Abstract
Muenke syndrome is an autosomal dominant craniosynostosis syndrome resulting from a defining point mutation in the FGFR3 gene. Muenke syndrome is characterized by coronal craniosynostosis (bilateral more often than unilateral), hearing loss, developmental delay and carpal and/or tarsal bone coalition. Tarsal coalition is a distinct feature of Muenke syndrome and has been reported since the initial description of the disorder in the 1990s. Although talocalcaneal coalition is the most common tarsal coalition in the general population, it has never been previously been reported in a patient with Muenke syndrome.
We present a 7-year-old female patient with Muenke syndrome and symptomatic talocalcaneal coalition. She presented at the age of 7 with limping, tenderness and pain in her right foot following a fall and strain of her right foot. She was treated with ibuprofen, shoe inserts, a CAM walker boot and stretching exercises without much improvement in symptoms. A Computed Tomography (CT) scan revealed bilateral talocalcaneal coalitions involving the middle facet. She underwent resection of the talocalcaneal coalitions, remaining pain-free postoperatively with an improvement in her range of motion, gait and mobility.
This report expands the phenotype of tarsal coalition in Muenke syndrome to include talocalcaneal coalition. A literature review revealed a high incidence of tarsal coalition in all FGFR related craniosynostosis syndromes when compared to the general population, a difference that is statistically significant. The most common articulation involved in all syndromic craniosynostoses associated with FGFR mutations is the calcaneocuboid articulation.
Keywords: Muenke syndrome, FGFR3 craniosynostosis, Tarsal fusion craniosynostosis, Tarsal coalition craniosynostosis, Talocalcaneal coalition, Syndromic craniosynostosis tarsal coalition, FGFR craniosynostosis tarsal fusion, Muenke syndrome tarsal fusion, Muenke syndrome tarsal coalition, The feet Muenke syndrome
INTRODUCTION
Tarsal coalition, a term used to describe a fibrous, cartilaginous, or bony connection between two or more tarsal bones, has an incidence of 2–13% in the general population [Solomon et al., 2003; Stormont et al., 1983]. In approximately 50% of cases, the coalition is bilateral [Kernbach 2010]. When tarsal coalition occurs in an isolated manner, the talocalcaneal articulation is most commonly affected, with an incidence of 48.1%, while the calcaneonavicular articulation is the second most common, involved in 43.6% of cases. Coalitions of the talonavicular, calcaneocuboid, cubonavicular, and naviculocuneiform tarsal bones, while reported, are extremely rare in the general population [Stormont et al., 1983; DelSel et al., 1959; Sullivan et al., 1996]. Tarsal coalitions are generally asymptomatic, and typically present as an incidental radiographic finding in an asymptomatic individual. In fact, the number of patients with symptomatic tarsal coalitions represents the minority [Bohne et al., 2001].
While tarsal coalition can occur in an isolated form, without any additional anomalies, it also occurs as a part of a number of genetic syndromes and conditions, including Nievergelt syndrome, phocomelia, fibular hemimelia and other gross limb anomalies [Pearlman et al., 1964; Leonard 1974; Austin 1951; O’Rahilly 1953]. Tarsal coalition also occurs as a part of several craniosynostosis syndromes caused by mutations in the Fibroblast Growth Factor Receptor(FGFR) genes, including Apert, Pfeiffer, Crouzon, Jackson-Weiss, and Muenke syndromes [Anderson et al., 1997a; Anderson et al., 1998a; Anderson et al., 1999; Jackson et al., 1976; Muenke et al., 1997]. Muenke syndrome is the most common of these genetic craniosynostosis syndromes, with an estimated incidence of 1 in 30,000 births [Boulet et al., 2008].
Muenke syndrome is an autosomal dominant craniosynostosis syndrome due to the defining point mutation, c.749C>G, in the FGFR3 gene, resulting in the missense mutation p. Pro250Arg [Muenke et al., 1997]. Muenke syndrome is characterized by coronal craniosynostosis (bilateral more often than unilateral), hearing loss, developmental delay and carpal and/or tarsal bone coalition. Tarsal coalition is a distinct feature of Muenke syndrome, as evidenced by its occasional description as: “coronal craniosynostosis with brachydactyly and carpal/tarsal coalition” [Graham et al., 1998]. Tarsal bone coalition has been reported since the initial description of the disorder in the 1990s and, in our review of the literature, is found to have an incidence of 25% in Muenke syndrome. The coalition usually involves the calcaneus and cuboid bones, a coalition that is actually quite rare in the general population. Other reported coalitions have involved the calcaneus and navicular bones and the cuboid and cuneiform bones [Muenke et al., 1997; Boulet et al., 2008; Graham et al., 1998]. Interestingly, although talocalcaneal coalition is the most common tarsal coalition in the general population, it has never been previously been reported in a patient with Muenke syndrome.
We report on a patient with Muenke syndrome who had symptomatic talocalcaneal coalition. The literature is reviewed with regards to all of the cases of Muenke syndrome in which feet were examined radiographically. Additionally, tarsal coalition in additional FGFR-related craniosynostosis syndromes is reviewed.
MATERIALS AND METHODS
This patient participated in our IRB-approved protocol on Muenke syndrome, with informed consent obtained. Patient information collected included: clinic notes, surgical history, past medical history, imaging and genetic testing. A Medline search was conducted to find all previously reported cases of Muenke syndrome from 1996 (time period of intial description of Muenke syndrome) to the present [2011]. The key words and patient terms searched included “Muenke syndrome,” “coronal synostosis,” “FGFR3 craniosynostosis,” “P250R,” “Pro250Arg,” and “syndromic craniosynostosis.” Additional searches were done to find all reports of tarsal and limb anomalies in patients with Apert syndrome, Crouzon syndrome, Pfeiffer syndrome, Jackson-Weiss syndrome, Crouzon syndrome with acanthosis nigricans and Beare-Stevenson syndrome.
