Abstract
The glucocorticoid induced receptor (GIR) is a stress-responsive gene that is abundantly expressed in forebrain limbic regions. GIR has been classified as a NPY-like receptor, however, physiological attributes have not been investigated. In the current study mice lacking GIR (−/−) were screened in various paradigms related to stress, anxiety, activity, memory, fear and reward. GIR −/− mice elicited behavioral insensitivity to the anxiogenic effects of restraint stress. However, hypothalamic pituitary adrenal (HPA) axis response to stress was not impacted by GIR deficiency. Increased preference for sucrose was observed in GIR −/− mice suggestive of modulation of reward-associated behaviors by the receptor. A delayed acquisition of spatial learning was also observed in GIR −/− mice. There were no effects of genotype on the modulation of anxiety-like behavior, activity, and fear conditioning-extinction. Our data extend previous studies on GIR regulation by glucocorticoids and provides novel evidence for a role of GIR in reward, learning and the behavioral outcomes of stress.
Keywords: GIR, glucocorticoid, stress, anxiety, reward, learning, hypothalamic pituitary adrenal axis
Introduction
The glucocorticoid-induced receptor (GIR) is a G protein-coupled receptor also referred to as JP05, GPR72 or GPR83. GIR was originally identified as a stress-responsive transcript from the murine T-cell line WEHI-7TG and normal thymocytes treated with glucocorticoids and forskolin (Baughman et al. 1991; Harrigan et al. 1989; Harrigan et al. 1991). Human and rat GIR transcripts cloned later revealed significant homology to the murine form (De Moerlooze et al. 2000; Wang et al. 2001). Based on an interaction between GIR and Neuropeptide Y (NPY) ligands, GIR was classified as an “NPY-Y2-like” receptor by our group (Sah et al. 2007). In addition, studies from our lab and others have reported a predominant expression of the GIR transcript in several stress-regulatory forebrain limbic regions of rodent brain (Pesini et al. 1998; Sah et al. 2005).
The functional role of GIR has not been well characterized. Recent studies using lentiviral GIR constructs have shown that GIR may participate in central thermoregulation (Dubins et al. 2012). GIR is regulated in the CNS following in vivo administration of dexamethasone, suggesting a potential role of this receptor in stress-associated physiology (Adams et al. 2003). In situ hybridization studies revealed a high expression of this receptor in forebrain regions such as the pre-frontal cortex, amygdala, hippocampus, as well as in various hypothalamic nuclei (Sah et al. 2005). Thus, GIR is well positioned to orchestrate stress regulation, emotional behaviors, learning-memory and reward.
To investigate the physiological contributes of GIR, we generated GIR knockout mice (GIR −/−) and conducted comprehensive phenotyping using paradigms associated with stress, anxiety, fear, reward, activity and learning-memory in comparison with wild-type littermates. We also assessed hypothalamic pituitary adrenal (HPA) axis response to acute restraint stress. The rationale for selection of these outcomes was based upon the abundance of GIR expression in areas regulating stress (hypothalamus, hippocampus, amygdala), anxiety and fear (amygdala, prefrontal cortex), reward (striatum) and learning/memory (hippocampus) as well as the regulation of GIR following glucocorticoids (Adams et al. 2003). GIR also bears pharmacological similarity to NPY receptors (Sah et al. 2007) that are known to orchestrate stress-associated outcomes (Eaton et al. 2007; Thorsell 2011; Wu et al. 2011).
While baseline anxiety, locomotor activity and conditioned fear-extinction were unaffected by the absence of GIR, a marked attenuation of stress-evoked anxiety was observed in two separate behavioral paradigms. GIR deficient mice also elicited an increased preference for sucrose and a delayed acquisition of spatial learning. However, stress evoked activation of HPA axis responses was not affected in mice lacking GIR. This constellation of behaviors is indicative of selective functional contributions of the glucocorticoid induced receptor in regulating spatial learning, reward and behavioral consequences of stress exposure.
Materials and Methods
Subjects and Design
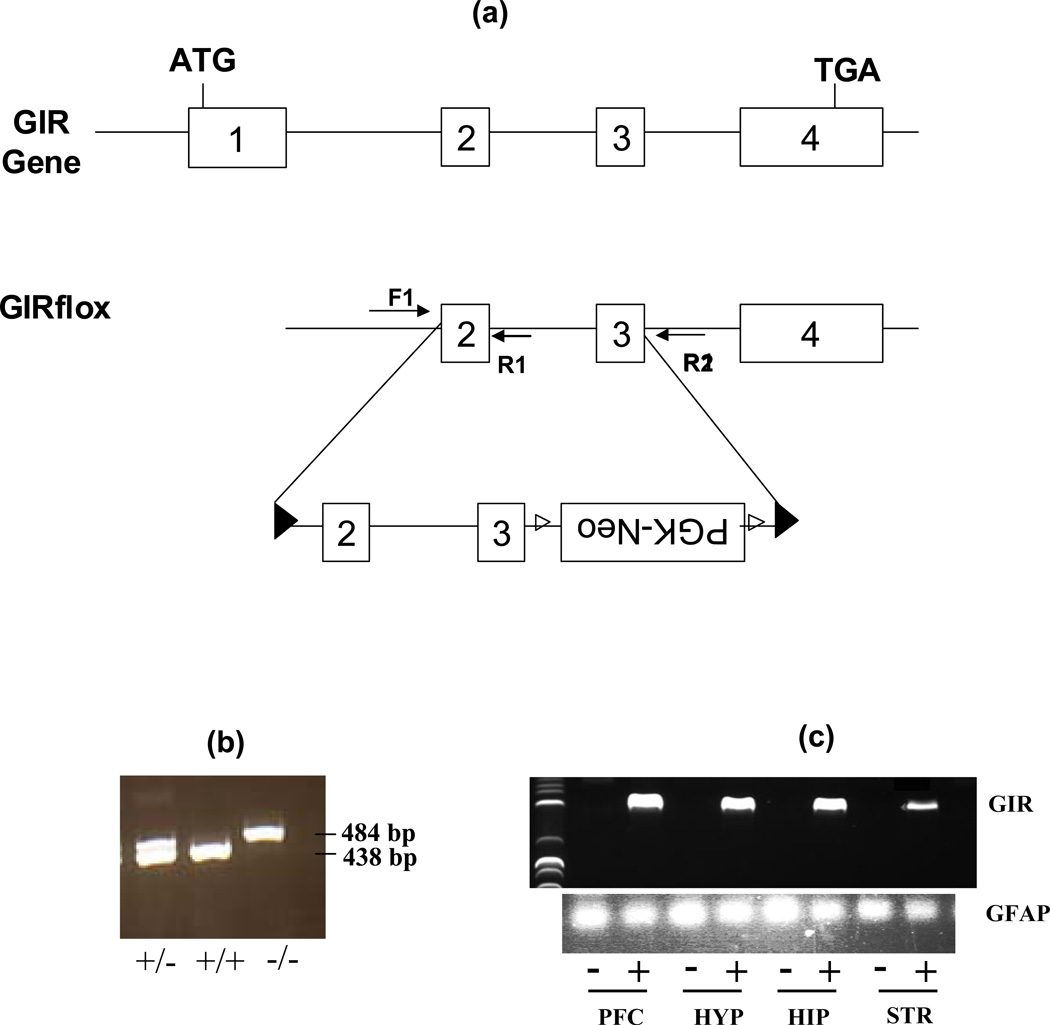
GIR knockouts were generated from GIRflox/flox mice developed by Organon-Lexicon Laboratories, UK. The mutant line was initially generated by homologous recombination in 129 derived ES cells. LoxP sites were inserted along with a selection cassette containing a neomycin resistance gene flanked by FRT sites (see Fig 1). GIRflox allele resulted from excision of the frt-flanked sequences by FLP recombinase, yielding LoxP sites spanning exons 2, 3 as well as intermediary intron. Chimeric mice were generated by injection of targeted clones into C57BL/6 blastocytes. To knockdown GIR, we crossed GIRflox/flox mice with EIIA-CRE mice on a C57BL/6 background to produce GIR−/− mice. Mice were housed under a 12 h light, 12h-dark cycle (lights on at 06:00 h) and constant temperature (23 −28 °C) conditions. Food and water were provided ad libitum. All genotyping for the GIR alleles was done by PCR on DNA extracted from tail biopsies using forward F1 (TGGAAATGCCACCCCAGAGC) and reverse R1 (AGACGGAGATGGGAGCATGC) and R2 (TGTGAGCACACACTCCCTGG) primers. All experiments reported here were performed on male littermates from heterozygous mice carrying wild type GIR alleles (GIR+/+) and knockouts (GIR−/−). Behavioral testing was performed between 8–16 weeks of age during the light cycle. All studies were conducted under a research protocol approved by the Institutional Animal Care and Use Committee in a vivarium accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC).
