate for 30 min followed by 7 min in 3% lead citrate with intermittent water rinses. Sections were examined at 60 kV in a Hitachi H-7500 transmission electron microscope (Pleasanton, CA). Neurons were identified based on size, morphology, and presence of large nucleoli and their identity was confirmed by presence of synapses and absence of heterochromatin. Images were collected at 4,000 × and 20,000 × magnification and digitized with an Epson Perfection V750 PRO scanner (Long Beach, CA). Total cytosolic area and mitochondrial number from at least twelve CA3 neurons per animal were quantified. Approximately 10–20 micrographs covering the complete neuronal soma were integrated with minimum overlap using MCID™ Analysis 7.0 software (InterFocus Imaging Ltd, UK). All micrographs were coded and analyzed by a third party blind to the experimental conditions. The results are expressed as total number of mitochondria per 100 µm2 cytosol.
2.10. Statistical Analysis
All the data are expressed as means ± SD with a sample size of 5–10 animals per group. Statistical significance for single dose GTA administration experiments was performed using a Kruskal-Wallis nonparametric ANOVA followed by Dunn’s post hoc test for multiple comparisons. For the 28 day daily acetate supplementation study the treatment group was compared to control using an unpaired, two-tailed, Mann-Whitney U-test. Significance was set at p ≤ 0.05. The statistical analysis was performed using GraphPad InStat statistical software (Ver. 3.10, San Diego, CA).
3. Results
3.1. Single-dose acetate supplementation reduces brain AMP levels without altering other nucleotides
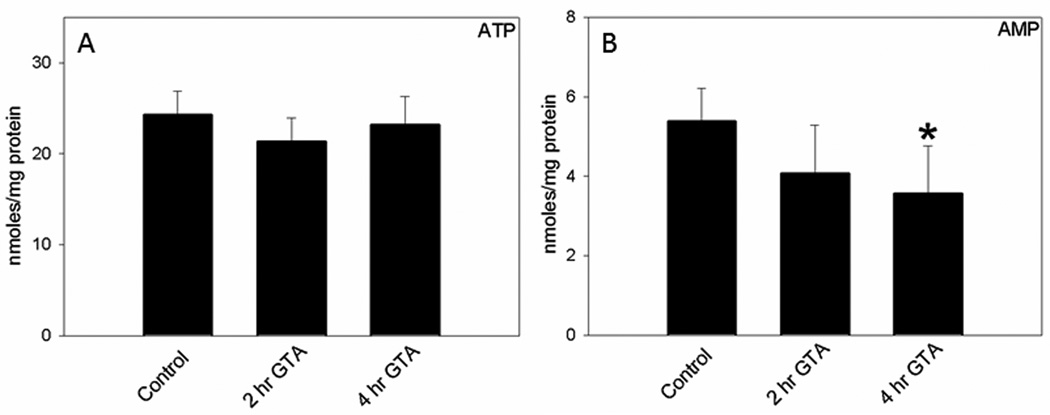
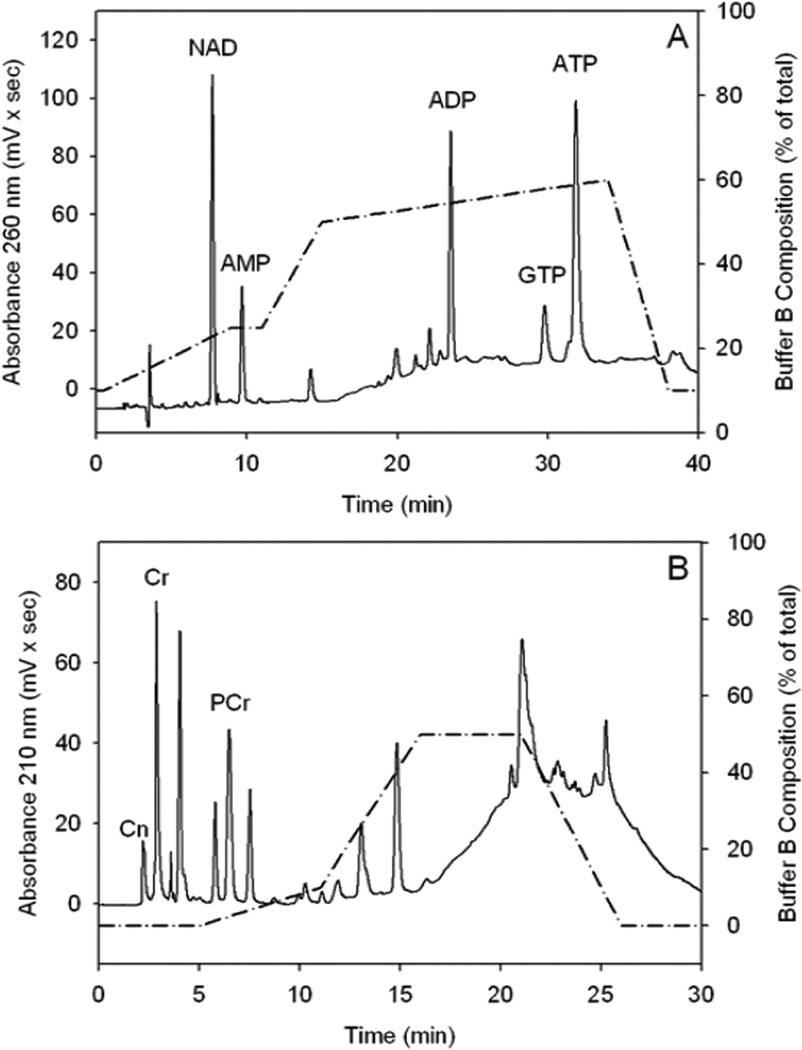
Because acetate supplementation is thought to stimulate brain bioenergetics by increasing acetyl-CoA metabolism, we measured brain nucleotide levels in control rats and rats treated with GTA for 2, and 4 hr. We found no differences in brain ATP (Figure 1A), ADP (15.3±3.4 – 17.9±1.1 nmoles/mg protein), NAD (5.6±0.8 – 6.6±0.7 nmoles/mg protein), or GTP (4.9±0.5 – 5.7±1.0 nmoles/mg protein). The control nucleotide values observed in this study are comparable to the reported values obtained using 6 kW (2 sec) microwave irradiation to stop brain metabolism (Delaney and Geiger, 1996). On the other hand, brain AMP levels were significantly decreased at 4 hr following treatment (Figure 1B). The calculated energy charge ratio [ECR, (ATP + ½ ADP) / (ATP + ADP + AMP)], which incorporates the complete adenylate pool remained unchanged between GTA treated (0.73 ± 0.06) and control rats (0.70 ± 0.02). The lack of changes in the ECR can be explained by the relatively smaller proportion of AMP compared to ATP and ADP. To further substantiate these findings nucleotide stability and recovery studies were performed using radio-labeled ATP, ADP, and AMP. Figure 2, panel A depicts the separation of the different nucleotides from brain tissue samples. The separation was reproducible with average retention times of 9.4 ± 0.2, 14.0 ± 0.2, 23.4 ± 0.1, 29.5 ± 0.2, and 31.5 ± 0.2 min for NAD, AMP, ADP, GTP, and ATP, respectively. Nucleotide standards showed a linear response over the range of 6.25–400 µM and were found to be stable for at least 3 months at 4° C. Radioactively labeled [14C] ATP, [14C] ADP, and [14C] AMP (90, 98, and 97% pure) were used to measure the extraction recovery of adenine nucleotides. Known amounts of radioactivity was added to the sample before extraction, the corresponding peaks after elution were collected, and the nucleotide recovery was measured using liquid scintillation counting (LS 6500, Beckman Coulter, Fullerton, CA). The total nucleotide recovery was 103.4 ± 3.2% and the individual recoveries for ATP, ADP, and AMP were 103.2 ± 2.4%, 103.1 ± 5.1%, and 112.0 ± 3.1% (n=6), respectively. This data suggests that the extraction of nucleotides was complete and extraction loss was minimal.
