Abstract
Objectives
Prior studies have reported increased risks of congenital heart defects (CHD) and pyloric stenosis (PS) after prenatal exposure to macrolide antibiotics. We sought to assess the association between maternal use of erythromycin and non-erythromycin macrolides and the risks of CHD and PS.
Study Design
Among participants in the Slone Epidemiology Center Birth Defects Study from 1994 to 2008, we identified 4132 infants with CHD and 735 with PS as cases, and 6952 infants without any malformation as controls. We estimated odds ratios (ORs) and 95% confidence intervals (CIs) associated with use of erythromycin or non-erythromycin macrolides in each trimester using conditional logistic regression and adjusting for risk factors for CHD and PS, fever, specific types of infections, and their associated treatments.
Results
During the first trimester, 0.4% and 0.7% of control women had used erythromycin and non-erythromycin macrolides, respectively. Compared to non-use during pregnancy, first-trimester exposure to erythromycin was not associated with an increased risk of CHD (OR, 1.3; 95% CI, 0.6–2.6) or PS (OR, 0.9; 95% CI, 0.3–3.0). The corresponding ORs for non-erythromycin macrolides were 0.7 (95% CI, 0.4–1.3) for CHD and 1.7 (95% CI, 0.6–4.6) for PS. We found no association between third-trimester exposure to erythromycin or non-erythromycin macrolides and the risk of PS. Hypothesis generation analyses did not identify appreciable associations between maternal use of macrolides and other common specific birth defects.
Conclusions
We found no meaningful associations between the risks of CHD, PS, and other common malformations in relation to use of macrolides in pregnancy.
Keywords: azithromycin, clarithromycin, congenital malformations, erythromycin, macrolides
Introduction
Macrolide antibiotics are frequently used for presumed or documented Gram-positive lower and upper respiratory infections, soft tissue infections, and Helicobacter pylori-related peptic ulcer. Moreover, for the treatment of chlamydia and other selected infections in pregnancy, erythromycin has been specifically recommended because other effective antibiotics for such infections are contraindicated for pregnant women.1 Because these indications are common and azithromycin and erythromycin are classified as FDA category B, macrolide antibiotics are the second most frequently used antibacterial class during pregnancy in the United States.2, 3 Macrolide antibiotics are often subdivided into erythromycin, the first-introduced macrolide, and non-erythromycin drugs, including clarithromycin and azithromycin, which have fewer effects on gastrointestinal motility than erythromycin.4
Exposure to erythromycin in early pregnancy has been associated with an increased risk of congenital heart defects (CHD) (odds ratios [OR], 1.84; 95% confidence interval [CI], 1.29–2.62); the association was largely attributed to unspecified CHD (OR, 3.57; 95% CI, 1.70–6.12).5 However, Cooper et al. did not find an increase in the risk of CHD after exposure to either erythromycin or non-erythromycin macrolides.6 The National Birth Defects Prevention Study (NBDPS) also published null findings for macrolides overall in relation to CHD, but they did not differentiate erythromycin from non-erythromycin macrolides.3
In 1999, infantile hypertrophic pyloric stenosis (PS) was linked to exposure to macrolide antibiotics in postnatal days 2–17,7 a finding confirmed in 2001.8 Kallen et al. reported an elevated risk of PS among the offspring of women who took erythromycin in early pregnancy (risk ratio, 3.03; 95% CI, 1.08–8.50).5 Cooper et al. did not replicate this association, but found an elevated risk of PS associated with exposure to non-erythromycin macrolides prescribed any time during pregnancy (OR, 2.77; 95% CI, 1.22–6.30).9 Using data from the Slone Birth Defects Study (1976–1998), Louik et al. found no association between PS and exposure to either type of macrolide antibiotics after the 32nd gestational week (OR, 0.7; 95% CI, 0.3–1.8 for erythromycin and OR, 1.1; 95% CI, 0.3–3.6 for non-erythromycin macrolides), but there were very few subjects exposed to non-erythromycin macrolides in this study.10, 11 These findings have raised concerns regarding maternal use of macrolide antibiotics in either early or late pregnancy.
Despite being commonly prescribed during pregnancy, the safety profile of macrolide antibiotics is yet to be determined, not only with regard to the hypothesized risks of CHD and PS, but also with regard to the range of other specific major birth defects. We therefore sought to test the hypotheses that the risks of CHD and PS are elevated among infants or fetuses exposed to erythromycin and/or non-erythromycin macrolides during pregnancy and, in exploratory analyses, to identify possible associations with other specific defects. The analyses utilized data from the Slone Epidemiology Center Birth Defects Study (BDS), an ongoing program of case-control surveillance of medications in relation to birth defects.
Materials and Methods
Study population
The BDS was established in 1976,12 and since that time has interviewed mothers of malformed infants ascertained through review of admissions and discharges at major referral hospitals and clinics in the greater metropolitan areas of Boston, Philadelphia, Toronto and San Diego, and through statewide birth defects registries in New York State (since 2004) and in Massachusetts (since 1998). For hospital-based surveillance, the subjects’ physicians are asked to confirm the diagnosis and mothers are asked to provide medical record releases in order to permit confirmation of the infant’s condition. Infants with isolated minor defects are excluded. Beginning in 1992, the BDS also enrolled a sample of mothers of non-malformed infants as controls: initially these infants were identified exclusively at study hospitals but, since 1998, the BDS also includes a population-based random sample of newborns in Massachusetts. The study has been approved by the Boston University Institutional Review Board (IRB) and the IRBs of all relevant participating institutions. It is fully compliant with the requirements of the Health Insurance Portability and Accountability Act. The current analysis was restricted to women interviewed between 1994 and 2008 because full ascertainment of our control group, non-malformed infants, was not underway until 1994. Among eligible subjects in the last decade, the mothers of 73 percent of malformed infants and 68 percent of non-malformed controls contacted agreed to an interview and provided informed consent.