Two-tailed p-tests were done to compare the frequency of tarsal coalition in the general population to the frequency of tarsal coalition in the following FGFR craniosynostosis syndromes: Apert syndrome, Crouzon syndrome, Pfeiffer syndrome, Jackson-Weiss syndrome and Muenke syndrome. General population frequency was obtained from an MRI study of 574 patients. This MRI study was a prospective study that reviewed ankle MRIs of 574 patients in a population requiring an MRI of the ankle [Nalaboff et al., 2008]. A total of six two-tailed p-tests were done to compare the frequency of tarsal coalition in the general population to the FGFR craniosynostosis syndromes (Apert, Crouzon, Pfeiffer, Jackson-Weiss and Muenke syndromes). A two-tailed p-test was also done to compare the frequency of tarsal coalition in the general population to the overall frequency of tarsal coalition in FGFR craniosynostosis syndromes.
RESULTS
Clinical Report
The patient was a 7-year-old female with Muenke syndrome. She was diagnosed at 4 months of age via FGFR3 testing which found the defining mutation causative of Muenke syndrome. Her parents have been tested for the defining mutation that causes Muenke syndrome and were negative, thus making this patient’s inheritance of the syndrome sporadic. Further family history is non-contributory, with no family members having features of Muenke syndrome, such as hearing loss, developmental delay, craniosynostosis or facial features of Muenke syndrome. She has the following features of Muenke syndrome: bicoronal craniosynostosis, developmental delay, hearing loss, high palate, and strabismus. Additionally she has hypertelorism, temporal bossing, and midfacial hypoplasia. She has had three operations including a frontal orbital advancement at 6 months of age and additional operations at 20 months of age and 3 years of age for recurrent deformities. She has a moderate motor delay and has been in occupational therapy for 4 years. She currently attends occupational therapy one time per week. All of her speech and language milestones were within normal limits. She does not have intellectual disability, with an IQ of 117. Her hearing loss is bilateral and mixed. She also experiences recurrent otitis media with effusion and has had four sets of tympanostomy tubes. Additional medical issues include torticollis for which she attends physical therapy and tonsillar hypertrophy for which she underwent a tonsilloadenoidectomy. She did not have radiographs of her hands or feet at the time of initial diagnosis at 4 months of age.
At the age of 7, she presented with right foot pain and limping which was exacerbated by exercise and walking. Six months prior, she had a fall and subsequent “strain” of her right foot by report, which “seemed like it took a long time to heal” perparental description. Radiographs of her right foot were taken immediately after this fall, and were read as negative for any kind of fracture or deformity.
On physical exam, there was no significant deformity. She had very widened feet, particularly in the area around her toes, as well as high arches. She was tender to palpation at the dorsal aspect of her right foot in the area of the 4th metatarsal head. Due to bilateral Achilles contractures noted on physical exam, her range of motion in both feet was limited on dorsiflexion, unable to dorsiflex beyond the neutral position bilaterally. She had normal range of motion to plantarflexion, inversion, and eversion bilaterally. Strength in her right foot was 4/5 (left was 5/5). She was initially treated with a trial of ibuprofen, shoe inserts to support her high arches, and home exercises. At 1.5 month follow-up, she was placed in a CAM walker boot (lower extremity boot composed of rigid plastic that provides support, protection and immobilization of the ankle), which helped with the toe walking. At 3 month follow-up, toe walking had continued, gait remained abnormal and plantar pain had improved. Radiographs of her right foot at this time were read as normal. 3 months subsequently, symptoms persisted and she had CT scans of both feet, which revealed large bilateral coalitions of her talus and calcaneus bones (talocalcaneal coalition), involving the middle facet (Fig. 1a–c, Fig. 2a–c). She underwent resection of bilateral talocalcaneal coalitions. At 1 year follow-up, her range of motion and gait had markedly improved. Additionally, she remains pain-free.
Figure 1.
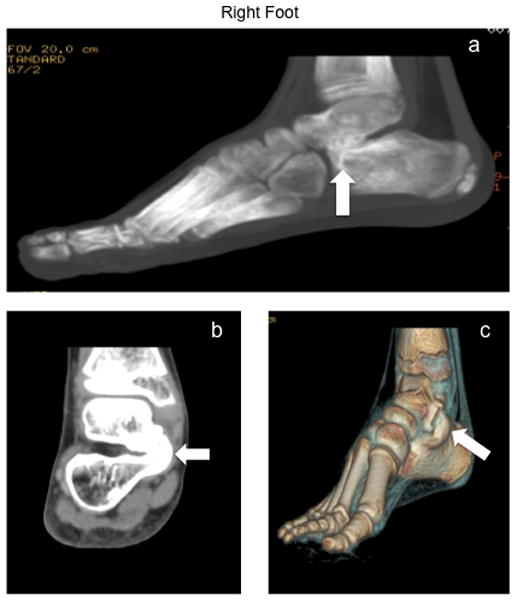
Figure 1a: Lateral Computed Tomography (CT) view of right foot showing the talocalcaneal coalition (white arrow)
Figure 1b: Axial Computed Tomography (CT) view of right foot showing the talocalcaneal coalition (white arrow)
Figure 1c: 3D Computed Tomography (CT) view of right foot showing the talocalcaneal coalition (white arrow)
Figure 2.
