Summary
Lipid bodies are eukaryotic structures for temporary storage of neutral lipids such as acylglycerols and steryl esters. Fatty acyl-CoA and cholesterol are two substrates for cholesteryl ester (CE) synthesis via the ACAT reaction. The intracellular parasite Toxoplasma gondii is incapable of sterol synthesis and unremittingly scavenges cholesterol from mammalian host cells. We previously demonstrated that the parasite expresses a cholesteryl ester-synthesizing enzyme, TgACAT1. In this paper, we identified and characterized a second ACAT-like enzyme, TgACAT2, which shares 56% identity with TgACAT1. Both enzymes are endoplasmic reticulum-associated and contribute to CE formation for storage in lipid bodies. While TgACAT1 preferentially utilizes palmitoyl-CoA, TgACAT2 has broader fatty acid specificity and produces more CE. Genetic ablation of each individual ACAT results in parasite growth impairment whereas dual ablation of ACAT1 and ACAT2 is not tolerated by Toxoplasma. ΔACAT1 and ΔACAT2 parasites have reduced CE levels, fewer lipid bodies, and accumulate free cholesterol, which causes injurious membrane effects. Mutant parasites are particularly vulnerable to ACAT inhibitors. This study underlines the important physiological role of ACAT enzymes to store cholesterol in a sterol-auxotrophic organism such as Toxoplasma, and furthermore opens up possibilities of exploiting TgACAT as targets for the development of antitoxoplasmosis drugs.
Keywords: Protozoa, toxoplasmosis, fat storage organelle, cholesterol homeostasis, acyl-CoA:cholesterol acyltransferase, ACAT inhibitors
Introduction
Cholesterol is a contradictory molecule: it is a vital part of membranes; however, its overabundance in cells leads to inflammation and oxidative damages. Mammalian cells have developed several protective mechanisms against cholesterol overloading (reviewed in Chang et al., 2006). Among the cholesterol homeostatic mechanisms are: i) the reverse cholesterol transport, which delivers cholesterol to extracellular lipoproteins, ii) the conversion of cholesterol into oxysterols, which triggers cholesterol catabolism, and iii) the esterification and storage of cholesterol within cytoplasmic inclusions. Cholesteryl esters (CE), i.e. cholesterol with long-chain fatty acids linked to the hydroxyl group, are less polar than free cholesterol, and represent the biologically inert storage forms, mainly confined to cytoplasmic lipid bodies. When needed, CE can then be de-esterified by hydrolysis and released from lipid bodies for the formation of new membranes. In general, the cycle of cholesterol esterification and hydrolysis provides the major short-term buffering of cholesterol levels in cells.
Among the members of the superfamily of membrane-bound acyltransferases, acyl-CoA:cholesterol acyltransferase enzymes (ACAT) are dedicated to the synthesis of CE. The sterol ester biosynthetic machinery is evolutionary conserved, and genes encoding sterol-esterifying enzymes have been identified in Vertebrates, plants, Invertebrates such as nematodes and insects, the fungi (yeast) branch and protozoa, but not in prokaryotes (reviewed in Athenstaedt and Daum, 2006). Higher eukaryotes express two enzymes, ACAT1 and ACAT2, which share 44-47% of amino acid homology. Concerning the substrates, both enzymes utilize cholesterol or oxysterols, but different acyl-CoA: 16:0, 18:1, 18:2 for ACAT1 and ACAT2, and 20:4 for ACAT1 (summarized in Chang et al., 2009). Mammalian ACAT 1 and ACAT2 are endoplasmic reticulum (ER)-resident proteins; however they differ in their tissue distribution and membrane topology. While ACAT1 is found in various cells and tissues, ACAT2 expression is restricted to hepatocytes and intestinal mucosal cells. The distinctive patterns of tissue distribution of ACAT1 and ACAT2 correlate with the separate functions of these enzymes: ACAT1 generates intracellular CE for storage in lipid bodies to maintain appropriate cholesterol availability in cell membranes whereas ACAT2 produces CE that are assembled in the ER and incorporated into apoB-containing lipoproteins to promote cholesterol absorption. Confirming these observations, ACAT1-deficient mice lack CE droplets but have no apparent defects in lipoprotein production or cholesterol absorption. In contrast, ACAT2-knockout mice produce apoB-containing lipoproteins with low CE content in the liver, and are protected from hypercholesterolemia. Similar to higher eukaryotic cells, Saccharomyces cerevisiae possesses two sterol O-acyltransferase-encoding genes, ARE1 and ARE2 (reviewed in Daum et al., 2007). The two gene products are located in the ER; they vary in their sterol substrate specificity (ergosterol for ARE2 and sterol precursors for ARE1) but have similar specificity towards fatty acids (Zweytick et al., 2000). The two yeast ARE have different physiological functions, depending on growth conditions. Interestingly, deletion of both ARE1 and ARE2 results in complete abolishment of sterol esterification, yet the double mutant remains viable.
Unlike mammals, protozoa are incapable of cholesterol de novo synthesis, and therefore their survival strictly depends on their ability to retrieve cholesterol from their environment. The intracellular protozoan parasite Toxoplasma gondii multiplies in a parasitophorous vacuole (PV) formed in the cytoplasm of mammalian cells. The parasite scavenges cholesterol from plasma low-density lipoproteins (LDL) by re-routing host lysosomes to its PV, and further internalizes cholesterol using membrane-associated transport proteins (Coppens et al., 2000; 2006; Ehrenman et al., 2010). Previous studies have reported the deleterious effects of well-known ACAT inhibitors on T. gondii (Sonda et al., 2001; Ohshiro and Tomoda, 2011), which has suggested endogenous ACAT activities in the parasite. First genetic evidence was provided by our earlier work, in which we identified two isoforms of an ACAT-related enzyme, designated TgACAT1α and TgACAT1β sharing 18% sequence identity with human ACAT1 (Nishikawa et al., 2005). Like their counterparts, the TgACAT1 isoforms reside in the ER and have a broad sterol substrate specificity to form CE. Host LDL and fatty acids scavenged by Toxoplasma can serve as ACAT activators, resulting in stimulation of CE synthesis and lipid droplet biogenesis in the parasite. Contrariwise, selected ACAT inhibitors dramatically alter the plasma membrane architecture of Toxoplasma, and consequently impair parasite growth. Together, this demonstrates the importance for T. gondii to maintain an optimal amount of intracellular cholesterol, and consequently its vulnerability towards interference with the cholesterol storage pathways.
Here, we have extended our studies on the molecular equipment used by Toxoplasma to synthesize CE, and reinvestigated the physiological importance of cholesterol storage for this parasite. We have identified and characterized a second ACAT-related gene product, named TgACAT2, and compared the activity and substrate specificity of TgACAT2 with the previously reported TgACAT1. We have genetically disrupted either ACAT1 or ACAT2 and analyzed the consequence of such deletions on parasite viability and CE content. Our results show that the activity of at least one ACAT enzyme is essential for CE formation and parasite viability. We conclude that TgACAT1 and TgACAT2 have non-redundant roles to store host-derived cholesterol in parasite lipid bodies, and that the storage cholesterol function is essential in the cholesterol auxotroph T. gondii.
Results
Cloning and molecular characterization of a second ACAT-related enzyme in Toxoplasma
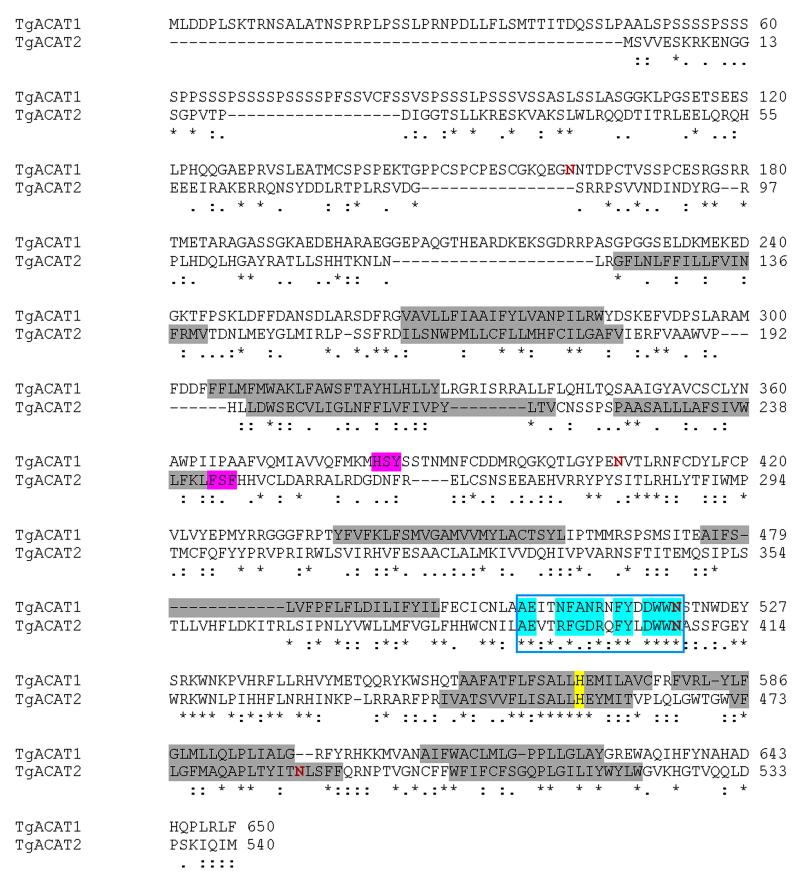
Through searches for sequence homology to signature motifs of O-acyltransferases in the Toxoplasma genome database (www.ToxoDB.org), we previously identified a gene coding for an acyl-CoA:cholesterol acyltransferase (ACAT)-like enzyme, named TgACAT1 (Nishikawa et al., 2005). Additional BLAST searches in the Toxoplasma genome database have divulged the existence of second ACAT enzyme (TGGT1_114790; location on chromosome VIII) that we named TgACAT2 based on order of discovery. The TgACAT2 ORF was amplified by PCR from a cDNA library. The ORF is 1,623 nucleotides long, consists of 15 exons and encodes a polypeptide of 540 amino acids, with a predicted molecular weight of 63.4-kDa (Fig. 1). Sequence comparison between the previously reported TgACAT1 and TgACAT2 indicates 55.7% identity (80.1% similar) in 433 aa overlap between the two parasite enzymes.