Figure 1.
Targeted disruption of the GIR gene in the mouse: (a) Wild-type GIR gene is shown spanning exons 1–4. Lower line shows the transgene used to generate GIR −/− mice with targeted deletion of exons 2 and 3. ; Insertion cassette with position of flox (▶) sites, PGK-neo frt(▷) sites and genotyping primers F1, R1 and R2 is indicated. (b) Primers (F1) and (R1) produced a 438 bp band in the wild type GIR (+/+) and heterozygous (+/−) mice but not in GIR knockout (−/−) mice. Primers (F1) and (R2) produced a 484 bp band in (−/−) and (+/−) mice but a (~2.7kb) product in +/+ mice (not see here). (c) Reverse-transcriptase polymerase chain reaction revealed the absence of GIR message in various forebrain regions of (−/−) mice as compared to (+/+) mice. Lower panel shows Glial fibrillary acidic protein (GFAP) as loading control. PFC=prefrontal cortex, HYP=hypothalamus, HIP=hippocampus, STR=striatum.
GIR detection by RT-PCR
GIR mRNA expression in various brain regions was investigated using reverse transcription-polymerase chain reaction (RT-PCR) experiments. Mice brains were obtained following cervical dislocation and specific brain regions were rapidly dissected out. Total RNA was isolated from each region by single step guanidine thiocyanate-phenol extraction using the TRI-REAGENT (Molecular Research Center, Cincinnati, OH) following the manufacturer instructions. The concentrations of RNA samples were determined by spectrophotometric measurements at 260 and 280 nm. First strand cDNA was synthesized from total RNA using a random hexamer primer (Promega, Madison, WI) following manufacturer’s instruction. Mouse GIR specific primers: 5’-ATGAAGGTTCCTCCTGTCCTG-3’ (forward) and 5’-GGTCCACTGCGATAGCTGTC-3’ (reverse) [accession number NM_010287] were synthesized (Integrated DNA Technologies, Coralville, IA). The reverse primer was selected from a region spanning exon 2 that was excised in knockout mice. Specific primers for GFAP used as the internal control were: 5’-GAA AAC CGC ATC ACC ATT CC-3’ (forward) and 5’-GCA TCT CCA CCG TCT TTA CC-3’ (reverse) [accession number NM_010277]. PCR was run for 35 cycles at 94 °C for 30 sec., 57 °C for 30 sec., 72 °C for 30 sec, followed by 1cycle at 72 °C at 10 min. PCR products were electrophoresed on 1.2 % agarose gel and stained with ethidium bromide.
Locomotor Activity
Twenty four hour home cage activity was measured using the SmartFrame stainless steel cage rack frame with infrared photo beam interruption sensors (7X and 15Y). Data was then analyzed using the HMM100 MotorMonitor software. To measure locomotor activity in the open field and the EPM, videos were analyzed using Clever System topscan software (Clever Sys Inc. Reston, VA).
Open Field (OF)
Mice were exposed to a dimly lit open field apparatus that consisted of a white 50 X 50 X 22cm plexiglas box. Each mouse was placed in the center and allowed to freely explore for 5 min. To investigate stress effects on open field behavior, mice were restrained in plastic restrainers for 30 min. Mice were returned to their home cage for 5 minutes to enable post restraint grooming and were then subjected to the open field exposure. The test was recorded from a camera mounted above the OF. Videos were scored for time spent in the border and center areas and distance travelled using Clever System Topscan software.
Elevated Plus Maze (EPM)
To study the expression of innate anxiety we assessed wild type and knockout mice on the EPM using validated procedures (Pellow et al. 1985). The apparatus comprised of a PVC maze with two open (66×5cm) and two enclosed (66 x5×15cm) arms. The arms radiated from a 5 cm central square. The entire apparatus was elevated 38 cm off the floor. For testing, each animal was placed on the center square of the maze facing the same open arm. To investigate stress effects on EPM behavior, mice were restrained in plastic restrainers. for 30 minutes. Mice were returned to their home cage for 5 minutes to enable post restraint grooming and were then subjected to the EPM test. Behavior was recorded from an overhead ceiling camera for 5 min. Video files were captured and saved for later scoring. Parameters were scored using the Topscan program Clever System (Clever Sys Inc. Reston, VA) for arm time and locomotor activity.
Morris Water Maze
The Morris water maze (MWM) was performed as previously described (Vorhees & Williams 2006), with minor modifications. The maze consisted of a circular, fiberglass pool (122 cm in diameter, 60 cm height; Rowland Fiberglass, Ingleside, TX) filled with room temperature tap water (43 cm deep). The water maze pool was enclosed by plastic curtains on two sides and white walls on the opposing sides. Extra-maze visual cues (42 cm×76 cm posters printed with contrasting shapes and patterns) were placed at N, S, E, and W positions around the pool. A clear glass platform (10.5 cm×10.5 cm square) was submerged 0.5 cm below the water surface. Mice were trained for 4 days with 3 randomized trials separated by at least 1 hr. For each learning trial, mice were placed into the water at one of four possible starting points with similar path lengths to the platform (e.g., SW,W, N, NE). The submerged platform was kept in the same quadrant of the pool for all of the trials. A trial was terminated and latency recorded when the mouse found and climbed onto the platform for 5 s. If the mouse did not reach the platform within 90 s, the trial was terminated, and the mouse was placed upon the platform for 5 s. On the fifth and final day of testing, a 90-s probe trial was performed with the mice starting from a novel position (NW) and the escape platform removed from the pool. Trials were digitally recorded using CleverSys TopScan software.
