Abstract
The human intruder test is a testing paradigm designed to measure rhesus macaques’ behavioral responses to a stressful and threatening situation. In the test, an unfamiliar human positions him/herself in various threatening positions relative to a caged macaque. This paradigm has been utilized for over twenty years to measure a variety of behavioral constructs, including fear and anxiety, behavioral inhibition, emotionality, and aggression. To date there have been no attempts to evaluate comprehensively the structure of the behavioral responses to the test. Our first goal was to identify the underlying latent factors affecting the different responses among subjects, and our second goal was determine if rhesus reared in different environments respond differently in this testing paradigm. To accomplish this, we first performed exploratory and confirmatory factor analyses on the behavioral responses of 3–4 month-old rhesus macaques, utilizing data from over 2,000 separate tests conducted between 2001–2007. Using the resulting model, we then tested to see whether early rearing experience affected responses in the test. Our first analyses suggested that most of the variation in infant behavioral responses to the human intruder test could be explained by four latent factors: “Activity,” “Emotionality,” “Aggression,” and “Displacement.” Our second analyses revealed a significant effect of rearing condition for each factor score (P < 0.001); most notable socially-reared animals had the lowest Activity score (P < 0.001), indoor mother-reared animals had the highest Displacement score (P < 0.001), and nursery-reared animals had the highest Emotionality (P < 0.001) and lowest Aggression scores (P < 0.001). These results demonstrate that this standardized testing paradigm reveals multiple patterns of response, which are influenced by an animal’s rearing history.
Keywords: human intruder test, emotionality, confirmatory factor analysis, rhesus monkeys, Macaca mulatta
1. Introduction
The human intruder test is a well-established and frequently used testing paradigm to measure behavioral response to a stressful and threatening situation in captive rhesus macaques (Macaca mulatta). Researchers have used this paradigm to measure a variety of different behavioral constructs, including anxiety and fear [Bethea et al. 2004; Corcoran et al. 2011; Davidson et al. 1993; Fox et al. 2008; Kalin et al. 2004; Kalin et al. 2001; Williamson et al. 2003], behavioral inhibition [Coleman et al. 2003; Kalin et al. 2007; Rogers et al. 2008], emotionality [Izquierdo and Murray 2004; Izquierdo et al. 2005], defensive behavior [Kalin and Shelton 1989; Kalin and Shelton 1998; Kalin et al. 2007; Kalin et al. 2005; Kalin et al. 1991a], and aggression [Minier et al. 2011]. However there has never been an attempt to evaluate comprehensively the structure of the behavioral responses to a human intruder test, and identify the underlying latent factors affecting the different responses among subjects.
Kalin and Shelton first established the human intruder test as a means of measuring defensive behavior in infant rhesus [Kalin and Shelton 1989]. The basic design of their test involves three conditions in which behavioral responses are scored. In the first condition, the test monkey is separated from its mother or conspecific and left alone in a testing room for 9–30 minutes, depending on the study. In the second condition, a human intruder enters the test room, stands 2.5 meters from the monkey and presents his profile without giving eye contact. In the final condition, the human intruder turns to face the subject, giving direct eye contact, which is considered to be the most aggressive and threatening condition of the paradigm. Although the order and length of time in each condition has varied, this basic three-condition paradigm has been utilized multiple times by Kalin and colleagues [Kalin and Shelton 1989; Kalin and Shelton 1998; Kalin et al. 2004; Kalin et al. 2007; Kalin et al. 2001; Kalin et al. 2008; Kalin et al. 1991a; Kalin et al. 1991b], and other researchers alike [Bethea et al. 2004; Coleman et al. 2003; Corcoran et al. 2011; Friedman et al. 1996; Izquierdo and Murray 2004; Izquierdo et al. 2005; Machado and Bachevalier 2008; Willette et al. 2007; Williamson et al. 2003]. Kalin and colleagues have occasionally modified this test to exclude the stare condition [Davidson et al. 1993; Kalin et al. 1998; Rogers et al. 2008], and have sometimes extended the profile condition and repeatedly entered and exited the room to prevent habituation to the intruder [Fox et al. 2008; Kalin et al. 2005; Rogers et al. 2008]. Capitanio and colleagues further modified this paradigm and created a four-condition human intruder paradigm [Capitanio 1999; Capitanio et al. 2006; Capitanio et al. 2011; Golub et al. 2009; Karere et al. 2009; Kinnally et al. 2010; Minier et al. 2011; Rommeck et al. 2011]. In this version of the human intruder test, the profile and stare conditions are each repeated twice, once with the intruder positioning himself 1 meter from the cage, and once at 0.3 meters from the cage. In their initial 1999 report the human intruder test was conducted in the animals’ home cage, while subsequent studies conducted the test in a separate testing room, much like the Kalin paradigm. However in contrast to the Kalin paradigm, in the Capitanio paradigm animals are typically separated from their home cage 5 hours prior to testing, and are somewhat familiar with the testing cage, while in the Kalin paradigm animals are left alone in the novel testing room for only 9–30 minutes before the intruder enters.
In their initial study, Kalin and Shelton indicated that noteworthy behaviors exhibited by the subject animals during the test included distress vocalizations (cooing), aggressive vocalizations (barking), and freezing behavior (remaining motionless). They characterized these behaviors as reflecting different patterns of defensive behavior in light of the threatening situation: cooing signals to a mother for help, barking is an aggressive response to inescapable danger, and freezing functions to avoid detection and prevent attack in the natural environment. Since this initial experiment, other researchers have included many additional behavioral responses in their ethogram, including body position, position in cage, aggressive behaviors, displacement behaviors, affiliative behaviors, stereotypic behaviors, self-directed behaviors, and many more (for a list of behaviors recorded by Capitanio and colleagues see Table 1).