Figure 1.
Quantification of brain ATP (panel A) and AMP levels (panel B) in control, and GTA-treated rats that were subjected to microwave fixation at 2, and 4 hr post treatment. Data represent mean ± SD (nmol/mg protein), n= 8, 7, and 9 for Control, 2, and 4 hr post GTA treatment, respectively. Statistical analysis was performed using Kruskal-Wallis nonparametric ANOVA followed by Dunn’s post hoc test and the statistical significance is reported comparing treatment duration to control values (*, p ≤ 0.05).
Figure 2.
Representative chromatograms illustrating the elution order of nucleotides (NAD, AMP, ADP, GTP, ATP, Panel A) and phosphagens (Cn, Cr, PCr, Panel B) from microwave-fixed brains. Abbreviations are: ATP, adenosine triphosphate; ADP, adenosine diphosphate; AMP, adenosine monophosphate; GTP, guanosine triphosphate; NAD, nicotinamide adenine dinucleotide; PCr, phosphocreatine; Cr, creatine; and Cn, creatinine. Solid lines represent the absorbance measured at 260 nm (Panel A) and 210 nm (Panel B) and the dashed line represent the gradient of solvent B (percent of total).
3.2. Single-dose acetate supplementation increases brain phosphocreatine levels
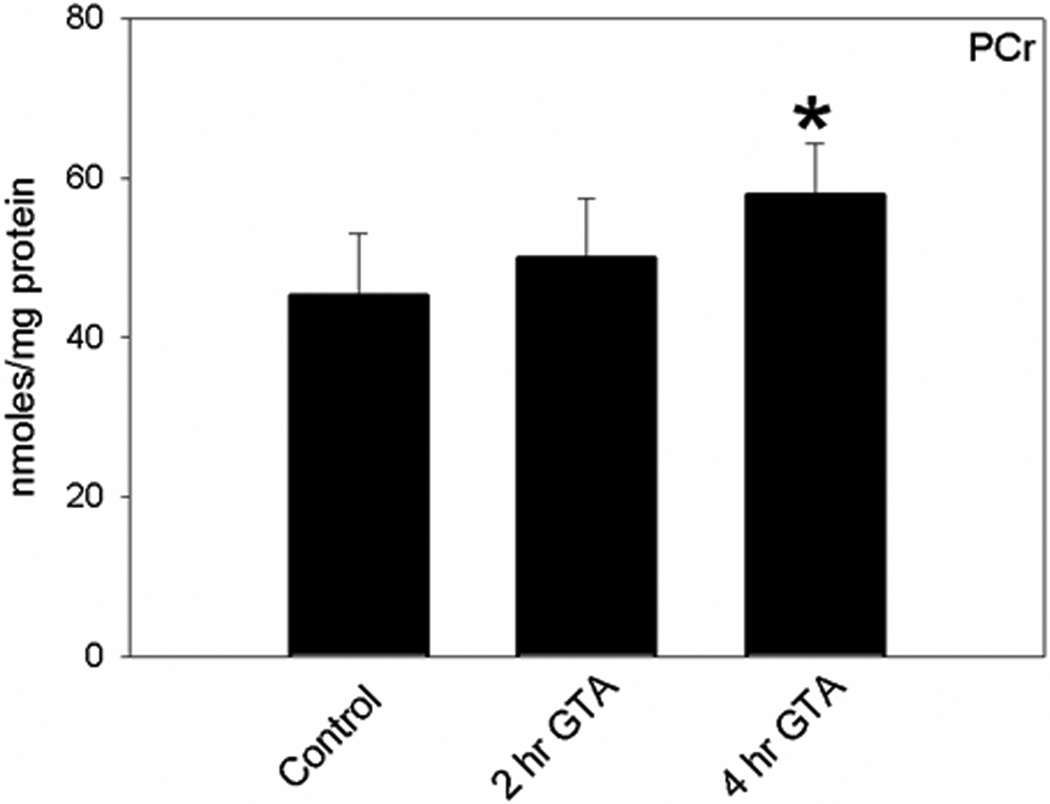
Because adenine nucleotides are tightly regulated by negative feedback mechanisms, under normal conditions their levels remain stable (Wallimann et al., 1992). Therefore, if acetate is utilized in the TCA cycle the generated energy is conserved in the form of PCr (Meyer et al., 1984). Thus, creatinine (Cn), Cr, and PCr (phosphagen) analysis was performed to determine the effect that acetate supplementation has on stimulating brain energy reserves. We found that acetate supplementation significantly increased brain PCr levels (Figure 3A) and the PCr/Cr ratio at 4 hr (0.54±0.07) compared to control (0.39±0.06). We found no difference in the concentration of brain Cr and Cn levels (106.3±13.7 – 119.5±7.7, and 4.0±0.4 – 4.8±1.1 nmoles/mg protein, respectively). In this separation, the concentration of the ion-pairing reagent (TBAP) was reduced to 2.3 mM. The addition of TBAP or the gradient elution did not affect the phosphagen separation. However both were essential to increase the retention time and ensure complete resolution of the phosphagens from sample contamination and other nucleotides (Figure 2, panel B). The averaged retention times of Cn, Cr, and PCr in brain tissue samples were 2.2 ± 0.0, 2.9 ± 0.0 and 6.5 ± 0.0 min, respectively. Phosphagen standards were prepared in water and a linear response was observed over the range of 37.50–1200 µM for Cr and 18.75–600 µM for Cn and PCr.
Figure 3.
Quantification of brain PCr levels in control and GTA-treated rats that were subjected to microwave fixation at 2, and 4 hr post treatment. Abbreviations are: PCr, phosphocreatine. Data represent mean ± SD (nmol/mg protein), n= 8, 7, and 9 for Control, 2, and 4 hr post GTA treatment, respectively. Statistical analysis was performed using Kruskal-Wallis nonparametric ANOVA followed by Dunn’s post hoc test and the statistical significance is reported comparing treatment duration to control values (*, p ≤ 0.05).