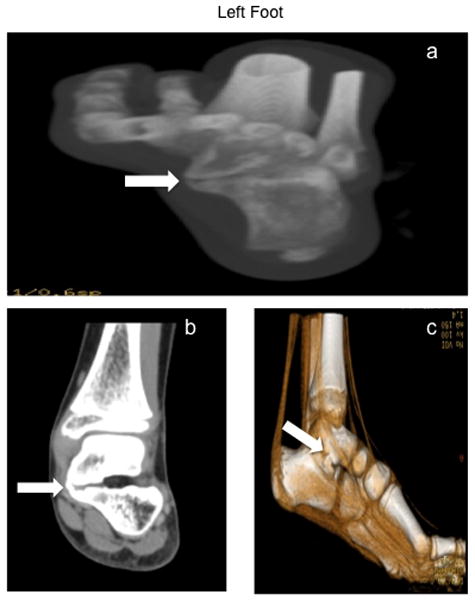
Figure 2a: Plantar axial Computed Tomography (CT) view of left foot showing the talocalcaneal coalition (white arrow)
Figure 2b: Dorsal axial Computed Tomography (CT) view of left foot showing the talocalcaneal coalition (white arrow)
Figure 2c: Plantar oblique 3D Computed Tomography (CT) view of left foot showing the talocalcaneal coalition (white arrow)
Comparison of Frequencies of Tarsal Coalition in General Population vs. FGFR Related Syndromic Craniosynostoses
Results of two-tailed p-tests to compare the frequency of tarsal coalition in the general population (66/574 = 11.5%) to the frequency of tarsal coalition in FGFR craniosynostosis syndromes showed that there was a statistically significant difference in the incidence of tarsal coalition in the general population when compared to the frequency of tarsal coalition in Apert syndrome (p= <0.0001), Jackson-Weiss syndrome (p=<0.0001), Muenke syndrome (p=0.0006) and Pfeiffer syndrome (p=<0.0001) (Table III). Statistical comparison of the frequency of tarsal coalition in the general population to that in Crouzon syndrome revealed no statistically significant difference (p=0.5078). Finally, statistical comparison of the frequency of tarsal coalition in the general population to the overall frequency of tarsal coalition in FGFR craniosynostosis syndromes revealed a statistically significant difference (p<=0.0001).
Table III.
Tarsal coalition in FGFR related craniosynostosis syndromes
| Syndrome | % affected (no. patients affected/no. examined): types of coalition reported | p-value (incidence of tarsal coalition in syndrome vs. general population) | Additional Limb Anomalies Described |
|---|---|---|---|
| Apert Syndrome (FGFR2) | 91% (91/100): progressive fusion of all tarsal bones, sparing talonavicular joint |
p= <0.0001 | Bony syndactyly, synonychia, short metatarsals, malformed metatarsals, metatarsal coalition, symphalangism, carpal bone coalition, clinodactyly, postaxial polydactyly, abnormal bone configuration, winging of scapula, short humeri, glenoid dysplasia, genua valga, elbow ankylosis/synostosis, hypoplastic changes of: scapula, humerus, radius, ulna, pelvis and femur. |
| Crouzon Syndrome (FGFR2) | 13% (3/24): calcaneocuboid |
p=0.5078 | Clinodactyly, carpal bone fusion, hypoplastic 4th metacarpal, short metacarpals, soft tissue syndactyly, brachydactyly, hypoplastic/absent phalanges, broad phalanges, pseudoepiphyses of 1st and 5th metacarpals, broad metatarsals, pseudoepiphysis of 1st and 5th metatarsals, dysplastic cuneiforms, symphalangism. |
| Jackson-Weiss Syndrome (FGR2) | 82% (23/28): calcaneocuboid, calcaneonavicular, navicular-1st cuneiform, navicular-medial cuneiform |
p=<0.0001 | Brachydactyly, cone shaped epiphyses, hypoplastic phalanges, carpal bone malsegmentation, bipartite lunate, short and broad metatarsals, broad phlanages, hallux varus, metatarsus adductus, metatarsal coalition, symphalangism, cutaneous syndactyly, short and broad toes, cone shaped epiphyses, malformed metatarsals, malformed phalanges |
| Muenke Syndrome (FGFR3) | 25% (27/109): calcaneocuboid, calcaneonavicular, cuboid-cuneiform, talocalcaneal |
p=0.0006 | Brachydactyly, clinodactyly, broad toes and thumbs, cone shaped epiphyses, thimble like phalanges, symphalangism, short, broad phalanges, hypoplastic/absent phalanges, partial syndactyly. |
| Pfeiffer Syndrome (FGFR1, FGFR2) | 50% (20/40): medial cuneiform- navicular, calcaneocuboid, cuneiform-cuneiform |
p=<0.0001 | Broad halluces, hallux varus, soft tissue syndactyly, broad medially deviated thumbs, metatarsus adductus, malformed metatarsals, symphalangism, hypoplastic/absent phalanges, metatarsal thinning, coxa valgus, shallow acetabulae, elbow ankylosis, elbow synostosis, triangular phalanges, clinodactyly. |
Table III Figures Obtained From:
Apert syndrome: [Anderson et al., 1999; Cohen et al., 1993b; Cohen et al., 1995; Schauerte et al., 1996; Mah et al 1991]
Crouzon syndrome: [Anderson et al., 1997a; Anderson et al., 1997; Craig et al., 1977; Proudman et al., 1994]
Jackson-Weiss syndrome: [Jackson et al., 1976; Ades et al., 1994; Heike et al., 2001; Li et al., 1994; Parket al., 1995]
Muenke syndrome: [This Report, Muenke et al., 1997; Trusen et al., 2003]
Pfeiffer syndrome: [Rossi et al., 2003, Gripp et al., 1998, Roscioli et al., 2000; Anderson et al., 1998a; Saldino et al., 1972; Asnes et al., 1969; Naveh et al., 1976; Hacketet al., 2006]
DISCUSSION
Our patient did not have radiographs of her hands or feet at the time of diagnosis of Muenke syndrome, as the hand and feet findings in Muenke syndrome are of academic interest, but have been historically thought of as inconsequential. A review of the literature of patients with Muenke syndrome in which foot radiographs were examined, including this report, showed that of 109 patients examined, 27 patients (25%) had tarsal coalition (Table I, Table II). This is statistically significantly higher than the incidence of tarsal coalition in the general population, even when using the highest estimated incidence of tarsal coalition in the general population as determined by the most sensitive imaging modality, magnetic resonance imaging (MRI) (p=0.0006) (Table III). The most common tarsal coalition in Muenke syndrome is calcaneocuboid coalition, with a prevalence of 59%; this type of tarsal coalition is rare in the general population, with a prevalence of 1.3%, occurring in 4 of 314 patients with tarsal coalition in the general population in one review (Table II) [DelSel et al., 1959].