Table 1.
Behaviors and Definitions for Human Intruder Test
| Behavior | Definition |
|---|---|
| States (duration) | |
| Sit | Hindquarters are on the perch or floor; includes shifting weight slightly one step |
| Lie | Relaxed posture with body resting on a horizontal surface |
| Stand | Torso in a stationary position and weight is supported by 3 or 4 legs; can include steps taken that only involve one or two feet |
| Active | Whole body movement; step, jump |
| Crouch | Ventral surface close to floor; head at or below the level of the shoulders |
| Sleep | Eyes closed |
| Rock/Sway | Unbroken rhythmic movements of the upper body while the animal is sitting |
| Hang | Holding onto ceiling or front mesh; all 4 limbs off of floor |
| Motor Stereotypy | Movement back and forth, repeatedly covering the same route |
| Cage position (duration) | |
| Front | The animal’s head is located in the front half of the testing cage (close to the intruder) |
| Events (frequency) | |
| Scratch | Common usage |
| Self-clasp | Hand or feet closed on fur or some body part |
| Self-bite | Discrete biting action, usually directed to limbs and often accompanied by a threat face |
| Stroke | Very gently bringing the hand or foot across the side of head or face |
| Self-manipulate | Masturbation, pulling or tugging or pushing at self |
| Self-groom | Using hands or lips to pick through or part its own fur |
| Suck | Insertion into mouth of fingers, toes, and other body parts |
| Back-flip | Tossing the body up and backwards in a circular motion in the air |
| Convulsive jerk | Sudden and somewhat violent contractions of the limbs and trunk |
| Cage shake/Bounce | Holding onto cage and shaking it, generating a lot of noise |
| Vocal coo | Medium-pitched, moderately intense, clear call |
| Vocal screech | Intense, very high-pitched |
| Vocal gecker | Staccato cackling sounds |
| Vocal bark | Gruff, abrupt, low-pitched vocalization |
| Vocal other | Other vocalizations not previously described |
| Lipsmack | Rapid lip movement usually with pursed lips, accompanied by a smacking sound |
| Threat | Scored with at least two or more of the following: open mouth stare, head bob, ear flaps, bark vocalizations |
| Fear grimace | Exaggerated grin with teeth showing |
| Yawn | Wide open mouth displaying teeth |
| Tooth grind | Loud gnashing of teeth |
| Environmental Explore | Discrete manipulation by hand or mouth with the physical environment or objects in the cage |
Kinnally and colleagues proposed that there are two latent factors responsible for the subjects’ responses to the human intruder test: activity and emotionality [Kinnally et al. 2010]. They created activity and emotionality scales based on the behaviors measured and demonstrated the reliability of these scales using Cronbach’s alpha. The scales were created using a priori predictions based on previous research; however, without performing an exploratory factor analysis to formally evaluate the structure of the data, it is possible they missed other latent variables involved in subjects’ behavioral response. A true exploratory factor analysis could be used to identify all of the underlying factors affecting an animal’s response, and simplify the diverse behavioral responses to the test. Knowing the different factors that underlie differing responses in a human intruder test can inform researchers as to what the test is measuring, and elucidate a broader range of research questions that the test could be used to answer.
In 2001, one of us (JPC) initiated a 25-hour BioBehavioral Assessment (BBA) program for 3–4 month old infants [Capitanio et al. 2006] at the California National Primate Research Center (CNPRC). This program was developed as a means to characterize the behavioral and physiological responses of the majority of animals available at the CNPRC. As a part of the BBA assessment program, animals take part in a human intruder test. Between 2001 and 2007, over 2,000 infants were assessed. In the present study we performed an exploratory factor analysis on human intruder data collected between 2001–2004 to identify the underlying latent variables affecting behaviors shown in response to the human intruder. Then we tested and confirmed the generality of this structure by performing a confirmatory factor analysis on a new set of animals tested between 2005–2007. After establishing a model, we evaluated the validity of the factors by testing for differences in factor scores between three major rearing conditions common to many primate facilities: outdoor social rearing, indoor mother rearing, and nursery rearing. Previous research has consistently shown rearing condition to affect multiple behavioral and physiological outcomes in the BBA program, including outcomes from tests other than the human intruder; for example, compared to socially-reared animals, indoor mother-reared animals tend to show more activity, and nursery-reared animals tend to show greater emotionality [e.g. Capitanio et al. 2006; Capitanio et al. 2005]. In the present report, we tested whether these response patterns were evident in the human intruder test.
2. Methods
2.1. BioBehavioral Assessment
The procedures involved in the BBA have previously been described in detail [Golub et al. 2009]. Each year since 2001 the majority of rhesus macaques born at the CNPRC that are available for testing were selected to take part in the 25-hour BBA program. Between the ages of approximately 90 and 120 days, infants were temporarily separated from their mothers and/or social partners and relocated to individual indoor cages for the 25-hour testing period. During this time, infants took part in multiple behavioral and physiological assessments, including a human intruder test.