Sucrose Preference Testing
Sucrose preference was investigated as described before (Mueller & Bale 2008) with modifications. Mice were habituated for 3 days to two water bottles. On the fourth day water was replaced with a sucrose solution (1, 3, 10 or 30%) in one of the bottles for seven days. Amount of water and sucrose solution consumed was measured daily, and the bottles were reversed to control for side preference. Sucrose preference was calculated based on the ratio of sucrose solution consumed and total liquid consumed in a 24hr period. Food intake, water intake and body weight were observed throughout the sucrose preference test.
Fear Conditioning and Extinction
All animals underwent a contextual conditioning paradigm to investigate fear conditioning and extinction using the Freeze Monitor apparatus (San Diego Instruments, San Diego, CA). On day 1, each mouse was placed in the shock chamber and allowed to acclimate for 5 min. On day 2, animals were placed back in the chamber and after a 30 sec period given 3 shocks of 0.5mA intensity, 1 sec duration administered 1 min apart. Animals were recorded for post shock freezing. The animals were placed in the chamber the next 5 days and recorded for 5 min without shocks to measure fear conditioning and extinction. Freezing was defined as the absence of all movement except that necessary for respiration (Fanselow 1980).
Restraint Stress and Neuroendocrine Response
Animals were restrained in plastic restrainers for 30 min. Tail blood was collected in unrestrained animals by tail vein nick at 30, 60 and 120 min from the time restraint stress was initiated. These time points were selected to measure peak or near peak levels of corticosterone as well as the termination of the response by feedback inhibition. Plasma was separated through centrifugation at 3000×g for 15 min and stored at −20 °C until radioimmunoassay. Plasma corticosterone was measured using the ImmuChem Double Antibody Corticosterone 125I RIA kit (MP Biomedicals, Orangeburg, NY) according to the manufacturer's protocol. All samples were run in duplicate and within the same assay. Intra-assay coefficient of variation was less than 10%. Corticosterone concentration was calculated using AssayZap software (Biosoft, Cambridge, UK).
Statistical Analysis
Behavior in the open field, elevated plus maze, water maze, and locomotor activity was measured using TopScan software (CleverSys. Inc., Reston, VA). For fear conditioning and extinction digitized images were acquired for later scoring of freezing. Planned comparisons with two-tailed Student’s t-test were used across all experiments to determine statistical significance between genotypes (p < 0.05). Repeated measures ANOVA was used to analyze HPA response following restraint, home cage locomotor activity, sucrose preference, fear conditioning-extinction and water maze acquisition over blocks of time. Prism software was used to analyze all data (GraphPad Software, Inc., La Jolla, CA).
Results
GIR −/− mice
As shown in Figure 1c, excision of exons 2–3 from the GIR gene led to the ablation of GIR transcript from several brain regions that have previously been reported to show high GIR mRNA expression by our group (Sah et al. 2005). Deletion of GIR did not produce any effects on reproducibility, litter size or gross physical abnormalities (data not shown).
Motor activity in the home cage is not impacted by GIR deficiency
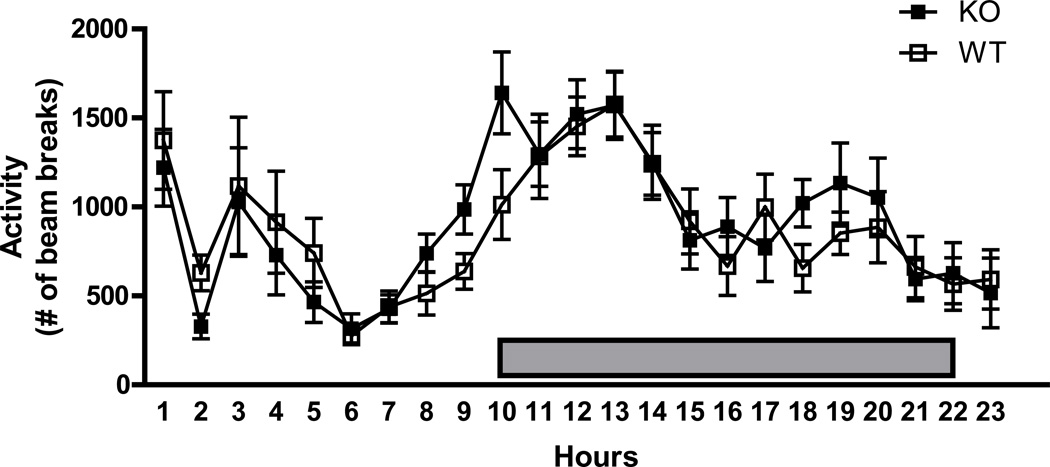
Home cage motor activity measured over 24 hr under baseline conditions of food and water availability did not show any significant differences between GIR +/+ and −/− mice (Fig 2). Two way ANOVA revealed a significant effect of time [F(22,667) = 7.17; p<0.05], with no effect of genotype [ F(1,667) = 0.55; p>0.05].
Figure 2.
Locomotor activity in the home cage measured over 24 hours in (+/+) and (−/−) mice showed no significant differences over the circadian cycle. Filled box indicates hours during the dark phase of the circadian cycle. Data shown are mean ± SEM; n= 15–16 /group.