Figure 1. Characteristic features of the predicted sequence of TgACAT2 showing the conserved critical motifs of the superfamily of membrane-bound O-acyltransferases.
Alignment of the predicted ORF of TgACAT2 and TgACAT1 (isoform alpha; GenBank accession #: AY562994) using the BioEdit program, revealing the presence of many identical (*), conserved (:) or semi-conserved (.) substitutions in these proteins. Similarly to TgACAT1, TgACAT2 displays an invariant histidine (yellow) positioned within a long hydrophobic region of the superfamily of the membrane-bound O-acyltransferases, a conserved fatty acid binding site (turquoise) and a putative cholesterol binding site including an invariant conserved serine (magenta). Potential transmembrane segments ( according to the transmembrane folding program : http://liao.cis.udel.edu/website/servers/TMMOD/scripts/frame.php?p=description) are highlighted in grey and putative N-glycosylation sites (according to the NetNGLyc 1.0 server) are depicted in red.
Like TgACAT1, TgACAT2 has all the major hallmarks of the ACAT family (Fig. 1). First, the protein displays the common features of the superfamily of membrane-bound O-acyltransferases (Hofmann, 2000; Cases et al., 1998) with an invariant histidine positioned within a long hydrophobic region (His456). Second, the specific motifs shared between ACAT and DGAT1 enzymes (Bouvier et al., 2000) are present in TgACAT2; these motifs include a putative invariant serine (Ser244) and the remarkably conserved motif [AExxRFGDRxFYxDWWN] corresponding to the fatty acid binding site, starting at Ala391. Third, the TgACAT2 sequence also harbors the putative cholesterol binding site [H/YSF] comprised of a serine (Ser244) residue surrounded by aromatic and/or basic amino acids (Guo et al., 2001) present in all the identified ACAT enzymes so far [Phe243SF]. Fig. S1 shows partial sequence alignment of TgACAT2 with the two human enzymes, ACAT1 and ACAT2, confirming the high degree of conservation of the ACAT motifs in the parasite sequence. Pairwise comparison of TgACAT2 with human ACAT1 or ACAT2 indicates 25.2% identity (58.8% similar) in 437 aa overlap and 25.8% identity (53.8% similar) in 535 aa overlap, respectively. The transmembrane region prediction program (http://ulrec3.unil.ch/software/TMPRED_form.html) favors seven membrane helix spanning domains for TgACAT2. A unique characteristic of TgACAT1 (isoform α only) is the presence of a unique hydrophilic serine-rich region at the N-terminus (~50% serine between amino acids 24-107), which was predicted to contribute to the overall stability of TgACAT1 (Nishikawa et al., 2005). However, this long serine stretch is not present in the TgACAT2 sequence.
Like TgACAT1, TgACAT2 localizes to the parasite ER
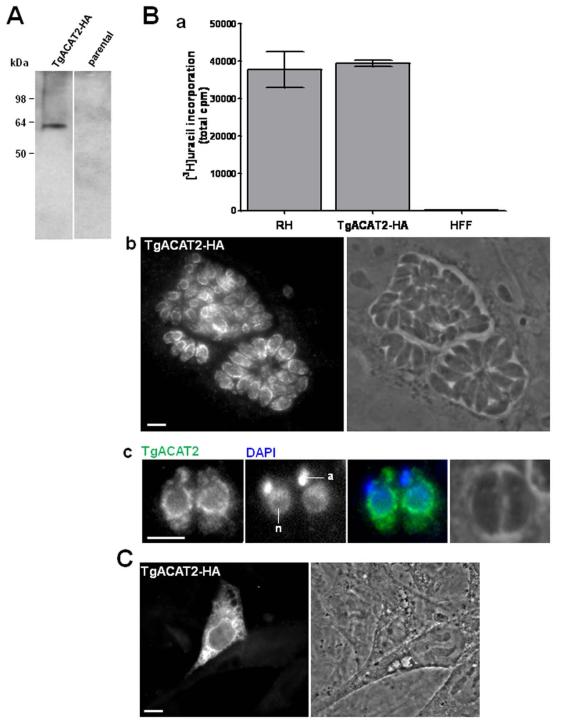
We next examined the intracellular localization of TgACAT2. For this purpose, we created a stable line of Toxoplasma expressing C-terminally HA-tagged TgACAT2 under control of the strong tubulin promotor. To verify the expression of exogenous TgACAT2-HA, we performed SDS-PAGE and immunoblot analysis using anti-HA antibodies from lysates of transgenic parasites, and detected a single band at ~62- kDa (Fig. 2A). Apparently, overexpression of TgACAT2 did not affect the parasite growth, as determined by uracil incorporation assay (Fig. 2B, panel a). The protein localization in TgACAT2-HA-expressing T. gondii was assayed by immunofluorescence assays (IFA) using anti-HA antibodies. Data in panel b in Fig. 2B illustrate a labeling of cortical tubular extensions and a perinuclear staining as well, as clearly visible on parasites at higher magnification (Fig. 2B, panel c). These observations indicate that TgACAT2 localizes to the ER, as previously shown for TgACAT1 (Nishikawa et al., 2005).
Figure 2. Expression and localization of TgACAT2-HA in transgenic Toxoplasma and mammalian cells.
A. Immunoblots of lysates of T. gondii stably expressing TgACAT2-HA and mock-transfected RH parasites (parental) revealed with anti-HA antibodies. B. Panel a: Uracil incorporation assay of TgACAT2-HA vs. RH parasites. HFF were infected with 5×106 parasites for 4-h prior to labeling with [3H]uracil for 2-h and subsequent quantification by scintillation counting. Panels b and c: Immunofluorescence assays (IFA) of TgACAT2-HA-expressing parasites using anti-HA antibody. DAPI staining for the apicoplast (a) and the nucleus (n). Bars are 5 μm. C. IFA of mouse embryonic fibroblasts (MEF) transiently transfected with a plasmid containing TgACAT2-HA prior to IFA using anti-HA antibody. Bar is 10 μm.
Expression of TgACAT2 in mammalian cells lacking ACAT activities stimulates lipid body biogenesis and restores cholesterol esterification
We next conducted experiments to probe the enzymatic activity of TgACAT2 by expressing the protein in a cell line of mouse embryonic fibroblasts (MEF) lacking its unique ACAT gene (ACAT1), and therefore the ability to form any steryl esters (MEF ACAT1−/− ; Meiner et al., 1996) in order to examine the potential ability of TgACAT2 to restore ACAT activity in the mutant cell, namely the resuming of cholesterol esterification activity. We first analyzed the intracellular localization of TgACAT2 when ectopically expressed in MEF wild-type (MEF ACAT1+/+). The cells were transfected with an expression plasmid containing TgACAT2-HA and immunolabeled with antibodies against HA to examine the distribution of TgACAT2. Expectedly, a fluorescence pattern corresponding to cortical and perinuclear ER was discernible (Fig. 2C). MEF ACAT1−/− were then transfected with the same plasmid, and IFA data using anti-HA antibodies show similar ER localization (Fig. 3A).
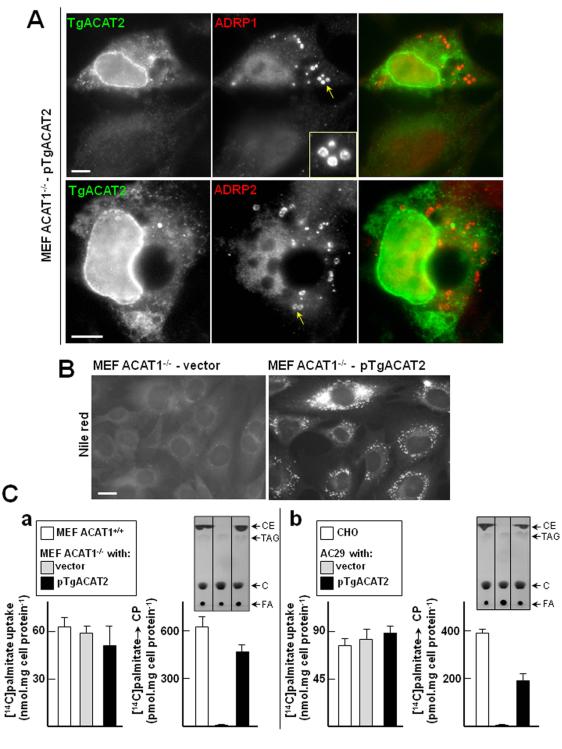
Figure 3. Stimulation of lipid body production and synthesis of CE in mammalian ACAT-deficient cells expressing TgACAT2.