The human intruder test took place in a testing cage in a room adjacent to the animals’ holding room, and occurred five hours after the initial relocation. The test comprised four one-minute conditions: “Profile-Far,” “Profile-Near,” “Stare-Far,” and “Stare-Near.” During the first condition the experimenter positioned herself ~1 meter in front of the cage and presented her left profile to the animal’s cage (Profile-Far). At the end of the minute, the experimenter moved to ~0.3 meters from the cage, while still maintaining a profile position (Profile-Near). After holding that position for a minute, the experimenter moved back to the 1 meter position and made direct eye contact with the animal (Stare-Far). One minute later the experimenter returned to the 0.3 meter position while maintaining direct eye contact for an additional minute (Stare-Near). The four-minute test session was videotaped and later coded by a single observer using “The Observer” [Noldus 1991]. Intra- and inter-observer reliability, checked annually, exceeds 85% agreement on behavior codes. All behaviors were recorded as states (durations that were mutually exclusive and exhaustive) or events (frequencies). Due to slight variations in length of observation periods, duration measures were converted to proportion of the total observation time, and frequencies of states and events were converted to rate per 60 s. Cage position (front vs back) was also recorded but only recordings of front of cage were used in the analysis, as front and back categories are also mutually exclusive and exhaustive. For a full list of behaviors recorded see Table 1.
2.2. Subjects
For the exploratory factor analysis we used all animals that had taken part in the BBA between 2001–2004, totaling 1,094 subjects. For the confirmatory factor analysis we used all animals that had taken part in the BBA between 2005–2007, totaling 923 subjects. All subjects were born at the CNPRC and were tested when they were between 89 and 133 days old (mean of 107.61 days, standard deviation of 10.18 days). Subjects were raised in one of three conditions. 1692 animals were socially-reared, 225 were indoor mother-reared, and 100 were nursery-reared. Socially-reared animals were raised with their biological or foster mother in either acre or 400 square foot outdoor enclosures that house anywhere from 15–200 animals. Indoor mother-reared and nursery-reared animals were raised indoors with no social group. Indoor mother-reared animals were housed in a cage with their biological or foster mother, and at most one additional adult and infant pair. Nursery-reared animals were individually housed until 3 weeks of age, at which time they were given visual access to an infant of the same age, with whom they were eventually paired at 5 weeks of age. All subjects were cared for in compliance with protocols approved by the Institutional Animal Care and Use Committee at the University of California Davis, and adhered to the requirements of the Animal Welfare Act and US Department of Agriculture regulations [Animal Welfare Act 1985], and the American Society of Primatologists’ Principles for the Ethical Treatment of Non Human Primates.
2.3. Data Analysis
Both exploratory and confirmatory factor analyses were performed using the Mplus 3.0 program [Muthén and Muthén 1998–2010]. Before performing the exploratory factor analysis, and because the test trials were only 1-min in duration, we modified the data so that rare behaviors (i.e., behavior that was recorded only for 15% or fewer of the animals) were recoded as 1-0 (1 if behavior was ever expressed by the individual, 0 if it was never expressed). A correlation table of all variables was created and we removed any variables that didn’t correlate with at least one other variable by at least 0.3. For our initial exploratory analysis we included every behavior four times, one for each condition of the test (for example, yawn was included as yawn profile-far, yawn profile-near, yawn stare-far, and yawn stare-near). In our preliminary analysis however, we found that multiple occurrences of any given behavior always appeared in the same factor. These results indicated that the behaviors were consistently expressed across conditions, and as a result we computed a mean for the rates or proportions of each behavior across all four conditions, and used these values in the formal analysis.
Using the averaged data we once again modified the data such that any behavior that was never recorded in 85% or more of the subjects was changed to 1-0, and we removed any variables that didn’t correlate with at least one other variable by 0.3. Exploratory factor analyses were performed using a robust weighted least square parameter estimator (WLSMV) and an oblique (promax) rotation for both four and five factor outcomes, determined by a scree plot of eigenvalues. For both models any variables that loaded less than 0.3 on any factors were removed [Child 2006].
Confirmatory factor analysis was performed for both four and five factor models using maximum likelihood estimation. Any variable that loaded above 0.3 on multiple factors was put into both factors in the confirmatory model. Models were evaluated for goodness of fit using the chi-square test, comparative fit index (CFI), Tucker-Lewis fit index (TLI), root mean square error of approximation (RMSEA), and weighted root mean square residuals (WRMR). For a mix of categorical and continuous variables, a CFI and TLI score > 0.90 is generally regarded as indicating adequate fit, a RMSEA score < 0.08 is regarded as indicating adequate fit [MacCallum et al. 1996], and a WRMR score < 1 is regarded as indicating good fit [Yu 2002].
After the best fitting model was selected, internal consistency of the scales was analyzed using standarized Cronbach’s alpha in R computational software [Team 2011]. Cronbach’s alpha is a measure of the internal consistency for a scale of multiple variables. However, when variables have restricted variability, or are skewed and not normally distributed, Cronbach’s alpha can appear artificially low despite variables measuring the same underlying construct [Enders and Bandalos 1999]. Almost all variables in the final model were either 1-0 outcomes with little variability, or were positively skewed counts. For this reason, our use of Cronbach’s alpha was focused primarily on determining whether internal consistency of the scales could be enhanced by removal of one or more variables.
