Abstract
The timing of puberty is controlled by many genes. The elements coordinating this process have not, however, been identified. Here we show that an epigenetic mechanism of transcriptional repression times the initiation of female puberty in rats. We identify silencers of the Polycomb group (PcG) as major contributors to this mechanism, and show that PcG proteins repress Kiss1, a puberty-activating gene. Hypothalamic expression of two key PcG genes, Eed and Cbx7, decreases and methylation of their promoters increases preceding puberty. Inhibiting DNA methylation blocks both events and results in pubertal failure. The pubertal increase in Kiss1 is accompanied by EED loss from the Kiss1 promoter and enrichment of histone H3 modifications associated with gene activation. Preventing the eviction of EED from the Kiss1 promoter disrupts pulsatile GnRH release, delays puberty, and compromises fecundity. Our results identify epigenetic silencing as a novel mechanism underlying the neuroendocrine control of female puberty.
INTRODUCTION
Much has been learned in recent years about the neuroendocrine mechanisms controlling the initiation of female reproductive function. It requires changes in the release of gonadotropin-releasing hormone (GnRH) from neurosecretory neurons mostly located in the medial basal hypothalamus of primates, and the preoptic region of rodents 1, 2. These changes are, in turn, determined by modifications in transsynaptic 3 and glial 4 inputs to the GnRH neuronal network. While the transsynaptic changes involve an increase in excitatory inputs and a reduction in inhibitory influences 1, the glial component is predominantly facilitatory, and exerted by both growth factors and small molecules that directly or indirectly stimulate GnRH secretion 4. The direct excitatory transsynaptic regulation of GnRH secretion is provided by at least three different neuronal subsets: kisspeptin neurons acting via GPR54 receptors 5, glutamatergic neurons acting mostly via AMPA receptors 6, 7, but also NMDA receptors 7, 8, and GABA acting via ionotropic GABAA receptors 9. The inhibitory counterpart of this circuitry depends principally on GABAergic neurons acting via GABAB metabotropic receptors 9, but also on opiatergic neurons that employ different peptides and a variety of different receptors for inhibitory neurotransmission [reviewed in 1].
As predicted by the complexity of this cellular network, several reports have suggested that no isolated pathway or cellular subset is solely responsible for the neuroendocrine control of puberty 10–12. Instead, initiation of this process may require regulatory gene networks controlled by a handful of “upstream” genes 10. Some of these central nodes have been identified, including the POU-domain gene Oct2, the homeodomain gene Ttf1/Nkx2.1, and a novel Zinc finger-containing gene termed EAP1 (Enhanced At Puberty1) 13. Although monogenic mutations, such as those affecting GNRHR 14, GPR54 15, 16, KiSS1 17, TAC3 and TACR3 18, result in pubertal failure, it does not appear that these are the only puberty-relevant genes as genome-wide association studies have shown that variants of more than 30 genes are associated with the age of menarche in humans 19.
It is thus apparent that the genetic underpinnings of puberty are multigenic, but this realization does not explain how inherited, permanent changes in DNA sequence can regulate gene expression dynamically, while also imposing an encompassing level of coordination and transcriptional plasticity to the gene networks involved. Here we develop the concept that a biological regulatory system that meets these requirements is epigenetics. Our results provide proof-of-principle for the view that the timing of female puberty is under the regulatory control of an epigenetic mechanism of transcriptional repression. We identify the Polycomb group (PcG) of transcriptional silencers 20 as integral parts of this repressive mechanism, and implicate two PcG genes (Cbx7 and Eed) as core components of the PcG complex operating in the prepubertal hypothalamus. Using the Kiss1 gene as a prototype of a gene whose products are directly involved in controlling GnRH output 21, we provide evidence for the view that the PcG complex represses the advent of reproductive maturity by targeting downstream genes involved in the stimulatory control of GnRH secretion at puberty.
RESULTS
Inhibition of DNA methylation results in pubertal failure
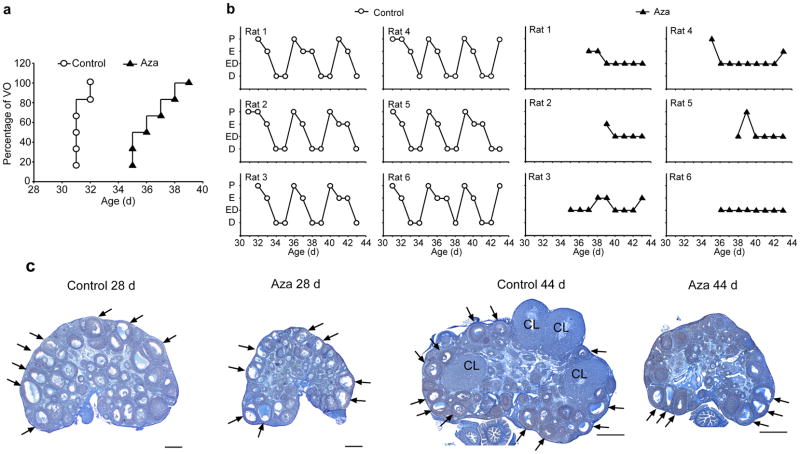
To gain insights into the potential contribution of DNA methylation to the regulation of puberty, we inhibited DNA methylation by treatment with 5′-Azacytidine (Aza), a well-established DNA methyl transferase (DNMT) inhibitor 22, 23. The treatment (2 mg/Kg BW/day, i.p) was initiated on postnatal day (PND) 22, which in the rat corresponds to the initiation of the early juvenile (EJ) phase of pubertal development 2. We first evaluated the effect of Aza on the timing of puberty and estrous cyclicity, by continuing the treatment until PND44, i.e., nearly two weeks after all control animals had reached puberty. In all subsequent studies, the animals were treated only for the duration of the juvenile period, i.e., from PND22 to PND28. Rats subjected to long-term Aza treatment had delayed vaginal opening (Fig. 1a), (mean age at vaginal opening: C= 31.33 ± 0.21, n=6 vs Aza= 36.67 ± 0.67 days; t=−7.628, p<0.001, Student t Test), failed to reach puberty as assessed by the lack of ovulation, and showed no estrous cyclicity, as determined by daily vaginal lavages after vaginal opening (Fig. 1b). These alterations did not appear to result from a general, non-specific effect of Aza, because the animals treated with the inhibitor weighed significantly more (20 g) than control animals at the time of vaginal opening, and had not achieved puberty at the time of euthanasia even though they weighed 35 g more than the weight reached by controls at the time of first ovulation (Supplementary Fig. 1). Morphological examination of the ovaries either at PND 28, which marks the transition between late juvenile (LJ) development and the initiation of puberty 2 or on PND 44 showed that the ovaries of Aza-treated rats had no overt abnormalities, but were developmentally delayed (Fig. 1c). By PND 28, these ovaries had only small antral follicles and were about half the size of a control ovary. At PND 44, the ovaries of Aza treated rats had antral follicles, but no corpora lutea, indicating that they had not ovulated, and consequently, puberty had failed to occur.
Figure 1. In vivo inhibition of DNA methylation results in pubertal failure.
(a) Female rats treated with 5-Azacytidine (Aza; i.p., 2mg/kg BW/day) from PND22 onwards have delayed vaginal opening. At the time when vaginal opening has occurred in all control animals (PND32), vaginal patency was not apparent in any of the Aza-treated rats. (b) Estrous cycle profiles showing that Aza treatment markedly disrupts estrous cyclicity. The phases of the estrous cycle depicted on the Y axis are proestrous (P), estrous (E), a transitional stage estrous-diestrous (ED), and diestrous (D). (c) Microphotographs illustrating the delay in ovarian maturation caused by Aza treatment during the juvenile phase of prepubertal development (PND 22 to 28) and the absence of ovulation, assessed by the lack of corpora lutea (CL) in ovaries from young adult (PND day 44) rats. Arrows point to examples of antral follicles. Scale bars = 100 μm.