A. IFA of MEF ACAT1−/− expressing TgACAT2-HA. MEF ACAT1−/− were transiently transfected with pTgACAT2-HA before double immunostaining using antibodies against HA (green) to identify transfected cells and ADRP (red) to label lipid bodies (inset). Bars are 10 μm. B. Fluorescence microscopy using Nile Red to visualize lipid bodies in MEF ACAT1−/− stably transfected with a plasmid containing TgACAT2-HA or vector alone. Bar is 20 μm. C. Quantitative measurement of CE synthesis in MEF ACAT1−/− (panel a) or AC29 cells (panel b) expressing TgACAT2-HA by thin layer chromatography (TLC). Palmitate uptake and incorporation into CE were monitored in parental cells (MEF and CHO) and transfected cells (MEF ACAT1−/− and AC29) with pTgACAT2-HA or vector only. Results of palmitate uptake or cholesteryl palmitate (CP) production expressed in nmol palmitate per mg cell protein or nmol palmitate incorporated into CP per mg cell protein, respectively, are means ± SD of three separate experiments. Differences between values of palmitate incorporated into CP in cells between TgACAT2- expressed cells and vector controls are statistically significant (*, P < 0.0005). Representative chromatograms are shown at the top of histograms for CE synthesis. C, cholesterol; FA, fatty acids; TAG, triglycerides.
The ability of TgACAT2 to esterify cholesterol was then evaluated by examining the re-emergence of lipid bodies in MEF ACAT1−/− upon expression of TgACAT2. The adipose differentiation-related protein (ADRP, also named perilipin 2 or adipophilin) is a major lipid body-coating protein that is particularly important for de novo formation and maturation of lipid bodies after their release from the ER (Murphy et al., 2012). MEF ACAT1−/− were transiently transfected with pTgACAT2-HA for 24-h, and to visualize potentially newly formed lipid droplets, cells were immunolabeled with two different antibodies against ADRP (anti-ADRP-1 and anti-ADRP2). Remarkably, in cells expressing TgACAT2, a fluorescent labeling of small spherical structures disseminated throughout the cytosol was apparent with either antibody against ADRP (Fig. 3A). These structures exhibited an annular staining that is typical for lipid body-coating proteins. This indicates that lipid body formation was reconstituted in MEF ACAT1−/− following expression of TgACAT2.
We next engineered a MEF ACAT1−/− cell line stably expressing TgACAT2-HA to characterize the cell lipid content. We first wanted to confirm the presence of canonical lipid bodies containing neutral lipids in expressors by incubating the cells with Nile Red, a fluorescent lipid body dye. Cells expressing the parasite enzyme displayed a bright punctuate fluorescent staining in contrast to mock-transfected cells, which confirms the induction of lipid body formation by TgACAT2 (Fig. 3B). To correlate the presence of lipid bodies with reconstituted cholesterol esterification activity mediated by TgACAT2, we monitored the synthesis of CE in MEF ACAT1−/− stably expressing TgACAT2-HA, compared to MEF wild type and mock-transfected cells. Radiolabeled palmitate as a fatty acyl substrate for cholesterol esterification was added to the medium for 2-h. First we verified the ability of the three cell lines to equally internalize same amounts of radioactive palmitate; no statistical difference for palmitate uptake was observed between the cell lines (Fig. 3C, panel a). Lipid fractions of the cells were then resolved by thin layer chromatography (TLC) and the levels of [14C]palmitate-labeled CE were quantified. Clearly, expression of TgACAT2 in MEF ACAT1−/− cells restored the ability of the cells to synthesize CE to almost MEF wild-type levels. To confirm these data, we transfected mammalian AC29 cells, another cell line lacking ACAT activities following chemical mutagenesis (Cadigan et al., 1988) with pTgACAT2 or the empty vector, to compare the production of cholesteryl palmitate with CHO wild-type cells (Fig. 3C, panel b). In accordance with our data collected from MEF ACAT1−/−, AC29 cells transfected with pTgACAT2 were also able to synthesize cholesteryl palmitate. Levels of cholesteryl palmitate in TgACAT2-transfected cells corresponded to about half to those of CHO wild-type cells.
Together, this demonstrates that TgACAT2 is capable of catalyzing the cholesterol esterification reaction and triggering the storage of CE in lipid bodies.
TgACAT1 and TgACAT2 utilize LDL-cholesterol as substrate, but differ in their fatty acid specificity
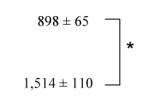
We next planned experiments to compare the enzymatic activities of TgACAT1 and TgACAT2 and determine the nature of substrates preferentially used by these enzymes. MEF ACAT1−/− transiently expressing either TgACAT1 or TgACAT2 tagged with HA were incubated with tritiated cholesterol associated with LDL in the medium for 2-h and the levels of [3H]cholesteryl esters were quantified (Table I). No differences were observed in the amounts of radioactive cholesterol taken up from the medium by the two transgenic cells. However, TLC analyses showed significantly higher levels of radioactive CE in TgACAT2-expressing MEF ACAT1−/− than TgACAT1-expressing MEF ACAT1−/−.
Table I.
Uptake of cholesterol by MEF ACAT1−/− cells transfected with TgACAT1 or TgACAT2, and incorporation into cholesteryl esters
| cholesterol uptake (cpm.μg cell protein−1) |
CE levels (cpm.μg cell protein−1) |
|
|---|---|---|
| MEF ACAT1−/− transfected with: | ||
| vector | 3,991 ± 601 | n.d. |
| TgACAT1 | 4,003 ± 510 |
|
| TgACAT2 | 3,758 ± 436 |
MEF ACAT1−/− were transfected with vector alone, or plasmids containing either TgACAT1 or TgACAT2. Cells were then incubated with [3H]cholesterol-LDL. Cholesterol uptake was monitored by scintillation counting. CE detection was performed by TLC analysis. The results are mean values ± SD from three incubations (*; P < 0.01). n.d., not detected.
We next performed studies to assess the fatty acyls that are preferentially used by either TgACAT. We selected oleate, palmitate, stearate and arachidonate that are known to be avidly taken up by Toxoplasma (Quittnat et al., 2004) and incorporated in CE (Lige et al., 2011): among total CE, cholesteryl oleate represents 42%, cholesteryl palmitate 26%, cholesteryl stearate 3.4% and cholesteryl arachidonate 2.6%). MEF ACAT1−/− cells transiently expressing either TgACAT1 or TgACAT2 were exposed to [3H]palmitic acid (16:0), [3H]stearic acid (18:0), [3H]oleic acid (18:1) or [3H]arachidonic acid (20:4) for 3-h prior to quantification of labeled CE produced by the two cell types. No difference in amounts of internalized fatty acids by TgACAT1- and TgACAT2-expressing cells was noticed (Table II). Cells expressing TgACAT1 preferentially incorporated palmitate and, to a lesser extent, oleate into cholesterol. We also detected the production of CE using radioactive stearate or arachidonate though the abundance of stearate- and arachidonate-labeled CE was ~6 and ~9-times less than CE containing oleate, respectively. By contrast, cells expressing TgACAT2 synthesized equal amounts of CE containing palmitate acid, stearate, oleate acid or arachidonate.
Table II.
Uptake of fatty acids by MEF ACAT1−/− cells transfected with TgACAT1 or TgACAT2, and incorporation of free fatty acids into cholesteryl esters
| FFA uptake (nmol.FFA mg cell protein−1) |
CE activity (nmol FFA incorporated into CE) |
||
|---|---|---|---|
| MEF ACAT1−/− transfected with: | |||
| vector | 16:0 | 130.1 | n.d. |
| 18:0 | 110.1 | n.d. | |
| 18:1 | 118.1 | n.d. | |
| 20:4 | 99.9 | n.d. | |
| TgACAT1 | 16:0 | 127.0 | 5.22 |
| 18:0 | 102.9 | 0.94 | |
| 18:1 | 100.4 | 2.33 | |
| 20:4 | 115.6 | 0.59 | |
| TgACAT2 | 16:0 | 98.4 | 6.10 |
| 18:0 | 119.1 | 5.32 | |
| 18:1 | 127.5 | 5.54 | |
| 20:4 | 111.3 | 4.99 | |
MEF ACAT1−/− were transfected with the vector alone, or plasmids containing either TgACAT1 or TgACAT2 for 24-h. Cells were then incubated with [3H]palmitic acid (16:0), [3H]stearic acid (18:0), [3H]oleic acid (18:1) or [3H]arachidonic acid (20:4) for 3-h. Free fatty acid (FFA) uptake was monitored by scintillation counting. CE formation was performed by TLC analysis. The results are mean values from incubations performed in duplicate with less than 10% variation. n.d., not detected.
Together, this suggests that both parasite enzymes utilize host-derived cholesterol as a substrate. TgACAT2 forms more CE than TgACAT1 in MEF ACAT1−/− cells and utilizes a broad range of fatty acyls. In contrast, TgACAT1 shows marked preference for palmityol-CA over other fatty acyl-CoA.