Cases
Hypothesis Testing
Cases consisted of 4,132 infants and fetuses with a diagnosis of CHD and 735 infants with PS. We excluded from analysis infants with chromosomal defects, known Mendelian inherited disorders, syndromes, DiGeorge sequence (associated with 22q deletion), and metabolic and functional disorders. CHD or PS complicated with other defects (but not as part of an identified chromosomal or Mendelian inherited syndrome) were included in the general analysis and studied separately in a secondary analysis.
Exploratory analyses
Other major defects examined included the following categories:1348 oral clefts, 1138 central nervous system (CNS) defects, 308 respiratory system defects, 1825 gastrointestinal system defects, 1099 genital system defects, 1511 urinary system defects, 1948 musculoskeletal system defects, and 385 others. Where there were sufficient numbers of subjects with specific defects, those were considered as well. The same exclusion criteria described above also applied to these case groups.
Controls
Our control group consisted of 6,952 infants without any malformation.
Interviews
Within 6 months of the subject’s delivery, trained study nurses unaware of study hypotheses interview mothers of study subjects. The 45–60 minute interview is detailed and structured and includes questions on maternal demographic characteristics, mothers’ medical histories, obstetric histories, maternal health behaviors and occupation, and a detailed history of the use of medication (including prescription, over-the-counter, and vitamin and herbal products) from two months before the date of the last menstrual period (LMP) through the entire pregnancy. Recall of medication exposures is enhanced by questions regarding indications for use (e.g., infections), and a list of specifically-named medications,13 which includes, among other antibiotics, erythromycin and non-erythromycin macrolides.
Mothers who report taking a particular medication are further asked to identify, as accurately as possible, the dates when use began and ended. Recall of the timing of medication use is enhanced by the use of a calendar that highlights the mother’s reported LMP date and her delivery date. Further, subjects are asked how certain they are about each of these dates. Interviewers record the certainty of each reported date as follows: (i) exact, if the exact date is reported, (ii) estimated, if a date is stated as an estimate, or (iii) sometime in a given month, if the day within a month is unknown. Mothers who cannot recall the month are considered to have unknown dates of exposure. Mothers are also asked details about their pattern of use of the particular medication, including duration (days of treatment), frequency of use (e.g., days per week or month), and specific doses. We defined exposure as systemic use of erythromycin or non-erythromycin macrolides.
Algorithm to classify timing of exposure
We developed an exposure classification algorithm taking into account recall uncertainty in reported timing of medication exposure.14 For uncertain start/stop dates reported as being sometime in a month, we considered the possible exposure period to be the widest interval consistent with that report (e.g., if a mother reported medication use sometime in May, we assigned May 1 as her start date and May 31 as her stop date). Based on reported duration of use, we classified a mother as being “likely exposed” in the window of interest if her medication use, independent of date certainty, had to at least partially include the window of interest; she was considered “possibly exposed” if her use could fall either within the window of interest or completely outside of it. To minimize misclassification of exposure, only women classified as “likely exposed” to each medication comprised our exposure group of primary interest.
Data analysis
For both hypothesis testing and the exploratory analyses, we used conditional logistic regression to estimate ORs and CIs for exposure to macrolide antibiotics in each trimester and the risk of specific birth defects. For the timing of pregnancy periods, we defined the LMP date based on early ultrasound examination; in the absence of that information, we relied on maternal report. We defined the estimated date of conception (EDC) as 14 days after the LMP date and the first trimester of pregnancy as 90 days following the EDC (encompassing the etiologically important period of vulnerability to the development of most structural birth defects). Since that period is not established for PS, we considered exposure in each trimester for that defect. The second and third trimesters were defined as 91st to 180th day and 181st day to the end of the pregnancy, respectively, following the EDC. It was postulated that erythromycin exposure might cause PS by interacting with motilin receptors, which are present in the fetus beginning at 32 weeks’ gestation.15 Therefore, in a sensitivity analysis for PS, we also examined exposure to erythromycin after the 32nd gestational week. We used multivariate conditional logistic regression to adjust for the following factors: (1) geographic area of participants’ residences and calendar year when they were ascertained (to account for secular trends and regional variations in use of antimicrobials as well as recruitment of cases with specific malformations); (2) risk factors for congenital malformations, including maternal age, race, education level, pre-pregnancy body mass index (BMI), family history of CHD or PS, diabetes mellitus, first trimester cigarette smoking, peri-conceptional folic acid supplement, and multiple pregnancy; (3) infection-related factors, including urinary tract, respiratory, or vaginal/yeast infection, sexually transmitted disease, other kinds of infection, and/or febrile events with/without treatment that occurred in the first trimester. Further adjustment for use of anticonvulsants, alcohol consumption in the first trimester, and history of infertility with/without treatment did not change our results appreciably. The reference category was no exposure to the medication of interest at any time and in any form (systemic or topical use) from 56 days before the LMP date through the end of pregnancy. To avoid unstable estimates, ORs with less than 5 exposed cases or 100 total cases were not calculated. Statistical analyses utilized SAS 9.2 (SAS Institute Inc., Cary, NC).
Sensitivity analyses
Because preterm deliveries, stillbirths and terminations may occur differentially among cases versus non-malformed controls and because they would have a shorter opportunity for exposure late in pregnancy, we conducted a sensitivity analysis that excluded these babies in the analysis for late exposure to macrolides (in the third trimester or after the 32nd gestational week).
If a mother reported having used an antibiotic but the type was not otherwise specified (antibiotic NOS), she would be considered a user of an antibacterial but not a user of a specific antibiotic. Therefore, the reference group for macrolide antibiotics use could include a few users of other antibiotics, including antibiotic NOS, some of whom might have actually used a macrolide antibiotic. In a sensitivity analysis, we excluded users of antibiotic NOS from the reference group.