After removing any variables as suggested by the Cronbach’s alpha analysis, final factor scores were created for each individual. First, scores for each variable that were included in the final analysis were z-scored across all subjects tested within a given birth year. Factor scores were then created by summing the z-scores for all variables that loaded on a given factor, using unit weighting. Finally, because factor scores were not normally distributed we evaluated correlations between factors using Kendall’s coefficient of concordance and using factor scores from all 2017 subjects. To determine the effect of rearing condition of human intruder response, we performed a Kruskal-Wallis one-way analysis of variance with rearing condition as the predictor variable, and human intruder factor score as the outcome variable. Separate analyses were performed for each of the factors determined in the previous analysis. Post hoc adjusted pairwise comparisons were then performed to test for significant differences between each rearing condition. Kruskal-Wallis tests and pairwise comparisons were performed using R computational software [Team 2011].
3. Results
Exploratory and Confirmatory Factor Analysis
Exploratory factor analysis yielded satisfactory four-factor and five-factor models. The confirmatory factor analysis revealed an adequate fit for the five-factor model based on two indices (χ2 (39) = 205.37, P < .00001; RMSEA = 0.068), and an inadequate fit based on the remaining three indices (CFI = 0.89; TLI = 0.88; WRMR = 1.55). Analysis on the four-factor model revealed an adequate fit for four of the indices of fit (χ2 (23) = 127.49, P < .00001; CFI = 0.93; TLI = 0.91; RMSEA = 0.07) and an inadequate fit for the remaining indicator (WRMR = 1.45). Consequently, we selected the four-factor model.
Analysis of internal consistency on the four factors (using standardized Cronbach’s alpha) revealed values of 0.63 for factor 1, 0.57 for factor 2, 0.48 for factor 3, and 0.59 for factor 4. By removing “vocal other” from factor 2 Cronbach’s alpha increased to 0.63, and by removing “self-clasp” from factor 3 Cronbach’s alpha increased to 0.58. With these adjustments the following four-factor model was finalized: Factor 1) Active, cage shake, environmental explore; Factor 2) Convulsive jerk, fear grimace, self-clasp, vocal coo; Factor 3) Threat, vocal bark, vocal other; Factor 4) Tooth grind, yawn. Table 2 presents, for each behavior, the standardized factor loading (with standard error [SE]), along with its implied communality (h2), which reflects the proportion of variance in each behavior that is accounted for by the factor. Correlational analysis revealed minor positive correlations between the factors ranging from 0.098 to 0.293. For the sake of discussion and ease of explanation, we have named the four factors in our model Activity, Emotionality, Aggression, and Displacement, respectively (see Table 2).
Table 2.
Confirmatory Factor Analysis Model
| Variables | F1 | F2 | F3 | F4 | h2 |
|---|---|---|---|---|---|
| Standardized factor loadings (SE) | |||||
| Activity | |||||
| Active | .637 (.034) | .0 | .0 | .0 | .406 |
| Cage Shake* | .453 (.043) | .0 | .0 | .0 | .205 |
| Environmental Explore | .683 (.033) | .0 | .0 | .0 | .467 |
| Emotionality | |||||
| Convulsive Jerk* | .0 | .891 (.083) | .0 | .0 | .794 |
| Fear Grimace | .0 | .684 (.108) | .0 | .0 | .468 |
| Self-Clasp* | .0 | .343 (.030) | .0 | .0 | .118 |
| Vocal Coo | .0 | .565 (.084) | .0 | .0 | .319 |
| Aggression | |||||
| Threat | .0 | .0 | .775 (.039) | .0 | .600 |
| Vocal Bark | .0 | .0 | .626 (.036) | .0 | .391 |
| Vocal Other* | .0 | .0 | .440 (.042) | .0 | .194 |
| Displacement | |||||
| Tooth Grind | .0 | .0 | .0 | .730 (.103) | .533 |
| Yawn* | .0 | .0 | .0 | .582 (.089) | .339 |
| Factor correlations (SE) | |||||
| F1 – Activity | 1.0 | ||||
| F2 – Emotionality | .293 (.015) | 1.0 | |||
| F3 – Aggression | .244 (.015) | .098(.015) | 1.0 | ||
| F4 – Displacement | .201 (.015) | .159 (.016) | .221 (.015) | 1.0 | |
Final four-factor model for behavioral response to human intruder test. For each behavior the factor loading (with standard error [SE]) is presented, along with its implied communality (h2), which reflects the proportion of variance in each behavior that is accounted for by the factor.