Toxoplasma lacking either ACAT1 or ACAT2 develops poorly and has reduced size body
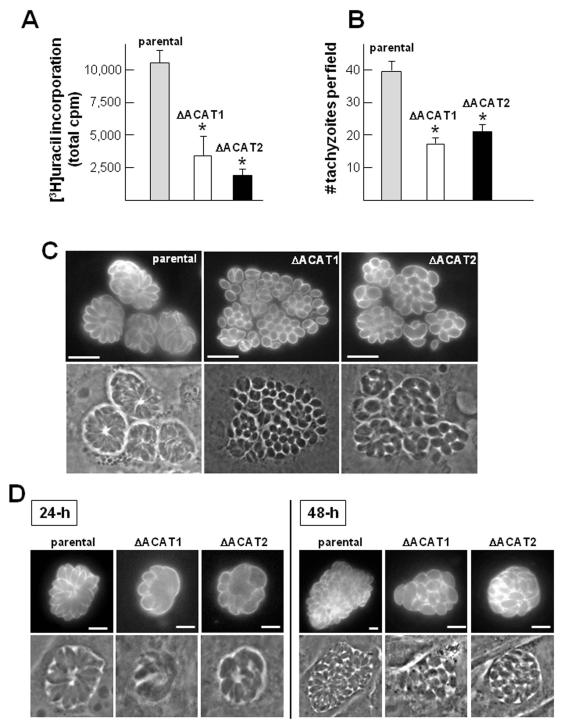
We previously reported the high vulnerability of Toxoplasma towards ACAT inhibitors (Nishikawa et al., 2005). To further assess the physiological importance of the ACAT activities in the parasite and provide more insights into TgACAT1 and TgACAT2 functions, we focused on genetically disrupting the ACAT1 or ACAT2 gene. A fusion PCR-based method was used for ACAT1 or ACAT2 deletion by their replacement with the selectable marker HXGPRT; successful homologous recombination was confirmed by PCR (Fig. S2). Despite their very slow growth in cultured cells compared to the parental strain (ΔKu80ΔHXGPRT or ΔKu80), clones lacking either ACAT1 or ACAT2 could be identified. The growth rate of the ΔACAT1 or ΔACAT2 strains in fibroblasts was quantified by uracil incorporation assays 24-h post-invasion (p.i.; Fig. 4A). In accordance with our functional data identifying TgACAT2 as the major contributor to CE formation in the parasite, the ΔACAT2 also showed the more dramatic growth defect with a growth rate lowered by ~4-fold as compared to a ~3-fold reduction in the ΔACAT1 mutant. To further characterize the observed phenotype, we next looked more specifically at the ability of mutant parasites to invade host cells by enumerating intracellular parasites 20-min p.i. (Fig. 4B). As compared to the parental strain both mutant parasite strains showed a ~50% reduced invasion efficiency. This suggests that the growth defect observed in the ΔACAT1 strain is largely due to impaired host cell invasion, while the deletion of TgACAT2 appears to have a broader effect, affecting the host cell invasion as well as other post-invasion events.
Figure 4. Growth and morphology of Toxoplasma lacking either ACAT1 or ACAT2.
A. Quantification of replication rate in vitro of the ΔACAT1 and ΔACAT1 strains compared to the ΔKu80 parental strain. [3H]uracil incorporation was assayed using fibroblasts infected with these three strains for 24-h. Values of uracil incorporation in the parasites expressed in cpm, are the means ± SD from three separate experiments. Differences between values obtained for the two ΔACAT strains relative to the parental strain are statistically significant (*, P<0.05). B. Quantification of invasion using the red/green assay for parasites stained with antibodies against the plasma membrane marker SAG1. Histograms represent internal parasites and data are means ± SD of triplicates, counting 20 randomly selected fields for each sample at 20× objective (*, P<0.05). C. IFA on ΔACAT1, ΔACAT2 andΔKu80 strains using anti-SAG1antibodies to visualize the global shape of the parasites 36-h p.i. D. Same IFA on ΔACAT1, ΔACAT2 andΔKu80 as described in C. Parasites were incubated in excess LDL (2 mg.ml−1) for 24-h or 48-h prior to fixation. Bars are 5 μm.
We then looked at the morphology of the mutant strains and their organization within the PV by optical microscopy. Mutant parasites were immunolabeled with antibodies against the plasma membrane marker SAG1 (Fig. 4C). Both ΔACAT strains were characterized by a very small and globular cell body as compared to the parental strain typified by a long and lunate cell body (dimension: ~1 × 2-μm for parasite mutants vs. 1.5 × 7-μm for control parasites), which might be due to abnormalities in their lipid membrane composition. In addition, parasite mutants were disordered in their PV and were rarely organized as typical ‘rosettes’, which may reflect asynchronous divisions. Complementation of the mutant strains with the respective coding sequences completely restored the growth deficiencies (Fig. S3 A-B), confirming that the observed defects were specific to the deletion of the ACAT1 or ACAT2 gene, and therefore to the reduced ACAT activities in either mutant.
Host-derived LDL is the major source of cholesterol for Toxoplasma (Coppens et al., 2000). Profusion of LDL in the culture medium leads to massive uptake of cholesterol by the parasite, suggestive of an uncontrolled uptake of this lipid by T. gondii. Nevertheless, as an efficient strategy to balance increasing cholesterol levels, the parasite is able to esterify cholesterol for subsequent storage in lipid bodies (Nishikawa et al., 2005). The number of lipid bodies correlates with the amounts of LDL in the medium. We hypothesized that exposure of our mutant parasite strains to excess exogenous cholesterol would lead to a toxic accumulation of free cholesterol causing membrane damages and parasite death. Hence, we next challenged the ΔACAT1 and ΔACAT2 strains by incubating them in medium containing 20-times more LDL than normal conditions and examined their morphology (Fig. 4D). As compared to control parasites, parasite mutants showed grosser morphological alterations including distortion of the parasite body shape compared to mutants incubated under control conditions (10% FBS with 0.1 mg LDL/ml) at 24-h p.i. Additionally, the applied stress seemed to induce defects in cytokinesis as evidenced by asynchronous divisions and slow individualization of nascent parasites. After 2 days incubation with excess LDL, the mutants had formed smaller and disorganized PVs as compared to the parental strain and appeared to have lost their capability to egress.
Together, these results reveal an essential function of TgACAT activities in controlling cholesterol levels in the parasite, which is required for parasite intracellular development and differentiation.
The ΔACAT1 strain shows plasma membrane defects whereas the ΔACAT2 strain accumulates lipids in the PV
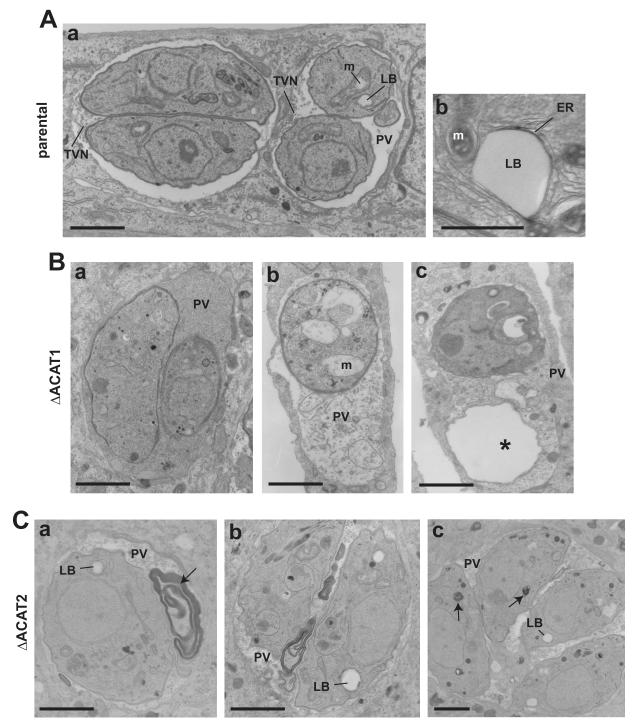
We next undertook EM studies to scrutinize the morphological abnormalities of parasites lacking ACAT1 or ACAT2 compared to control parasites. Panel A of Fig. 5 shows two PVs of the parental strain of Toxoplasma characterized by an electron-dense cytoplasm. Several lipid bodies surrounded by ER elements in close proximity to the mitochondrion were visible. Besides the presence of a tubulo-vesicular network of membranes, putatively ascribed to promote nutrient delivery to the parasites (Sibley et al., 1995), few structures were present within the PV. In contrast, the ΔACAT1 strain had an electron-lucent cytoplasm and accumulated dense material and membranous structures into the PV, likely originating from the parasite cytoplasm upon rupture of the plasma membrane (Fig. 5B). The presence of parasite “ghosts” lacking cytoplasmic constituents supports this assumption. Interestingly, this phenotype has been also observed for Toxoplasma treated with ACAT inhibitors (Nishikawa et al., 2005). In addition, some parasite organelles were dramatically altered such as the mitochondrion showing swollen cristae. By comparison, the ΔACAT2 strain exhibited large deposits of osmiophilic material, likely lipids, both in the PV lumen and the cytoplasm (Fig. 5C). This may reflect gross lipid disorders as a consequence of a perturbation of the balance between CE and free cholesterol.
Figure 5. Ultrastructure Toxoplasma lacking either ACAT1 or ACAT2.
A-C. Transmission electron microscopy comparing the ultrastructure of the parental strain with ΔACAT1 and ΔACAT2 parasites. A. Panel a illustrates two PV with parental parasites with well-identifiable organelles and the tubulo-vesicular network (TVN). Panel b shows a classical lipid body surrounded by ER elements and close to mitochondria (m). B. Panels a to c highlight the presence of abnormal material debris in the PV lumen of the ΔACAT1 strain. Morphology of mitochondria was altered (panel b) and ghost parasites were visible (asterisk in panel c). C. Panels a to c illustrate abnormal accumulation of osmiophilic materials (arrows) in the vacuolar space or within the cytoplasm for the ΔACAT2 strain. Small residual lipid bodies (LB) were visible. Scale bars are 0.5 μm.