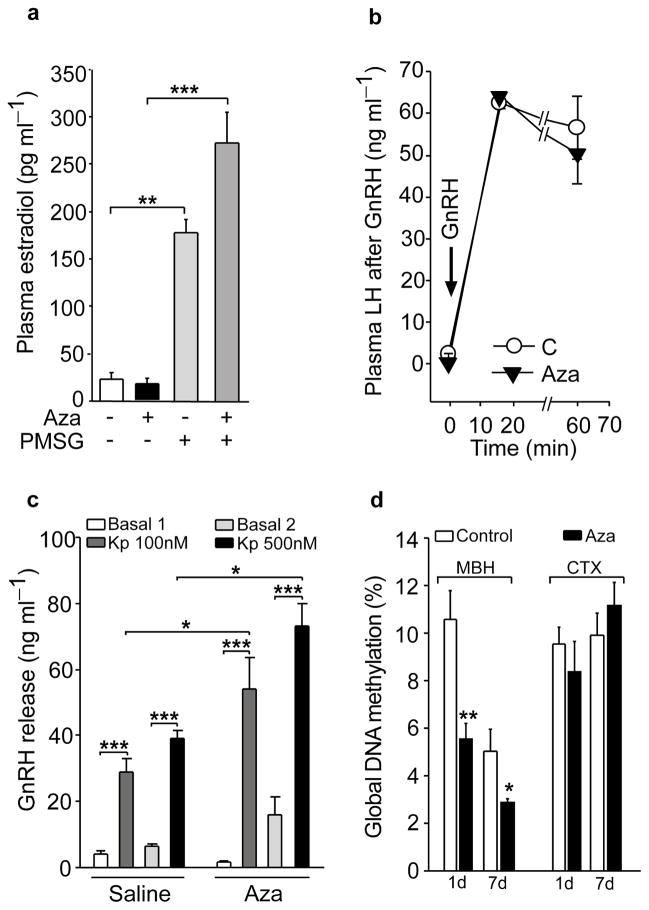
To determine the site where Aza may be acting to prevent the advent of puberty, we first examined the competence of the ovary to respond to gonadotropins with estradiol production. We treated rats with Aza from PND 22 to 28, administered a single s.c injection of pregnant mare serum gonadotropin (PMSG, 8 IU/rat) on PND 26, and collected trunk blood for estradiol measurement on PND 28. The Aza treatment did not inhibit, and even enhanced, the estradiol response of the ovary to PMSG (Fig. 2a). This outcome suggested that the delay in puberty is due to a central or pituitary, instead of an ovarian defect. Consistent with this interpretation, basal plasma LH levels were lower in 28-day-old Aza treated rats than vehicle-treated controls (C= 1.89 ± 0.41 ng ml−1, n=19 vs Aza= 0.55 ± 0.16 ng ml−1, n=23; t=3.23, p=0.002, Student t Test), and Aza treated rats had a greatly diminished LH response to ovariectomy, performed on PND 24 and assessed on PND 28 (C=52.56 ± 6.93 ng ml−1, n=6 vs Aza=7.43 ± 2.76 ng ml−1, n=6; t=6.48, p<0.001, Student t Test). Despite this deficiency, the pituitary response to in vivo administration of GnRH on PND 28 was normal in Aza treated rats (Fig. 2b), indicating the absence of a pituitary defect. To evaluate the ability of GnRH neurons to respond to a physiological neuroendocrine stimulus, medial basal hypothalamic (MBH) fragments from PND 28 rats, which contain the median eminence-arcuate nucleus (ME-ARC) region, were exposed to kisspeptin, a potent GnRH secretagogue 24. The ME-ARC from Aza treated rats responded to kisspeptin with significantly more GnRH release than vehicle-treated controls (Fig. 2c), suggesting cellular hyper-responsiveness presumably due to a deficiency in endogenous kisspeptin production.
Figure 2. In vivo inhibition of DNA methylation prevents puberty by disrupting developmental events upstream from the GnRH-pituitary-ovarian axis.
(a) Treatment with Aza (2mg/kg BW/day/7days, PND 22–28) does not prevent, but instead augments, the estradiol response of the ovary to stimulation with gonadotropins (PMSG, 8 IU/rat, given as a single i.p injection on PND 26; two days before blood collection)(F3,31=47.81, p<0.001, One-Way ANOVA followed by the SNK multiple comparison test, n=8 animals/group). (b) GnRH (6.5 μg/kg i.p) injection increases plasma LH similarly in control and Aza treated animals (there is a statistically significant difference between time points within each treatment, F2,7=277.23, p<0.001, but not between treatments, F1,7=0.46, p=0.52 with no significant interaction between time points and treatments, F2,47=0.56, p=0.58, Two Way Repeated Measures ANOVA, n=8 rats/group). (c) Hyper-response of the GnRH system to kisspeptin, as determined by the in vitro release of GnRH from ME-ARC fragments (derived from PND 28 rats) incubated with either 100 or 500 nM of Kisspeptin-10 (Kp) (there is a statistically significant interaction between treatment group and Kp dose, F3,47=5.93, p=0.007, Two Way Repeated Measures ANOVA, n=6 rats/group). (d) The Aza treatment reduces global DNA methylation in the female rat hypothalamus but not in the cerebral cortex (CTX) as compared with diluent-treated controls (C) (In MBH: One day of treatment t=3.65, p=0.004, 7 days of treatment t=2.98, p=0.044. In CTX no statistical significance was found, t=0.803, p=0.441 and t=−0.928, p=0.375 one and seven day treatment respectively, Student t Test, n=6 rats/group). Columns or circles are means and vertical lines represent S.E.M. *p<0.05; **p<0.01; ***p<0.001
Because these results implied a hypothalamic site of Aza action, we carried out a study to verify the ability of Aza to reduce DNA methylation in the hypothalamus. We measure global DNA methylation in the MBH and cerebral cortex at two intervals (one and seven days) after initiating the Aza treatment. At both time points DNA methylation was significantly reduced in the MBH, but not the cerebral cortex (Fig. 2d). This difference may be due to the lack of a fully functional blood brain barrier in the MBH, a feature that allows significant transfer of macromolecules from the bloodstream to the hypothalamic parenchyma 25, 26.
To determine if Aza may have delayed puberty by affecting hormonal systems other than that controlling the hypothalamic-pituitary-ovarian axis, we measured serum prolactin (PRL), because PRL is produced independently from LH, delays puberty when secreted at subnormal levels, and disrupt estrus cyclicity when produced in excess 2. We found that serum PRL levels were identical in diluent and Aza-treated rats(C=1.9 ± 0.4 ng ml−1, n=8 vs Aza=1.8 ± 0.3 ng ml−1, n=7; t=0.433, p=0.67, Student t Test). To examine still another endocrine system, we measured serum corticosterone, an adrenal steroid known to delay the onset of puberty in rats when secreted in excess in response to stress 2. Instead of being elevated, corticosterone levels were reduced in 5-Aza treated rats (C=232.8 ± 49.5 ng ml−1, n=7 vs Aza=47.1 ± 11.8 ng ml−1, n=7; t=9.66, p<0.001, Student t Test), possibly due to a diminished ovarian estradiol output 27. Thus, the delay of puberty caused by Aza treatment is not due to either deregulation of PRL secretion or corticosterone hypersecretion. Altogether, these results suggest that inhibition of DNA methylation delays puberty by unleashing a repressive mechanism that, operating within the neuroendocrine brain, keeps puberty in check by inhibiting genes (such as Kiss1) required for puberty to occur.
A repressive complex is silenced at puberty
To search for potential repressor genes that may become more methylated in the hypothalamus at puberty, we used DNA methylation arrays to interrogate the MBH at different pubertal stages (EJ, LJ and LP) (data available at http://www.ncbi.nlm.nih.gov/gds/?term=GSE38505). EJ and LJ were defined earlier; the LP (late proestrus) phase of puberty corresponds to the day of the first preovulatory surge of gonadotropins, which in the rat can be considered as mid-puberty 2. Using the bioinformatics methods described in the on-line Supplementary Information section, we observed that genes with a pattern of changing methylation at either the LJ or LP phases of puberty were functionally enriched for a cluster of chromatin/histone modification terms. Several of these genes are components of a common regulatory domain as they were either members of the PcG silencing complex (Cbx7, Cbx8, Phc3, Ring1B/Rnf2, and Yy1) or encoded proteins that interact with PcG proteins (Rybp, Csnk2b, Kdm2b). With the exception of Ring1B/Rnf2, all of these genes exhibited a general pattern of increased promoter methylation at puberty (Supplementary Fig. 2), suggesting that the PcG silencing complex may undergo functionally important epigenetic changes with the advent of puberty.