Anxiogenic effects of stress are attenuated in GIR −/− mice with no effect on baseline anxiety
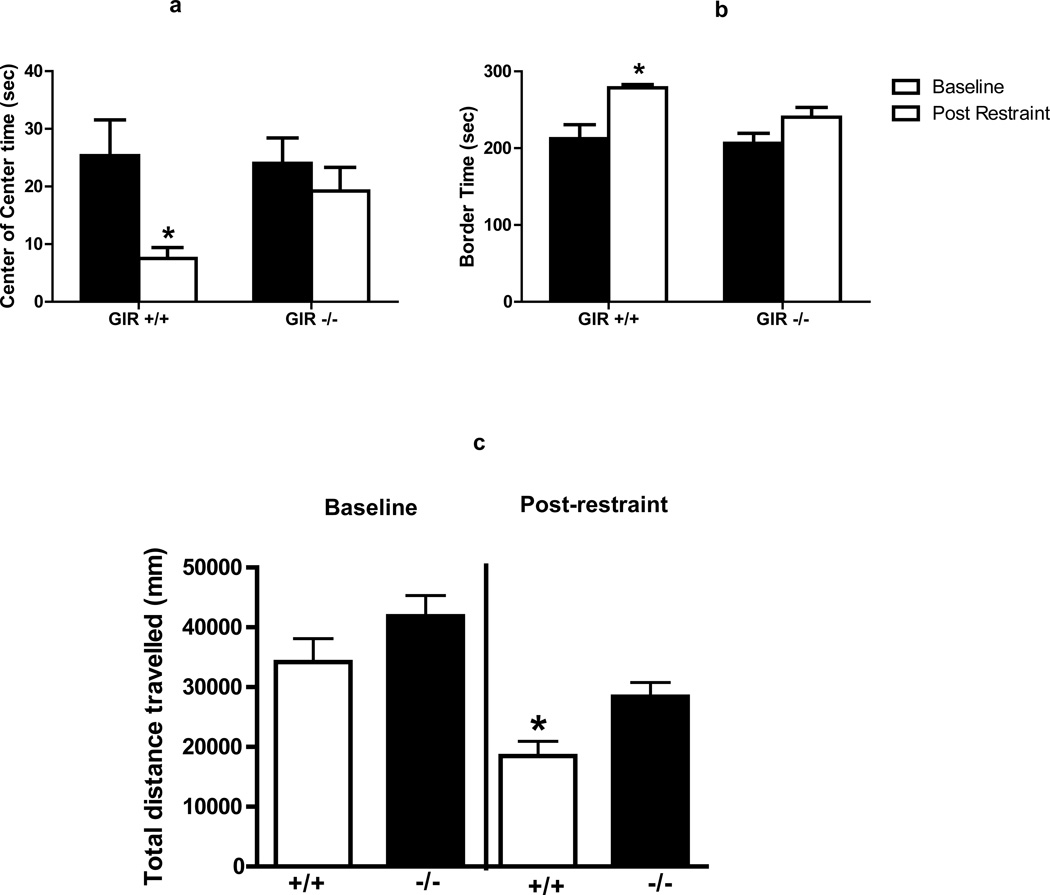
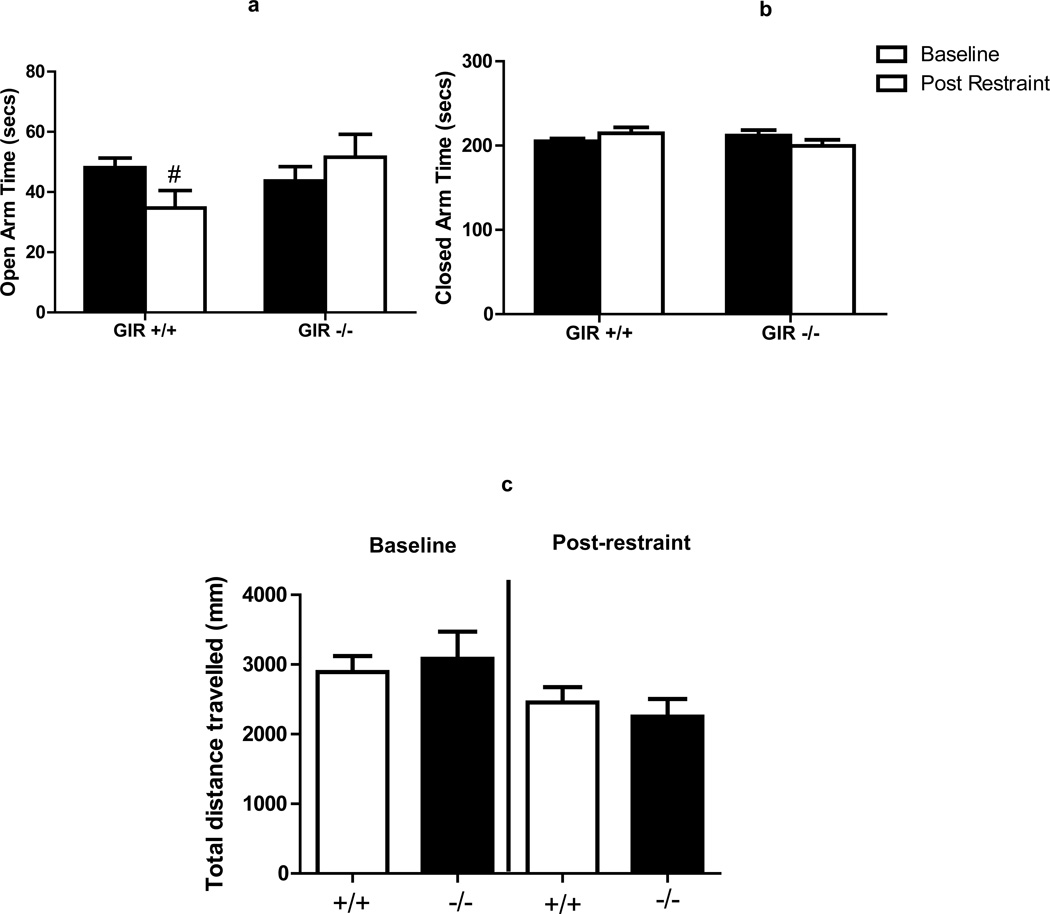
To assess the effects of GIR deficiency on anxiety, we measured anxiety-like behavior in two separate paradigms: the open field and elevated plus maze, both under no stress (baseline) conditions and following restraint stress. Exposure to the open field or the elevated plus maze under baseline conditions with no prior stress exposure produced no significant differences in anxiety-related outcomes between genotypes (baseline groups in Fig 3 and 4). However, exposure to 30 minutes of restraint stress prior to the open field or EPM produced significant differences in behavior between wild type and knockout mice. As shown in Fig 3A, GIR +/+ mice spent significantly less time in central area of the open field as compared to the no stress condition [t(14) =2.735; p<0.05]. Also, stressed +/+ mice spent significantly more time in the border areas as compared to the no stress group [t(14)=3.528; p<0.05]. In contrast there were no significant differences in time spent in center [t(14)=0.798; p>0.05) and border [t(14)=1.867; p>0.05] areas between stress and no stress groups of GIR−/− mice. Testing on the EPM revealed more subtle effects of restraint stress on behavior than those observed in the open field. Thus, GIR +/+ mice showed reduced open arm time following stress that trended towards significance [t(20)=1.79; p=0.08], while GIR −/− mice showed no differences between stress and no stress cohorts (t(20)=0.779; p=0.44).
Figure 3.
GIR knock out (−/−) mice are resilient to stress evoked anxiety. Open field testing for time spent in center of center area (a), border (b) and motor activity during the total test period (c) for wild type (+/+) and GIR knock out (−/−) mice. A highly significant anxiogenic effect of restraint was observed as decreased center time (a) and increased border time (b) only in the wild-type mice but was absent in GIR −/− mice, (* p<0.05 versus other conditions). There was no genotype difference at baseline. Motor activity showed no significant differences between genotypes at baseline. GIR −/− mice showed increased activity post stress as compared to +/+ mice (p<0.05); Data are mean ± SEM; n= 8–9/group.
Figure 4.
GIR knock out mice (−/−) do not elicit the anxiogenic effects of restraint stress in the elevated plus maze (EPM) test. Time spent in Open (a), Closed (b) arms and motor activity during the total test period (c) for GIR wild type (+/+) and knock out (−/−) mice are shown. Exposure to restraint produced reduced open arm time in +/+ mice (# p=0.08 versus baseline) GIR −/− mice showed no difference in open arm time between baseline and restraint stress. Closed arm time and motor activity were not impacted by stress or genotype. (Panels b and c); Data are mean ± SEM; n= 8–9 baseline n=12–13 restraint stress group.