3.3. Single dose and long-term acetate supplementation do not alter brain glycogen content
Another energy reserve available to brain is astrocytic glycogen. During acetate supplementation, preferential utilization of acetate by astrocytes may result in reduced glucose utilization or channeling of unused glucose to glycogen synthesis. Under increased metabolic demand astrocytes support neuronal energy metabolism through glycogenolysis and increasing the availability of glucose for neurons (Dinuzzo et al., 2012). Thus, we measured brain glycogen levels after 4 hr of single GTA dose and 28 days of daily GTA administration. We did not find any significant changes in brain glycogen levels with 4 hr (4.02±0.67 µmol/g) and 28 GTA (4.12±0.73 µmol/g) administrations as compared to respective controls (3.78±0.84 and 3.77±0.95 µmol/g). The whole brain glycogen levels obtained in this study are comparable to published values found in other animal models (3.3–12 µmol/g) (Choi and Gruetter, 2003; Cruz and Dienel, 2002; Herzog et al., 2008; Morgenthaler et al., 2009). Regional variation in brain glycogen levels do occur with highest levels found in pons/medulla and cerebellum and lowest in cortex and striatum (Brown, 2004; Herzog et al., 2008; Kong et al., 2002). Grey matter is also known to have higher glycogen content compared to white matter (Brown, 2004).
3.4. Long-term acetate supplementation does not alter hippocampal neuron mitochondrial number or whole brain cardiolipin content
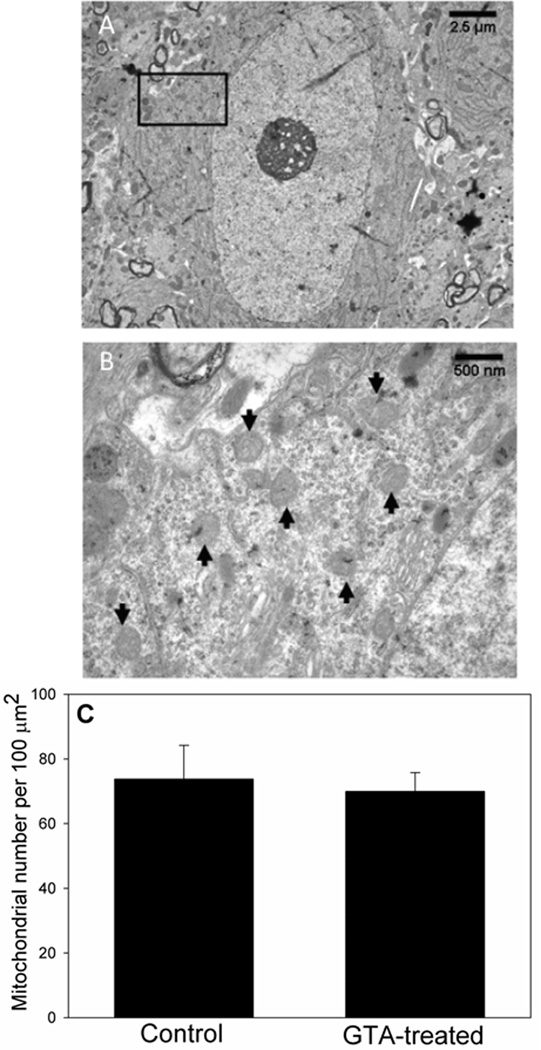
It has been shown that 4 weeks of ketogenic diet treatment in rats increases the number of mitochondria in hippocampal neurons (Bough et al., 2006; Nylen et al., 2009). Since the primary effect of both, ketogenic diet and acetate supplementation is thought to be through increasing acetyl-CoA metabolism we measured the effect that long-term acetate supplementation had on mitochondrial biogenesis. Thus, we quantified mitochondrial number in hippocampal neurons (CA3 region) of rats treated with GTA or water for 28 days using electron microscopy. A representative electron micrograph of a hippocampal neuron in the CA3 region at a magnification of 4,000 × and 20,000 × is shown in Figures 4A and 4B, respectively. Quantification of these images showed that there was no change in the number of neuronal mitochondria per 100 µm2 area when comparing controls to GTA-treated rats (Figure 4C). To further quantify global changes in brain mitochondrial mass, we measured whole brain cardiolipin levels and cardiolipin fatty acid content in rats treated with either water or GTA daily for 28 days. In eukaryotes, cardiolipin is exclusively found in the inner mitochondrial membrane and thus is a marker for mitochondrial biosynthesis (Daum, 1985; Schlame, 2007). We found that treatment did not alter brain cardiolipin content which showed means of 96.9 ± 18.9 and 88.8 ± 20.1 nmol/mg protein in control and treated rats, respectively (Table 1). Further, treatment with GTA did not alter the fatty acid content of mitochondrial cardiolipin as determined using gas-liquid chromatography (Table 1). Collectively, these data suggest that acetate supplementation unlike the ketogenic diet has no effect on neuronal mitochondrial biogenesis.
Figure 4.
Number of mitochondria in hippocampal CA3 neurons in control and rats treated with GTA. Panel A shows a representative electron micrograph of a CA3 neuron at 4,000X, and panel B represents a 20,000 × magnification of that region. Arrows indicate examples of mitochondria contained within this reference panel. Panel C shows the quantification of mitochondria found in a 100 µm2 cytosol area. Data presented as the mean ± SD (n=5). Statistical analysis was performed using two-tailed unpaired Mann-Whitney U-test with the threshold for statistical significance set at p ≤ 0.05.
Table 1.
Brain cardiolipin fatty acid composition and content in control and GTA-treated rats.
| Fatty Acid | Control | GTA-Treated | Control | GTA-Treated |
|---|---|---|---|---|
| nmol/mg protein | Mole % | |||
| Palmitate (16:0) | 34.6 ± 4.4 | 35.9 ± 7.5 | 9.0 ± 2.8 | 9.1 ± 1.5 |
| Stearate (18:0) | 62.2 ± 13.0 | 61.2 ± 10.4 | 15.4 ± 1.8 | 15.7 ± 3.1 |
| Oleate (18:1n-9) | 106.0 ± 30.7 | 114.8 ± 28.7 | 25.7 ± 4.6 | 28.4 ± 3.3 |
| Linoleate (18:2n-6) | 23.3 ± 6.4 | 23.0 ± 8.0 | 5.8 ± 1.2 | 5.6 ± 1.5 |
| γ- linolenate (18:3n-6) | 5.5 ± 0.9 | 4.9 ± 0.8 | 1.4 ± 0.4 | 1.3 ± 0.4 |
| Eicosenoate (20:1n-9) | 10.1 ± 3.0 | 9.0 ± 2.1 | 2.6 ± 0.8 | 2.3 ± 0.5 |
| Dihomo-γ-linolenate (20:3n-6) | 5.0 ± 1.2 | 4.9 ± 1.1 | 1.2 ± 0.2 | 1.2 ± 0.1 |
| Arachidonate (20:4n-6) | 53.7 ± 23.7 | 54.8 ± 20.0 | 12.7 ± 4.9 | 13.3 ± 3.2 |
| Lignocerate (24:0) | 4.5 ± 2.1 | 4.3 ± 1.5 | 1.0 ± 0.4 | 1.1 ± 0.5 |
| Adrenate (22:4n-6) | 8.2 ± 3.1 | 6.1 ± 3.3 | 2.0 ± 0.5 | 1.5 ± 0.6 |
| Nervonate (24:1n-9) | 4.2 ± 0.7 | 3.8 ± 0.7 | 1.1 ± 0.2 | 1.0 ± 0.2 |
| Docosahexaenoate (22:6n-3) | 20.0 ± 9.4 | 18.5 ± 8.0 | 4.6 ± 2.0 | 4.5 ± 1.4 |
| Total fatty acid content | 404.5 ± 75.9 | 399.3 ± 79.5 | ||
| Cardiolipin content | 96.6 ± 18.9 | 88.8 ± 20.1 | ||
Values represent the means ± SD from control (n = 6) and GTA-treated (n = 10) rats in units of nmol/mg protein and mole percent.