Results
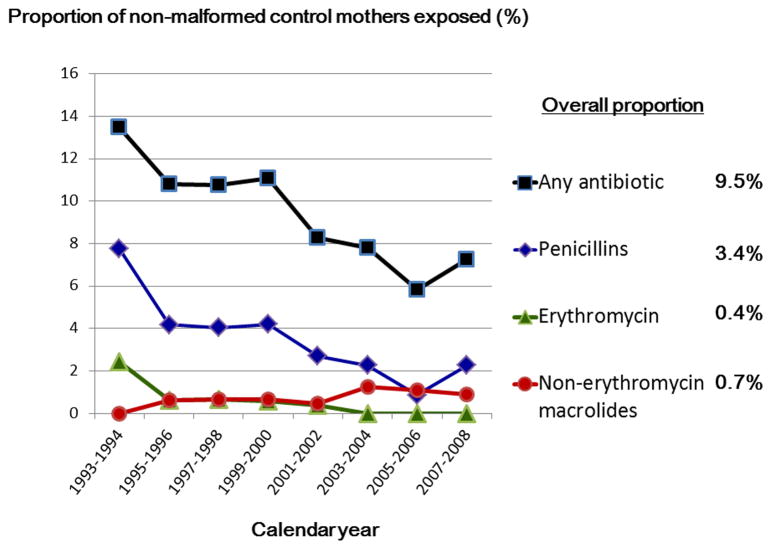
Among the control group, 9.5% of women reported having used at least one antibacterial in the first trimester, the most common type being penicillins (3.4%), followed by macrolide antibiotics (1.1% overall; 0.4% for erythromycin and 0.7% for non-erythromycin macrolides); 4.1% reported having taken an antibiotic NOS. From 1994 to 2008, there was a decreasing trend in erythromycin use and an increasing trend in use of non-erythromycin macrolides during pregnancy (Figure 1).
Figure 1. Prevalence of antibiotic use in the first trimester by calendar years of mothers’ last menstrual period date.
- The figure presents trends for those study centers that contribute data throughout study period, i.e., Boston and Philadelphia area.
- Note: 4.1% of women reported having used an antibiotic but the type could not be specified.
Hypothesis testing: CHD and PS
The distributions of selected characteristics are shown for cases and controls in Table 1. Geographic area of participants’ residences and calendar time were highly associated with the relative frequency of CHD and PS cases because of secular trends and regional variations in recruitment of study subjects with different malformations (data not shown). Therefore all the estimates presented below were adjusted for both factors.
Table 1.
Baseline characteristics among mothers of infants with congenital heart defects, pyloric stenosis, and a control group of mothers with non-malformed infants
| Variables | Levels | CHD Cases (N=4132) | PS Cases (N=735) | Controls (N=6952), N (%) | ||
|---|---|---|---|---|---|---|
| N (%) | OR (95% CI) a | N (%) | OR (95% CI) a | |||
| Mother’s age | <20 | 238 (5.8) | 1.2 (0.9–1.4) | 37 (5.0) | 1.2 (0.8–1.7) | 394 (5.7) |
| 20–24 | 627 (15.2) | 1.2 (1.1–1.4) | 122 (16.6) | 1.5 (1.2–1.9) | 888 (12.8) | |
| 25–29 | 1098 (26.6) | 1.0 (0.9–1.1) | 194 (26.4) | 1.2 (0.9–1.4) | 1703 (24.5) | |
| 30–34 | 1341 (32.5) | 1.0 (Ref) | 241 (32.8) | 1.0 (Ref) | 2460 (35.4) | |
| 35–39 | 679 (16.4) | 1.1 (0.9–1.2) | 121 (16.5) | 1.1 (0.9–1.4) | 1254 (18.0) | |
| >=40 | 149 (3.6) | 1.2 (0.9–1.5) | 20 (2.7) | 1.1 (0.7–1.8) | 253 (3.6) | |
| Mother’s race/ethnicity | White | 2890 (69.9) | 1.0 (Ref) | 618 (84.1) | 1.0 (Ref) | 5144 (74.0) |
| Black | 299 (7.2) | 0.9 (0.8–1.1) | 32 (4.4) | 0.5 (0.3–0.7) | 489 (7.0) | |
| Hispanic | 594 (14.4) | 1.0 (0.8–1.2) | 50 (6.8) | 0.9 (0.7–1.3) | 844 (12.1) | |
| Asian, pacific islander | 214 (5.2) | 1.4 (1.2–1.6) | 23 (3.1) | 0.5 (0.3–0.7) | 336 (4.8) | |
| Others | 135 (3.3) | 1.8 (1.4–2.5) | 12 (1.6) | 0.6 (0.3–1.1) | 139 (2.0) | |
| Mother’s education | <13 | 1395 (33.8) | 1.0 (Ref) | 267 (36.3) | 1.0 (Ref) | 1887 (27.1) |
| 13–15 | 1034 (25.0) | 0.8 (0.7–0.9) | 194 (26.4) | 0.7 (0.6–0.9) | 1699 (24.4) | |
| >=16 | 1699 (41.1) | 0.7 (0.6–0.8) | 274 (37.3) | 0.6 (0.5–0.7) | 3363 (48.4) | |
| Mother’s Prepregnancy BMI | <18.5 | 232 (5.6) | 0.9 (0.8–1.1) | 45 (6.1) | 0.9 (0.7–1.3) | 423 (6.1) |
| 18.5–24.9 | 2334 (56.5) | 1.0 (Ref) | 432 (58.8) | 1.0 (Ref) | 4340 (62.4) | |
| 25–29.9 | 865 (20.9) | 1.1 (1.0–1.3) | 145 (19.7) | 1.1 (0.9–1.4) | 1334 (19.2) | |
| 30–34.9 | 369 (8.9) | 1.5 (1.2–1.7) | 67 (9.1) | 1.6 (1.2–2.1) | 475 (6.8) | |
| 35–39.9 | 143 (3.5) | 1.5 (1.2–2.0) | 30 (4.1) | 2.2 (1.4–3.4) | 167 (2.4) | |
| >=40 | 79 (1.9) | 1.9 (1.4–2.6) | 6 (0.8) | 0.9 (0.4–2.3) | 91 (1.3) | |
| Diabetes Mellitus | None | 3747 (90.7) | 1.0 (Ref) | 698 (95.0) | 1.0 (Ref) | 6618 (95.2) |
| Diagnosed before pregnancy | 101 (2.4) | 5.6 (3.6–8.7) | 4 (0.5) | 1.4 (0.5–4.1) | 37 (0.5) | |
| Diagnosed during pregnancy | 280 (6.8) | 1.8 (1.5–2.2) | 33 (4.5) | 1.4 (0.9–2.0) | 290 (4.2) | |
| Folic acid supplementation | None | 166 (4.0) | 1.0 (Ref) | 42 (5.7) | 1.0 (Ref) | 248 (3.6) |
| Any peri-conceptional use b | 2434 (58.9) | 1.0 (0.8–1.2) | 434 (59.0) | 0.8 (0.5–1.1) | 4431 (63.7) | |
| Family history of CHD c | None | 3469 (84.0) | 1.0 (Ref) | -- | 6318 (90.9) | |
| First degree | 308 (7.5) | 2.0 (1.7–2.5) | 267 (3.8) | |||
| Second degree | 259 (6.3) | 1.9 (1.6–2.4) | 264 (3.8) | |||