Variable treated as dichotomous present/absent
Rearing Condition and Factor Scores
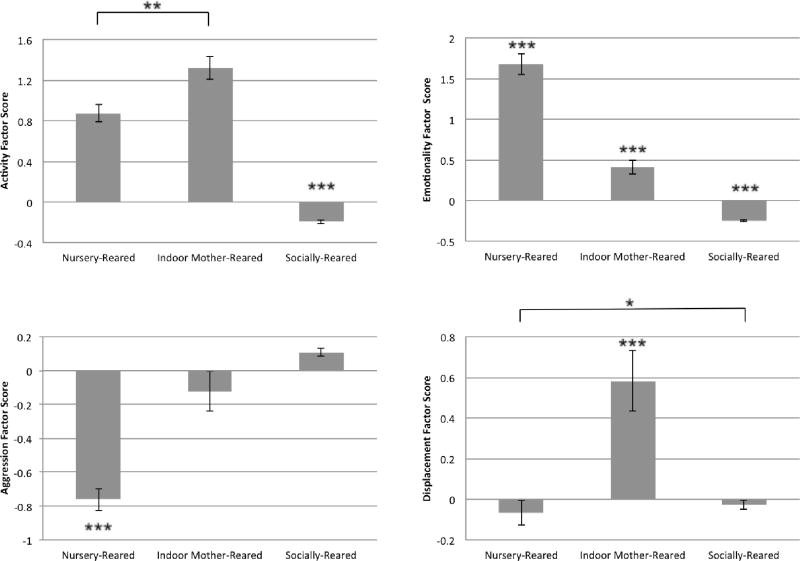
Analyses demonstrated a significant effect of rearing condition on Activity score (Kruskal-Wallis: H = 303.9, df = 2, P < 0.001), Emotionality score (H = 469.0 df = 2, P < 0.001), Aggression score (H = 103.8 df = 2, P < 0.001), and Displacement score (H = 30.6, df = 2, P < 0.001) (Figure 1). Further pairwise analyses of the Activity factor revealed that outdoor socially-reared animals had significantly lower activity scores than both nursery-reared (P < 0.001) and indoor mother-reared animals (P < 0.001), and that nursery-reared animals had significantly lower activity scores than indoor mother-reared animals (P < 0.01). Analyses of the Emotionality factor revealed that outdoor socially-reared animals had significantly lower emotionality scores than both indoor mother-reared (P < 0.001) and outdoor socially-reared animals (P < 0.001), and that indoor mother-reared animals had significantly lower emotionality scores than nursery-reared animals (P < 0.001). For the Aggression factor, nursery-reared animals had significantly lower aggression scores than both indoor mother-reared (P < 0.001) and outdoor socially-reared animals (P < 0.001). Finally, pairwise analyses of the Displacement factor revealed that indoor mother-reared animals had significantly higher displacement scores than both nursery-reared (P < 0.001) and outdoor socially-reared animals (P < 0.001), and that nursery-reared animals had significantly lower displacement scores than socially-reared animals (P < 0.05) (Figure 1).
Figure 1.
Mean Factor Scores by Rearing Condition
Mean Activity, Emotionality, Aggression, and Displacement factor scores for nursery-reared, indoor mother-reared, and socially-reared monkeys. Error bars represent one standard error. Asterisk indicate significant differences in factor scores based on pairwise comparisons after Kruskal-Wallis tests. * P < 0.05, ** P < 0.01, *** P < 0.001
4. Discussion
Exploratory and Confirmatory Factor Analysis
Utilizing data from over 1,000 animals, exploratory factor analysis of behavioral data from the human intruder test yielded a model that suggests four underlying latent factors can explain the variation in behavioral responses: Activity, Emotionality, Aggression, and Displacement. In addition, we were able to replicate and confirm this four-factor model using a confirmatory factor analysis on human intruder data from nearly 1,000 additional subjects – four of the five measures of model fit confirmed that this four-factor model was an appropriate model for the observations. Such confirmation of the model increases confidence that this structure is a replicable description of the underlying factors that contribute to an individual’s response to our version of the human intruder test.
Although the human intruder test has been used extensively as a test of behavioral reaction to separation and threat, a full analysis of the factors that contribute to behavioral variation in response to this test has never been performed. Not surprisingly, the four factors that we extracted are very similar to the behavioral constructs that have been investigated by other researchers over the past 20 years (see below).
The Activity factor comprises “activity,” “cage shake,” and “environmental explore.” In contrast to highly active animals, animals that show very little of these three behaviors may be behaviorally inhibited. Both behavioral inhibition and freezing behavior are extremely common measures for the human intruder test. In their initial study, Kalin and Shelton described the freezing behavior seen in many of their subjects as a defensive behavior to avoid detection under challenging circumstances. In our version of the human intruder test, we didn’t specifically measure freezing behavior; freezing was not a prominent response because the intruder was much closer to the animals in our study than in tests used by others. Nevertheless, a low score on Activity likely means an animal is inactive and non-exploratory, which may be effectively similar to behavioral inhibition and freezing.
The Emotionality factor comprises “convulsive jerk,” “fear grimace,” “self-clasp,” and “vocal coo.” Convulsive jerk is a part of the gecker response among infants which, much like the vocal coo, functions to call attention to an infant’s mother in times of distressful situations [Patel and Owren 2007]. A fear grimace is a signal of subordination that is typically seen in frightened animals as a means of appeasing or preventing aggression. Self-clasp is a behavior that is typically only seen in rhesus monkeys reared without a mother [Capitanio 1986], but like the other behaviors that loaded on this factor, it is often expressed at high rates when the animal is in stressful situations [Cross and Harlow 1965]. In summary all four behaviors can be seen as an emotional response to a challenging situation. It is important to note, however, that three of these behaviors (convulsive jerk, self-clasp, and vocal coo), are seen at a much higher rate in infants, and likely would not be seen in an adult response to a human intruder.
The Aggression factor comprises “threat,” “vocal bark,” and “vocal other,” all of which are often seen in aggressive encounters. While “vocal other” is not necessarily an aggressive behavior, it can include grunts and other aggressive vocalizations. We therefore believe this factor represents an underlying tendency for the subject to respond aggressively to the human intruder.
The final factor, Displacement, comprises only two behaviors: “tooth grind” and “yawn.” Both of these behaviors are known in rhesus macaques to be displacement behaviors: behaviors that are functionally unrelated to current activity, and occur when an animal is in a state of conflict, frustration, or high anxiety [Maestripieri et al. 1992; Schino et al. 1996]. It thus appears that this final factor may be measuring anxiety in the subject.