4. Discussion
Acetate supplementation increases plasma acetate by 100-fold (19.3±4.72 mM) in rats (Reisenauer et al., 2011) and brain acetate by 17-fold (8.5 µmol/g brain ~10.6 mM) in mice (Mathew et al., 2005) treated with a single oral dose of 6 and 5.8 g/Kg body weight glyceryl triacetate, respectively. Acetate crosses the blood brain barrier (Deelchand et al., 2009) and is preferentially taken up by astrocytes through facilitated transport via monocarboxylate transporters (MCT) (Waniewski and Martin, 1998) prior to activation to acetyl-CoA by either cytosolic or mitochondrial acetyl-CoA synthetase enzymes (Hallows et al., 2006). In support of this, acetate supplementation increases brain acetyl-CoA levels by 2.2-fold (5.7±1.9 µg/g tissue ~8.7±2.9 µM) within 30 min and remains elevated out to 4 hr following treatment (Reisenauer et al., 2011). Acetyl-CoA is a substrate for various pathways including fatty acid synthesis, protein acetylation, and the TCA cycle. Regarding protein acetylation, single and multiple oral doses of GTA increase acetylation of histone and non-histone proteins that in turn alters inflammatory gene expression (Soliman and Rosenberger, 2011; Soliman et al., 2012b; Soliman et al., 2012c). However, the influence of acetate supplementation on brain energy stores has not been tested. In this study we measured the effect that a single and multiple oral doses of glyceryl triacetate had on brain nucleotides, phosphagens, and mitochondrial biogenesis, respectively. We found that 4 hr after oral GTA administration, brain PCr levels were increased significantly compared to controls (Figure 3), consistent with the increase in brain acetyl-CoA. However, we did not observe changes in brain ATP (Figure 1A), ADP, GTP, or NAD levels (data not shown).
The metabolic half-life of ATP is only a few milliseconds and is controlled by a balance of cellular energy supply and demand (Erecinska and Silver, 1989). In our experiments, rats were not challenged by noxious stimuli nor was there an increase in the energy demand due to disease pathology. Thus, we speculate that the energy generated as a result of mitochondrial acetyl-CoA metabolism would be stored in the form of PCr. ATP and other adenylate metabolites are key regulators of metabolic pathways that maintain cellular homeostasis and thus their levels are tightly regulated (Wallimann et al., 1992). Phosphocreatine, on the other hand is metabolically inert with regard to biochemical regulation and serves as a reservoir of energy (Wallimann et al., 1992). During increased metabolic demand or diseased states, the brain can utilize PCr to maintain ATP levels (Meyer et al., 1984). Following injury, a decrease in PCr levels precedes ATP loss (Lowry et al., 1964; Norberg et al., 1975) and thus it is important to monitor and maintain both ATP and PCr levels. In this regard, acetate supplementation partly restores brain ATP levels in a rat model of traumatic brain injury (Arun et al., 2010), however PCr levels were not measured in this study and ATP restoration may be secondary to the neuroprotective effects of acetate. This study substantiates these findings by demonstrating that acetate supplementation can directly influence basal PCr levels. Since labeled acetate is known to enter the glial glutamine pool it may be argued that the observed changes in PCr levels in this study predominantly reflect changes in astrocytes. However, mitochondrial creatine kinase, the enzyme responsible for PCr formation is selectively expressed in neurons but not in astrocytes whereas the cytosolic form of creatine kinase is expresses by both cell types (Tachikawa et al., 2004). Thus analysis of changes in PCr levels in individual cell types in vitro is required to determine which cell type contributes toward an increase in PCr levels.
After 4 hr of acetate supplementation there was a modest but significant reduction in brain AMP levels (Figure 1B) which coincide with the increase in PCr levels. The creatine-phosphate shuttle is responsible for the transfer of high energy phosphate between PCr/Cr and ATP/ADP, however AMP is not involved in this transfer (Meyer et al., 1984). Since we did not observe significant changes in the ATP, ADP or Cr levels, there appears to be a discrepancy between increases in PCr levels and its biochemical relationship to other components of the creatine kinase system. This may partially be explained by increase in free [Mg2+] as a result of ATP hydrolysis induced by microwave irradiation (Srivastava et al., 2012) and subsequent changes in the equilibrium constant of the creatine kinase reaction (Lawson and Veech, 1979). Nonetheless, given the increase in PCr and the tight control over cellular ATP and ADP levels under normal conditions suggests that the reduction in AMP may be the result of an overall increase in cellular energy supply. Alternatively, increased acetyl-CoA may result in a situation where AMP can be metabolized to adenosine. In brain, adenosine levels are in the picomolar range while AMP levels are in the nanomolar range (Delaney and Geiger, 1996), which suggests that a small change in AMP can lead to a dramatic increase in brain adenosine. Further, it has been demonstrated that acetate uptake in neurons induces intracellular acidosis resulting in stimulation of the sodium hydrogen exchanger and the sodium potassium ATPase which consume ATP and result in adenosine release (Zamzow et al., 2006). Adenosine is known for its anti-inflammatory and neuroprotective properties (Cunha, 2005; Ribeiro, 2005). In this regard, acetate supplementation attenuates neuroglia activation and prevents loss of choline-acetyl transferase immunoreactivity in rats subjected to neuroinflammation (Reisenauer et al., 2011), suggesting that increasing mitochondrial energy reserves and adenosine levels may contribute to the neuroprotective and anti-inflammatory effects of acetate supplementation. Since acetyl-CoA is a ubiquitous substrate for various biochemical pathways there can be multiple mechanisms by which acetate supplementation can attenuate inflammation and offer neuroprotection. Although increase in energy is beneficial, a direct link to the anti-inflammatory effect of acetate supplementation remains unknown. In brain, ATP and adenosine are known to have a role in modulating inflammation through purinergic receptor signaling (Di Virgilio et al., 2009). Altered purine levels and receptor expression have the potential of modulating purinergic signaling which can disrupt the inflammatory response. Thus, understanding the effects that acetate supplementation has on purinergic signaling will allow us to integrate acetate-induced changes in energy metabolism with the potential to alter purinergic signaling. Future studies exploring the link between reduced AMP levels and alterations in purinergic signaling are required to test this hypothesis.