| Third degree | 92 (2.2) | 1.6 (1.1–2.2) | 103 (1.5) | |||
| Family history of PS c | None | -- | 644 (87.6) | 1.0 (Ref) | 6909 (99.4) | |
| First degree | 58 (7.9) | 24.2 (13.6–42.8) | 24 (0.3) | |||
| Second degree | 23 (3.1) | 14.4 (6.7–30.8) | 15 (0.2) | |||
| Third degree | 10 (1.4) | 12.2 (3.6–41.5) | 4 (0.1) | |||
| Primiparous | No | 2860 (69.2) | 1.0 (Ref) | 506 (68.8) | 1.0 (Ref) | 4833 (69.5) |
| Yes | 1272 (30.8) | 1.0 (0.9–1.1) | 229 (31.2) | 1.0 (0.8–1.2) | 2119 (30.5) | |
| Multiple pregnancy | Single | 3908 (94.6) | 1.0 (Ref) | 698 (95.0) | 1.0 (Ref) | 6749 (97.1) |
| Multiple | 224 (5.4) | 2.5 (2.0–3.2) | 37 (5.0) | 2.1 (1.4–3.2) | 203 (2.9) | |
| Smoking in the 1st trimester d | Nonsmoker | 2376 (57.5) | 1.0 (Ref) | 351 (47.8) | 1.0 (Ref) | 4034 (58.0) |
| Smoked during 1st trimester | 830 (20.1) | 1.2 (1.0–1.3) | 217 (29.5) | 1.7 (1.4–2.1) | 1207 (17.4) | |
| Ex-smoker/smoke outside the 1st trimester | 926 (22.4) | 1.0 (0.9–1.1) | 167 (22.7) | 1.1 (0.9–1.4) | 1711 (24.6) | |
| Infection in the 1st trimester d,e | UTI | 199 (4.8) | 1.2 (1.0–1.5) | 33 (4.5) | 1.1 (0.8–1.7) | 272 (3.9) |
| Respiratory infection | 908 (22.0) | 1.2 (1.0–1.3) | 158 (21.5) | 1.1 (0.9–1.3) | 1403 (20.2) | |
| Yeast/vaginal | 255 (6.2) | 1.0 (0.8–1.2) | 57 (7.8) | 1.1 (0.8–1.5) | 421 (6.1) | |
| STD | 77 (1.9) | 1.0 (0.7–1.4) | 11 (1.5) | 1.0 (0.5–1.9) | 130 (1.9) | |
| Others | 313 (7.6) | 1.0 (0.9–1.2) | 74 (10.1) | 1.1 (0.9–1.5) | 510 (7.3) | |
| Febrile event in the 1st trimester | None | 3313 (80.2) | 1.0 (Ref) | 574 (78.1) | 1.0 (Ref) | 5601 (80.6) |
| Fever >=101 F in the 1st trimester | 97 (2.3) | 1.3 (1.0–1.8) | 19 (2.6) | 1.7 (1.0–2.8) | 130 (1.9) | |
| Fever <101 F in the 1st trimester | 121 (2.9) | 1.1 (0.8–1.4) | 23 (3.1) | 1.1 (0.7–1.7) | 201 (2.9) | |
Ref: reference group, CHD: congenital heart defects, PS: pyloric stenosis, Ref: reference group, BMI: body mass index, UTI: urinary tract infection, STD: sexually transmitted disease
Based on conditional logistic regression, adjusted for region of participants’ residences and calendar year of ascertainment
Any folic acid supplementation during −1~+1 month around last menstrual period (LMP)
Family history of CHD when infants with CHD served as cases; and family history of PS when infants with PS served as cases
First trimester of pregnancy was defined as 90 days after estimated date of conception (14 days after LMP)
Reference group for each type of infection was no infection under comparison in the first trimester
As shown in Table 2, we found no association between first trimester maternal use of macrolides as a class and the risk of CHD (OR, 0.9; 95% CI, 0.6–1.3) or PS (OR, 1.3; 95% CI, 0.6–2.8). For erythromycin, the risk of CHD was 1.3 (95% CI, 0.6–2.6) and for PS it was 0.9 (95% CI, 0.3–3.0). The corresponding ORs for non-erythromycin macrolides were 0.7 (95% CI, 0.4–1.3) for CHD and 1.7 (95% CI, 0.6–4.6) for PS. We also found no meaningful association between the risk of PS and exposure to macrolides overall or to erythromycin or non-erythromycin macrolides in the second and third trimesters (Table 2). Restriction to data after 1998 (excluding the data overlapping with our prior study of PS10) did not materially affect our results (data not shown). Examining specific cardiac defects within the overall CHD category, we also observed no meaningful associations. For example, the OR of septal defects was 1.3 (95% CI, 0.6–3.0) for erythromycin and 0.8 (95% CI, 0.4–1.4) for non-erythromycin macrolides (Table 3). In the sensitivity analysis for etiologically relevant timing of exposure for PS, no associations were found between either erythromycin or non-erythromycin macrolides after the 32nd gestational week. Excluding preterm deliveries, stillbirths and therapeutic abortions did not appreciably affect our risk estimates for late-pregnancy exposure to macrolide antibiotics or to the macrolide subgroups (data not shown). Exclusion of users of antibiotic NOS from the analysis or restriction to isolated CHD and PS (without other major malformations) also did not materially affect the results (data not shown).