The PcG silencing complex is considered as a master regulator of genomic programs, because it acts at different stages of development to define which sets of genes are active and which ones are quiescent 20, 28. The PcG system is composed of three repressive complexes (PRC1, PRC2 and PhoRC) working together to bring about gene silencing 20, 28. The PRC1 complex (Supplementary Fig. 3) includes a group of proteins termed CBX, because they contain a highly conserved chromodomain (CBX) at their amino terminus 29. The mammalian homologs of Drosophila polycomb proteins are CBX2, 4, 6, 7, and 8 28. In different cells, the PRC1 complex may contain different CBX proteins 30. The PRC2 complex includes four core subunits: enhancer of Zeste (EZH1, EZH2), suppressor of Zeste [SUZ12], and the WD40 domain proteins EED and P55 (RBBP4 and RBBP7) 28 (Supplementary Fig. 3). PhoRC, the third PcG complex, contains two proteins, Pho and its homologue Phol, which bind directly to DNA 28. In mammals, these proteins are encoded by the Yy1 gene (Supplementary Fig. 3), which has both repressive and activating functions 31.
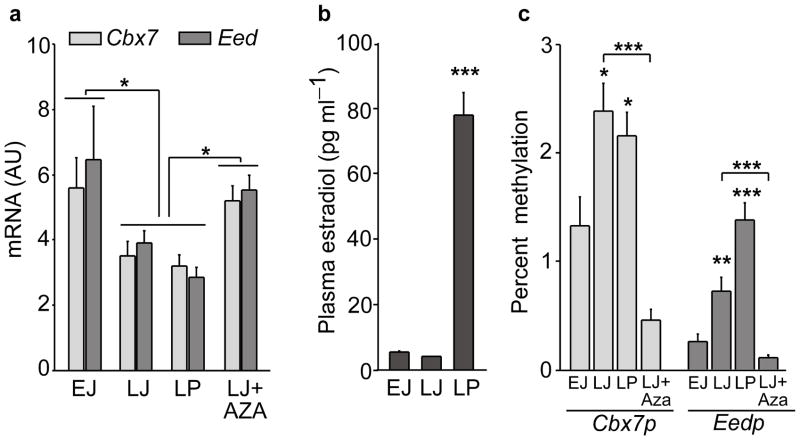
To determine if the changes in promoter DNA methylation of PcG genes suggested by the arrays are accompanied by altered gene expression, we focused our attention on the PcG complex and measured by quantitative (q) PCR the mRNA abundance of most members of this complex in the MBH at the time of puberty. We observed that only expression of two PcG genes required for appropriate PcG function 29, 32, the PRC1 member Cbx7 and the PRC2 member Eed (not represented in the DNA arrays), decreases at LJ, i.e., at the time when puberty is initiated in the female rat 2. This reduction was maintained throughout puberty (Fig. 3a), seemingly unaffected by the peripubertal increase in serum estradiol levels (Fig. 3b), but was prevented by the administration of Aza (Fig. 3a). Among the other members of the PcG complex examined, only Yy1 mRNA levels decreased significantly at puberty, but this decrease occurred much more gradually (Supplementary Fig. 3). No reduction in mRNA levels of any member of the PcG complex, including Cbx7 and Eed, was seen in the preoptic area (POA) region during puberty (Supplementary Fig. 3), indicating that the pubertal decrease in Cbx7 and Eed expression is MBH-specific.
Figure 3. The initiation of puberty is accompanied by increased promoter methylation and decreased expression of two PcG genes required for PcG-mediated gene silencing in the medial basal hypothalamus (MBH).
(a) Cbx7 and Eed mRNA expression decrease in the MBH at the initiation of puberty and this decrease is prevented by treatment with Aza. EJ = early juvenile; LJ = late juvenile; LP = late proestrus, the day of the first preovulatory surge of gonadotropins. (For Cbx7 expression: F3,31= 3.72, p=0.022 and for Eed expression: F3,31= 6.07, p=0.003, One Way ANOVA, n=6–10 rats per group). (b) Changes in plasma estradiol levels at the time of female puberty (F2,17=109.18, p<0.001, One Way ANOVA, n=6 rats/group). (c) Methylation of the promoter regions of Cbx7 and Eed (Cbx7p and Eedp, respectively) increases in the MBH with the advent of puberty as determined by mCytosine immunoprecipitation followed by real time PCR of immunoprecipitated DNA. Treatment with Aza prevents the increase in promoter methylation. The results are expressed as percent methylation (signal from mC immunoprecipitated DNA/signal from input DNA x 100) (Cbx7p methylation: F3,18=17.6, p<0.001 and Eedp methylation: F3,18=23.22, p<0.001, One Way ANOVA, n=4–5 rats per group). Columns are means and vertical bars are S.E.M. *p<0.05; **p<0.01; ***p<0.001 vs. EJ or Aza groups (in all cases ANOVA was followed by the SNK multiple comparison test for unequal replications).
We observed that the advent of puberty is associated with increased promoter methylation of both Cbx7 and Eed (Fig. 3c), and that this change was prevented by Aza (Fig. 3c). The regions examined for changes in methylation are shown in Supplementary Fig. 4. The increase in DNA methylation did not result from changes in circulating estradiol levels, because it was distinct at LJ, when plasma estradiol levels are still low, and remained unabated on the day of the first preovulatory surge of gonadotropins (Fig. 3c), when plasma estradiol levels are massively increased (Fig. 3b). Altogether, these results suggest that the onset of female puberty is accompanied by active epigenetic repression of the PcG silencing system in the MBH of female rats.
The Kiss1 gene is a downstream target of PcG repression
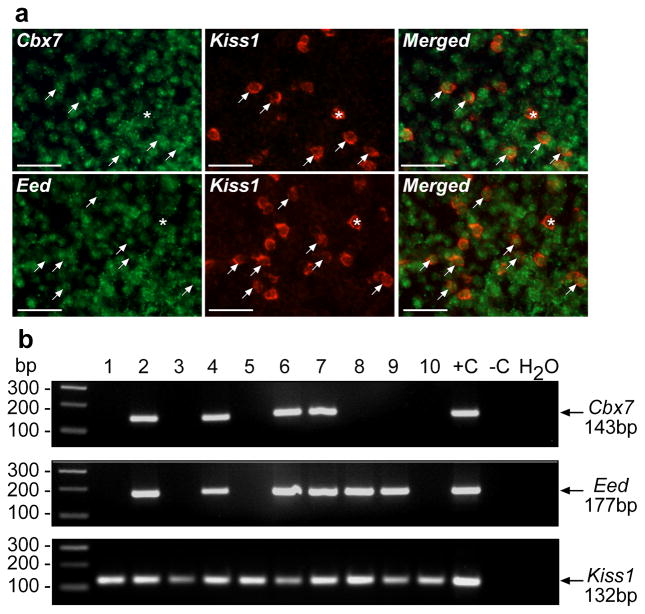
In all mammalian species so far studied the first neuroendocrine manifestation of puberty is a diurnal increase in pulsatile GnRH and hence LH release [reviewed in 2]. This mode of GnRH secretion has been postulated to be driven by a subset of ARC neurons, called KNDy neurons 33, 34, because they produce Kisspeptin, NKB and Dynorphin 33. Since kisspeptin and NKB work coordinately within the population of KNDy neurons to stimulate GnRH secretion, their encoding genes (Kiss1 and Tac2) can be considered as components of a unique class of puberty-activating genes. Using Kiss1 as a prototype of this class, we carried out studies to determine if Cbx7 and Eed are expressed in kisspeptin neurons of the ARC. Double fluorescent in situ hybridization (Fig. 4a) and single-cell PCR (Fig. 4b) of eGFP-tagged kisspeptin neurons 35 showed that these neurons contain both Eed and Cbx7 mRNAs.
Figure 4. The Cbx7 and Eed genes are expressed in kisspeptin neurons of the ARC.