Table I.
Tarsal Coalition in Muenke Syndrome
| # patients examined | Tarsal Fusion | Calcaneocuboid | Calcaneonavicular | Cuboid-cuneiform | Talocalcaneal | NS | Symptomatic | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | Almeida et al., 2009 | |||||
| 1 | 1 | 1 | Barbosa et al., 2008 | |||||
| 1 | 1 | 1 | 1 | 1 | Didolkar et al., 2009* | |||
| 7 | 1 | 1 | Doherty et al., 2007 | |||||
| 5 | 1 | 1 | 1 | Graham et al., 1998* | ||||
| 2 | 0 | Gripp et al., 1998 | ||||||
| 1 | 0 | Grosso et al., 2003 | ||||||
| 2 | 0 | Hughes et al., 2001 | ||||||
| 23 | 3 | 2 | 1 | Lajeunie et al., 1999 | ||||
| 4 | 0 | 6 | Lowry et al., 2001 | |||||
| 16 | 6 | Muenke et al., 1997 | ||||||
| 27 | 5 | 3 | Renier et al., 2000 | |||||
| 1 | 0 | Robin et al., 1998 | ||||||
| 1 | 0 | Roscioli et al., 2001 | ||||||
| 8 | 2 | 2 | Trusen et al., 2003 | |||||
| 1 | 0 | Abdel-Salam et al., 2010 | ||||||
| 7 | 5 | 5 | de Jong et al., 2011 | |||||
| 1 | 1 | 1 | This report |
These patients had >1 coalition, involving the calcaneocuboid and calcaneonavicular coalitions (Graham), and the calcaneonavicular and cuboid-cuneiform coalition (Didolkar).
NS = Not Specified
Table II.
Prevalence of tarsal coalitions in Muenke syndrome
| Type of Tarsal Coalition | % | n |
|---|---|---|
| Calcaneocuboid | 59 | 16 |
| Calcaneonavicular | 26 | 7 |
| Cuboid-cuneiform | 4 | 1 |
| Talocalcaneal | 4 | 1 |
| Not Specified | 15 | 4 |
| >1 Fusion | 7 | 2 |
27 of 109 (25%) patients examined had tarsal coalitions.
Based on data from: Abdel-Salam et al., 2010; Almeida et al., 2009; Barbosa et al., 2008; Didolkar et al., 2009; Doherty et al., 2007; Graham et al., 1998; Gripp et al., 1998; Grosso et al., 2003; Hughes et al., 2001; Lajeunie et al., 1999; Muenke et al., 1997; Renier et al., 2000; Robin et al., 1998; Roscioli et al., 2001; Trusen et al., 2003; de Jong et al., 2011 and this report
Symptomatic tarsal coalition in Muenke syndrome is quite rare. Of all patients with Muenke syndrome reported with tarsal coalitions, two (including our patient) have been symptomatic, requiring evaluation and treatment. The other reported patient with a symptomatic coalition was a 10 year-old female with a several year history of migratory arthralgias of her hands, feet, hips, elbows and shoulders. She had bilateral non-osseus calcaneonavicular and osseus cuboid-lateral cuneiform coalitions bilaterally [Didolkar et al., 2009]. She underwent resection of the calcaneonavicular coalition in her right foot, but continued to experience arthralgias. Our patient adds to the reports of cases of symptomatic tarsal coalition in Muenke syndrome.
Six months after initial presentation, our patient was identified to have bilateral talocalcaneal coalitions on CT, which were missed on radiographs at initial time of her ankle strain, the event that likely unmasked the underlying coalition. Additionally, her coalitions were missed again on a second set of radiographs 3 months after her initial presentation. Talocalcaneal coalitions are indeed difficult to diagnose. The symptoms are insidious, and often the coalition is missed on X-ray because the subtalar joint surfaces overlap and are oblique [Sullivan et al., 1996; Takakura et al., 1991]. CT imaging is a modality that is not only a valuable mean to diagnose talocalcaneal coalition, as demonstrated in this case, but also reveals the type of coalition (osseus, cartilaginous, fibrous), the extent of the coalition, and can additionally aid with identification of other abnormalities and/or coalitions [Stormont et al., 1983; Schenkel et al., 2010].
This patient presentation is unique when compared to talocalcaneal coalition as it presents in the general population in a number of ways. Our patient presented at the age of 7, which is early compared to the usual age of presentation in isolated talocalcaneal coalition. Patients with talocalcaneal coalition usually present from 12–16 years of age, when the coalition ossifies. In one series of 47 patients with talocalcaneal coalition aged 5–54 years old, the average age at diagnosis was 17.3 years; 30 patients were adolescents, 6 were in their 20s and 3 were in their 40s [Takakura et al., 1991]. Only two patients were younger than 10 years old, while one patient was in his 50s. Indeed, talocalcaneal coalition is quite rare at these extremes of age (younger than 10 and above 50). Our patient also had concomitant Achilles contractures, which is unusual. This is likely a secondary affect of the coalition itself, and the toe walking, rather than directly related to Muenke syndrome.