Table 2.
Maternal exposure to macrolide antibiotics and risk of congenital heart defects or pyloric stenosis
| Non-malformed controls (N=6952), N (%) | Cases of CHD (N=4132) | Cases of PS (N=735) | |||
|---|---|---|---|---|---|
| N (%) | OR (95% CI) d | N (%) | OR (95% CI) d | ||
| Any Macrolides | |||||
| No exposure during pregnancy | 6655 (95.7) | 3948 (95.5) | 1.0 (Ref) | 693 (94.3) | 1.0 (Ref) |
| Exposure in the 1st trimester a | 70 (1.0) | 46 (1.1) | 0.9 (0.6–1.3) | 12 (1.6) | 1.3 (0.6–2.8) |
| Exposure in the 2nd trimester a | 74 (1.1) | 47 (1.1) | 1.1 (0.7–1.7) | 7 (1.0) | 1.3 (0.5–3.0) |
| Exposure in the 3rd trimester a,c | 71 (1.0) | 47 (1.1) | 1.0 (0.6–1.6) | 11 (1.5) | 1.3 (0.6–2.9) |
| Erythromycin | |||||
| No exposure during pregnancy | 6828 (98.2) | 4064 (98.4) | 1.0 (Ref) | 717 (97.6) | 1.0 (Ref) |
| Exposure in the 1st trimester a | 28 (0.4) | 18 (0.4) | 1.3 (0.6–2.6) | 4 (0.5) | 0.9 (0.3–3.0) |
| Exposure in the 2nd trimester a | 27 (0.4) | 15 (0.4) | 0.9 (0.4–2.0) | 4 (0.5) | 1.5 (0.4–4.8) |
| Exposure in the 3rd trimester a,c | 20 (0.3) | 15 (0.4) | 1.1 (0.5–2.6) | 5 (0.7) | 1.5 (0.5–5.1) |
| Non-erythromycin macrolides | |||||
| No exposure during pregnancy | 6773 (97.4) | 4013 (97.1) | 1.0 (Ref) | 711 (96.7) | 1.0 (Ref) |
| Exposure in the 1st trimester b | 43 (0.6) | 29 (0.7) | 0.7 (0.4–1.3) | 8 (1.1) | 1.7 (0.6–4.6) |
| Exposure in the 2nd trimester b | 48 (0.7) | 32 (0.8) | 1.2 (0.7–2.0) | 3 (0.4) | - |
| Exposure in the 3rd trimester b,c | 51 (0.7) | 32 (0.8) | 1.0 (0.6–1.7) | 7 (1.0) | 1.5 (0.6–3.8) |
Ref: reference group, OR: odds ratio, CI: confidence interval
ORs comparing likely exposure to erythromycin in the window of interest with no exposure to erythromycin during pregnancy based on an algorithm previously published 14
ORs comparing likely exposure to non-erythromycin macrolides in the window of interest with no exposure to non-erythromycin macrolides during pregnancy based on an algorithm previously published 14
Excluding preterm deliveries, stillbirths and terminated abortions did not change the estimates materially
ORs adjusted for region of participants’ residences and calendar year when they were ascertained, maternal age, race, education level, pre-pregnancy BMI, family history of congenital malfomarions, diabetes mellitus, first trimester cigarette smoking, peri-conceptional folic acid supplement, multiple pregnancy, urinary tract, respiratory, or vaginal/yeast infection, sexually transmitted disease, and other kinds of infection, and/or febrile events that occurred in the first trimester
Table 3.