An alternative energy reserve available to brain is glycogen, which is primarily localized in astrocytes as cytoplasmic granules. Although brain glycogen is primarily localized in astrocytes the enzyme glycogen synthase involved in glycogen synthesis is expressed in neurons as well as astrocytes. The expression of glycogen phosphorylase, the enzyme responsible for glycogen breakdown, is primarily astrocytic (Brown, 2004). Since acetate is preferentially utilized by astrocytes it is possible that acetate utilization may divert glucose towards glycogen synthesis in astrocytes or spare glucose for neuronal utilization. During insulin-induced hypoglycemic conditions, brain glycogen levels fall in areas with high metabolic rate, suggesting that brain glycogen is used to support brain energetics (Brown, 2004). Thus we measured brain glycogen levels in animals treated with GTA for 4 hr and for 28 days. Interestingly, single dose and long-term acetate supplementation did not alter brain glycogen levels. Estimated glycogen turnover rates are between 5–10 hr (Choi and Gruetter, 2003; Morgenthaler et al., 2009) which explains the absence of changes in glycogen levels with 4 hr of GTA treatment. Further a 28 day treatment regimen is enough to observe any significant changes in glycogen synthesis. However absence of changes in glycogen with long term-treatment suggests that GTA does not affect brain glycogen synthesis or during this long period the reserve energy is utilized for other biochemical pathways. Same is true with acetate utilization, since acetyl-CoA is a ubiquitous intermediate it may be utilized in other metabolic pathways like protein acetylation. In this regard we have shown that acetate supplementation increases global brain histone acetylation in vivo and in vitro in astrocyte and microglial cultures (Soliman et al., 2012a; Soliman et al., 2012b). Nonetheless, acetate utilization by astrocytes may reduce glial glucose utilization and increase neuronal glucose availability.
Alternatively, it is possible that under significantly high acetate levels achieved with acetate supplementation, neurons may utilize acetate for energy production. The molecular basis for astrocyte-specific uptake of acetate has only been tested by two studies with conflicting results. Rat cortical astrocytes generate labeled CO2 18 times faster than cortical synaptosomes suggesting that acetate is preferentially utilized by astrocytes compared to neurons (Waniewski and Martin, 1998). Differences in acetate utilization pathways between the two cell types may be due to the synthesis of alternative TCA cycle intermediates in astrocytes verses neurons or concentration dependent selective acetate uptake by astrocytes via monocarboxylic acid-like transporters. Neurons are known to express monocarboxylic acid transporters (Pierre and Pellerin, 2005) and the activity of acetyl-CoA synthetase, the enzyme responsible for acetate utilization, is higher in synaptosomes (Waniewski and Martin, 1998) suggesting that neurons can also utilize acetate. This is substantiated by studies demonstrating that neuronal MCT2 transports acetate with affinities comparable to that of astrocytic MCT1 (Km ~1.6–2.5 mM) (Rae et al., 2012), although acetate transport by MCT2 may be limited in presence of lactate similar to that found with the sodium-dependent MCT1 (Moschen et al., 2012). On the other hand, Brand et al. demonstrated that acetate can be metabolized by both the cell types and that in neurons acetate-derived labeled carbon is recovered as glutamate and aspartate while in astrocytes as glutamine (Brand et al., 1997). The major difference between the experimental paradigms of the two studies is the concentration of labeled acetate used, 0.2 and 5 mM, respectively. Moreover, in vivo glial metabolism studies utilize labeled acetate at either physiological (~0.2 mM) or much lower levels which are well below normal glucose levels where acetate utilization is primarily astrocytic. Brain acetate levels achieved with GTA administration are approximately 10 mM (8.5 µmol/g brain) (Mathew et al., 2005), twice the normal glucose levels (5 mM). Thus, at such high acetate concentrations the excess acetate may be utilized by neurons, considering that synaptosomes have significantly higher acetyl-CoA synthetase activity compared to astrocytes (Waniewski and Martin, 1998). Therefore, at this point it is unclear whether the in vivo effects of acetate supplementation observed in the current study are predominantly astrocytic or neuronal. Future, in vitro studies measuring the effects of acetate on individual cell types will allow us to make the necessary distinction.
Because acetate supplementation and ketogenic diet share a common metabolic intermediate, acetyl-CoA, it is important to understand the influence acetate supplementation has on mitochondrial biogenesis. Ketogenic diet being a high-fat low-carbohydrate diet enhances hepatic β-oxidation of free fatty acids and increases circulating levels of β-hydroxy butyrate (BHB), the predominant ketone body, from 0.2 mM to 3.9 mM in mouse plasma (Yudkoff et al., 2005). In brain, the uptake of ketones across the BBB is mediated by monocarboxylic acid transporters where they are taken up by both neurons and astrocytes (Cremer, 1971; Melo et al., 2006). Once inside the cell, BHB bypasses the glycolytic steps enters the mitochondria and is converted into acetoacetate, acetoacetyl-CoA, and finally acetyl-CoA based on NAD+ availability (White and Venkatesh, 2011). Ketone bodies formed as a result of 4 weeks on a ketogenic regimen increase the number of mitochondria in hippocampal neurons (Bough et al., 2006). However, we found that a 28 day treatment with GTA did not alter hippocampal CA3 neuronal mitochondrial number (Figure 4). Post-synaptic inter-neurons in the CA3 region receive axonal input from entorhinal cortex and dentate gyrus and relay information to CA1 cell bodies. Since acetate is preferentially utilized by glia (Waniewski and Martin, 1998), absence of changes in neuronal mitochondrial number does not exclude changes in astrocytic mitochondria. Thus to detect global changes in mitochondrial mass, we quantified cardiolipin levels and cardiolipin fatty acid content in whole brain. However, we did not find any significant changes in whole brain mitochondrial mass or cardiolipin fatty acid composition (Table 1). This suggests that acetate supplementation does not significantly alter the synthesis of new mitochondria in whole brain however changes in their size and distribution in specific brain compartment cannot be ruled out. The current study was focused at comparing the known effects of ketogenic diet on mitochondrial biogenesis in hippocampal neurons with that of acetate supplementation. However, an in depth investigation of how acetate supplementation affects mitochondrial biogenesis in astrocytes, considering acetate is preferentially utilized by astrocytes, is required.
One of the proposed mechanisms by which the ketogenic diet increases mitochondrial biogenesis is activation and increased expression of the transcription factor, peroxisome proliferator-activated receptor gamma co-activator 1 alpha (PGC-1α) (Wallace et al., 2010). PGC-1α is considered as the master regulator of mitochondrial biogenesis and is regulated by reversible acetylation. Sirtuin 1 (Sirt1) is a NAD+-dependent cytosolic protein deacetylase that activates PGC-1α by removing the acetyl group (Rodgers et al., 2005). Since ketone bodies are utilized exclusively inside the mitochondria they spare cytosolic NAD+ which is normally consumed during glycolytic metabolism (Wallace et al., 2010). This activates Sirt1 resulting in deacetylation of PGC-1α and induction of mitochondrial biogenesis. Further, limited carbohydrate availability with ketogenic diet reduces insulin levels and enhances glucagon secretion both of which through independent signaling pathways up-regulate PGC-1α expression and thus induce mitochondrial biogenesis (Wallace et al., 2010). Acetate supplementation, on the other hand, does not reduce carbohydrate availability, elevates cytoplasmic and mitochondrial acetyl-CoA levels, and as suggested earlier may increase neuronal glucose availability. Thus, cytosolic acetyl-CoA through protein acetylation may maintain the normal acetylation state of PGC-1α and prevent induction of mitochondrial biogenesis. Although acetate like ketone bodies may also spare cytosolic NAD+ and activate Sirt1, Sirt1-mediated deacetylation of acetyl-CoA synthetase would further facilitate acetyl-CoA formation (Wilhelm and Hirrlinger, 2012) and maintain normal mitochondrial biogenesis. The exact mechanisms that regulate acetate utilization in different cellular compartments are still unclear however this may partially explain the differential effects of ketogenic diet and acetate supplementation on mitochondrial biogenesis. Therefore, while the two treatments share similarity at increasing acetyl-CoA levels this study suggests that acetate supplementation unlike the ketogenic diet does not result in mitochondrial biogenesis.