Since motor activity can be impacted by anxiogenic and stressful stimuli, GIR mice were also screened for activity following exposure to the open field and EPM. No effect of genotype on activity was observed on exposure to the open field or EPM under baseline conditions (Fig 3C and 4C). Prior exposure to restraint stress reduced activity in +/+ mice in the open field. In comparison, GIR −/− mice elicited significantly higher activity in the open field following stress as compared to +/+ mice [t(15)=3.198; p<0.05] (Fig 3C). Activity in the EPM following stress exposure was not affected by genotype.
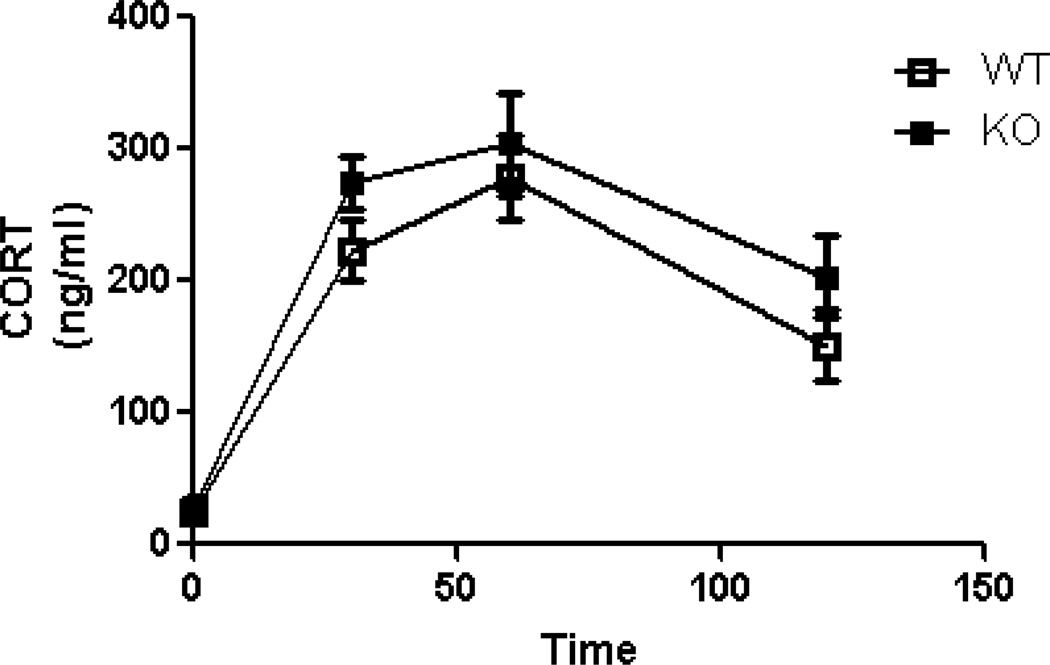
Hypothalamic pituitary adrenal axis response following restraint stress is not affected by the absence of GIR
Restraint stress produced a robust increase in plasma levels of corticosterone (Fig 5). Two way repeated measures ANOVA revealed a significant effect of time [F (3,51) = 39.11; p < 0.05] with no genotype [F (1,51) =3.18; p > 0.05] effect. Analysis of area under the curve revealed no significant difference between GIR +/+ and −/− mice (p>0.05).
Figure 5.
Exposure to acute restraint stress produces similar neuroendocrine response in GIR +/+ and −/− mice. Plasma corticosterone (CORT) concentrations to a 30-min restraint challenge are shown as a time course. There were no differences between +/+ and −/− mice. Data are mean ± SEM ; n =8/group.
Increased preference for sucrose in GIR −/− mice
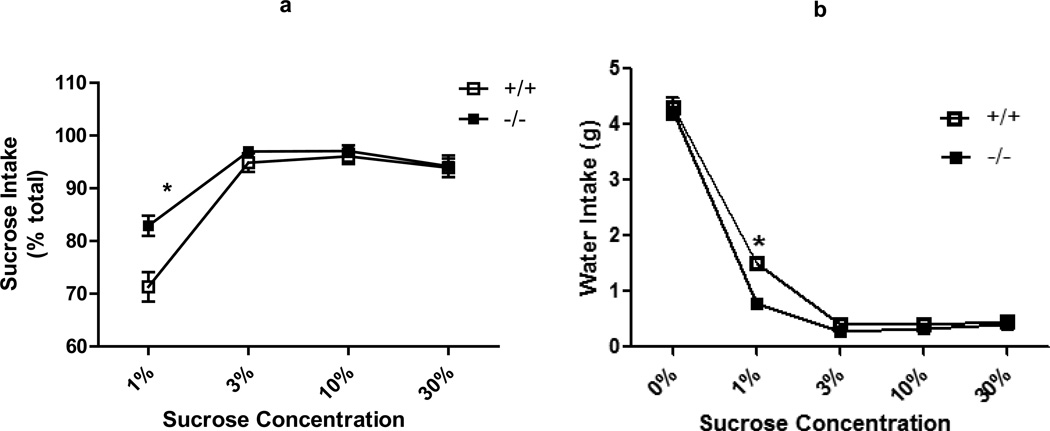
Hedonic state of GIR −/− and +/+ mice was assessed using a sucrose preference task. GIR −/− mice displayed an increased intake of 1% sucrose compared with +/+ male offspring (Fig 6A). A significant effect of genotype [F (1,45) = 5.985; p<0.05], sucrose concentration [F (3,45) = 60.54; p<0.05] and an interaction between genotype×concentration [F (3,45) = 4.933; p<0.05] was observed. Posthoc analysis revealed a significant effect at 1% concentration (p<0.05). Increasing sucrose concentration to 3, 10 or 30% ameliorated the increased preference of GIR −/− mice. Water intake during 1% sucrose exposure was significantly lower in −/− mice compared to +/+ (Fig 6B), however, total fluid consumption was not different between +/+ and −/− mice (data not shown).
Figure 6.