Use of macrolides during first trimester and specific major congenital malformations
| Total no. | Exposure to any macrolides in 1st trimester (N=204) a | Exposure to erythromycins in 1st trimester (N=81) a | Exposure to non-erythromycin macrolides in 1st trimester (N=124) a | ||||
|---|---|---|---|---|---|---|---|
| N (%) | OR (95% CI) | N (%) | OR (95% CI) | N (%) | OR (95% CI) | ||
| No malformations | 6952 | 70 (1.0) | 1.0 (Ref) | 28 (0.4) | 43 (0.6) | ||
| CHD | 4132 | 46 (1.1) | 0.9 (0.6–1.3) | 18 (0.4) | 1.3 (0.6–2.6) | 29 (0.7) | 0.7 (0.4–1.3) |
| Conotruncal | 903 | 5 (0.6) | 0.4 (0.2–1.2) | 1 (0.1) | 4 (0.4) | ||
| Septal defects | 2811 | 33 (1.2) | 0.9 (0.6–1.5) | 12 (0.4) | 1.3 (0.6–3.0) | 21 (0.7) | 0.8 (0.4–1.4) |
| RVOTO | 464 | 5 (1.1) | 0.8 (0.3–2.2) | 4(0.9) | 2 (0.4) | ||
| LVOTO | 652 | 8 (1.2) | 1.0 (0.4–2.3) | 2(0.3) | 6 (0.9) | 1.2 (0.4–3.3) | |
| Pyloric stenosis | 735 | 12 (1.6) | 1.3 (0.6–2.8) | 4(0.5) | 0.9 (0.3–3.0) | 8 (1.1) | 1.7 (0.6–4.6) |
| Oral clefts | 1348 | 22 (1.6) | 1.2 (0.7–2.2) | 8(0.6) | 1.6 (0.6–4.4) | 13 (1.0) | 1.0 (0.5–2.1) |
| CL/P | 877 | 16 (1.8) | 1.5 (0.8–3.0) | 7(0.8) | 2.0 (0.7–6.0) | 8 (0.9) | 1.1 (0.5–2.7) |
| CP | 471 | 6 (1.3) | 1.1 (0.4–2.8) | 1 (0.2) | 5 (1.1) | 1.2 (0.4–3.3) | |
| CNS defects | 1138 | 11 (1.0) | 0.8 (0.4–1.6) | 6 (0.5) | 1.2 (0.4–3.6) | 6 (0.5) | 0.7 (0.3–1.8) |
| NTD | 465 | 5 (1.1) | 0.8 (0.3–2.2) | 4 (0.9) | 2 (0.4) | ||
| CNS NOS | 599 | 6 (1.0) | 0.8 (0.3–2.1) | 2 (0.3) | 4 (0.7) | ||
| Respiratory system defects | 308 | 6 (1.9) | 0.9 (0.3–2.4) | 4 (1.3) | 2 (0.6) | ||
| Respiratory system defects NOS | 250 | 6 (2.4) | 1.0 (0.4–2.8) | 4 (1.6) | 2 (0.8) | ||
| GI defects | 1825 | 21 (1.2) | 1.0 (0.6–1.7) | 9 (0.5) | 1.0 (0.4–2.4) | 13 (0.7) | 1.0 (0.5–1.9) |
| Genital system defects | 1099 | 13 (1.2) | 0.8 (0.4–1.6) | 4 (0.4) | 9 (0.8) | 0.7 (0.3–1.6) | |
| Hypospadias & epispadias b | 557 | 5 (0.9) | 0.4 (0.1–1.2) | 2 (0.4) | 3 (0.5) | ||
| Genital system defects NOS | 208 | 6 (2.9) | 2.8 (1.0–7.7) | 2 (1.0) | 4 (1.9) | ||
| Urinary system defects | 1511 | 18 (1.2) | 1.0 (0.5–1.7) | 7 (0.5) | 1.2 (0.5–3.0) | 11 (0.7) | 0.8 (0.4–1.7) |
| Renal collecting system anomalies | 958 | 9 (0.9) | 0.7 (0.3–1.5) | 4 (0.4) | 5 (0.5) | 0.5 (0.2–1.4) | |
| Musculoskeletal system defects | 1948 | 16 (0.8) | 0.8 (0.4–1.4) | 6 (0.3) | 0.7 (0.3–1.9) | 9 (0.5) | 0.7 (0.3–1.5) |
| Clubfoot | 562 | 5 (0.9) | 0.6 (0.2–1.6) | 3 (0.5) | 2 (0.4) | ||
CHD: congenital heart defects, CNS: central nervous system defects, NTD: neural tube defects, GI: gastrointestinal system defects, LVOTO: left ventricular outflow tract obstruction, RVOTO: right ventricular outflow tract obstruction, CL/P: cleft lip with/ without cleft palate, CP: cleft palate alone, GI: gastrointestinal system Reference group: no exposure to the antibiotic of interest during pregnancy
ORs adjusted for region of participants’ residences and calendar year when they were ascertained, maternal age, race, education level, pre-pregnancy BMI, family history of congenital malformations, diabetes mellitus, first trimester cigarette smoking, peri-conceptional folic acid supplement, multiple pregnancy, urinary tract, respiratory, or vaginal/yeast infection, sexually transmitted disease, and other kinds of infection, and/or febrile events that occurred in the first trimester; ORs with less than 5 exposed cases or 100 total cases were not estimated.
Likely exposure in the first trimester based on our algorithm 14
Restricted to male infants
Role of confounding by risk factors and by indication
ORs adjusted for geographic region of participants’ residences and year of ascertainment were only marginally influenced by further adjustment for risk factors and infection variables, including exposures to other specified antibiotics (as a group that included penicillins, cephalosporins, sulfonamides, and quinolones); analyses were conducted for first trimester exposures for CHD and for all three trimesters for PS. We found no associations between the risk of CHD or PS and the use of other specific antibacterials (data not shown).
Exploratory analyses
Examination of macrolide use in the first trimester in relation to major birth defects revealed largely null findings except for a borderline association between exposure to macrolides overall and unspecified genital system defects (Table 3).
Comment
We did not find an association between the risk of either CHD or PS and prenatal exposure to erythromycin or non-erythromycin macrolides. In addition, our analyses did not identify any appreciably increased risks of other specific congenital malformations following antenatal exposure to macrolide antibiotics.