The accurate determination of high-energy phosphates requires rapid brain fixation to instantaneously stop metabolism. Head-focused microwave irradiation is a quick technique to rapidly inactivate enzymes while preserving the structural integrity of the brain. In this study we used 6 kW output power with a 2 sec exposure, as described (Delaney and Geiger, 1996). Consequently, the nucleotide content obtained in this study closely resembles the reported values obtained using comparable microwave conditions (Delaney and Geiger, 1996; Gupta and Dettbarn, 2003). Nonetheless, brain ATP levels obtained by microwave fixation are lower, and ADP and AMP levels are subsequently higher than those obtained by freeze blowing or in-situ funnel freezing techniques (Ponten et al., 1973; Veech et al., 1973). In addition to differential inactivation of nucleotide metabolizing enzymes (Nelson, 1973), microwave irradiation can directly break the high energy phosphate bond in ATP and result in an approximate 30 % ATP loss when compared to the freeze blowing methods (Srivastava et al., 2012). The phosphate bond in PCr is however stable to thermal degradation and creatine kinase, the enzyme that converts PCr to creatine, is efficiently inactivated by microwave irradiation (Nelson, 1973) resulting in PCr values similar to those obtained with freeze blowing (Srivastava et al., 2012).
In conclusion, acetate supplementation increases brain PCr and reduces brain AMP levels with no effect on other nucleotide and glycogen levels or neuronal mitochondrial number. These data suggest that brain can utilize acetate for energy production, and in contrast to the ketogenic diet long-term acetate supplementation has no effect on neuronal mitochondrial biogenesis.
Highlights.
Direct evidence for acetate supplementation stimulating brain bioenergetics
Following 4 hr of acetate supplementation rat brain phosphocreatine levels increase
In parallel it reduces brain AMP levels without altering other nucleotides
Long-term acetate supplementation does not alter mitochondrial biogenesis
Acknowledgements
This publication was made possible by a Grant from the National Center for Research Resources (NCRR), a component of the National Institute of Health (NIH) (# P20RR017699).
Abbreviations
- GTA
glyceryl triacetate
- TCA
tricarboxylic acid
- ECR
energy charge ratio
- PCr
phosphocreatine
- Cr
creatine
- Cn
creatinine
- PBS
phosphate buffered saline
- TBAP
tetrabutylammonium phosphate
- KH2PO4
mono-basic potassium phosphate
- HPLC
high performance liquid chromatography
- TLC
thin layer chromatography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interests with the publication of this manuscript.
References
- Arun P, Ariyannur PS, Moffett JR, Xing G, Hamilton K, Grunberg NE, Ives JA, Namboodiri AM. Metabolic acetate therapy for the treatment of traumatic brain injury. J Neurotrauma. 2010;27:293–298. doi: 10.1089/neu.2009.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestrino M, Lensman M, Parodi M, Perasso L, Rebaudo R, Melani R, Polenov S, Cupello A. Role of creatine and phosphocreatine in neuronal protection from anoxic and ischemic damage. Amino Acids. 2002;23:221–229. doi: 10.1007/s00726-001-0133-3. [DOI] [PubMed] [Google Scholar]
- Balestrino M, Rebaudo R, Lunardi G. Exogenous creatine delays anoxic depolarization and protects from hypoxic damage: dose-effect relationship. Brain Res. 1999;816:124–130. doi: 10.1016/s0006-8993(98)01131-7. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, Shaw R, Smith Y, Geiger JD, Dingledine RJ. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60:223–235. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brand A, Richter-Landsberg C, Leibfritz D. Metabolism of acetate in rat brain neurons, astrocytes and cocultures: metabolic interactions between neurons and glia cells, monitored by NMR spectroscopy. Cell Mol Biol. 1997;43:645–657. [PubMed] [Google Scholar]
- Brewer GJ, Wallimann TW. Protective effect of the energy precursor creatine against toxicity of glutamate and beta-amyloid in rat hippocampal neurons. J Neurochem. 2000;74:1968–1978. doi: 10.1046/j.1471-4159.2000.0741968.x. [DOI] [PubMed] [Google Scholar]
- Brissette CA, Houdek HM, Floden AM, Rosenberger TA. Acetate supplementation reduces microglia activation and brain interleukin-1beta levels in a rat model of Lyme neuroborreliosis. J Neuroinflammation. 2012;9:249. doi: 10.1186/1742-2094-9-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM. Brain glycogen re-awakened. J Neurochem. 2004;89:537–552. doi: 10.1111/j.1471-4159.2004.02421.x. [DOI] [PubMed] [Google Scholar]
- Choi IY, Gruetter R. In vivo 13C NMR assessment of brain glycogen concentration and turnover in the awake rat. Neurochem Int. 2003;43:317–322. doi: 10.1016/s0197-0186(03)00018-4. [DOI] [PubMed] [Google Scholar]
- Cremer JE. Incorporation of label from D-β-hydroxy(14 C)butyrate and (3-14 C)acetoacetate into amino acids in rat brain in vivo. Biochem J. 1971;122:135–138. doi: 10.1042/bj1220135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz NF, Dienel GA. High glycogen levels in brains of rats with minimal environmental stimuli: implications for metabolic contributions of working astrocytes. J Cereb Blood Flow Metab. 2002;22:1476–1489. doi: 10.1097/01.WCB.0000034362.37277.C0. [DOI] [PubMed] [Google Scholar]
- Cunha RA. Neuroprotection by adenosine in the brain: From A(1) receptor activation to A (2A) receptor blockade. Purinergic Signal. 2005;1:111–134. doi: 10.1007/s11302-005-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum G. Lipids of mitochondria. Biochim Biophys Acta. 1985;822:1–42. doi: 10.1016/0304-4157(85)90002-4. [DOI] [PubMed] [Google Scholar]