Our patient is unique as she represents the first reported case of talocalcaneal coalition in Muenke syndrome and the second reported case of symptomatic tarsal coalition in Muenke syndrome. It is possible that there may be other patients with Muenke syndrome who have talocalcaneal coalition, which have gone undiagnosed due to difficulties with visualization of the coalition on X-ray or due to the fact that extremity imaging is not a routine part of management in Muenke syndrome. We are not suggesting that radiographs be obtained in all patients with a diagnosis of Muenke syndrome. However, in patients with Muenke syndrome and symptoms of extremity pain, limping, arthralgias, abnormal gait and/or other similar symptoms, extremity imaging should be considered due to the known hand and foot anomalies associated with this condition. This is also true of patients with similar clinical findings and symptoms who have Apert, Pfeiffer, Crouzon and Jackson-Weiss syndromes. What this case demonstrates, is the importance of clinical judgment and evaluation in the context of a relatively common genetic condition. Knowing what to look for, for example, on radiographs can help guide the clinician to the correct diagnosis, with the goal of the best possible care of the patient.
FOOT ANOMALIES IN FGFR RELATED SYNDROMIC CRANIOSYNOSTOSES
Seven clinically distinct craniosynostosis syndromes are caused by mutations in three FGR genes(FGFR1, FGFR2, FGFR3). These syndromes include Apert syndrome, Crouzon syndrome, Pfeiffer syndrome, Muenke syndrome, Jackson-Weiss syndrome, Beare-Stevenson syndrome, and Crouzon syndrome with acanthosis nigricans. A literature review of tarsal coalitions in craniosynostosis syndromes due to mutations in all FGFR genes, the majority of which occur in FGFR2, revealed that tarsal coalitions have been reported in Apert, Pfeiffer, Crouzon, Jackson-Weiss and Muenke syndrome (FGFR3) (Table III). Fisher’s t-test revealed that tarsal coalitions are significantly overrepresented in FGFR-related craniosynostosis syndromes compared to the general population (p<0.0001) (Table III). Tarsal coalitions have not yet been reported in Beare-Stevenson syndrome (FGFR2) or Crouzon syndrome with acanthosis nigricans (also called “Crouzonodermoskeletal syndrome”) (FGFR3) [Barge-Schaapveld DQ et al., 2011; Di Rocco F et al., 2011; Schweitzer et al., 2001; Cohen et al., 1999].
Apert syndrome, associated with mutations in FGFR2, is a craniosynostosis syndrome in which complex, severe bony syndactyly of the hands and feet is a consistent clinical feature [Wilkie et al., 1995]. It has the highest incidence of tarsal coalition among all of the craniosynostosis syndromes, reported in 91% of cases reviewed (Table III). In all feet, with age there is progressive and sequential coalition of the tarsals and metatarsals, commonly sparing the talonavicular joint, such that 100% of adults examined with Apert syndrome are found to have tarsal coalitions (cases reviewed in this report giving the 91% incidence included all age ranges) [Schauerte et al., 1966; Mah et al., 1991]. This talonavicular sparing is also a feature of those cases with multiple tarsal coalitions in Pfeiffer syndrome [Saldino et al., 1972]. The limb anomalies in Apert syndrome extend beyond tarsal coalition, are more severe in the upper limb, and are the most severe of all the craniosynostosis syndromes (Table III). It is the illegimitate autocrine activation of mutated mesenchymal FGFR2c p.P253 specifically by Fibroblast Growth Factor 10 (FGF10) ligand, that is likely responsible for the syndactyly in Apert syndrome; the p.P253R mutation accounts for 33% of cases of Apert syndrome and is associated with more severe syndactyly when compared to the other causative mutation of Apert syndrome, p.S252W, which accounts for 66% of cases [Ibrahimi et al., 2004, Hajihosseini et al., 2009; Wilkie et al., 1995]. Further, in a mouse model of Apert syndrome, knockdown of FGF10 in these micerescues some of the skeletal anomalies [Hajihosseini et al., 2009].
Pfeiffer syndrome is associated with mutations in FGFR1 or FGFR2 [Muenke et al., 1994; Rutland et al., 1995]. In addition to craniosynostosis of the coronal suture, patients with Pfeiffer syndrome characteristically have broad, medially deviated halluces and variable soft tissue syndactyly [Rossi et al., 2003]. Tarsal coalition was reported in 50% of cases reviewed (Table III). Multiple different mutations in the FGFR2 gene cause Crouzonsyndrome, which is characterized clinically by craniosynostosis commonly involving the coronal suture and prominent eyes (proptosis), secondary to early coalition of the skull sutures [Jabs et al., 1994]. Patients with Crouzon syndrome have long been thought to lack limb anormalities; it is in fact reported that there are no limb abnormalities in this condition [Reardon et al., 1995]. However, radiographic analysis has shown that patients with Crouzon syndrome have carpal coalitions, tarsal coalitions and other limb anomalies (Table III). In 24 cases reviewed, three patients had tarsal coalitions (13%), all involving the calcaneocuboid articulation. One of these patients was symptomatic, requiring surgical intervention for a calcaneocuboid coalition, a coalition that is generally thought of as inconsequential in the general population [Craig et al., 1977]. Crouzon syndrome is the only FGFR-related craniosynostosis described herein, in which the incidence of tarsal coalition is not statistically significant when compared to the general population (p=0.5078) (Table III). This may be due to a lack of reporting or a lack of radiographic investigations in this patient population. The clinical findings of craniosynostosis, tarsal and metatarsal coalitions, with short, broad, medially deviated great toes in the absence of hand anomalies, are characteristic of patients with Jackson-Weiss syndrome, a craniosynostosis syndrome caused by multiple mutations in FGFR2 [Jabs et al., 1994; Graham et al., 1998]. A review of the literature revealed an occurrence of tarsal coalition in 23 out of 28 patients (82%) reported.