In their initial study, Kalin and Shelton proposed that the human intruder test measured two different behavioral strategies to threat; behavioral inhibition and aggression [Kalin and Shelton 1989]. Recently Kinnally and colleagues suggested the response to the human intruder test was determined in part by two latent variables, activity and emotionality [Kinnally et al. 2010]. In our four-factor analysis we similarly found and confirmed factors for activity (which we believe may be the opposite of behavioral inhibition), aggression, and emotionality, as well as an anxiety factor. Our results, obtained using a very large sample, give further support and confirmation to the pattern of results found by others. Because we found that the responses in the human intruder test can be characterized along four dimensions, our analysis suggests that this is a useful paradigm to measure effectively multiple constructs that reflect broad domains of psychological functioning.
We acknowledge that there are still unanswered questions regarding the human intruder test. Most importantly, we have performed this analysis on data from 3–4 month old infants, and the behavioral repertoire and predominant behaviors of an infant are somewhat different from those of adult animals [Corcoran et al. 2011]. It is possible that if a similar factor analysis were performed on human intruder test results from adult rhesus, a different factor scheme would emerge, and different behavioral constructs might be evident. It is also important to note that while our factor analysis has informed our understanding of the underlying constructs that characterize the behavioral responses in the human intruder test, we are still unsure of the predictive power of these factors. Further research is needed to evaluate whether human intruder factor scores are predictive of patterns of behavioral functioning at later ages.
Rearing Condition and Factor Scores
Results demonstrated that socially-reared animals had the lowest Activity score, indoor mother-reared animals had the highest Displacement score, and nursery-reared animals had the highest Emotionality score and lowest Aggression score. This pattern of results is broadly consistent with rearing group differences found for other measures in the BBA program, as described in the Introduction – indoor mother-reared animals show greater activity, and nursery-reared animals are generally very emotionally reactive [Capitanio et al. 2006]. The present results demonstrate that an animal’s history and rearing condition affect how it experiences the human intruder paradigm and influence its response to a threatening human stimulus.
When interpreting these differences in factor scores it is necessary to recognize that while every subject experienced the exact same testing procedure, they did not share the same familiarity with the testing environment; nursery-reared and indoor mother-reared animals were raised in cages similar to those utilized in the tests, while socially-reared animals were raised in large outdoor enclosures drastically different from indoor housing cages. Socially-reared animals therefore may have experienced the most challenging and novel testing environment, and interestingly responded with the lowest Activity factor scores. As previously discussed, a low Activity score may represent behavioral inhibition or freezing, both common defense mechanisms for extremely challenging environments. Consequently, we must recognize that while all animals experienced the exact same physical testing environment, socially-reared animals may have interpreted this to be a relatively higher level of challenge and threat.
In addition to being placed in a novel physical environment, the human intruder test challenged subjects by separating them from their familiar social environment; nursery-reared animals were separated from the social partner with whom they had shared a cage since 5 weeks of age, mother-reared animals were separated from their mother, and socially-reared animals were separated from their entire social troupe. Often, the initial response of infants to a social separation is the protest response, indicated by vigorous activity and calling [Bowlby 1960; Seay et al. 1962], however this response may be inhibited when animals are in a highly challenging environment, as indicated by the low activity scores observed in our socially-reared animals. Social separation may theoretically be the least traumatic for nursery-reared animals, who have previous experience being separated from their social partner. Unlike mother-reared and socially-reared animals, who have typically never been separated from their mother before the BioBehavioral Assessment, nursery-reared animals are frequently separated from their social partner for short periods of time, and spend the first 5 weeks of life without an in-cage social partner. This difference in separation-related experiences may explain why both mother-reared and socially-reared animals scored significantly higher Displacement factor scores than nursery-reared animals. Interestingly socially-reared animals did not receive as high displacement scores as mother-reared animals; this may be because of the greater unfamiliarity with the indoor conditions for the socially-reared animals, leading to a more inhibited response overall.
Finally, it is important to recognize that prior to the human intruder test, animals from the different rearing conditions underwent extremely different experiences interacting with humans. Nursery-reared animals had the most frequent and positive human interactions; human caretakers fed the infants from an early age, and often held and carried them during various medical checks and cage cleaning. Therefore it is not surprising that nursery-reared animals received the lowest Aggression factor scores; anecdotally it was noted that they were instead attracted to and interested in interacting with the intruder. This interest however may have been problematic, as nursery-reared infants often appeared distraught when the intruder did not reciprocate attention, as they are accustomed in the nursery. Many nursery-reared animals responded by displaying convulsive jerks and vocal coos towards the intruder (an emotional response traditionally directed at the infant’s mother), and this likely explains why nursery-reared animals received the highest Emotionality factor score of all three rearing conditions.
In conclusion these results demonstrate the importance of recognizing an animal’s history when interpreting their response to a human intruder. Although every subject in a study may receive the exact same human intruder test, the level of stress and challenge experienced by each subject depends on their previous experience with each aspect of the paradigm – the indoor nature of the testing, familiarity with humans, etc. Overall our results demonstrate the human intruder test to be a valid paradigm to measure an animal’s activity, emotionality, aggression, and displacement in response to a challenging environment, however they also demonstrate the importance in recognizing and accounting for differences in animal rearing and history when interpreting the test results.
Acknowledgments
This project was funded by the National Center for Research Resources (R24RR019970 to JPC and P51RR000169 to CNPRC) and is currently supported by the Office of Research Infrastructure Programs/OD (R24OD010962 and P51OD011157, respectively). The authors thank Laura Del Rosso and Laura Calonder for technical assistance, Brenda McCowan and Joy Mench, for their edits and helpful suggestions in manuscript preparation, Andrea Gottlieb for her assistance in data analysis, and the animal care and veterinary staffs of CNPRC for support.