- Deelchand DK, Shestov AA, Koski DM, Ugurbil K, Henry PG. Acetate transport and utilization in the rat brain. J Neurochem. 2009;109(Suppl 1):46–54. doi: 10.1111/j.1471-4159.2009.05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney SM, Geiger JD. Brain regional levels of adenosine and adenosine nucleotides in rats killed by high-energy focused microwave irradiation. J Neurosci Methods. 1996;64:151–156. doi: 10.1016/0165-0270(95)00119-0. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Ceruti S, Bramanti P, Abbracchio MP. Purinergic signalling in inflammation of the central nervous system. Trends Neurosci. 2009;32:79–87. doi: 10.1016/j.tins.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Dinuzzo M, Mangia S, Maraviglia B, Giove F. The role of astrocytic glycogen in supporting the energetics of neuronal activity. Neurochem Res. 2012;37:2432–2438. doi: 10.1007/s11064-012-0802-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnett M, Harris RC, Orme CE. Reverse-phase ion-pairing high-performance liquid chromatography of phosphocreatine, creatine and creatinine in equine muscle. Scand J Clin Lab Invest. 1991;51:137–141. doi: 10.1080/00365519109091099. [DOI] [PubMed] [Google Scholar]
- Erecinska M, Silver IA. ATP and brain function. J Cereb Blood Flow Metab. 1989;9:2–19. doi: 10.1038/jcbfm.1989.2. [DOI] [PubMed] [Google Scholar]
- Gupta RC, Dettbarn WD. Prevention of kainic acid seizures-induced changes in levels of nitric oxide and high-energy phosphates by 7-nitroindazole in rat brain regions. Brain Res. 2003;981:184–192. doi: 10.1016/s0006-8993(03)03034-8. [DOI] [PubMed] [Google Scholar]
- Hallows WC, Lee S, Denu JM. Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc Natl Acad Sci U S A. 2006;103:10230–10235. doi: 10.1073/pnas.0604392103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer DF, Unverferth DV, Kelley RE, Harvan PA, Altschuld RA. Extraction and measurement of myocardial nucleotides, nucleosides, and purine bases by high-performance liquid chromatography. Anal Biochem. 1988;169:300–305. doi: 10.1016/0003-2697(88)90288-6. [DOI] [PubMed] [Google Scholar]
- Herzog RI, Chan O, Yu S, Dziura J, McNay EC, Sherwin RS. Effect of acute and recurrent hypoglycemia on changes in brain glycogen concentration. Endocrinology. 2008;149:1499–1504. doi: 10.1210/en.2007-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch FL. Cardiolipins and biomembrane function. Biochim Biophys Acta. 1992;1113:71–133. doi: 10.1016/0304-4157(92)90035-9. [DOI] [PubMed] [Google Scholar]
- Houtkooper RH, Vaz FM. Cardiolipin, the heart of mitochondrial metabolism. Cell Mol Life Sci. 2008;65:2493–2506. doi: 10.1007/s00018-008-8030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klivenyi P, Ferrante RJ, Matthews RT, Bogdanov MB, Klein AM, Andreassen OA, Mueller G, Wermer M, Kaddurah-Daouk R, Beal MF. Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nat Med. 1999;5:347–350. doi: 10.1038/6568. [DOI] [PubMed] [Google Scholar]
- Kong J, Shepel PN, Holden CP, Mackiewicz M, Pack AI, Geiger JD. Brain glycogen decreases with increased periods of wakefulness: implications for homeostatic drive to sleep. J Neurosci. 2002;22:5581–5587. doi: 10.1523/JNEUROSCI.22-13-05581.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson JW, Veech RL. Effects of pH and free Mg2+ on the Keq of the creatine kinase reaction and other phosphate hydrolyses and phosphate transfer reactions. J Biol Chem. 1979;254:6528–6537. [PubMed] [Google Scholar]
- Long EK, Rosenberger TA, Picklo MJ., Sr Ethanol withdrawal increases glutathione adducts of 4-hydroxy-2-hexenal but not 4-hydroxyl-2-nonenal in the rat cerebral cortex. Free Radic Biol Med. 2010;48:384–390. doi: 10.1016/j.freeradbiomed.2009.10.048. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV, Hasselberger FX, Schulz DW. Effect of Ischemia on Known Substrates and Cofactors of the Glycolytic Pathway in Brain. J Biol Chem. 1964;239:18–30. [PubMed] [Google Scholar]
- Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev. 2009;59:293–315. doi: 10.1016/j.brainresrev.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R, Arun P, Madhavarao CN, Moffett JR, Namboodiri MA. Progress toward acetate supplementation therapy for Canavan disease: glyceryl triacetate administration increases acetate, but not N-acetylaspartate, levels in brain. J Pharmacol Exp Ther. 2005;315:297–303. doi: 10.1124/jpet.105.087536. [DOI] [PubMed] [Google Scholar]
- Matthews RT, Ferrante RJ, Klivenyi P, Yang L, Klein AM, Mueller G, Kaddurah-Daouk R, Beal MF. Creatine and cyclocreatine attenuate MPTP neurotoxicity. Exp Neurol. 1999;157:142–149. doi: 10.1006/exnr.1999.7049. [DOI] [PubMed] [Google Scholar]
- Matthews RT, Yang L, Jenkins BG, Ferrante RJ, Rosen BR, Kaddurah-Daouk R, Beal MF. Neuroprotective effects of creatine and cyclocreatine in animal models of Huntington's disease. J Neurosci. 1998;18:156–163. doi: 10.1523/JNEUROSCI.18-01-00156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo TM, Nehlig A, Sonnewald U. Neuronal-glial interactions in rats fed a ketogenic diet. Neurochem Int. 2006;48:498–507. doi: 10.1016/j.neuint.2005.12.037. [DOI] [PubMed] [Google Scholar]
- Menzies RA, Gold PH. The turnover of mitochondria in a variety of tissues of young adult and aged rats. J Biol Chem. 1971;246:2425–2429. [PubMed] [Google Scholar]
- Meyer RA, Sweeney HL, Kushmerick MJ. A simple analysis of the "phosphocreatine shuttle". Am J Physiol. 1984;246:C365–C377. doi: 10.1152/ajpcell.1984.246.5.C365. [DOI] [PubMed] [Google Scholar]
- Minchin MC, Beart PM. Compartmentation of amino acid metabolism in the rat dorsal root ganglion; a metabolic and autoradiographic study. Brain Res. 1975;83:437–449. doi: 10.1016/0006-8993(75)90835-5. [DOI] [PubMed] [Google Scholar]
- Morais VA, De Strooper B. Mitochondria dysfunction and neurodegenerative disorders: cause or consequence. J Alzheimers Dis. 2010;20(Suppl 2):S255–S263. doi: 10.3233/JAD-2010-100345. [DOI] [PubMed] [Google Scholar]
- Morgenthaler FD, Lanz BR, Petit JM, Frenkel H, Magistretti PJ, Gruetter R. Alteration of brain glycogen turnover in the conscious rat after 5h of prolonged wakefulness. Neurochem Int. 2009;55:45–51. doi: 10.1016/j.neuint.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschen I, Broer A, Galic S, Lang F, Broer S. Significance of short chain fatty acid transport by members of the monocarboxylate transporter family (MCT) Neurochem Res. 2012;37:2562–2568. doi: 10.1007/s11064-012-0857-3. [DOI] [PubMed] [Google Scholar]
- Nelson SR. Effects of microwave irradiation on enzymes and metabolites in mouse brain. Radiat Res. 1973;55:153–159. [PubMed] [Google Scholar]
- Nisoli E, Carruba MO. Nitric oxide and mitochondrial biogenesis. J Cell Sci. 2006;119:2855–2862. doi: 10.1242/jcs.03062. [DOI] [PubMed] [Google Scholar]