For the hypotheses we tested related to CHD and PS, our results are consistent with two recently published large studies. For CHD, an American cohort study found no association between the risk of CHD overall and maternal use of erythromycin or non-erythromycin macrolides during pregnancy,6 nor did a Norwegian study find elevated risks of CHD overall or atrial/ventricular septal defects in infants exposed to erythromycin in the first trimester.16 NBDPS also reported null findings for macrolide antibiotic use overall in relation to subgroups of CHD.3 For PS, our group had previously reported a lack of association with erythromycin use in either early (gestational weeks 1–25) or late pregnancy (after the 32nd gestational week).10 Findings from the current study, which added data collected from 1998 to 2008, indicate that exposure to erythromycin or non-erythromycin macrolides in either early or late pregnancy is not associated with an increased risk of PS; the results remained consistent when we excluded the data that had been reported in the prior study.
This apparent lack of teratogenic effect should be interpreted with caution. First, we had small samples of exposed subjects with many specific defects, limiting our conclusions to defects that tend to occur most commonly. Further, there are several alternative explanations for our null findings. First, given that power was limited for some comparisons, our CIs around many estimates were relatively wide and we cannot rule out modest associations (for example, the upper bound of the 95%CI for the association between third trimester erythromicin use and PS was 5.1). As noted above, however, our results are consistent with at least three other large studies. Secondly, non-differential exposure misclassification (either in the subject’s ability to identify the specific drug or the gestational timing of the exposure) can bias the results towards the null. This possibility is minimized by the use of a highly structured questionnaire.13 Further, we applied strict criteria for certainty of exposure, excluding from the exposed group those subjects who were unsure about the exact date of exposure.14 Also, in subanalyses that excluded mothers who reported using an unknown antibiotic during pregnancy, our results remained close to the null. Third, this is an interview-based study where women were asked about actual use of a medication. While this design minimizes exposure misclassification due to non-adherence (a problem in designs that rely on records of prescriptions written and/or filled), recall bias may occur if case mothers recall their use of macrolides during pregnancy differently than mothers of the non-malformed controls. However, this bias was unlikely to play an important role as we did not identify meaningful associations.
The two prior studies reporting increased risks of CHD and PS among macrolide antibiotic users found the associations in the context of multiple comparisons, a fact noted by the respective authors.5, 9 The numbers of users of these drugs were relatively small and the CIs wide. For example, Cooper et al. reported an elevated risk of PS among mothers taking non-erythromycin macrolides based on only 6 exposed cases.9
In addition to CHD and PS, we also examined exposure to macrolides in relation to other common malformation groups and, where numbers allowed, relatively common specific congenital malformations; the results were largely null. The possibility that any macrolide exposure in the first trimester may increase the risk of unspecified genital system defects may well be a chance finding given its identification in the setting of multiple comparisons (both exposure and outcome involved broad and heterogenous categories of “any macrolide” and “genital defects overall”, respectively).
In conclusion, we could not replicate the previously-described associations between macrolides as a class, and more specifically erythromycin or non-erythromycin macrolides, and the risk of CHD and/or PS, nor did we identify meaningful increases in risks for many of specific major congenital malformations. While active surveillance on the safety of macrolide use during pregnancy should be continued, we find no evidence supporting major teratogenic effects of macrolide antibiotics in human fetuses.
Acknowledgments
We thank Dawn Jacobs, RN, MPH, Fiona Rice, MPH, Rita Krolak, RN, Kathleen Sheehan, RN, Moira Quinn, RN, Clare Coughlin, RN, Nancy Rodriquez-Sheridan, Carolina Meyers, Joan Shander and Paula Wilder for their assistance in data collection; Nastia Dynkin for computer programming; the staff of the Massachusetts Department of Public Health Center for Birth Defects Research and Prevention, Dr. Charlotte Druschel and the New York State Health Department, and Drs. Christina Chambers and Kenneth Jones of the University of California, San Diego, as well as the medical and nursing staff at all participating hospitals for assistance with case ascertainment: Baystate Medical Center, Beth Israel Deaconess Medical Center, Boston Medical Center, Brigham & Women’s Hospital, Brockton Hospital, Cambridge Hospital, Caritas Good Samaritan Medical Center, Charlton Memorial Hospital, Children’s Hospital, Emerson Hospital, Falmouth Hospital, Haverhill-Hale Hospital, Jordan Hospital, Kent Hospital, Lawrence General Hospital, Lowell General Hospital, Melrose-Wakefield Hospital, Metro West Medical Center-Framingham, Mt. Auburn Hospital, New England Medical Center, Newton-Wellesley Hospital, North Shore Medical Center, Rhode Island Hospital, Saints Memorial Medical Center, South Shore Hospital, Southern New Hampshire Medical Center, St. Elizabeth’s Medical Center, St. Luke’s Hospital, St. Vincent Hospital, UMASS Memorial Health Care, Women & Infants’ Hospital, Abington Memorial Hospital, Albert Einstein Medical Center, Alfred I. duPont Hospital for Children, Bryn Mawr Hospital, Chester County Hospital, Children’s Hospital of Philadelphia and their Clinical and Translational Research Center (grant UL1-RR-024134), Christiana Care Health Services, Community Hospital, Crozer-Chester Medical Center, Doylestown Hospital, Frankford Hospital, Hahnemann University Hospital, The Hospital of the University of Pennsylvania, Lankenau Hospital, Lancaster General Hospital, Lehigh Valley Hospital, Nanticoke Memorial Hospital, Pennsylvania Hospital, Sacred Heart Hospital, St. Christopher’s Hospital for Children, St. Mary Medical Center, Temple University Health Sciences Center, Reading Hospital & Medical Center, Thomas Jefferson University Hospital, Grand River Hospital, Guelph General Hospital, Hamilton Health Sciences Corporation, The Hospital for Sick Children, Humber River Regional Hospital-Church Site, Humber River Regional Hospital-Finch Site, Joseph Brant Memorial Hospital, Lakeridge Health Corporation, London Health Sciences Center, Mt. Sinai Hospital, North York General Hospital, Oakville Trafalgar Memorial Hospital, Scarborough Hospital–General Division, Scarborough Hospital–Grace Division, St. Joseph’s Health Centre-London, St. Joseph’s Health Centre-Toronto, St. Joseph’s Healthcare-Hamilton, St. Michael’s Hospital, Sunnybrook & Women’s College Health Sciences Center, Toronto East General Hospital, Toronto General Hospital, Trillium Health Center, William Osler Heath Centre, York Central Hospital, York County Hospital, Alvarado Hospital, Balboa Naval Medical Center, Camp Pendleton Naval Hospital, Children’s Hospital and Health Center, Kaiser Zion Medical Center, Palomar Medical Center, Pomerado Hospital, Scripps Mercy Hospital, Scripps Memorial Hospital-Chula Vista, Scripps Memorial Hospital-Encinitas, Scripps Memorial Hospital-La Jolla, Sharp Chula Vista Hospital, Sharp Coronado Hospital, Sharp Grossmont Hospital, Sharp Mary Birch Hospital, Tri-City Medical Center, and UCSD Medical Center.; we particularly thank all the mothers who participated in the study.