References
- Animal Welfare Act. Animal Welfare Act, Food Secuirity Act of 1985, Subtitle F—Animal Welfare [Internet] 1985 [cited 2011]. Available from: http://awic.nal.usda.gov/nal_display/index.php?info_center=3&tax_level=3&tax_subject=182&topic_id=1118&level3_id=6735&level4_id=0.
- Bethea CL, Streicher JM, Coleman K, Pau FKY, Moessner R, Cameron JL. Anxious behavior and fenfluramine-induced prolactin secretion in young rhesus macaques with different alleles of the serotonin reuptake transporter polymorphism (5HTTLPR) Behavior Genetics. 2004;34(3):295–307. doi: 10.1023/B:BEGE.0000017873.61607.be. [DOI] [PubMed] [Google Scholar]
- Bowlby J. GRIEF AND MOURNING IN INFANCY AND EARLY-CHILDHOOD. Psychoanalytic Study of the Child. 1960;15(1):9–52. [Google Scholar]
- Capitanio J. Behavioral Pathology. In: Mitchell G, Erwin J, editors. Comparative Primate biology: volume 2a Behavior, conservation and ecology. New York: 1986. [Google Scholar]
- Capitanio JP. Personality dimensions in adult male rhesus macaques: Prediction of behaviors across time and situation. American Journal of Primatology. 1999;47(4):299–320. doi: 10.1002/(SICI)1098-2345(1999)47:4<299::AID-AJP3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mason WA, Mendoza SP, DelRosso L, Roberts JA. Nursery Rearing and Biobehavioral Organzation. Nursery Rearing of Nonhuman Primates in the 21st Century. 2006:191–214. [Google Scholar]
- Capitanio JP, Mendoza SP, Cole SW. Nervous temperament in infant monkeys is associated with reduced sensitivity of leukocytes to cortisol’s influence on trafficking. Brain Behavior and Immunity. 2011;25(1):151–159. doi: 10.1016/j.bbi.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Mason WA, Maninger N. Rearing environment and hypothalamic-pituitary-adrenal regulation in young rhesus monkeys(Macaca mulatta) Developmental Psychobiology. 2005;46(4):318–330. doi: 10.1002/dev.20067. [DOI] [PubMed] [Google Scholar]
- Child D. The essentials of factor analysis. London; New York: Continuum; 2006. [Google Scholar]
- Coleman K, Dahl RE, Ryan ND, Cameron JL. Growth hormone response to growth hormone-releasing hormone and clonidine in young monkeys: Correlation with behavioral characteristics. Journal of Child and Adolescent Psychopharmacology. 2003;13(3):227–241. doi: 10.1089/104454603322572561. [DOI] [PubMed] [Google Scholar]
- Corcoran CA, Pierre PJ, Haddad T, Bice C, Suomi SJ, Grant KA, Friedman DP, Bennett AJ. Long-term effects of differential early rearing in rhesus macaques: Behavioral reactivity in adulthood. Developmental Psychobiology. 2011;54(5):546–555. doi: 10.1002/dev.20613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross HA, Harlow HF. Prolonged and progressive effects of partial isolation on the behavior of macaque monkeys. Journal of Experimental Research in Personality. 1965;1(1):39–49. [Google Scholar]
- Davidson RJ, Kalin NH, Shelton SE. Lateralized response to diazepam predicts temperamental style in rhesus-monkeys. Behavioral Neuroscience. 1993;107(6):1106–1110. doi: 10.1037//0735-7044.107.6.1106. [DOI] [PubMed] [Google Scholar]
- Enders CK, Bandalos DL. The Effects of Heterogeneous Item Distributions on Reliability. Applied Measurement in Education. 1999;12(2):133–150. [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Trait-Like Brain Activity during Adolescence Predicts Anxious Temperament in Primates. Plos One. 2008;3(7) doi: 10.1371/journal.pone.0002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EM, Reyes TM, Coe CL. Context-dependent behavioral effects of interleukin-1 in the rhesus monkey (Macaca mulatta) Psychoneuroendocrinology. 1996;21(5):455–468. doi: 10.1016/0306-4530(96)00010-8. [DOI] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Widaman KF, Capitanio JP. Iron Deficiency Anemia and Affective Response in Rhesus Monkey Infants. Developmental Psychobiology. 2009;51(1):47–59. doi: 10.1002/dev.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA. Combined unilateral lesions of the amygdala and orbital prefrontal cortex impair affective processing in rhesus monkeys. Journal of Neurophysiology. 2004;91(5):2023–2039. doi: 10.1152/jn.00968.2003. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. Journal of Neuroscience. 2005;25(37):8534–8542. doi: 10.1523/JNEUROSCI.1232-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Defensive behaviors in infant rhesus-monkeys - environemtal cues and neurochemical regulation. Science. 1989;243(4899):1718–1721. doi: 10.1126/science.2564702. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Ontogeny and stability of separation and threat-induced defensive behaviors in rhesus monkeys during the first year of life. American Journal of Primatology. 1998;44(2):125–135. doi: 10.1002/(SICI)1098-2345(1998)44:2<125::AID-AJP3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. Journal of Neuroscience. 2004;24(24):5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. Role of the primate orbitofrontal cortex in mediating anxious temperament. Biological Psychiatry. 2007;62(10):1134–1139. doi: 10.1016/j.biopsych.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ, Kelley AE. The Primate Amygdala Mediates Acute Fear But Not the Behavioral and Physiological Components of Anxious Temperament. The Journal of Neuroscience. 