The occurrence of tarsal coalition in syndromic craniosynostoses associated with mutations in FGFR genes is undoubtedly related to the unique roles in physiologic processes and the unique patterns of expression of the FGFR genes, genes that play roles in various physiological processes including cell proliferation, differentiation, migration, apoptosis, and pattern formation [Goldfarb et al., 1996; Robin 1999]. This is evidenced by the high incidence of tarsal coalition in these craniosynostosis syndromes when compared to the general population, a difference that is statistically significant (Table III). Overall, it appears that the most common articulation involved in all syndromic craniosynostoses associated with FGFR mutations is the calcaneocuboid articulation. It is our hope that this review covered tarsal coalitions as they occur in syndromic craniosynostoses, and brings to attention the importance of this feature, particularly to the orthopedist, as well as to other practitioners who see patients with these diagnoses. As these patients have anomalies that span beyond the head and face, their care requires a multidisciplinary approach.
Acknowledgments
We would like to express our gratitude to the patient described in this article and the patient’s family for their willingness to participate in our study and for their informed consent to participate in our study and to publish this report. We would also like to thank Dr. M. Michael Cohen Jr. for his critical review of this manuscript. This research was supported by the Division of Intramural Research at the National Human Genome Research Institute (National Institutes of Health, Department of Health and Human Services, United States of America).
Footnotes
None of the listed authors of this manuscript have any relevant conflicts of interest to disclose.
References
- Ades LC, Mulley JC, Senga IP, Morris LL, David DJ, Haan EA. Jackson-Weiss syndrome: clinical and radiological findings in a large kindred and exclusion of the gene from 7p21 and 5qter. Am J Med Genet. 1994;51:121–130. doi: 10.1002/ajmg.1320510208. [DOI] [PubMed] [Google Scholar]
- Anderson PJ, Hall CM, Evans RD, Jones BM, Hayward RD. Hand anomalies in Crouzon syndrome. Skeletal Radiol. 1997;26:113–115. doi: 10.1007/s002560050203. [DOI] [PubMed] [Google Scholar]
- Anderson PJ, Hall CM, Evans RD, Hayward RD, Jones BM. The feet in Crouzon syndrome. J Craniofac Genet Dev Biol. 1997a;17:43–47. [PubMed] [Google Scholar]
- Anderson PJ, Hall CM, Evans RD, Jones BM, Hayward RD. The feet in Pfeiffer syndrome. J Craniofac Surg. 1998a;9:98–102. doi: 10.1097/00001665-199801000-00018. [DOI] [PubMed] [Google Scholar]
- Anderson PJ, Hall CM, Evans RD, Hayward RD, Jones BM. The feet in Apert’s syndrome. J Pediatr Orthop. 1999;19:504–507. doi: 10.1097/00004694-199907000-00015. [DOI] [PubMed] [Google Scholar]
- Asnes RS, Morehead CD. Pfeiffer syndrome. Birth Defects: Original Article Series. 1969;5:198–203. [Google Scholar]
- Austin FH. Synphalangism and related fusions of tarsal bones. Radiology. 1951;56:882. doi: 10.1148/56.6.882. [DOI] [PubMed] [Google Scholar]
- Barge-Schaapveld DQ, Brooks AS, Lequin MH, van Spaendonk R, Vermeulen RJ, Cobben JM. Beare-Stevenson syndrome: two Dutch patients with cerebral abnormalities. Pediatr Neurol. 2011;44:303–307. doi: 10.1016/j.pediatrneurol.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Bohne WH. Tarsal coalition. Curr Opin Pediatr. 2001;13:29–35. doi: 10.1097/00008480-200102000-00005. [DOI] [PubMed] [Google Scholar]
- Boulet SL, Rasmussen SA, Honein MA. A population-based study of craniosynostosis in metropolitan Atlanta, 1989–2003. Am J Med Genet Part A. 2008;146A:984–991. doi: 10.1002/ajmg.a.32208. [DOI] [PubMed] [Google Scholar]
- Cohen MM, Kreiborg S. Skeletal abnormalities in the Apert syndrome. Am J Med Genet. 1993b;47:624–632. doi: 10.1002/ajmg.1320470509. [DOI] [PubMed] [Google Scholar]
- Cohen MM, Kreiborg S. Hands and feet in the Apert syndrome. Am J Med Genet Part A. 1995;57:82–96. doi: 10.1002/ajmg.1320570119. [DOI] [PubMed] [Google Scholar]
- Cohen MM., Jr Let’s call it “Crouzonodermoskeletal syndrome” so we won’t be prisoners of our own conventional terminology. Am J Med Genet. 1999;84:74. [PubMed] [Google Scholar]
- Craig CL, Goldberg MJ. Calcaneocuboid coalition in Crouzon’s syndrome craniofacial dysostosis): report of a case and review of the literature. J Bone Joint Surg Br. 1977;59:826–827. [PubMed] [Google Scholar]
- DelSel JM, Grand NE. Cubonavicular synostosis: A rare tarsal anomaly. J Bone Joint Surg. 1959;41B:149. doi: 10.1302/0301-620X.41B1.149. [DOI] [PubMed] [Google Scholar]