(a) Double fluorescent in situ hybridization showing the presence of Cbx7 and Eed mRNA transcripts (green color) in Kiss1 mRNA containing neurons (red color). Arrows point to double positive neurons; asterisks denote Kiss1-mRNA positive neurons without detectable Cbx7 or Eed mRNA. Scale bar= 20μm. (b) Single cell-PCR of eGFP-tagged kisspeptin neurons 35 demonstrating the presence of Cbx7 and Eed mRNAs in these cells.
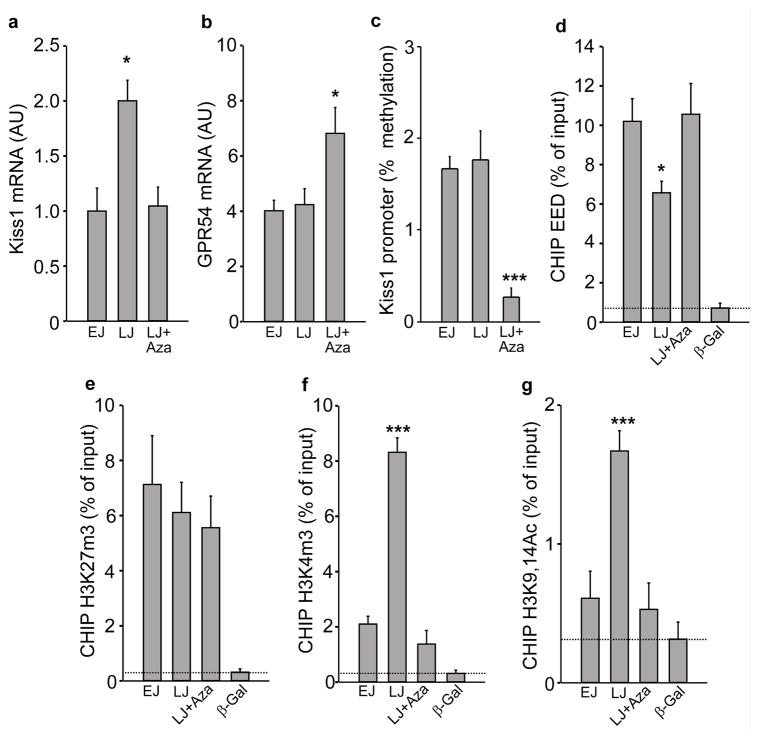
Measurement of the mRNAs encoding kisspeptin and its receptor GPR54 in the MBH demonstrated that Kiss1 mRNA abundance increases in this brain region between EJ and LJ, and that inhibition of DNA methylation prevented this change (Fig. 5a). In contrast Gpr54 mRNA levels remained unaltered in LJ animals as compared with EJ rats, but were significantly increased by the inhibition of DNA methylation (Fig. 5b). These results suggest that the hyper-responsiveness of the GnRH neuronal network to kisspeptin seen in Aza-treated rats may be related, at least in part, to a reduced endogenous production of kisspeptin in the presence of upregulated levels of its GPR54 receptor. Methylation of the Kiss1 promoter remained unchanged in the MBH of LJ animals as compared to EJ rats (Fig. 5c), indicating that the increase in Kiss1 mRNA abundance observed at the end of juvenile development is not caused by alterations in promoter methylation. Although Aza decreased Kiss1 promoter methylation (Fig. 5c), it obliterated the pubertal increase in Kiss1 mRNA levels, suggesting that inhibition of DNA methylation prevents the pubertal increase in Kiss1 expression by mechanisms other than changes in Kiss1 promoter methylation.
Figure 5. Increased Kiss1 expression in the MBH at the initiation of puberty is accompanied by eviction of EED from the Kiss1 promoter and changes in associated repressive and activating histone modifications, without changes in DNA methylation.
(a) Kiss1 mRNA abundance increases in the MBH at LJ and this increase is prevented by inhibition of DNA methylation (F2,21=7.11, p=0.005),. (b) Gpr54 mRNA abundance does not change in the MBH at the onset of puberty, but increases after inhibition of DNA methylation (F2,21=4.78, p=0.021). (c) Methylation of the Kiss1 promoter in the MBH does not change at puberty but it is diminished by inhibition of DNA methylation (F2,13=13.93, p<0.001). (d) EED is evicted from the Kiss1 promoter at the initiation of puberty and this eviction fail to occur in Aza treated animals (F3,19=17.98, p<0.001). (e) The association of H3K27m3 to the Kiss1 promoter does not decrease at LJ and is not affected by Aza (F3,22=3.45, p=0.037). (f) The abundance of H3K4m3 increases at LJ and the increase is prevented by inhibition of de novo DNA methylation (F3,23=76.09, p<0.001). (g) The association of H3K9,14Ac also increases at LJ and this increase fails to occur in Aza treated rats (F3,23=12.43, p<0.001). For all panels n=6–8 animals/group, except panel c (n=4–5). Columns are means and vertical bars are S.E.M. *p<0.05; ***p<0.001 vs. EJ (in all cases One Way ANOVA was followed by the SNK multiple comparison test for unequal replications). Antibodies to β–Galactosidase (β–Gal) (a protein not expressed in the rat) was used as negative control; dotted line depicts minimum sensitivity level of the technique.
Because MBH expression of both Cbx7 and Eed appears to be DNA methylation-dependent, and considering that EED is essential for PcG action 32, we selected Eed for further analysis. In silico analysis of the Kiss1 promoter (Supplementary Fig. 5a) demonstrated that it contains several motifs found to be present in PcG target genes, including the core motif for YY1 binding (CCAT), the GAF (GAGAG) and extended MPho (CNGCCATNDNND) motifs 36, the two BMI1 binding motifs (CCTTCC and GGNNNGNG) reported by Meng et al. 37, and the binding motif for HOTAIR, a long noncoding RNA, recently shown to serve as an anchor for PcG binding to gene promoters 38. To determine if these motifs have biological significance, we performed gene promoter assays and observed that EED indeed represses Kiss1 promoter activity and that this repressive effect is enhanced by YY1 (Supplementary Fig. 5b). Next, we carried out ChIP assays to determine: a) If EED is recruited to the Kiss1 promoter in the MBH, and b) if this relationship changes during the onset of puberty. We observed that the EED protein was associated with the Kiss1 promoter in EJ and this association decreased at LJ, the time of initiation of puberty (Fig. 5d). Consistent with the notion that inhibition of DNA methylation leads to increased expression of PcG genes, which then repress downstream targets genes, the pubertal loss of EED association to the Kiss1 promoter failed to occur in Aza treated rats (Fig. 5d). The chromatin status of the Kiss1 promoter also changed at the time of puberty. Whereas the content of H3K27me3, a PcG-dependent repressive histone modification 39, 40 did not decrease significantly in LJ animals (Fig. 5e), the abundance of two activating histone modifications, H3K4me3 and H3K9,14ac 39, 41, 42 increased markedly at this time (Fig. 5f,g). Treatment with Aza, which prevented the eviction of EED from the Kiss1 promoter at LJ, also prevented the LJ increase in both H3K4me3 and H3K9,14ac abundance (Fig. 5f,g). To determine if the chromatin landscape of the Kiss1 promoter continues to change as the pubertal process unfolds, we measured both H3K27me3 and its opposing counterpart H3K4me3 43 at LP, when the preovulatory surge of gonadotropins take place, and found a significant decrease in H3K27me3 levels, accompanied by persistently elevated levels of H3K4me3 (Supplementary Fig. 6a). Altogether these results are compatible with the notion that a repressive PcG-depending tone on the Kiss1 gene is lifted at the onset of puberty, and the status of the associated chromatin shifts from an inhibitory to an activating configuration, leading to activation of the Kiss1 gene (Supplementary Fig. 6b)