Both ΔACAT1 and ΔACAT2 strains have reduced levels in CE and accumulate free cholesterol
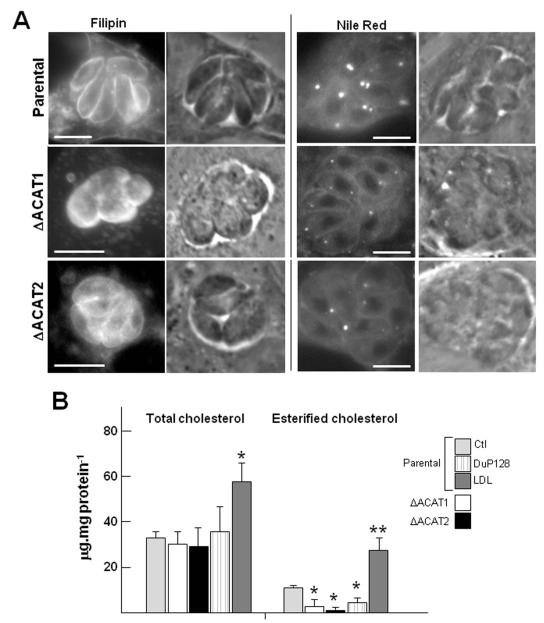
Our morphological analyses strongly suggest a key role for ACAT enzymes to adjust proper levels of free cholesterol in Toxoplasma. Hence, we next analyzed the sterol composition of the ΔACAT1 and ΔACAT2 strains to determine the relative contribution of TgACAT1 and TgACAT2 to the overall parasite CE synthesis. Sterols located in membranes or stored in lipid bodies were first visualized by fluorescence microscopy using filipin and Nile Red, respectively (Fig. 6A). Compared to control parasites, the cellular distribution of free cholesterol was unaltered in ΔACAT1 and ΔACAT2 parasites as a positive filipin signal was observed on the plasma membrane. Though, the ΔACAT parasites showed a dramatic decrease in the number and size of lipid bodies as illustrated by a weak Nile Red fluorescence signal.
Figure 6. Cholesterol and CE content in Toxoplasma lacking either ACAT1 or ACAT2.
A. Fluorescence microscopic observations of ΔACAT1, ΔACAT2 and parental strains to visualize membrane cholesterol using filipin and lipid bodies using Nile Red. Bars are 5 μm. B. Quantitative measurement of total cholesterol (free and esters forms) and CE alone in ΔACAT1, ΔACAT2 and parental strains. Total cholesterol content was determined on parasites exposed to various culture conditions for 24-h: the parental strain cultivated in normal medium (10% FCS; Ctl), in the presence of the ACAT inhibitor DuP128 or in excess cholesterol in the medium (LDL); and the ΔACAT1 and ΔACAT2 parasites cultivated in normal conditions. Results of total cholesterol content expressed in μg sterol molecules per mg cell protein, are the means ± SD from three separate experiments. Differences between values obtained for the parental strain grown in excess LDL and normal medium are statistically significant (*, P<0.05). To monitor cholesterol esterification activity, parasites were exposed to radioactive palmitate for 2-h prior to lipid extraction and separation on TLC plates. Results of lipid incorporation into CE expressed in μg palmitate incorporated into CE per mg cell protein, are means ± SD of three separate experiments. These data were compared to parental parasites under control conditions and were statistically significant (*, P<0.05; **, P<0.01).
Next, we performed lipid analyses to assess the content of free cholesterol and CE in the ΔACAT1 and ΔACAT2 strains as compared to the parental strain. No significant difference in the total amount of cholesterol (free + esterified cholesterol) was observed between the three strains (Fig. 6C). The total cholesterol content also remained unchanged in the parental strain exposed to the ACAT inhibitor DuP128. Expectedly, incubation in excess LDL led to a significant increase of total cholesterol amounts (by 170%) in the parental strain as a result of avid uptake of cholesterol-LDL by T. gondii.
To determine the ratio of free cholesterol vs. cholesterol that has been esterified in our strains, we incubated the parasites with radioactive palmitate prior to lipid extraction and TLC to monitor the synthesis of cholesteryl palmitate (Fig. 6C). In the parental strain, the fraction of cholesteryl palmitate constituted about one third of the total cholesterol (10.9 μg cholesteryl palmitate.mg protein−1 vs. 33.5 μg total cholesterol.mg protein−1). The synthesis of cholesteryl palmitate was significantly decreased in the ΔACAT strains and dropped to 2.49 μg.mg protein−1 and 1.25 μg.mg protein−1 in the ΔACAT1 and ΔACAT2 strains respectively (P < 0.033). By comparison, the production of cholesteryl palmitate was reduced to 4.4 μg.mg protein−1 in parasites treated with DuP128. This indicates that the amount of free cholesterol was elevated by 130%, 140% and 125% in the ΔACAT1 strain, ΔACAT2 strain and DuP128- treated parasites, respectively, as compared to the untreated parental strain. Expectedly, a substantial production of cholesteryl palmitate by the parasites exposed to excess LDL was observed; however, the amount of free cholesterol in these parasites remained in the same range as calculated for parasites cultivated in normal medium.
Loss of either ACAT gene renders the mutant parasites particularly vulnerable to ACAT inhibitor treatment
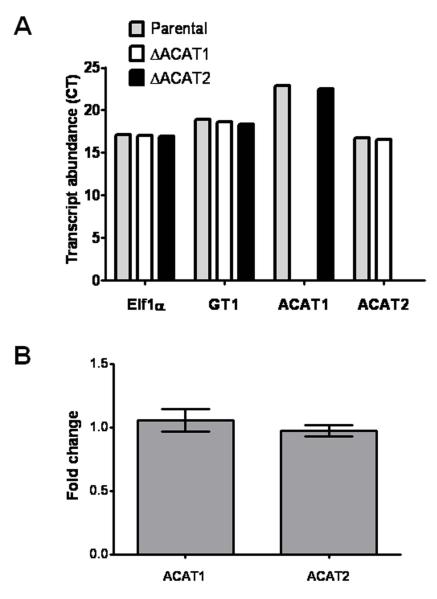
In order to completely abolish the synthesis of CE by the parasite, we attempted to generate a double knockout strain. However, our multiple attempts to delete the ACAT2 gene in the ΔACAT1 parasites and vice-versa, were unsuccessful, suggesting that expression of at least one an ACAT gene is required to ensure the parasite survival. We therefore performed quantitative real-time PCR to test for transcriptional co-regulation of the two ACAT genes. Expectedly, the transcript of TgACAT2, being the major ACAT enzyme in Toxoplasma, was more abundant and appeared at cycle 16, as compared to TgACAT1 which was visible at cycle 22 (Fig. 7A). Besides, the deletion of ACAT1 showed no changes in gene expression of the ACAT2 gene, and vice versa (Fig. 7B). In agreement with our functional data showing that the two ACAT enzymes are involved in the production of different pools of CE in the parasite, this further confirms that the two genes are non-redundant, and are not able to complement each other, which corroborates our observation that the double deletion is lethal.
Figure 7. Transcriptional profiles of TgACAT1 and TgACAT2.
RT-qPCR 100 ng total RNA of ΔACAT1, ΔACAT2 and parental strains were subjected to first-strand cDNA synthesis using random hexamer primers. Histograms show transcript abundance (A) or fold change (B) that were calculated by normalization with the housekeeping gene transcripts (TgElf1α and TgGT1).
Hence we used a pharmacological approach to further decrease the CE production in each single ΔACAT strain. Mutant parasites were incubated for 24-h with two different ACAT inhibitors, DuP128 or CI976, and parasite growth was monitored by uracil incorporation assays (Table III). As compared to the parental parasites treated with DuP128 or CI976, the ΔACAT1 and ΔACAT2 mutants displayed significantly higher sensitivity to treatment with ACAT inhibitors. For example, upon DuP128 exposure, ΔACAT1, ΔACAT2 and the parental strain showed growth reduction of 63%, 75% and 53% respectively, as compared to untreated parental parasites. A similar effect was observed with CI976 as the growth rate of the ΔACAT1, ΔACAT2 and the parental strain was reduced by 53%, 66 %, and 20% respectively, as compared to untreated parental parasites. After exposure to these inhibitors for 24-h, both mutant parasites showed signs of atrophy and were barely identifiable inside host cells.
Table III.
Effect of cholesterol esterification inhibitors on viability of parasites lacking TgACAT1 or TgACAT2
| Strains | Parental | ΔACAT1 [3H]uracil incorporation in cpm (% of growth relative to untreated parental strain) |
ΔACAT2 |
|---|---|---|---|
| Treatment: | |||
| - no drug | 51,902 ± 3,053 | 29,008 ± 2,027** | 19,482 ± 1,113** |
| (100%) | (56%) | (38%) | |
| - 5 μM DuP128 | 24,506 ± 5,291** | 19,123 ± 426** | 12,820 ± 1,134** |
| (47%) | (37%) | (25%) | |
| - 10 μM CI976 | 41,607 ± 3,648* | 24,480 ± 325** | 17,731 ± 972** |
| (80%) | (47%) | (34%) |
Radioactive incorporation assays on HFF infected with the parental, ΔACAT1 or ΔACAT2 strains. Cells were infected for 24-h, then treated with the cholesterol esterification inhibitors, DuP128 (5 μM) or CI976 (10 μM), or no drug for additional 24-h prior to uracil incorporation assays to monitor parasite growth. Results are the means ± SD of three different assays. Parasite growth was significantly impaired compared to untreated parental parasites (referred as 100% growth)
P<0.05
P<0.005.
Altogether, these data highlight an essential function of cholesterol esterification in Toxoplasma, which cannot tolerate the simultaneous loss of both ACAT activities, and establish a protective role for TgACAT1 and TgACAT2 to overcome the accumulation of cytotoxic free cholesterol.