- Di Rocco F, Collet C, Legeai-Mallet L, Arnaud E, Le Merrer M, Hadj-Rabia S, Renier D. Crouzon syndrome with acanthosis nigricans: a case-based update. Childs Nerv Syst. 2011;27:349–354. doi: 10.1007/s00381-010-1347-z. [DOI] [PubMed] [Google Scholar]
- Didolkar MM, Vinson EN, Gaca AM. A young patient with polyarthralgia and hearing loss: a case report of Muenke syndrome. Skeletal Radiol. 2009;38:1011–1014. doi: 10.1007/s00256-009-0716-8. [DOI] [PubMed] [Google Scholar]
- Graham JM, Jr, Braddock SR, Mortier GR, Lachman R, Van Dop C, Jabs EW. Syndrome of coronal craniosynostosis with brachydactyly and carpal/tarsal coalition due to Pro250Arg mutationin FGFR3 gene. Am J Med Genet. 1998;77:322–329. doi: 10.1002/(sici)1096-8628(19980526)77:4<322::aid-ajmg14>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Gripp KW, Stolle CA, McDonald-McGinn DM, Markowitz RI, Bartlett SP, Katowitz JA, Muenke M, Zackai EH. Phenotype of the fibroblast growth factor receptor 2 Ser351Cys mutation: Pfeiffer syndrome type III. Am J Med Genet. 1998;78:356–360. doi: 10.1002/(sici)1096-8628(19980724)78:4<356::aid-ajmg10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Goldfarb M. Functions of fibroblast growth factors in vertebrate development. Cytokine Growth Factor Rev. 1996;7:311–325. doi: 10.1016/s1359-6101(96)00039-1. [DOI] [PubMed] [Google Scholar]
- Hackett A, Rowe L. FGFR1 Pfeiffer syndrome without craniosynostosis: an additional case report. Clin Dysmorphol. 2006;15:207–210. doi: 10.1097/01.mcd.0000220608.40155.d4. [DOI] [PubMed] [Google Scholar]
- Hajihosseini MK, Duarte R, Pegrum J, Donjacour A, Lana-Elola E, Rice DP, Sharpe J, Dickson C. Evidence that Fgf10 contributes to the skeletal and visceral defects of an apert syndrome mouse model. Dev Dyn. 2009;238:376–385. doi: 10.1002/dvdy.21648. [DOI] [PubMed] [Google Scholar]
- Heike C, Seto M, Hing A, Palidin A, Hu FZ, Preston RA, Ehrlich GD, Cunningham M. Century of Jackson-Weiss syndrome: Further definition of clinical and radiographic findings in “lost” descendants of the original kindred. Am J Med Genet. 2001;100:315–324. doi: 10.1002/ajmg.1266. [DOI] [PubMed] [Google Scholar]
- Ibrahimi OA, Zhang F, Eliseenkova AV, Linhardt RJ, Mohammadi M. Biochemical analysis of pathogenic ligand-dependent FGFR2 mutations suggests distinct pathophysiological mechanisms for craniofacial and limb abnormalities. Hum Mol Genet. 2004;13:2313–2324. doi: 10.1093/hmg/ddh235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs EW, Li X, Scott AF, Meyers G, Chen W, Eccles M, Mao JI, Charnas LR, Jackson CE, Jaye M. Jackson-Weiss and Crouzon syndromes are allelic with mutations in fibroblast growth factor receptor 2. Nat Genet. 1994;8:275–279. doi: 10.1038/ng1194-275. [DOI] [PubMed] [Google Scholar]
- Jackson CE, Weiss L, Reynolds WA, Forman TF, Peterson JA. Craniosynostosis midface hypoplasia, and foot abnormalities: an autosomal dominant phenotype in a large Amish kindred. J Pediatr. 1976;88:963–968. doi: 10.1016/s0022-3476(76)81050-5. [DOI] [PubMed] [Google Scholar]
- Kernbach KJ. Tarsal coalitions: etiology, diagnosis, imaging, and stigmata. Clin Podiatr Med Surg. 2010;27:105–117. doi: 10.1016/j.cpm.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Leonard MA. The inheritance of tarsal coalition and its relationship to spastic flat foot. J Bone Joint Surg Br. 1974;56:520–526. [PubMed] [Google Scholar]
- Li X, Lewanda AF, Eluma F, Jerald H, Choi H, Alozie I, Proukakis C, Talbot CC, Vander-Kolk C, Bird LM, Jones MC, Cunningham M, Clarren SK, Pyeritz RE, Weissenbach J, Jackson CE, Jabs EW. Two craniosynostotic syndrome loci, Crouzon and Jackson-Weiss, map to chromosome 10q-q26. Genomics. 1994;22:418–424. doi: 10.1006/geno.1994.1403. [DOI] [PubMed] [Google Scholar]
- Mah J, Kasser J, Upton J. The foot in Apert syndrome. Clin Plast Surg. 1991;18:391–398. [PubMed] [Google Scholar]
- Muenke M, Schell U, Hehr A, Robin NH, Losken HW, Schinzel A, Pulleyn LJ, Rutland P, Reardon W, Malcolm S, Winter RM. A common mutation in the fibroblast growth factor receptor 1 gene In Pfeiffer syndrome. Nat Genet. 1994;8:269–274. doi: 10.1038/ng1194-269. [DOI] [PubMed] [Google Scholar]
- Muenke M, Gripp KW, McDonald-McGinn DM, Gaudenz K, Whitaker LA, Bartlett SP, Markowitz RI, Robin NH, Nwokoro N, Mulvihill JJ, Losken HW, Mulliken JB, Guttmacher AE, Wilroy RS, Clarke LA, Hollway G, Ades LC, Haan EA, Mulley JC, Cohen MM, Bellus GA, Francomano CA, Moloney DM, Wall SA, Wilkie AO, Zackai EH. A unique point mutation in the fibroblast growth factor receptor 3 gene (FGFR3) defines a new craniosynostosis syndrome. Am J Hum Genet. 1997;60:555–564. [PMC free article] [PubMed] [Google Scholar]