Overexpressing EED compromises reproductive capacity
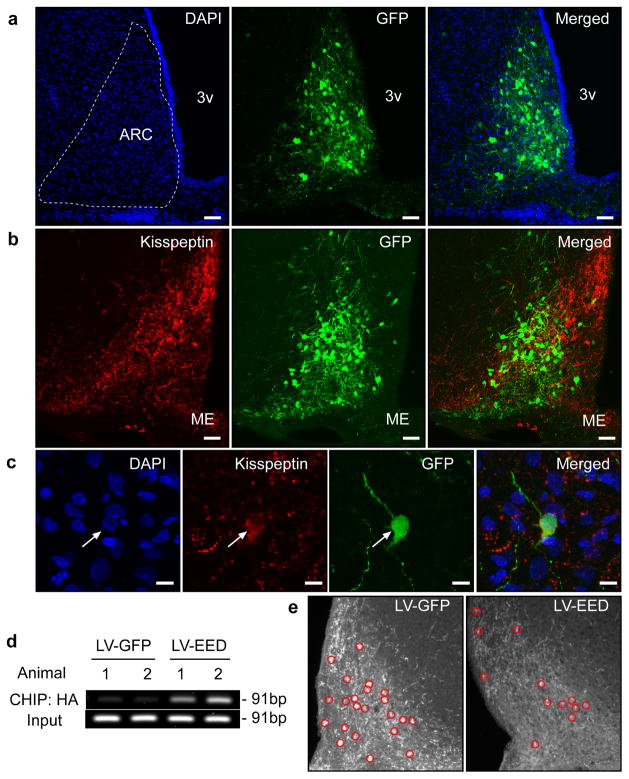
If PcG proteins are physiologically involved in the neuroendocrine control of female puberty via repression of the Kiss1 gene in the ARC, preventing the pubertal decrease in PcG gene expression that occurs in this hypothalamic region at the onset of puberty would be expected to delay the pubertal process. Because Eed is required for the silencing activity of the PcG complex 32, we chose to overexpress EED in the ARC of immature female rats. We cloned the coding region of rat Eed tagged with a hemagglutinin epitope into a lentivirus vector (LV) that also expresses eGFP (Supplementary Fig. 7a). After confirming the production of the HA-tagged protein by western blot (Supplementary Fig. 7b) we stereotaxically delivered this construct (termed LV-EED) bilaterally into the hypothalamus of 26-day-old female rats, targeting the ARC. Control animals were injected with a construct expressing only eGFP (LV-eGFP). Immunohistofluorescent analysis of the sites of injection using antibodies against eGFP was used to identify the transduced cells (Fig. 6a). Kisspeptin neurons, also identified by immunohistofluorescence, were one of the cell populations transduced by the virus (Fig. 6b,c). ChIP analysis of DNA extracted from microdissected ARC tissue containing the transduced cells revealed that the LV-produced EED-HA protein had been recruited to the Kiss1 promoter (Fig. 6d). The number of detectable immunopositive kisspeptin cells per section decreased 25% in LV-EED injected animals (LV-GFP= 21.4 ± 1.98, n=31 vs LV-EED= 15.9 ± 1.22, n=6; t=3.65, p<0.001, Student t Test), and the abundance of kisspeptin immunoreactive material per cell was reduced by 30% in LV-EED-injected animals as compared to control rats injected with LV-GFP (LV-GFP= 141.4 ± 1.89, n=102 vs LV-EED= 95.2 ± 2.7, n=94; t=14.35, p<0.001, Student t Test) (Fig. 6e), indicating that EED overexpression compromises kisspeptin production in about 50% of the ARC population of kisspeptin neurons. This inhibition is consistent with our in vitro results showing a repressive effect of EED on Kiss1 promoter activity (Supplementary Fig. 5b).
Figure 6. EED delivered to the ARC of immature female rats is recruited to the Kiss1 promoter and represses kisspeptin expression.
Double immunostaining for GFP (Green) and Kisspeptin (Red) in the ARC of an LV-EED injected 44 day-old rat. (a) Cell nuclei of the ARC region identified by DAPI staining (blue) and transduction of ARC neurons by the LV-EED construct. (b) Kisspeptin positive neurons transduced by LV-EED. (c) Higher magnification view of a kisspeptin neuron transduced with the LV-EED construct. Scale bars: 100μm (a–b), 10μm (c). (d) CHIP assay from two controls (LV-GFP) and two experimental (LV-EED) animals showing association of EED-HA to a genomic region that includes the Kiss1 transcription start site as determined by PCR amplification of DNA immunoprecipitated with antibodies recognizing the HA epitope tagging EED. (e) Detection of kisspeptin neuron cell bodies in the ARC after micro-injection of either LV-GFP or LV-EED. Red circles identify kisspeptin positive cell bodies.
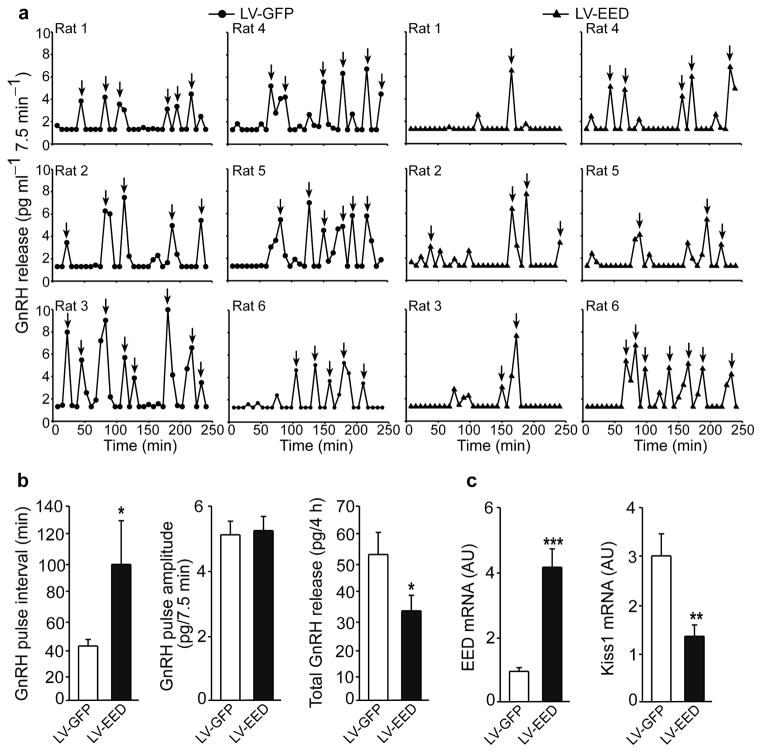
To determine if overexpression of EED alters pulsatile GnRH release from the hypothalamus we delivered LV-EED or LV-GFP to the ARC of a group of 22-day-old female rats, dissected the ARC-ME region seven days later, and incubated the tissues for 3h in Krebs-Ringer bicarbonate buffer, sampling the medium every 7.5 min, to measure GnRH output 44. The ARC-ME of animals injected with LV-GFP showed a robust pattern of pulsatile GnRH release with episodes of secretion occurring every 41 ± 2.7 min (Fig. 7a,b). In contrast, GnRH pulse frequency was reduced (p<0.05; Mann-Whitney Rank Sum test) to one pulse every 98 ± 30.7min in LV-EED injected rats (Fig. 7a,b). GnRH pulse amplitude was not affected (Fig. 7b), but total GnRH output was reduced in the LV-EED treated group (Fig. 7b). Measurement of Eed and Kiss1 mRNAs at the end of the incubation demonstrated that the ARC-ME of animals receiving LV-EED had 4 times more Eed mRNA than the ARC-ME of animals injected with LV-GFP (Fig. 7c), and that – consistent with the immunohistochemistry data – Kiss1 mRNA was reduced by 50% (Fig. 7c).
Figure 7. EED delivered to the ARC of immature female rats inhibits GnRH pulse frequency without changing pulse amplitude.
Seven days after stereotaxic delivery of lentiviral particles, ARC-ME explants derived from animals expressing GFP (LV-GFP, circles) or EED (LV-EED, triangles) were incubated for 4h in Krebs-Ringer-Bicarbonate buffer with the medium changed every 7.5min for assessment of pulsatile GnRH release. (a) Individual profiles of GnRH release from LV-GFP and LV-EED injected rats, n = 6 rats/group. Arrows indicate individual pulses. (b) Left panel: GnRH pulse frequency was significantly (t=25.5, p=0.026, Mann-Whitney Rank Sum test) reduced in LV-EED injected animals, as determined by an increase in interpulse interval; Middle panel: pulse amplitude remained unchanged (t=-0.307, p=0.765, Student t Test); Right panel: Total GnRH output/4h incubation was significantly (t=2.61, p=0.026, Student t test) reduced in the LV-EED treated animals. (c) Eed mRNA content was 4-fold higher (t=−5.77, p<0.001) and Kiss1 mRNA content 2 times lower (t=3.17, p=0.01) in LV-EED injected animals than in LV-GFP injected rats (Student t test, in all cases n=6 rats/group). Columns are means and vertical bars are S.E.M..