Discussion
Toxoplasma contains several species of CE with predominance of cholesteryl oleate and palmitate (Lige et al., 2011). The parasite is able to esterify its own cholesterol by ACAT-like enzyme previously characterized (Nishikawa et al., 2005). In this paper, we have extended our investigations on cholesterol storage in Toxoplasma by identifying a second O-acyltransferase member that catalyzes the synthesis of CE. Sterols are essential lipid components of eukaryotic membranes, which harbor multiple essential functions ranging from signal transduction to protein lipidation. Likewise, Toxoplasma contains cholesterol in its membrane; however, the parasite does not have the enzymatic machinery to synthesize the steroid core and relentlessly takes up sterols from the host cell (Coppens et al., 2000). The preferential source of cholesterol for Toxoplasma is plasma LDL internalized into late endosomes and lysosomes. When LDL is present in the medium, the parasite seizes cholesterol and transports the lipid into the ER, where cholesterol becomes esterified prior to storage in lipid bodies. In Toxoplasma, lipid bodies represent a reservoir for exogenously acquired lipids that are surplus to the immediate requirements of membrane formation. In medium poor in LDL, T. gondii consumes its stores of cholesterol, and slows down its cycle of replication (Coppens et al., 2000; Nishikawa et al., 2005). The presence of two ACAT enzymes both involved in the esterification and storage of cholesterol may relieve Toxoplasma from an unremitting salvage of cholesterol and enable it to survive episodic variations of LDL-cholesterol concentrations in the environment.
The presence of more than one sterol esterification enzyme in Toxoplasma is not a priori out-of-the-ordinary since yeast and mammalian cells also have two ACAT-related enzymes. However, while yeast or mammalian cells use their enzymes to perform different physiological activities (reviewed in Daum et al., 2007 and Athenstaedt and Daum, 2006), Toxoplasma, at first glance, appears to employ TgACAT1 and TgACAT2 to execute the same activity: the catalysis of cholesterol esterification and compartmentalization of CE within cytoplasm inclusions. In support of this idea, we showed that disruption of either ACAT1 or ACAT2 gene leads to reduced intracellular levels of CE and sparse lipid bodies. Nevertheless, our studies identified two slight differences in TgACAT1 and TgACAT2 activities. First, our results derived from heterologous expression of each parasite enzyme in ACAT-deficient mammalian cells showed that TgACAT2 forms more CE than TgACAT1, suggesting that TgACAT2 could be the major contributor of CE for the parasite. Supporting this notion are the higher level of expression of the TgACTA2 transcript as compared to TgACAT1 (cycle 16 vs. 22), and the more severe growth defect observed in the ΔACAT2 strain as compared to ΔACAT1 parasites (75% for ΔACAT2 vs. 65% for ΔACAT1 compared to the parental strain). Interestingly, the observed growth defect could be mostly (TgACAT1) or partially (TgACAT2) attributed to a reduced invasion efficiency, which was lowered to ~47% and ~54%, respectively. Apparently, deletion of parasite ACAT results in altered cycles of cholesterol esterification and accumulation of free cholesterol, which causes an abnormal membrane lipid composition and indirectly affects host cell invasion. Moreover, the deletion of TgACAT2 appears to have a broader effect, also impairing post-invasion events, demonstrating the greater overall role of TgACAT2 for the parasite viability. Second, TgACAT1 and TgACAT2 differ in their specificity for fatty acyl substrates. TgACAT1 has inherent higher acyl donor specificity than TgACAT2 and prefers palmitic acid over other fatty acids, which is the most predominant fatty acid found in T. gondii (Tomavo et al., 1989; Quittnat et al., 2004; Nishikawa et al., 2005; Naget et Boothroyd, 1989; Chaudhary et al., 2005, Mazumdar and Striepen, 2007; Ramakrishnan et al., 2012). One possible explanation for differences in fatty acyl selection between the parasite enzymes could be that, as proposed for mammalian ACAT (Joyce et al., 2000), subtle differences in the enzyme topology in the ER membranes that may influence the access to fatty acids of TgACATs.
In microorganisms, the biogenesis and accumulation of lipid bodies is an adaptive response to nutrient depletion that marks the cessation of growth and the entry into a quiescent phase. The number and the size of lipid bodies in Toxoplasma vary between the different parasite developmental stages and environmental conditions (Dubey, 1998). The oocyst form of Toxoplasma, e.g. sporozoite, which can survive in soil for years without dividing, contains larger stores of lipids than the replicative intracellular tachyzoite form that is adapted to live in nutrient-rich habitats as offered by mammalian cell interiors. Our data reveal, nevertheless, that storage cholesterol synthesis is essential for the survival of the tachyzoite form. For example, pharmacological inhibition of ACAT activities in the parasite results in poor survival. The loss of individual ACAT gene induces a similar phenotype in the parasite. Interestingly, though causing a severe growth defect, the loss of individual ACAT genes and the consequent changes in CE pools are tolerated by Toxoplasma, suggesting a plasticity in cholesterol metabolism and a partial redundancy of the two ACAT genes. However, our qRT-PCR analyses did not show a co-regulation at the transcriptional level of the two parasite ACAT genes. Furthermore, simultaneous loss of both ACAT genes seems lethal to the parasite, as demonstrated by our incapability to generate a double deletion mutant and the complete growth arrest upon treatment of the single mutant strains with ACAT inhibitors. Hence, TgACAT1 and TgACAT2 appear to be incapable of fully complementing each other, and the expression of at least one ACAT gene is absolutely required for the parasite survival.
Since a lack of production of plasma LDL-cholesterol by the mammalian host seems improbable, why is Toxoplasma so vulnerable to impairment of ACAT activities? A possible explanation might lie in the fact that the parasite scavenges LDL-cholesterol by uncontrolled uptake mechanisms, and engorges itself with cholesterol proportionally to the amount of this lipid in the environment. In case of large amounts of intracellular LDL-cholesterol, the parasite has no other option than detoxifying cholesterol by conversion into CE which have lower biological toxicity than free sterols, and thereby provide a safe sink for cholesterol. This is supported by the observed phenotypes of the ΔACAT parasites showing a ruptured plasma membrane, which suggests that these mutants might have become osmotically fragile upon excess free cholesterol in the plasma membrane. In comparison, yeast lacking ARE1 and ARE2 have no growth defect due to their ability to prevent an accumulation of free cholesterol in the ER via a stringent regulation of ergosterol de novo synthesis as well as ergosterol export from the ER using two ABC transporters (Sandager et al., 2002; Jacquier and Schneiter, 2012). Mutant yeast lacking these ABC transporters cannot grow normally, demonstrating the importance of sterol egress for sterol homeostasis (Wilcox et al., 2002). So far, an analogous mechanism of cholesterol efflux could not be identified in Toxoplasma, offering an alternative explanation for the higher sensitivity of the parasite towards interference with cholesterol homeostatic pathways.
Another possibility might be that lipid bodies in Toxoplasma may have more sophisticated functions than just temporary lipid and energy storage. In multicellular organisms, lipid bodies fulfill numerous additional roles including lipid transport, steroid sequestration, protein deposit, membrane trafficking and signaling (reviewed in Murphy, 2012). Hence, malfunctioning lipid bodies can increase the risk of developing diseases in humans, e.g., lipodystrophy in case of too little lipid body production; obesity, hepatic steatosis and atherosclerosis in case of overproduction of lipid bodies; several cancers and Parkinson’s and Alzheimer’s disease associated with abnormal lipid body function. Interestingly, a Toxoplasma strain lacking a Niemann-Pick, type C1-related protein (NCR1) forms abundant and large lipid bodies in comparison to wild-type parasites (Lige et al., 2011). An overall increase in the CE content has been detected in the ΔNCR1 strain, and yet these mutants are more virulent in vitro and in vivo than wild-type parasites. This information highlights that the regulation of lipid metabolism including the synthesis of CE in Toxoplasma may be more complex than expected for a unicellular organism.
Relatively little information on lipid body content in protozoa is available in the literature. The ciliated Tetrahymena pyriformis contains triacylglycerols (TAG; Borowitz and Blum, 1976) and can synthesize CE from cholesterol retrieved from the medium (Billheimer et al., 1989). The flagellate Trypanosoma brucei accumulates lipid bodies and a unique kinase has been localized at the surface of these structures (Flaspohlet et al., 2010). The apicomplexan Plasmodium falciparum expresses a single member of the MBOAT family that is involved in TAG synthesis and storage in lipid bodies (Vielemeyer et al., 2004; Palacpac et al., 2004a; Coppens and Vielemeyer, 2005), and that is essential for the parasite viability (Palacpac et al., 2004b). Plasmodium does not store cholesterol retrieved from the host cell. Toxoplasma contains three biosynthetic enzymes TgDGAT1, TgACAT1 and TgACAT2 that contribute to the storage of neutral lipids (Quittnat et al., 2004; Nishikawa et al., 2005; this study). We showed previously that TgDGAT1 is an essential enzyme and highlighted here the importance of ACAT activities for the parasite. Future research will be needed to provide insights into the biochemical properties of lipid bodies (e.g., protein content) and the interactions of these essential structures with cellular process (e.g., interorganelle communication) to uncover unique pathways in the life cycle of neutral lipids in Toxoplasma.
Experimental Procedures
Chemicals and antibodies
All chemicals were obtained from either Sigma Chem. Co. (St. Louis, MO) or Fisher (Waltham, MA) unless indicated otherwise. Solvents and standards for chromatography were of the highest analytical grade. Silica gel 60 TLC plates were from EM Science (Gibbstown, NJ). CI976 and DuP128 were kindly provided by A. Rodriguez (Johns Hopkins University). Low-density lipoprotein particles (LDL: density 1.019 to 1.063 g.ml−1) were isolated from human serum and prepared as described (Coppens et al., 2000). Radiolabeled reagents included: [1,2-3H]cholesterol (sp act: 58 Ci.mmol−1), [5,6-3H]uracil (sp act: 45 Ci.mmol−1), [9,10-3H]oleic acid (sp act: 12 Ci.mmol−1), [1-14C]palmitic acid (sp act: 57 mCi.mmol−1), [9,10-3H]palmitic acid (sp act: 57 mCi.mmol−1), [9,10-3H]stearic acid (sp act: 55 mCi.mmol−1) purchased from Moravek Biochemicals, Inc. (Brea, CA), and [5,6,8,9,11,12,14,15-3H]arachidonic acid (sp act: 210 mCi.mmo−1l) from Dupont NEN Corporation (Boston, MA). Primary antibodies used in this study were mouse anti-SAG1 generously given by J.F. Dubremetz (University of Montpellier), rabbit anti-adipose differentiation-related proteins (or ADFP) from Novus Biologicals, LLC (Littleton, CO), and mouse anti-HA from Babco (Richmond, CA).