Increased sucrose preference in GIR −/− mice. (a) Mice were trained to consume different concentrations of sucrose (1, 3, 10 or 30%). Significant increase in consumption was observed in GIR −/− mice at 1% sucrose compared to +/+ mice. (b) Water intake was significantly reduced during 1% sucrose availability in −/− mice. Data is shown as mean ± SEM for amount consumed. n=8–9/group
GIR deficient mice elicit delayed acquisition of spatial learning in the Morris water maze but normal fear acquisition conditioning and extinction
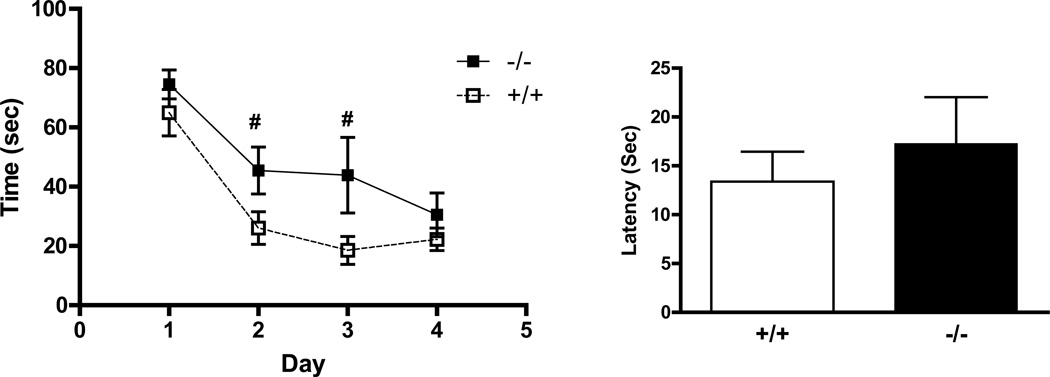
The observed abundance of GIR in areas such as the hippocampus, cortex and amygdala led us to investigate its effects on two distinct paradigms of associative learning: the Morris water maze and contextual fear conditioning and extinction. Deficits were observed in the acquisition of spatial learning during training on the Morris maze (Fig 7A). Over 4 days of training, latency to reach the platform decreased in wild-type controls and GIR knockouts, but markedly less in the knockouts. Repeated measures ANOVA revealed a significant effect of genotype [F (1,56) = 9.08; p< 0.05] and time [F (3,16) = 14.38 p< 0.05]. This indicates delayed spatial learning in −/− mice. Although latency to escape was compromised in GIR −/− mice for day 2 and 3 [difference in latency time showed trends, Day 2 (p=0.06) and Day 3 (p=0.08) versus GIR +/+ mice], they learned the task by day 4 similar to wild type mice, suggesting the absence of severe deficits in these mice. On the testing day, no differences were observed in finding the platform area during the probe trial between GIR −/− and +/+ mice (p > 0.05) (Fig 7B).
Figure 7.
Compromised spatial learning in GIR −/− mice in the Morris water maze (MWM) test. (a) Attenuation in the decline of latency to find the platform was observed in GIR −/− mice (overall genotype effect, F =9.08; p<0.05). Analysis of individual days showed a reduced decline in latency time to find platform in −/− mice (# Day 2: p=0.06; # Day 3: p=0.08 versus GIR +/+ mice). (b) The groups did not differ in probe trial testing 24 hr after the last training session. Data are mean ± SEM of 3 trials per day for time to reach the goal during 4 days of training (n=8–9 /group)
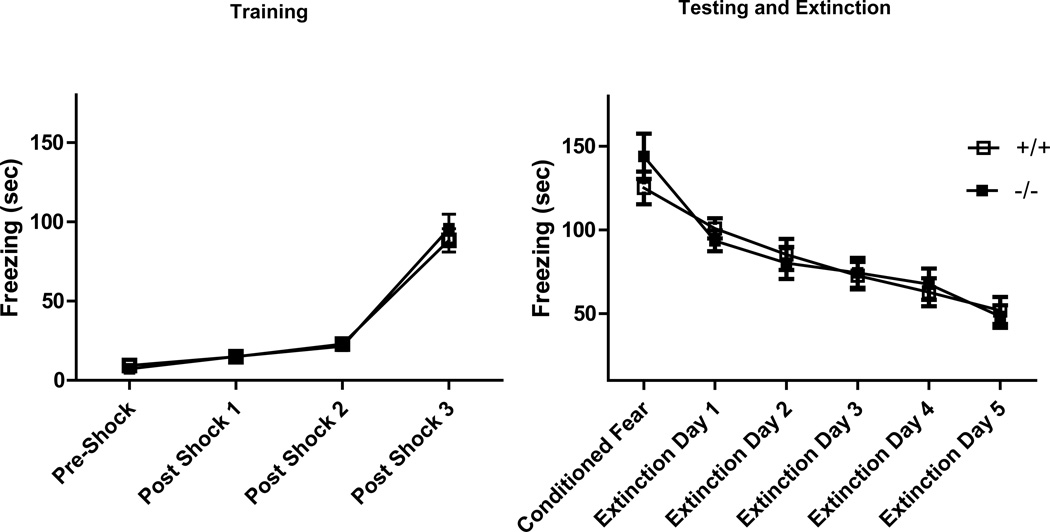
In a contextual fear conditioning paradigm, GIR knockout mice showed similar acquisition of fear during training (Fig 8). Measurement of conditioned fear 24 h post training upon re-exposure to context revealed no differences between genotypes (p>0.05). Extinction of fear over five consecutive days revealed a significant effect of time [F (5,137) =22.09; p<0.05] without a genotype effect [F (1,137) =0.764; p>0.05].
Figure 8.
Fear conditioning paradigm. GIR −/− mice and +/+ mice showed similar levels of freezing in fear acquisition during training (left panel), conditioned fear and extinction of fear (right panel). Data is shown as mean ± SEM for time spent freezing; n= 12–13 /group
Discussion
We report selective phenotypic traits in GIR-deficient mice, suggesting a behavioral insensitivity to stress, increased reward preference and delayed acquisition of spatial memory. These traits were observed in the absence of genotype effects on the regulation of locomotor activity, anxiety, fear, and hypothalamic pituitary adrenal axis response and are therefore specific. These findings extend previous reports on GIR regulation following administration of glucocorticoids and psychostimulants (Adams et al. 2003; Wang et al. 2001).
Based on our previous work on GIR distribution in rodent brain (Sah et al. 2005), we hypothesized its role in stress integration and regulation of emotional behavior in addition to learning/memory and reward. GIR expression is predominant in areas regulating motor activity such as the caudate putamen and nucleus accumbens. However, no differences were noted in baseline locomotor activity in the home cage or following novelty on exposure to the elevated plus maze or the open field. This suggests that GIR is dispensable for the control of motor activity at least under baseline conditions and following novelty. Presence of GIR in areas such as the amygdala, hippocampus and prefrontal cortex suggested a role in orchestration of emotional outcomes such as anxiety and fear. GIR deficient mice elicited similar responsiveness to anxiety-evoking effects of the open field and the EPM suggesting that GIR is not recruited in emotional behaviors under basal no-stress conditions.
A significant genotype effect was observed in the sucrose preference test, a behavioral measure for hedonic and reward responses, with GIR deficiency resulting in increased preference for sucrose. GIR mRNA shows predominant expression in the nucleus accumbens, olfactory tubercle, and ventromedial caudate putamen in mouse brain. Distribution in the mouse is also prevalent in the amygdalostriatal transition area and the interstitial nucleus of the posterior limb of the anterior commissure which represent the anatomical continuation of the ventral striatum into the amygdala (Pesini et al. 1998). These are primary targets of the mesolimbic and nigrostriatal dopamine system, suggesting a potential association of GIR with hedonic and reward-related behaviors. When tested for sucrose preference, GIR −/− mice showed a stronger preference at the 1% sucrose solution suggesting an increased basal sensitivity to hedonic rewards. These responses may potentially arise due to a regulatory influence of GIR on reward systems especially the mesolimbic dopaminergic system. The difference in preference in GIR deficient mice was ameliorated with increasing concentration of sucrose, suggesting that GIR was dispensable at increased reward value. Preference for lower concentration of sucrose in the absence of altered preference at higher concentrations has also been reported by other studies (Mueller & Bale 2008).