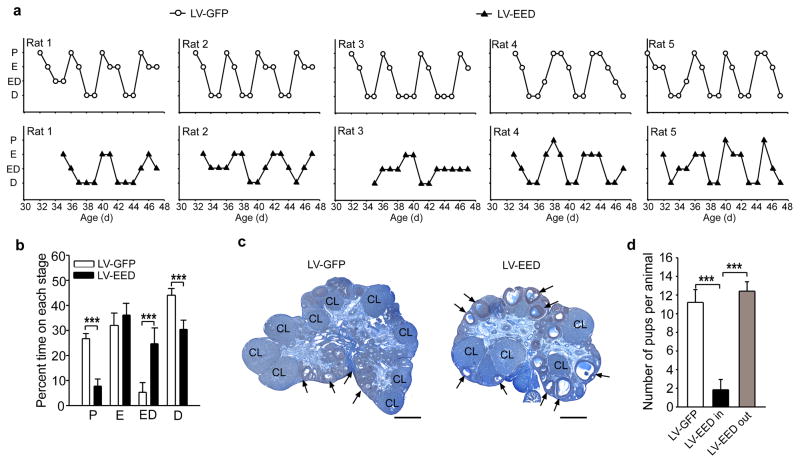
In keeping with these observations, an additional experiment showed that the age at first ovulation, assessed by the detection of cornified cells (first estrus) in vaginal smears followed by two consecutive days of leukocytes,(a condition highly correlated with ovulation) was delayed several days in LV-EED-injected rats (age at first ovulation: LV-GFP= 33 ± 0.31, n=5 vs LV-EED= 37.8 ±0.6, n=6; t=−16.12, p<0.001, Student t Test), and estrous cyclicity was disrupted (Fig. 8a,b). Examination of the ovaries at 50 days of age showed that LV-EED injected animals had some corpora lutea indicating that they had ovulated, but also exhibited an excess of antral follicles that had not reach the periovulatory stage (Fig. 8c). In contrast, LV-GFP-injected rats had an abundance of corpora lutea indicative of repeated ovulations (Fig. 8c). In a third experiment, the LV-GFP and LV-EED constructs were delivered to the ARC of 22-day-old rats and after all animals in the LV-GFP injected group showed three complete estrous cycles, all the animals were exposed to a fertile male for five days. We observed that rats in which the LV-EED construct was correctly targeted to the ARC had fewer pups or failed to deliver a litter upon exposure to a fertile male (Supplementary Table 3), in contrast to the >90% fertility observed in LV-GFP-injected controls (Fig. 8d). Thus, preventing the reduction in Eed expression that occurs in the ARC at the onset of puberty compromises GnRH pulsatile release, delays the pubertal process, disrupts estrous cyclicity, reduces ovulation, and decreases fecundity. Altogether, these results are consistent with the interpretation that the onset of female puberty is controlled by a PcG-dependent repressive mechanism involving silencing of the Kiss1 gene in kisspeptin neurons of the MBH.
Figure 8. EED delivered to the ARC of immature female rats delays puberty, disrupts estrous cycle and impairs fertility.
(a) Estrous cycles of LV-GFP (circles) and LV-EED (triangles) injected animals. (b) Disruption of estrous cyclicity by LV-EED. Results are shown as percent time spent at each particular phase during a 21-day period. P = proestrous (t=11.96, p<0.001), E = estrous (t=−1.52, p=0.159), ED = transitional stage estrous-diestrous (t=−6.077, p<0.001), D = diestrous (t=7.203, p<0.001) (Student t Test, n= 5 rats/group). (c) Example of ovaries from LV-GFP and LV-EED injected rats collected on PND44. CL indicates corpora luea and arrows point to examples of antral follicles. Scale bars = 100μm. (d) Fertility rate measured as the number of pups delivered after exposing LV-GFP and LV-EED injected females to a fertile male. LV-EED in, injections correctly placed into the ARC; LV-EED out, misplaced injections located outside the ARC. (F2,14=23.92, p<0.001, One Way ANOVA. n=5 rats/group). Columns are means and vertical bars are S.E.M. ***p<0.001 (ANOVA was followed by the SNK multiple comparison test for unequal replications to compare more than two groups).
DISCUSSION
The potential contribution of epigenetics to the regulation of puberty has never been addressed. In the present report, we provide evidence that an epigenetic mechanism of transcriptional repression, operating within the neuroendocrine brain, plays a significant role in the timing of female puberty. Our results identify the PcG system of transcriptional silencing 20, 28 as a central element of this repressive mechanism. Hypothalamic expression of Cbx7 and Eed, two PcG genes required for PcG action 29, 32, decreases preceding the onset of puberty, and this change is associated with increased DNA methylation of their 5′-flanking regions. Conversely, pharmacological inhibition of DNA methylation prevented the pubertal increase in Eed and Cbx7 DNA methylation, reversed the low peripubertal Eed and Cbx7 mRNA levels to elevated early juvenile values, and delayed puberty. This delay was not due to a non-specific or toxic effect of the inhibitor, because the animals failed to reach puberty despite exhibiting a body weight much greater than that attained by control rats at puberty. Moreover, it was not caused by changes in the secretion of two different hormones, PRL and corticosterone, which in deficiency (PRL) or excess (corticosterone) have been previously shown to delay puberty in the rat [reviewed in 2].
Within the hypothalamic-pituitary-ovarian axis, inhibition of DNA methylation did not affect the capacity of the ovary to respond to gonadotropin stimulation with estrogen release, and failed to alter the pituitary gonadotropin response to GnRH, suggesting a central site of action. Direct assessment of the GnRH response to kisspeptin, a major GnRH secretagogue 24, revealed that GnRH neurons of Aza-treated animals are hyper-responsive, instead of unresponsive, to kisspeptin. Although 5-Aza, like other DNMT inhibitors, may also act via mechanisms other than DNA methylation 45, 46, our results are consistent with the interpretation that pharmacological inhibition of DNA methylation prevents a methylation event scheduled to occur at the onset of puberty. Without ruling out GnRH neurons as direct targets of epigenetic control 47, our results suggest that: a) the pubertal delay caused by inhibition of DNA methylation involves cellular subsets functionally connected to the GnRH neuronal network, and b) the deficit may result from the activation of repressive genes whose expression would normally decrease at puberty. By inference, these repressors would be expected to negatively control the expression of downstream genes which need to be activated for puberty to occur.
A search for such repressors using DNA methylation arrays suggested that the initiation of puberty was accompanied by changing promoter methylation of several members of the PcG repressive complex and genes encoding proteins that interact with the PcG system. If this change is predictive of opposite changes in gene expression, one would expect to find decreased hypothalamic expression of PcG genes either during puberty or immediately antedating the initiation of this event. Measuring the expression of most PcG components in the MBH by qPCR demonstrated an early decrease in Cbx7 and Eed mRNA abundance preceding the initiation of puberty, and a significant drop in Yy1 expression at mid-puberty. EED is a PRC2 component required for PcG action 32. The decrease in Cbx7 and Eed expression occurred independently from changes in ovarian estrogen output, as it was essentially complete before the pubertal increase in circulating estrogen levels.
Because the kisspeptin-GPR54 system is critical for both puberty and adult reproductive function 15, 16, the Kiss1 gene can be considered as a prototype of the class of genes that need to be activated for puberty to occur. Accordingly, we used the Kiss1 gene to test the hypothesis that these puberty activating genes may be subjected to PcG repressive control. The increase in Kiss1 mRNA abundance that occur in the hypothalamus at the time of puberty was prevented, instead of enhanced by inhibition of DNA methylation, suggesting that a secondary mechanism set in motion by the loss of DNA methylation is responsible for the reduction in Kiss1 expression. A significant component of this mechanism appears to be the PcG silencing complex as the prepubertal association of EED to the Kiss1 promoter, which diminishes at the onset of puberty, is prevented by inhibition of DNA methylation.