Mammalian cell lines, culture conditions and parasite propagation
The mammalian cell lines used in this study are the primary HFF (ATCC CRL-1635), CHO cells (ATCC CCL-163), the AC29 mutant lacking ACAT activity from mutagenized CHO cells (Cadigan et al., 1988) generously given by Dr Ta-Yuan Chang (Dartmouth Medical School) and the MEF derived either from ACAT1+/+ or ACAT1−/− mice generated by targeted gene disruption (Meiner et al., 1996) kindly provided by Dr Robert Farese (University of California). HFF and CHO cells were cultivated in Dulbecco’s modified Eagle medium and the MEF cultures in RPMI 1640 medium. Both media were supplemented with 10% FCS, 2 mM glutamine and penicillin/streptomycin (100 units.ml−1 per 100 μg.ml−1). The RH strain tachyzoite of Toxoplasma gondii was used throughout this study and was propagated in vitro by serial passages in monolayers of HFF (Roos et al., 1994). Intracellular parasites were purified from host cells by density gradient using Nycodenz and isopycnic centrifugation as detailed previously (Coppens et al., 2000). To evaluate parasite viability, the incorporation of [3H]Uracil incorporated into the parasites was measured as described (Roos et al., 1994). Invasion assays were performed as previously described (Huynh et al., 2003). Twenty-four-well plates were infected with 107 parasites per well and incubated for 20 min at 37°C before fixation. Slides were differentially stained with anti-SAG1 antibodies for as per the red-green invasion assay.
Cloning of full-length cDNA encoding TgACAT2 and transient transfection of TgACAT2-HA in Toxoplasma
BLAST searches in the Toxoplasma database (www.ToxoDB.org) identified a second ACAT homolog in T. gondii (TgACAT2: TGGT1_114790). The 1.6-kb ORF was amplified from a T. gondii cDNA library generously provided by V.B. Carruthers (University of Michigan) using the primers F-ACAT2 and R-HAACAT2, and cloned into plasmid pYFP (BglII/AvrII) allowing the expression of C-terminally HA-tagged TgACAT2-HA under control of the tubulin promoter (see Table SI for the sequences of primers used in this study). The obtained cDNA fragment was subsequently sequenced and deposited in GenBank under the accession number JX441364. To study the subcellular localization of ACAT2 in Toxoplasma, extracellular parasites (~107, RH strain) were transfected with TgACAT2-HA by electroporation as described (Soldati and Boothroyd, 1997) and the protein localization was determined by immunofluorescence using mouse-anti-HA antibodies.
Plasmid constructs for expression in mammalian cells for functional studies
TgACAT2-HA was cloned into the mammalian expression plasmid pcDNA3.1− (EcoRI/HindIII) using the primers ACAT-ecor/ACAT-Hin resulting in the expression of a C-terminally HA-tagged protein under control of the pCMV promoter. The construct was transfected into ACAT1-deficient cells using the lipofectamine method as described (Quittnat et al., 2004). Transfected cell lines were selected under neomycin (800 μg/ml) selection, after 2 days or 2 weeks for transient or stable expression of TgACAT2-HA respectively. In one assay, a plasmid containing TgACAT1-HA (Nishikawa et al., 2005) was introduced into ACAT1- deficient cells for transient expression to monitor CE production in comparison MEF ACAT1−/− expressing TgACAT2-HA. The transfection % was measured by counting fluorescent cells expressing TgACAT1-HA or TgACAT2-HA detected by IFA using anti-HA antibodies. Comparable % transfection corresponding to 24 ± 3% and 27 ± 5 % were observed between TgACAT1- and TgACAT2-expressing MEF ACAT1−/−, respectively.
Genetic disruption of TgACAT1a or TgACAT2 gene
To create the ΔACAT1 or ΔACAT2 strain, we PCR amplified 600bp 5′- and 3′-UTR fragments of TgACAT1 (primer pairs ACAT1-P1/ACAT1-P4 and ACAT1-P5/ACAT1-P2) and TgACAT2 (primer pairs Acat2-P1/Acat2-P4 and Acat2-P5/Acat2-P2) as well as the hypoxanthine-xanthine-guanine phosphoribosyltransferase (HXGPRT) selectable marker cassette (primer pairs ACAT1-P3/ACAT1-P6 or Acat2-P3/Acat2-P6) and subsequently fused the 3 fragments via PCR as described (Lige et al., 2011). About 107 extracellular tachyzoites of the Δku80 strain (Huynh et al., 2009) were transfected with ~50μg of the knockout construct and transgenic parasites were selected using mycophenolic acid and xanthine as described (Donald et al., 1996). Stable parasites were cloned and screened for homologous crossover and the consequent absence of the respective ACAT gene locus by the different PCR reactions depicted in Fig. S2.
Complementation of the ΔACAT1 and ΔACAT2 strains by negative selection of HXGPRT
To generate the complementation construct the above mentioned 5′- and 3′-UTR fragments of ACAT1 and ACAT2 were fused adjacent to the respective gene coding sequences. The 3 individual PCR fragments were obtained by PCR using the primer pairs ACAT-P1/A1comp-4, A1comp-3/A1comp-6 and A1comp-5/ ACAT-P2 for ACAT1, as well as the primer combinations Acat2P1/A2comp-4, A2comp-3/A2comp-6 and A2comp-5/Acat2P2 for ACAT2. ~50μg of the complementation constructs were transfected into the corresponding knockout strain by electroporation and stable integration achieved by selection with 2- hydroxy-6-mercaptopurine (0.85 mg.ml−1), Resistant parasites were analyzed for homologous integration of the coding sequence by screening PCR using the primer combinations shown in Fig. S3.
Quantitative real-time PCR
Total RNA was isolated from the ΔKU80, ΔACAT1, and ΔACAT2 parasites using the RNeasy Mini Kit prior to reverse transcription of 100 ng RNA each using random hexamer primers. qPCR was performed using primers listed in Table S1. Transcript abundance was calculated by comparison of the ct values with the housekeeping transcripts (TGGT1_124740, GT1; TGGT1_037840, Elf1a).
Lipid measurements
Cholesterol concentration: Total cholesterol content was measured by an enzymatic and colorimetric assay using a reagent mixture from Roche Diagnostics Corp. (Indianapolis, IN). CE synthesis: Mammalian cells or parasites were incubated for 2-h in medium containing 0.2 mM radioactive FA bound to albumin (1% BSA, FA:BSA molar ratios of 2:1) or with 1 mg.ml−1of [3H]cholesterol incorporated into LDL to monitor the formation of radioalabeled CE. Cell isolation, lipid extraction and separation by TLC using petroleum ether/diethyl ether/acetic acid (80:20:1) were performed as described (Coppens et al., 1995).
Light and electron microscopy studies
Light and epifluorescence microscopy were performed on infected cells seeded on sterile coverslips in 24-well culture dishes. IFA on parasites or mammalian cells were performed as previously described (Coppens and Joiner, 2003) using primary antibodies against HA (1:1000) or ADFP (1:100), and fluorescently labeled secondary antibodies (Invitrogen): anti-mouse and anti-rabbit IgG antibodies conjugated to either Alexa 488 or Alexa 594 diluted at 1:2000. For detection of cholesterol using filipin or lipid bodies using Nile Red by fluorescence microscopy, intravacuolar parasites were fixed in paraformaldehyde, and treated as described (Quittnat et al., 2004; Coppens and Joiner, 2003). Slides were analyzed using a Nikon Eclipse E800 microscope equipped with a Spot RT CCD Camera and images were processed using the Image-Pro-Plus software (Media Cybernetics, Silver Spring, MD) before assembly using Adobe Photoshop (Adobe Systems, Mountain View, CA). For ultrastructural observation of the ΔACAT strains by thin-section transmission electron microscopy (EM), infected cells were fixed in 2.5% glutaraldehyde (Electron Microscopy Sciences, Hatfield, PA) and processed as described (Coppens and Joiner, 2003). Ultrathin sections of infected cells were stained by lead citrate and uranyl acetate before examination with a Philips CM120 EM (Eindhoven, the Netherlands) under 80 kV.
Protein determination
Protein content was determined by the bicinchoninic acid assay (Smith et al., 1995), using serum albumin as a standard.
Statistical analysis
For comparison of means, P was determined by analysis of variance against control (ANOVA 2).
Supplementary Material
Acknowledgements
The authors are thankful to the technical staff at the Microscopy Facility at Johns Hopkins University for their excellent assistance in electron microscopy techniques. This work was supported by the American Heart Association (grant GIA 0755368U to I.C.) and the National Institutes of Health (grant R01 AI060767 to I.C.).
Footnotes
Supplemental information
This section includes three Figures and one Table.