2001;21(6):2067–2074. doi: 10.1523/JNEUROSCI.21-06-02067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ. Brain regions associated with the expression and contextual regulation of anxiety in primates. Biological Psychiatry. 2005;58(10):796–804. doi: 10.1016/j.biopsych.2005.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Fox AS, Rogers J, Oakes TR, Davidson RJ. The serotonin transporter genotype is associated with intermediate brain phenotypes that depend on the context of eliciting stressor. Molecular Psychiatry. 2008;13(11):1021–1027. doi: 10.1038/mp.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Rickman M, Davidson RJ. Individual differences in freezing and cortisol in infant and mother rhesus monkeys. Behavioral Neuroscience. 1998;112(1):251–254. doi: 10.1037//0735-7044.112.1.251. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Takahashi LK. Defensive behaviors in infant rhesus-monkeys -ontogeny and context-dependent selective expression. Child Development. 1991a;62(5):1175–1183. [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Turner JG. Effects of alprazolam on fear-related behavioral, hormonal, and catecholamine responses in infant rhesus-monkeys. Life Sciences. 1991b;49(26):2031–2044. doi: 10.1016/0024-3205(91)90646-s. [DOI] [PubMed] [Google Scholar]
- Karere GM, Kinnally EL, Sanchez JN, Famula TR, Lyons LA, Capitanio JP. What is an “Adverse” Environment? Interactions of Rearing Experiences and MAOA Genotype in Rhesus Monkeys. Biological Psychiatry. 2009;65(9):770–777. doi: 10.1016/j.biopsych.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnally EL, Karere GM, Lyons LA, Mendoza SP, Mason WA, Capitanio JP. Serotonin pathway gene-gene and gene-environment interactions influence behavioral stress response in infant rhesus macaques. Development and Psychopathology. 2010;22(1):35–44. doi: 10.1017/S0954579409990241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCallum RC, Browne MW, Sugawara HM. Power analysis and determination of sample size for covariance structure modeling. Psychological Methods. 1996;1(2):130–149. [Google Scholar]
- Machado CJ, Bachevalier J. Behavioral and hormonal reactivity to threat: Effects of selective amygdala, hippocampal or orbital frontal lesions in monkeys. Psychoneuroendocrinology. 2008;33(7):926–941. doi: 10.1016/j.psyneuen.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D, Schino G, Aureli F, Troisi A. A modest proposal - displacement activities as an indicator of emotions in primates. Animal Behaviour. 1992;44(5):967–979. [Google Scholar]
- Minier DE, Tatum L, Gottlieb DH, Cameron A, Snarr J, Elliot R, Cook A, Elliot K, Banta K, Heagerty A. Human-directed contra-aggression training using positive reinforcement with single and multiple trainers for indoor-housed rhesus macaques. Applied Animal Behaviour Science. 2011;132(3–4):178–186. [Google Scholar]
- Muthén LK, Muthén BO. Mplus Version 3.01. Los Angeles, CA: Muthén & Muthén; 1998–2010. [Google Scholar]
- Noldus LPJJ. The Observer: a software system for collection and analysis of observational data. Behavioral Research Methods, Instruments, and Computers. 1991;23:415–429. [Google Scholar]
- Patel ER, Owren MJ. Acoustics and behavioral contexts of “gecker” vocalizations in young rhesus macaques (Macaca mulatta) Journal of the Acoustical Society of America. 2007;121(1):575–585. doi: 10.1121/1.2390662. [DOI] [PubMed] [Google Scholar]
- Rogers J, Shelton SE, Shelledy W, Garcia R, Kalin NH. Genetic influences on behavioral inhibition and anxiety in juvenile rhesus macaques. Genes Brain and Behavior. 2008;7(4):463–469. doi: 10.1111/j.1601-183X.2007.00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommeck I, Capitanio JP, Strand SC, McCowan B. Early Social Experience Affects Behavioral and Physiological Responsiveness to Stressful Conditions in Infant Rhesus Macaques (Macaca mulatta) American Journal of Primatology. 2011;73(7):692–701. doi: 10.1002/ajp.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schino G, Perretta G, Taglioni AM, Monaco V, Troisi A. Primate displacement activities as an ethopharmacological model of anxiety. Anxiety. 1996;2(4):186–191. doi: 10.1002/(SICI)1522-7154(1996)2:4<186::AID-ANXI5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Seay B, Hansen E, Harlow HF. MOTHER-INFANT SEPARATION IN MONKEYS. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1962;3(3–4):123–132. doi: 10.1111/j.1469-7610.1962.tb02047.x. [DOI] [PubMed] [Google Scholar]
- Team RDC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- Willette AA, Lubach GR, Coe CL. Environmental context differentially affects behavioral, leukocyte, cortisol, and interleukin-6 responses to low doses of endotoxin in the rhesus monkey. Brain Behavior and Immunity. 2007;21(6):807–815. doi: 10.1016/j.bbi.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DE, Coleman K, Bacanu SA, Devlin BJ, Rogers J, Ryan ND, Cameron JL. Heritability of fearful-anxious endophenotypes in infant rhesus macaques: A preliminary report. Biological Psychiatry. 2003;53(4):284–291. doi: 10.1016/s0006-3223(02)01601-3. [DOI] [PubMed] [Google Scholar]
- Yu C-Y. Evaluating Cutoff Criteria of Model Fit Indices for Latent Variable Models with Binary and Continuous Outcomes. Los Angeles: University of California, Los Angeles; 2002. [Google Scholar]