It is now clear that PcG mediated gene silencing requires H3K27me3; a modification catalyzed by PRC2. H3K27me3 then provides a “docking” site for the CBX components of PRC1 to form a repressive complex 20, 28. In turn, YY1 recruits PRC2 and PRC1 proteins, in addition to H3K27me3, to gene promoters to enhance transcriptional silencing 32. The eviction of EED from the Kiss1 promoter at the onset of the pubertal process would predict a concomitant loss of H3K27me3 at this time. Instead, H3K27me3 content decreased at LP, i.e., by mid-puberty. Contrasting with this protracted pattern of change, the abundance of H3K4me3 and acetylated histone 3 (H3 K9/14ac), two histone marks associated with gene activation 39, 41, increased markedly at LJ, i.e., at the initiation of puberty. Because H3K4me3 is a histone mark that opposes the repressive actions of H3K27me3 43, we examined the association of H3K4me3 to the Kiss1 promoter at mid-puberty, and found it to remain as elevated as in LJ. This developmental profile is consistent with the pattern of bivalent association observed for H3K27me3 and H3K4me3 in the promoter of genes mildly de-repressed during development 48. The evolving presence of both marks on the Kiss1 promoter at puberty is also consistent with the concept of “bivalent” domains 48, i.e., the simultaneous presence of repressive and activating histone modifications 48, 49 in the regulatory region of genes thought to be poised for activation in response to developmental cues 50. Noteworthy, the pubertal increase in the association of activating histone marks to the Kiss1 promoter failed to occur in Aza treated rats. Because the pubertal EED eviction also fails to occur in these animals, the simplest explanation is that persistent EED occupancy diminishes accessibility of activating histone marks to the Kiss1 promoter.
Directly supporting the overall validity of a PcG-dependent repressive mechanism holding in check the initiation of puberty is the pubertal delay observed when the decline in hypothalamic Eed expression that occurs during normal puberty is prevented via targeted lentivirus-mediated gene delivery. Over-expression of Eed in the ARC of the hypothalamus, which contains the KNDy neurons required for pulsatile GnRH release 34 reduced the number of neurons expressing detectable levels of immunoreactive kisspeptin, the content of immunoreactive kisspeptin per cell, and the abundance of Kiss1 mRNA in the ARC. Importantly, it reduced pulsatile GnRH release, delayed puberty, and disrupted estrous cyclicity. Although the animals receiving lentiviral particles carrying the EED gene were still able to ovulate, the estrus cycle profiles displayed by these animals suggested that they were ovulating sporadically. This inference is supported by the finding that these animals exposed to a fertile male delivered an average of 2 pups as compared with 12 pups delivered by rats receiving either a control virus or a virus expressing EED but targeted outside the ARC.
By showing that the neuroendocrine control of female puberty involves the participation of a repressive mechanism of epigenetic regulation, our results provide a novel insight into the integrative mechanisms utilized by the neuroendocrine brain to control the initiation of mammalian puberty. As such, they are consistent with the concept that the pubertal process is not only dependent on genetic determinants, but also on developmentally regulated changes in epigenetic information. They also raise the possibility that human syndromes of idiopathic precocious and delayed puberty of central origin may have a previously unappreciated epigenetic component.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/natureneuroscience/.
Supplementary Material
Supplementary Figure 1. In vivo inhibition of DNA methylation results in pubertal failure despite of the animals reaching body weights much greater than the body weight attained by controls at the time of puberty
Supplementary Figure 2. DNA methylation arrays reveal change in promoter/CpG island methylation of members of the PcG silencing complex in the MBH at puberty
Supplementary Figure 3. Expression of two PcG genes required for PcG-mediated gene silencing decreases in the MBH, but not in the POA at the time of puberty
Supplementary Figure 4. Segments of the 5-flanking region of Cbx7, Eed and Kiss1 targeted for DNA methylation and ChIP analysis
Supplementary Figure 5 EED decreases Kiss1 promoter activity in vitro
Supplementary Figure 6. The abundance of the antagonistic histone modifications H3K27me3 and H3K4me3 associated with the Kiss1 promoter changes in opposite directions as puberty unfolds
Supplementary Figure 7. Preparation and in vitro testing of a lentivirus construct encoding an HA-tagged EED protein
Supplementary Table 1. Primers used in this study
Supplementary Table 2. Antibodies used for Western blots or ChIP assays
Supplementary Table 3. Number of pups per litter after exposing LV-GFP and LV-EED injected animals to a fertile male for five days
Acknowledgments
We thank Maria E. Costa for technical assistance with the in situ hybridization procedures and the preparation of ovaries for morphological observation. This work was supported by US National Institute of Health (NIH: HD025123-ARRA and 8P51OD011092), and US National Science Foundation (NSF: IOS1121691) to S.R.O and by NIH: NS43330, DK68098 to O.K.R, and Marie Curie International Outgoing Fellowship within the 7th European Community Framework Programme (FP7-PEOPLE-2010-IOF) to J.M.C.
Footnotes
AUTHOR CONTRIBUTIONS
A.L and S.R.O designed the project and wrote the paper. A.L was involved in all aspects of the study. S.R.O coordinated the project, and performed the intrahypothalamic injections of viruses, including the imaging studies. A.Loche and J.M.C. ran global methylation arrays, ChIP and targeted methylation assays. O.R and M.B. performed the single cell PCR experiments. G.K. measured mRNA levels by qPCR assays. J.G.K. determined the number of Kiss1 neurons in the ARC. H.W. designed the Perl script for the detection of HOTAIR consensus motifs, and analyzed the data from DNA methylation arrays. G.P.P advised us on methylation assays.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Plant TM, Witchel SF. Puberty in nonhuman primates and humans. In: Neill JD, editor. The Physiology of Reproduction. 3. Academic Press/Elsevier; San Diego: 2006. pp. 2177–2230. [Google Scholar]
- 2.Ojeda SR, Skinner MK. Puberty in the rat. In: Neill JD, editor. The Physiology of Reproduction. 3. Academic Press/Elsevier; San Diego: 2006. pp. 2061–2126. [Google Scholar]
- 3.Kordon C, Drouva SV, Martínez de la Escalera G, Weiner RI. Role of classic and peptide neuromediators in the neuroendocrine regulation of luteinizing hormone and prolactin. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. 2. Vol. 1. Raven Press; New York: 1994. pp. 1621–1681. [Google Scholar]
- 4.Ojeda SR, Lomniczi A, Sandau U. Contribution of glial-neuronal interactions to the neuroendocrine control of female puberty. Eur J Neurosci. 2010;32:2003–2010. doi: 10.1111/j.1460-9568.2010.07515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Messager S, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suter KJ. Control of firing by small (S)-alpha-amino-3-hydroxy-5-methyl-isoxazolepropionic acid-like inputs in hypothalamic gonadotropin releasing-hormone (GnRH) neurons. Neuroscience. 2004;128:443–450. doi: 10.1016/j.neuroscience.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 7.Iremonger KJ, Constantin S, Liu X, Herbison AE. Glutamate regulation of GnRH neuron excitability. Brain Res. 2010;1364:35–43. doi: 10.1016/j.brainres.2010.08.071. [DOI] [PubMed] [Google Scholar]
- 8.Christian CA, Pielecka-Fortuna J, Moenter SM. Estradiol suppresses glutamatergic transmission to gonadotropin-releasing hormone neurons in a model of negative feedback in mice. Biol Reprod. 2009;80:1128–1135. doi: 10.1095/biolreprod.108.075077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herbison AE, Moenter SM. Depolarising and hyperpolarising actions of GABA(A) receptor activation on gonadotrophin-releasing hormone neurones: towards an emerging consensus. J Neuroendocrinol. 2011;23:557–569. doi: 10.1111/j.1365-2826.2011.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ojeda SR, et al. Minireview: The neuroendocrine regulation of puberty: Is the time ripe for a systems biology approach? Endocrinology. 2006;147:1166–1174. doi: 10.1210/en.2005-1136. [DOI] [PubMed] [Google Scholar]