High expression of GIR in areas such as the hippocampus, prefrontal cortex and amygdala led us to investigate GIR −/− mice in tests of spatial as well as fear learning and memory. Compromised spatial learning was observed in mice lacking GIR evident by an increased latency during training phase. However, GIR compromised mice showed equivalent latency on the final training day, suggesting that they did learn the task eventually. Since performance during probe testing was similar in both groups it is apparent that spatial reference memory is not regulated by GIR. Our previous work indicated abundant expression of GIR in scattered cells within the CA1 and dentate gyrus granule cell layers consistent with large interneurons, possibly GABAergic (Sah et al. 2005). Studies suggest an important role of inhibitory hippocampal interneurons in spatial learning (Moser 1996). Thus, GIR may regulate inhibitory transmission in the hippocampus impacting acquisition of spatial cues and learning. GIR-mediated spatial learning appears to be highly specific since no effects of GIR deficiency were observed on contextual fear associated learning. No differences in freezing responses during contextual fear acquisition, conditioning and extinction between GIR +/+ and −/− mice indicate that fear-associated responses do not require GIR. Fear-regulatory hippocampal mechanisms involve interplay with other regions such as the amygdala and the PFC and these coordinated circuits may not recruit GIR function.
Most relevant findings related to GIR function were stress-associated outcomes. Multiple lines of evidence from previous studies indicate association of GIR with glucocorticoid-related effects. Overlap in localization of the glucocorticoid receptor (GR) or mineralocorticoid receptor with GIR mRNAs occurs in several brain regions (Sah et al. 2005; Adams et al. 2003; Pesini et al. 1998) indicating a capacity for GIR regulation pursuant to glucocorticoid secretion. GIR was initially cloned as a stress-responsive transcript and is significantly down regulated following administration of the synthetic glucocorticoid, dexamethasone in the hypothalamus and hippocampus, areas implicated in stress response and regulation (Adams et al. 2003). An important aim of the current study was to investigate whether GIR is implicated in outcomes impacted by prior exposure to stress such as behavior, and whether it is required in hypothalamic pituitary adrenal activation following stress. Our data show that GIR impacts behavioral sensitivity to stress but does not participate in HPA axis response following an acute stressor. Neuroendocrine and behavioral responses to stress can be dissociated (Koob et al. 1993) and it is likely that GIR participates in the behavioral response without impacting neuroendocrine outcomes. Using two separate tests of innate anxiety we demonstrate that GIR deficient mice showed resilience to the anxiogenic effects of restraint stress. Stress-evoked anxiogenic effects in wild type mice were more robust in the open field than in the elevated plus maze. The open field test may be more aversive to the mice than the EPM, resulting in greater stress-evoked behavioral responses in this test, at least in our hands. Reduced activity of GIR +/+ mice in the open field following stress was reversed in GIR deficient mice indicative of improved exploration. Stress-induced suppression of activity and exploration has been reported earlier (Klenerova et al. 2009; Windle et al. 2006) and is reversed by anxiolytic and stress-modulatory agents. Effects of GIR knockdown on anxiety-like behaviors and motor activity were not observed in the absence of prior stress exposure, suggesting that GIR may be recruited following stress. Glucocorticoids promote anxiogenic responses that are abolished by adrenalectomy and treatment with blockers of glucocorticoid synthesis (Calvo et al. 1998; Calvo & Volosin 2001). Given that GIR expression is modulated by glucocorticoids (Adams et al. 2003), it is likely that this receptor mediates downstream effects of glucocorticoids and perhaps orchestrates the central effects of glucocorticoids in situations; such as following stress when their levels would be expected to rise. We believe this is an important finding given that no evidence exists to date on the functional contributes of GIR and supports its nomenclature as a “stress-responsive” receptor. Moreover, it is of interest to note that GIR −/− mice demonstrated attenuated stress-evoked responses as well as an increased drive for a rewarding stimulus, sucrose. Stress and reward stimulatory pathways have been reported to associate and interact especially in forebrain regions such as the accumbens, amygdala and hypothalamus (Christiansen et al. 2011; Ulrich-Lai et al. 2010). GIR expression is abundant in these regions and may regulate responses related to both stress and reward.
It is important to discuss the role of GIR in behavioral insensitivity to stress, compromised spatial acquisition and reward in light of its classification by our group as a NPY-like receptor subtype (Sah et al. 2007). NPY and its receptors have been implicated in the regulation of stress, anxiety and reward (Brown et al., 2000; Eaton et al. 2007; Thorsell 2011; Wu et al. 2011). It is intriguing to note that transgenic rats overexpressing NPY elicit a similar behavioral phenotype as observed by us in GIR knock-out mice: a profound behavioral insensitivity to stress evoked anxiety as well as a deficit in spatial learning, in the absence of any effects on HPA responses or innate anxiety (Thorsell et al. 2000). Previous studies from our group showed highest sequence homology as well as similarities in ligand-binding characteristics between GIR and Y2 receptor. Genetic ablation of the NPY-Y2 receptor subtype results in improved stress coping ability and reduced anxiety (Tschenett et al. 2003). Additionally, NPY administration in the accumbens produces a robust increase in operant responding, possibly mediated by increased dopamine levels (Quarta et al. 2011; Brown et al. 2000). Localization of NPY and its receptors in relation to GIR will be important as specific GIR antibodies become available. Our current data supporting similarities in behavioral phenotypes of GIR and NPY/NPY receptors in genetic models, as well as previous work from our group showing an interaction between GIR and NPY compounds provides a strong rationale for pursuing future mechanistic studies in this direction.
The present study provides the first evidence of functional contributions of the glucocorticoid induced receptor in the modulation of behavioral effects of stress, spatial learning and reward. GIR-modulation of stress-evoked anxiety appears to be independent of GIR regulation of HPA axis response. Our data indicate that this receptor may be a down-stream player in glucocorticoid-mediated behavioral outcomes. GIR-deficient mice express specific behavioral phenotypes and will prove useful in investigation of mechanistic contributions of GIR especially in stress, reward and memory associated paradigms.
Acknowledgements
We would like to acknowledge Dr. Brian Henry for providing GIR-flox mice. This work was supported by NIH grant MH083213 and VA Merit Award BX001075-01 to Dr. Renu Sah. Technical assistance of Charles M Dolgas, Brad Chambers and Benjamin Packard is also acknowledged.