Sources of financial support:
This project is supported by grant R01 HD046595-04 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD).
Footnotes
Original publication statement:
The abstract of this article has been presented at 26th International Conference on Pharmacoepidemiology and Therapeutic Risk Management, 19–22 Aug 2010, Brighton, UK. The paper and data presented here have not been published elsewhere and there are no similar papers in press or under review.
Conflict of interest:
The authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.1998 guidelines for treatment of sexually transmitted diseases. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47:1–111. [PubMed] [Google Scholar]
- 2.Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, Hernandez-Diaz S. Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am J Obstet Gynecol. 2011;205:51, e1–8. doi: 10.1016/j.ajog.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crider KS, Cleves MA, Reefhuis J, Berry RJ, Hobbs CA, Hu DJ. Antibacterial medication use during pregnancy and risk of birth defects: National Birth Defects Prevention Study. Arch Pediatr Adolesc Med. 2009;163:978–85. doi: 10.1001/archpediatrics.2009.188. [DOI] [PubMed] [Google Scholar]
- 4.Klein JO. History of macrolide use in pediatrics. Pediatr Infect Dis J. 1997;16:427–31. doi: 10.1097/00006454-199704000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Kallen BA, Otterblad Olausson P, Danielsson BR. Is erythromycin therapy teratogenic in humans? Reprod Toxicol. 2005;20:209–14. doi: 10.1016/j.reprotox.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Cooper WO, Hernandez-Diaz S, Arbogast PG, et al. Antibiotics potentially used in response to bioterrorism and the risk of major congenital malformations. Paediatr Perinat Epidemiol. 2009;23:18–28. doi: 10.1111/j.1365-3016.2008.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honein MA, Paulozzi LJ, Himelright IM, et al. Infantile hypertrophic pyloric stenosis after pertussis prophylaxis with erythromcyin: a case review and cohort study. Lancet. 1999;354:2101–5. doi: 10.1016/s0140-6736(99)10073-4. [DOI] [PubMed] [Google Scholar]
- 8.Mahon BE, Rosenman MB, Kleiman MB. Maternal and infant use of erythromycin and other macrolide antibiotics as risk factors for infantile hypertrophic pyloric stenosis. J Pediatr. 2001;139:380–4. doi: 10.1067/mpd.2001.117577. [DOI] [PubMed] [Google Scholar]
- 9.Cooper WO, Ray WA, Griffin MR. Prenatal prescription of macrolide antibiotics and infantile hypertrophic pyloric stenosis. Obstet Gynecol. 2002;100:101–6. doi: 10.1016/s0029-7844(02)02001-x. [DOI] [PubMed] [Google Scholar]
- 10.Louik C, Werler MM, Mitchell AA. Erythromycin use during pregnancy in relation to pyloric stenosis. Am J Obstet Gynecol. 2002;186:288–90. doi: 10.1067/mob.2002.119718. [DOI] [PubMed] [Google Scholar]
- 11.Louik C, Mitchell AA. Prenatal prescription of macrolide antibiotics and infantile hypertrophic pyloric stenosis. Obstet Gynecol. 2003;101:816. doi: 10.1016/s0029-7844(03)00053-x. author reply 16–7. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell AA, Rosenberg L, Shapiro S, Slone D. Birth defects related to bendectin use in pregnancy. I. Oral clefts and cardiac defects. JAMA. 1981;245:2311–4. [PubMed] [Google Scholar]
- 13.Mitchell AA, Cottler LB, Shapiro S. Effect of questionnaire design on recall of drug exposure in pregnancy. Am J Epidemiol. 1986;123:670–6. doi: 10.1093/oxfordjournals.aje.a114286. [DOI] [PubMed] [Google Scholar]
- 14.Yau WP, Lin KJ, Werler MM, Louik C, Mitchell AA, Hernandez-Diaz S. Drug certainty-response in interview-based studies. Pharmacoepidemiol Drug Saf. 2011;20:1210–6. doi: 10.1002/pds.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peeters T, Matthijs G, Depoortere I, Cachet T, Hoogmartens J, Vantrappen G. Erythromycin is a motilin receptor agonist. Am J Physiol. 1989;257:G470–4. doi: 10.1152/ajpgi.1989.257.3.G470. [DOI] [PubMed] [Google Scholar]
- 16.Romoren M, Lindbaek M, Nordeng H. Pregnancy outcome after gestational exposure to erythromycin-a population-based register study from Norway. Br J Clin Pharmacol. 2012 Mar 30; doi: 10.1111/j.1365-2125.2012.04286.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]