- 11.Krewson TD, et al. Chromosomes 6 and 13 harbor genes that regulate pubertal timing in mouse chromosome substitution strains. Endocrinology. 2004;145:4447–4451. doi: 10.1210/en.2004-0543. [DOI] [PubMed] [Google Scholar]
- 12.Seminara SB, Crowley WF., Jr Perspective: the importance of genetic defects in humans in elucidating the complexities of the hypothalamic-pituitary-gonadal axis. Endocrinology. 2001;142:2173–2177. doi: 10.1210/endo.142.6.8261. [DOI] [PubMed] [Google Scholar]
- 13.Ojeda SR, et al. The transcriptional control of female puberty. Brain Res. 2010;1364:164–174. doi: 10.1016/j.brainres.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bedecarrats GY, Kaiser UB. Mutations in the human gonadotropin-releasing hormone receptor: insights into receptor biology and function. Semin Reprod Med. 2007;25:368–378. doi: 10.1055/s-2007-984743. [DOI] [PubMed] [Google Scholar]
- 15.Seminara SB, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 16.de Roux N, et al. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lapatto R, et al. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007;148:4927–4936. doi: 10.1210/en.2007-0078. [DOI] [PubMed] [Google Scholar]
- 18.Topaloglu AK, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2008;41:354–358. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elks CE, et al. Thirty new loci for age at menarche identified by a meta-analysis of genome-wide association studies. Nat Genet. 2010;42:1077–1085. doi: 10.1038/ng.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simon JA, Kingston RE. Mechanisms of polycomb gene silencing: knowns and unknowns. Nat Rev Mol Cell Biol. 2009;10:697–708. doi: 10.1038/nrm2763. [DOI] [PubMed] [Google Scholar]
- 21.Han SK, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 23.Ghoshal K, Bai S. DNA methyltransferases as targets for cancer therapy. Drugs Today (Barc) 2007;43:395–422. doi: 10.1358/dot.2007.43.6.1062666. [DOI] [PubMed] [Google Scholar]
- 24.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herde MK, Geist K, Campbell RE, Herbison AE. Gonadotropin-releasing hormone neurons extend complex highly branched dendritic trees outside the blood-brain barrier. Endocrinology. 2011;152:3832–3841. doi: 10.1210/en.2011-1228. [DOI] [PubMed] [Google Scholar]
- 26.Broadwell RD, Brightman MW. Entry of peroxidase into neurons of the central and peripheral nervous systems from extracerebral and cerebral blood. J Comp Neurol. 1976;166:257–283. doi: 10.1002/cne.901660302. [DOI] [PubMed] [Google Scholar]
- 27.Nowak KW, Neri G, Nussdorfer GG, Malendowicz LK. Effects of sex hormones on the steroidogenic activity of dispersed adrenocortical cells of the rat adrenal cortex. Life Sci. 1995;57:833–837. doi: 10.1016/0024-3205(95)02015-b. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz YB, Pirrotta V. Polycomb silencing mechanisms and the management of genomic programmes. Nat Rev Genet. 2007;8:9–22. doi: 10.1038/nrg1981. [DOI] [PubMed] [Google Scholar]
- 29.Gil J, Bernard D, Martinez D, Beach D. Polycomb CBX7 has a unifying role in cellular lifespan. Nat Cell Biol. 2004;6:67–72. doi: 10.1038/ncb1077. [DOI] [PubMed] [Google Scholar]
- 30.Otte AP, Kwaks TH. Gene repression by Polycomb group protein complexes: a distinct complex for every occasion? Curr Opin Genet Dev. 2003;13:448–454. doi: 10.1016/s0959-437x(03)00108-4. [DOI] [PubMed] [Google Scholar]
- 31.Shrivastava A, Calame K. An analysis of genes regulated by the multi-functional transcriptional regulator Yin Yang-1. Nucleic Acids Res. 1994;22:5151–5155. doi: 10.1093/nar/22.24.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woo CJ, Kharchenko PV, Daheron L, Park PJ, Kingston RE. A region of the human HOXD cluster that confers polycomb-group responsiveness. Cell. 2010;140:99–110. doi: 10.1016/j.cell.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wakabayashi Y, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30:3124–3132. doi: 10.1523/JNEUROSCI.5848-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151:3479–3489. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gottsch ML, et al. Molecular Properties of Kiss1 Neurons in the Arcuate Nucleus of the Mouse. Endocrinology. 2011;152:4298–4309. doi: 10.1210/en.2011-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sing A, et al. A vertebrate Polycomb response element governs segmentation of the posterior hindbrain. Cell. 2009;138:885–897. doi: 10.1016/j.cell.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 37.Meng S, et al. Identification and characterization of Bmi-1-responding element within the human p16 promoter. J Biol Chem. 2010;285:33219–33229. doi: 10.1074/jbc.M110.133686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chu C, Qu K, Zhong FL, Artandi SE, Chang HY. Genomic maps of long noncoding RNA occupancy reveal principles of RNA-chromatin interactions. Mol Cell. 2011;44:667–678. doi: 10.1016/j.molcel.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z, et al. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet. 2008;40:897–903. doi: 10.1038/ng.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruthenburg AJ, Li H, Patel DJ, Allis CD. Multivalent engagement of chromatin modifications by linked binding modules. Nat Rev Mol Cell Biol. 2007;8:983–994. doi: 10.1038/nrm2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 42.Greer EL, et al. Members of the H3K4 trimethylation complex regulate lifespan in a germline-dependent manner in C. elegans. Nature. 2010;466:383–387. doi: 10.1038/nature09195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sawarkar R, Paro R. Interpretation of developmental signaling at chromatin: the Polycomb perspective. Dev Cell. 2010;19:651–661. doi: 10.1016/j.devcel.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 44.Bilger M, Heger S, Brann DW, Paredes A, Ojeda SR. A conditional, tetracycline-regulated increase in gamma amino butyric acid production near LHRH nerve terminals disrupts estrous cyclicity in the rat. Endocrinology. 2001;142:2102–2114. doi: 10.1210/endo.142.5.8166. [DOI] [PubMed] [Google Scholar]
- 45.Schaefer M, Hagemann S, Hanna K, Lyko F. Azacytidine inhibits RNA methylation at DNMT2 target sites in human cancer cell lines. Cancer Res. 2009;69:8127–8132. doi: 10.1158/0008-5472.CAN-09-0458. [DOI] [PubMed] [Google Scholar]
- 46.Komashko VM, Farnham PJ. 5-azacytidine treatment reorganizes genomic histone modification patterns. Epigenetics. 2010;5 doi: 10.4161/epi.5.3.11409. [DOI] [PubMed] [Google Scholar]
- 47.Kurian JR, Keen KL, Terasawa E. Epigenetic changes coincide with in vitro primate GnRH neuronal maturation. Endocrinology. 2010;151:5359–5368. doi: 10.1210/en.2010-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bernstein BE, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 49.Young MD, et al. ChIP-seq analysis reveals distinct H3K27me3 profiles that correlate with transcriptional activity. Nucleic Acids Res. 2011;39:7415–7427. doi: 10.1093/nar/gkr416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bernstein BE, Meissner A, Lander ES. The mammalian epigenome. Cell. 2007;128:669–681. doi: 10.1016/j.cell.2007.01.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. In vivo inhibition of DNA methylation results in pubertal failure despite of the animals reaching body weights much greater than the body weight attained by controls at the time of puberty
Supplementary Figure 2. DNA methylation arrays reveal change in promoter/CpG island methylation of members of the PcG silencing complex in the MBH at puberty
Supplementary Figure 3. Expression of two PcG genes required for PcG-mediated gene silencing decreases in the MBH, but not in the POA at the time of puberty
Supplementary Figure 4. Segments of the 5-flanking region of Cbx7, Eed and Kiss1 targeted for DNA methylation and ChIP analysis
Supplementary Figure 5 EED decreases Kiss1 promoter activity in vitro
Supplementary Figure 6. The abundance of the antagonistic histone modifications H3K27me3 and H3K4me3 associated with the Kiss1 promoter changes in opposite directions as puberty unfolds
Supplementary Figure 7. Preparation and in vitro testing of a lentivirus construct encoding an HA-tagged EED protein
Supplementary Table 1. Primers used in this study
Supplementary Table 2. Antibodies used for Western blots or ChIP assays
Supplementary Table 3. Number of pups per litter after exposing LV-GFP and LV-EED injected animals to a fertile male for five days