Footnotes
The authors declare no conflict of interest
References
- Adams F, Grassie M, Shahid M, Hill DR, Henry B. Acute dexamethasone administration reduces levels of orphan GPCR glucocorticoid-induced receptor (GIR) mRNA in rodent brain: potential role in HPA axis function. Mol. Brain Res. 2003;117:39–46. doi: 10.1016/s0169-328x(03)00280-8. [DOI] [PubMed] [Google Scholar]
- Baughman G, Harrigan MT, Campbell NF, Nurrish SJ, Bourgeois S. Genes newly identified as regulated by glucocorticoids in murine thymocytes. Mol. Endocrinology. 1991;5:637–644. doi: 10.1210/mend-5-5-637. [DOI] [PubMed] [Google Scholar]
- Brown CM, Coscina DV, Fletcher PJ. The rewarding properties of neuropeptide Y in perifornical hypothalamus vs. nucleus accumbens. Peptides. 2000;21:1279–1287. doi: 10.1016/s0196-9781(00)00270-9. [DOI] [PubMed] [Google Scholar]
- Calvo N, Martijena ID, Molina VA, Volosin M. Metyrapone pretreatment prevents the behavioral and neurochemical sequelae induced by stress. Brain Res. 1998;800:227–235. doi: 10.1016/s0006-8993(98)00515-0. [DOI] [PubMed] [Google Scholar]
- Calvo N, Volosin M. Glucocorticoid and mineralocorticoid receptors are involved in the facilitation of anxiety-like response induced by restraint. Neuroendocrinol. 2001;73:261–271. doi: 10.1159/000054643. [DOI] [PubMed] [Google Scholar]
- Christiansen AM, Dekloet AD, Ulrich-Lai YM, Herman JP. "Snacking" causes long term attenuation of HPA axis stress responses and enhancement of brain FosB/deltaFosB expression in rats. Physiol Behav. 2011;103:111–116. doi: 10.1016/j.physbeh.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moerlooze L, Williamson J, Liners F, Perret J, Parmentier M. Cloning and chromosomal mapping of the mouse and human genes encoding the orphan glucocorticoid-induced receptor (GPR83) Cytogene. & Cell Gene. 2000;90:146–150. doi: 10.1159/000015650. [DOI] [PubMed] [Google Scholar]
- Dubins JS, Sanchez-Alavez M, Zhukov V, Sanchez-Gonzalez A, Moroncini G, Carvajal-Gonzalez S, Hadcock JR, Bartfai T, Conti B. Downregulation of GPR83 in the hypothalamic preoptic area reduces core body temperature and elevates circulating levels of adiponectin. Metabolism. 2012 doi: 10.1016/j.metabol.2012.03.015. (epub) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton K, Sallee FR, Sah R. Relevance of neuropeptide Y (NPY) in Psychiatry. Curr Top Med Chem. 2007;7:1645–1659. doi: 10.2174/156802607782341037. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Conditional and unconditional components of post shock freezing. Pavlov J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Harrigan MT, Baughman G, Campbell NF, S Isolation and characterization of glucocorticoids- and cyclic AMP-induced genes in T lymphocytes. Mol. & Cell. Biol. 1989;9:3438–3446. doi: 10.1128/mcb.9.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrigan MT, Campbell NF, Bourgeois S. Identification of a gene induced by glucocortocoids in murine T-cells: a potential G protein-coupled receptor. Mol. Endocrinol. 1991;5:1331–1338. doi: 10.1210/mend-5-9-1331. [DOI] [PubMed] [Google Scholar]
- Klenerova V, Krejci I, Sida P, Hlinak Z, Hynie S. Modulatory effects of oxytocin and carbetocin on stress-induced changes in rat behavior in the open-field. J Physiol Pharmacol. 2009;60:57–62. [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC, Pich EM, Menzaghi F, Baldwin H, Miczek K, Britton KT. The role of corticotropin-releasing factor in behavioral responses to stress. Ciba Found Symp. 1993;172:277–289. doi: 10.1002/9780470514368.ch14. [DOI] [PubMed] [Google Scholar]
- Moser EI. Altered inhibition of dentate granule cells during spatial learning in an exploration task. J Neurosci. 1996;16:1247–1259. doi: 10.1523/JNEUROSCI.16-03-01247.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File S, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Meth. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Pesini P, Detheux M, Parmentier M, Hokfelt T. Distribution of a glucocorticoid-induced orphan receptor (JP05) mRNA in the central nervous system of the mouse. Mol. Brain Res. 1998;57:281–300. doi: 10.1016/s0169-328x(98)00099-0. [DOI] [PubMed] [Google Scholar]
- Quarta D, Leslie CP, Carletti R, Valerio E, Caberlotto L. Central administration of NPY or an NPY-Y5 selective agonist increase in vivo extracellular monoamine levels in mesocorticolimbic projecting areas. Neuropharmacol. 2011;60:328–335. doi: 10.1016/j.neuropharm.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Sah R, Parker SL, Sheriff S, Eaton K, Balasubramaniam A, Sallee FR. Interaction of NPY compounds with the rat glucocorticoid-induced receptor (GIR) reveals similarity to the NPY-Y2 receptor. Peptides. 2007;28:302–309. doi: 10.1016/j.peptides.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah R, Pritchard LM, Richtand NM, Ahlbrand R, Eaton K, Sallee FR, Herman JP. Expression of the glucocorticoid-induced receptor mRNA in rat brain. Neuroscience. 2005;133:281–292. doi: 10.1016/j.neuroscience.2005.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsell A. Central neuropeptide Y in anxiety- and stress-related behavior and in ethanol intake. Ann N Y Acad Sci. 2011;1148:136–140. doi: 10.1196/annals.1410.083. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Michalkiewicz M, Dumont Y, Quirion R, Caberlotto L, Rimondini R, Mathe AA, Heilig M. Behavioral insensitivity to restraint stress, absent fear suppression of behavior and impaired spatial learning in transgenic rats with hippocampal neuropeptide Y overexpression. Proc. Natl. Acad. of Sci. U.S.A. 2000;97:12852–12857. doi: 10.1073/pnas.220232997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschenett A, Singewald N, Carli M, Balducci C, Salchner P, Vezzani A, Herzog H, Sperk G. Reduced anxiety and improved stress coping ability in mice lacking NPY-Y2 receptors. Eur. J. Neurosci. 2003;18:143–148. doi: 10.1046/j.1460-9568.2003.02725.x. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Christiansen AM, Ostrander MM, Jones AA, Jones KR, Choi DC, Krause EG, Evanson NK, Furay AR, Davis JF, Solomon MB, de Kloet AD, Tamashiro KL, Sakai RR, Seeley RJ, Woods SC, Herman JP. Pleasurable behaviors reduce stress via brain reward pathways. Proc Natl Acad Sci U S A. 2010;107:20529–20534. doi: 10.1073/pnas.1007740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Herman JP, Pritchard LM, Spitzer RH, Ahlbrand RL, Kramer GL, Petty F, Sallee FR, Richtand NM. Cloning, expression and regulation of a glucocorticoid-induced recpeotr in rat brain: effect of repetitive amphetamine. Journal of Neuroscience. 2001;21:9027–9035. doi: 10.1523/JNEUROSCI.21-22-09027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle RJ, Gamble LE, Kershaw YM, Wood SA, Lightman SL, Ingram CD. Gonadal steroid modulation of stressinduced hypothalamo-pituitaryadrenal activity and anxiety behavior: role of oxytocin. Endocrinology. 2006;147:2423–2431. doi: 10.1210/en.2005-1079. [DOI] [PubMed] [Google Scholar]
- Wu G, Feder A, Wegener G, Bailey C, Saxena S, Charney D, Mathe AA. Central functions of neuropeptide Y in mood and anxiety disorders. Expert Opin Ther Targets. 2011;15:1317–1331. doi: 10.1517/14728222.2011.628314. [DOI] [PubMed] [Google Scholar]