- Nalaboff KM, Schweitzer ME. MRI of tarsal coalition: frequency, distribution, and innovative signs. Bull NYU Hosp Jt Dis. 2008;66:14–21. [PubMed] [Google Scholar]
- Naveh Y, Friedman A. Pfeiffer syndrome: report of a family and review of the literature. J Med Genet. 1976;13:277–280. doi: 10.1136/jmg.13.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rahilly R. A survey of carpal and tarsal anomalies. J Bone Joint Surg Am. 1953;35:626–642. [PubMed] [Google Scholar]
- Park WJ, Meyers GA, Li X, Theda C, Day D, Orlow SJ, Jones MC, Jabs EW. Novel FGFR2 mutations in Crouzon and Jackson-Weiss syndromes show allelic heterogeneity and phenotypic variability. Hum Mol Genet. 1995;4:1229–1233. doi: 10.1093/hmg/4.7.1229. [DOI] [PubMed] [Google Scholar]
- Pearlman HS, Edkin RE, Warren RF. Familial tarsal and carpal synostosis with radial-head subluxation (Nievergelt’s syndrome) J Bone Joint Surg Am. 1964;46:585–592. [PubMed] [Google Scholar]
- Proudman TW, Moore MH, Abbott AH, David DJ. Noncraniofacial manifestations of Crouzon’s disease. J Craniofac Surg. 1994;5:218–224. doi: 10.1097/00001665-199409000-00004. [DOI] [PubMed] [Google Scholar]
- Reardon W, Winter RM. The molecular pathology of syndromic craniosynostosis. Mol Med Today. 1995;1:432–437. doi: 10.1016/s1357-4310(95)90837-4. [DOI] [PubMed] [Google Scholar]
- Robin NH. Molecular genetic advances in understanding craniosynostosis. Plast Reconstr Surg. 1999;103:1060–1070. [PubMed] [Google Scholar]
- Roscioli T, Flanagan S, Mortimore RJ, Kumar P, Weedon D, Masel J, Lewandowski R, Hyland V, Glass IA. Premature calvarial synostosis and epidermal hyperplasia (Beare-Stevenson syndrome-like anomalies) resulting from a P250R missense mutation in the gene encoding fibroblast growth factor receptor 3. Am J Med Genet. 2001;101:187–194. doi: 10.1002/ajmg.1369. [DOI] [PubMed] [Google Scholar]
- Rossi M, Jones RL, Norbury G, Bloch-Zupan A, Winter RM. The appearance of the feet in Pfeiffer syndrome caused by FGFR1 P252R mutation. Clin Dysmorphol. 2003;12:269–274. doi: 10.1097/00019605-200310000-00012. [DOI] [PubMed] [Google Scholar]
- Rutland P, Pulleyn LJ, Reardon W, Baraitser M, Hayward R, Jones B, Malcolm S, Winter RM, Oldridge M, Slaney SF, Poole MD, Wilkie AOM. Identical mutations in the FGFR2 gene cause both Pfeiffer and Crouzon syndrome phenotypes. Nat Genet. 1995;9:173–176. doi: 10.1038/ng0295-173. [DOI] [PubMed] [Google Scholar]
- Saldino RM, Steinbach HL, Epstein CJ. Familial acrocephalosyndactyly (Pfeiffer Syndrome) Am J Roentgenol. 1972;116:609–622. doi: 10.2214/ajr.116.3.609. [DOI] [PubMed] [Google Scholar]
- Schauerte EW, St Aubin PM. Progressive synostosis in Apert’s syndrome (acrocephalosyndactyly), with a description of the roentgenographic changes in the feet. Am J Roentgenol. 1966;97:67–73. doi: 10.2214/ajr.97.1.67. [DOI] [PubMed] [Google Scholar]
- Schenkel D, Degraauw J, Degraauw C. Talocalcaneal coalition in a 15 year-old female basketball player. J Can Chiropr Assoc. 2010;54:222–228. [PMC free article] [PubMed] [Google Scholar]
- Schweitzer DN, Graham JM, Lachman RS, Jabs EW, Okajima K, Przylepa KA, Shanske A, Chen K, Neidich JA, Wilcox WR. Subtle radiographic findings of achondroplasia in patients with Crouzon syndrome with acanthosis nigricans due to an Ala391Glu substitution in FGFR3. Am J Med Genet. 2001;98:75–91. [PubMed] [Google Scholar]
- Solomon LB, Rühli FJ, Taylor J, Ferris L, Pope R, Henneberg M. A dissection and computer tomograph study of tarsal coalitions in 100 cadaver feet. J Orthop Res. 2003;21:352–358. doi: 10.1016/S0736-0266(02)00131-6. [DOI] [PubMed] [Google Scholar]
- Stormont DM, Peterson HA. The relative incidence of tarsal coalition. Clin Orthop Relat Res. 1983;181:28–36. [PubMed] [Google Scholar]
- Sullivan JA. The child’s foot. In: Morissey RT, Weinstein SL, editors. Lovell and Winters Pediatric Orthopedics. Philadelphia: Lippincott-Raven; 1996. pp. 4epp. 1077–1135. [Google Scholar]
- Takakura Y, Sugimoto K, Tanaka Y, Tamai S. Symptomatic talocalcaneal coalition. Its clinical significance and treatment. Clin Orthop Relat Res. 1991;269:249–256. [PubMed] [Google Scholar]
- Trusen A, Beissert M, Collmann H, Darge K. The pattern of skeletal anomalies in the cervical spine, hands and feet in patients with Saethre-Chotzen syndrome and Muenke-type mutation. Pediatr Radiol. 2003;33:168–172. doi: 10.1007/s00247-002-0823-3. [DOI] [PubMed] [Google Scholar]
- Wilkie AO, Slaney SF, Oldridge M, Poole MD, Ashworth GJ, Hockley AD, Hayward RD, David DJ, Pulleyn LJ, Rutland P, Malcolm S, Winter RM, Reardon W. Apert syndrome results from localized mutations of FGFR2 and is allelic with Crouzon syndrome. Nat Genet. 1995;9:165–172. doi: 10.1038/ng0295-165. [DOI] [PubMed] [Google Scholar]