- Norberg K, Quistorff B, Siesjo BK. Effects of hypoxia of 10–45 seconds duration on energy metabolism in the cerebral cortex of unanesthetized and anesthetized rats. Acta Physiol Scand. 1975;95:301–310. doi: 10.1111/j.1748-1716.1975.tb10054.x. [DOI] [PubMed] [Google Scholar]
- Novelli A, Reilly JA, Lysko PG, Henneberry RC. Glutamate becomes neurotoxic via the N-methyl-D-aspartate receptor when intracellular energy levels are reduced. Brain Res. 1988;451:205–212. doi: 10.1016/0006-8993(88)90765-2. [DOI] [PubMed] [Google Scholar]
- Nylen K, Velazquez JL, Sayed V, Gibson KM, Burnham WM, Snead OC., 3rd The effects of a ketogenic diet on ATP concentrations and the number of hippocampal mitochondria in Aldh5a1(-/-) mice. Biochim Biophys Acta. 2009;1790:208–212. doi: 10.1016/j.bbagen.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettegrew JW, Panchalingam K, Klunk WE, McClure RJ, Muenz LR. Alterations of cerebral metabolism in probable Alzheimer's disease: a preliminary study. Neurobiol Aging. 1994;15:117–132. doi: 10.1016/0197-4580(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Pierre K, Pellerin L. Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J Neurochem. 2005;94:1–14. doi: 10.1111/j.1471-4159.2005.03168.x. [DOI] [PubMed] [Google Scholar]
- Ponten U, Ratcheson RA, Salford LG, Siesjo BK. Optimal freezing conditions for cerebral metabolites in rats. J Neurochem. 1973;21:1127–1138. doi: 10.1111/j.1471-4159.1973.tb07567.x. [DOI] [PubMed] [Google Scholar]
- Radin NS. Extraction of tissue lipids with a solvent of low toxicity. Methods Enzymol. 1981;72:5–7. doi: 10.1016/s0076-6879(81)72003-2. [DOI] [PubMed] [Google Scholar]
- Rae C, Fekete AD, Kashem MA, Nasrallah FA, Broer S. Metabolism, compartmentation, transport and production of acetate in the cortical brain tissue slice. Neurochem Res. 2012;37:2541–2553. doi: 10.1007/s11064-012-0847-5. [DOI] [PubMed] [Google Scholar]
- Reisenauer CJ, Bhatt DP, Mitteness DJ, Slanczka ER, Gienger HM, Watt JA, Rosenberger TA. Acetate supplementation attenuates lipopolysaccharide-induced neuroinflammation. J Neurochem. 2011;117:264–274. doi: 10.1111/j.1471-4159.2011.07198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro JA. What can adenosine neuromodulation do for neuroprotection? Curr Drug Targets CNS Neurol Disord. 2005;4:325–329. doi: 10.2174/1568007054546090. [DOI] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Rouser G, Siakotos AN, Fleischer S. Quantitative analysis of phospholipids by thin-layer chromatography and phosphorus analysis of spots. Lipids. 1966;1:85–86. doi: 10.1007/BF02668129. [DOI] [PubMed] [Google Scholar]
- Schlame M. Assays of cardiolipin levels. Methods Cell Biol. 2007;80:223–240. doi: 10.1016/S0091-679X(06)80011-7. [DOI] [PubMed] [Google Scholar]
- Soliman M, Rosenberger TA. Acetate supplementation increases brain histone acetylation and inhibits histone deacetylase activity and expression. Mol Cell Biochem. 2011;352:173–180. doi: 10.1007/s11010-011-0751-3. [DOI] [PubMed] [Google Scholar]
- Soliman ML, Combs CK, Rosenberger TA. Modulation of inflammatory cytokines and mitogen-activated protein kinases by acetate in primary astrocytes. J Neuroimmune Pharmacol. 2012a doi: 10.1007/s11481-012-9426-4. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman ML, Puig KL, Combs CK, Rosenberger TA. Acetate reduces microglia inflammatory signaling in vitro. J Neurochem. 2012b;123:555–567. doi: 10.1111/j.1471-4159.2012.07955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman ML, Smith MD, Houdek HM, Rosenberger TA. Acetate supplementation modulates brain histone acetylation and decreases interleukin-1beta expression in a rat model of neuroinflammation. J Neuroinflammation. 2012c;9:51. doi: 10.1186/1742-2094-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Kashiwaya Y, Chen X, Geiger JD, Pawlosky R, Veech RL. Microwave irradiation decreases ATP, increases free [Mg(2+)], and alters in vivo intracellular reactions in rat brain. J Neurochem. 2012;123:668–675. doi: 10.1111/jnc.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachikawa M, Fukaya M, Terasaki T, Ohtsuki S, Watanabe M. Distinct cellular expressions of creatine synthetic enzyme GAMT and creatine kinases uCK-Mi and CK-B suggest a novel neuron-glial relationship for brain energy homeostasis. Eur J Neurosci. 2004;20:144–160. doi: 10.1111/j.1460-9568.2004.03478.x. [DOI] [PubMed] [Google Scholar]
- Veech RL, Harris RL, Veloso D, Veech EH. Freeze-blowing: a new technique for the study of brain in vivo. J Neurochem. 1973;20:183–188. doi: 10.1111/j.1471-4159.1973.tb12115.x. [DOI] [PubMed] [Google Scholar]
- Wallace DC, Fan W, Procaccio V. Mitochondrial energetics and therapeutics. Annu Rev Pathol. 2010;5:297–348. doi: 10.1146/annurev.pathol.4.110807.092314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the 'phosphocreatine circuit' for cellular energy homeostasis. Biochem J. 1992;281(Pt 1):21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waniewski RA, Martin DL. Preferential utilization of acetate by astrocytes is attributable to transport. J Neurosci. 1998;18:5225–5233. doi: 10.1523/JNEUROSCI.18-14-05225.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H, Venkatesh B. Clinical review: ketones and brain injury. Crit Care. 2011;15:219. doi: 10.1186/cc10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm F, Hirrlinger J. Multifunctional roles of NAD(+) and NADH in astrocytes. Neurochem Res. 2012;37:2317–2325. doi: 10.1007/s11064-012-0760-y. [DOI] [PubMed] [Google Scholar]
- Wyss MT, Magistretti PJ, Buck A, Weber B. Labeled acetate as a marker of astrocytic metabolism. J Cereb Blood Flow Metab. 2011;31:1668–1674. doi: 10.1038/jcbfm.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Nissim I, Horyn O, Lazarow A, Luhovyy B, Wehrli S, Nissim I. Response of brain amino acid metabolism to ketosis. Neurochem Int. 2005;47:119–128. doi: 10.1016/j.neuint.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Zamzow CR, Bose R, Parkinson FE. The effect of acidosis on adenosine release from cultured rat forebrain neurons. Brain Res Mol Brain Res. 2006;1082:23–31. doi: 10.1016/j.brainres.2006.01.115. [DOI] [PubMed] [Google Scholar]