References
- Athenstaedt K, Daum G. The life cycle of neutral lipids: synthesis, storage and degradation. Cell Mol Life Sci. 2006;63:1355–1369. doi: 10.1007/s00018-006-6016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billheimer JT, Landrey JR, Conner RL. The presence of acyl-CoA: cholesterol acyltransferase in Tetrahymena pyriformis W. Comp Biochem Physiol B. 1989;92:675–680. doi: 10.1016/0305-0491(89)90248-4. [DOI] [PubMed] [Google Scholar]
- Borowitz MJ, Blum JJ. Triacylglycerol turnover in Tetrahymena pyriformis. Relation to phospholipid synthesis. Biochim Biophys Acta. 1976;424:114–124. doi: 10.1016/0005-2760(76)90056-4. [DOI] [PubMed] [Google Scholar]
- Bouvier-Nave P, Benveniste P, Oelkers P, Sturley SL, Schaller H. Expression in yeast and tobacco of plant cDNAs encoding acyl CoA:diacylglycerol acyltransferase. Eur J Biochem. 2000;267:85–96. doi: 10.1046/j.1432-1327.2000.00961.x. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Heider JG, Chang TY. Isolation and characterization of Chinese hamster ovary cell mutants deficient in acyl-coenzyme A:cholesterol acyltransferase activity. J Biol Chem. 1988;263:274–282. [PubMed] [Google Scholar]
- Cases S, Smith SJ, Zheng YW, Myers HM, Lear SR, Sande E, et al. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc Natl Acad Sci USA. 1998;95:13018–13023. doi: 10.1073/pnas.95.22.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TY, Li BL, Chang CC, Urano Y. Acyl-coenzyme A:cholesterol acyltransferases. Am J Physiol Endocrinol Metab. 2009;297:E1–9. doi: 10.1152/ajpendo.90926.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TY, Chang CC, Ohgami N, Yamauchi Y. Cholesterol sensing, trafficking, and esterification. Annu Rev Cell Dev Biol. 2006;22:129–157. doi: 10.1146/annurev.cellbio.22.010305.104656. [DOI] [PubMed] [Google Scholar]
- Chaudhary K, Donald RG, Nishi M, Carter D, Ullman B, Roos DS. Differential localization of alternatively spliced hypoxanthine-xanthine-guanine phosphoribosyltransferase isoforms in Toxoplasma gondii. J Biol Chem. 2005;280:22053–22059. doi: 10.1074/jbc.M503178200. [DOI] [PubMed] [Google Scholar]
- Coppens I, Dunn JD, Romano JD, Pypaert M, Zhang H, Boothroyd JC, Joiner KA. Toxoplasma gondii sequesters lysosomes from mammalian hosts in the vacuolar space. Cell. 2006;125:261–274. doi: 10.1016/j.cell.2006.01.056. [DOI] [PubMed] [Google Scholar]
- Coppens I, Joiner KA. Host but not parasite cholesterol controls Toxoplasma cell entry by modulating organelle discharge. Mol Biol Cell. 2003;14:3804–3820. doi: 10.1091/mbc.E02-12-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppens I, Levade T, Courtoy PJ. Host plasma low density lipoprotein particles as an essential source of lipids for the bloodstream forms of Trypanosoma brucei. J Biol Chem. 1995;270:5736–5741. doi: 10.1074/jbc.270.11.5736. [DOI] [PubMed] [Google Scholar]
- Coppens I, Sinai AP, Joiner KA. Toxoplasma gondii exploits host low-density lipoprotein receptor-mediated endocytosis for cholesterol acquisition. J Cell Biol. 2000;149:167–180. doi: 10.1083/jcb.149.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppens I, Vielemeyer O. Insights into unique physiological features of neutral lipids in Apicomplexa: from storage to potential mediation in parasite metabolic activities. Int J Parasitol. 2005;35:597–615. doi: 10.1016/j.ijpara.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Daum G, Wagner A, Czabany T, Athenstaedt K. Dynamics of neutral lipid storage and mobilization in yeast. Biochimie. 2007;89:243–248. doi: 10.1016/j.biochi.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Donald RG, Carter D, Ullman B, Roos DS. Insertional tagging, cloning, and expression of the Toxoplasma gondii hypoxanthine-xanthine-guanine phosphoribosyltransferase gene. Use as a selectable marker for stable transformation. J Biol Chem. 1996;271:14010–14019. doi: 10.1074/jbc.271.24.14010. [DOI] [PubMed] [Google Scholar]
- Dubey JP. Advances in the life cycle of Toxoplasma gondii. Int J Parasitol. 1998;28:1019–1024. doi: 10.1016/s0020-7519(98)00023-x. [DOI] [PubMed] [Google Scholar]
- Ehrenman K, Sehgal A, Lige B, Stedman TT, Joine KA, Coppens I. Novel roles for ATP-binding cassette G transporters in lipid redistribution in Toxoplasma. Mol Microbiol. 2010;76:1232–1249. doi: 10.1111/j.1365-2958.2010.07169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaspohler JA, Jensen BC, Saveria T, Kifer CT, Parsons M. A novel protein kinase localized to lipid droplets is required for droplet biogenesis in trypanosomes. Eukaryot Cell. 2010;9:1702–1710. doi: 10.1128/EC.00106-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Cromley D, Billheimer JT, Sturley SL. Identification of potential substrate-binding sites in yeast and human acyl-CoA sterol acyltransferases by mutagenesis of conserved sequences. J Lipid Res. 2001;42:1282–1291. [PubMed] [Google Scholar]
- Hofmann K. A superfamily of membrane-bound O-acyltransferases with implications for wnt signaling. Trends Biochem Sci. 2000;25:111–112. doi: 10.1016/s0968-0004(99)01539-x. [DOI] [PubMed] [Google Scholar]
- Huynh MH, Carruthers VB. Tagging of endogenous genes in a Toxoplasma gondii strain lacking Ku80. Eukaryot Cell. 2009;8:530–539. doi: 10.1128/EC.00358-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh MH, Rabenau KE, Harper JM, Beatty WL, Sibley LD, Carruthers VB. Rapid invasion of host cells by Toxoplasma requires secretion of the MIC2-M2AP adhesive protein complex. EMBO J. 2003;22:2082–2090. doi: 10.1093/emboj/cdg217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquier N, Schneiter R. Mechanisms of sterol uptake and transport in yeast. J Steroid Biochem Mol Biol. 2012;129:70–78. doi: 10.1016/j.jsbmb.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Joyce CW, Shelness GS, Davis MA, Lee RG, Skinner K, Anderson RA, Rudel LL. ACAT1 and ACAT2 membrane topology segregates a serine residue essential for activity to opposite sides of the endoplasmic reticulum membrane. Mol Biol Cell. 2000;11:3675–3687. doi: 10.1091/mbc.11.11.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lige B, Jayabalasingham B, Zhang H, Pypaert M, Coppens I. Role of an ancestral d-bifunctional protein containing two sterol-carrier protein-2 domains in lipid uptake and trafficking in Toxoplasma. Mol Biol Cell. 2009;20:658–672. doi: 10.1091/mbc.E08-05-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lige B, Romano JD, Bandaru VV, Ehrenman K, Levitskaya J, Sampels V, et al. Deficiency of a Niemann-Pick, type C1-related protein in Toxoplasma is associated with multiple lipidoses and increased pathogenicity. PLoS Pathog. 2011;7:e1002410. doi: 10.1371/journal.ppat.1002410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar J, Striepen B. Make it or take it: fatty acid metabolism of apicomplexan parasites. Eukaryot Cell. 2007;6:1727–1735. doi: 10.1128/EC.00255-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar J, H. Wilson E, Masek K, A. Hunter C, Striepen B. Apicoplast fatty acid synthesis is essential for organelle biogenesis and parasite survival in Toxoplasma gondii. Proc Natl Acad Sci U S A. 2006;103:13192–13197. doi: 10.1073/pnas.0603391103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiner VL, Cases S, Myers HM, Sande ER, Bellosta S, Schambelan M, et al. Disruption of the acyl-CoA:cholesterol acyltransferase gene in mice: evidence suggesting multiple cholesterol esterification enzymes in mammals. Proc Natl Acad Sci USA. 1996;93:14041–14046. doi: 10.1073/pnas.93.24.14041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DJ. The dynamic roles of intracellular lipid droplets: from archaea to mammals. Protoplasma. 2012;249:541–585. doi: 10.1007/s00709-011-0329-7. [DOI] [PubMed] [Google Scholar]
- Nagel SD, Boothroyd JC. The major surface antigen, P30, of Toxoplasma gondii is anchored by a glycolipid. J Biol Chem. 1989;264:5569–5574. [PubMed] [Google Scholar]
- Nishikawa Y, Quittnat F, Stedman TT, Voelker DR, Choi JY, et al. Host cell lipids control cholesteryl ester synthesis and storage in intracellular Toxoplasma. Cell Microbiol. 2005;7:849–867. doi: 10.1111/j.1462-5822.2005.00518.x. [DOI] [PubMed] [Google Scholar]
- Ohshiro T, Tomoda H. Isoform-specific inhibitors of ACATs: recent advances and promising developments. Future Med Chem. 2011;3:2039–2061. doi: 10.4155/fmc.11.158. [DOI] [PubMed] [Google Scholar]
- Palacpac NM, Hiramine Y, Seto S, Hiramatsu R, Horii T, Mitamura T. Evidence that Plasmodium falciparum diacylglycerol acyltransferase is essential for intraerythrocytic proliferation. Biochem Biophys Res Commun. 2004b;321:1062–1068. doi: 10.1016/j.bbrc.2004.07.070. [DOI] [PubMed] [Google Scholar]
- Palacpac NM, Hiramine Y, Mi-ichi F, Torii M, Kita K, Hiramatsu R, et al. Developmental-stage-specific triacylglycerol biosynthesis, degradation and trafficking as lipid bodies in Plasmodium falciparum-infected erythrocytes. J Cell Sci. 2004a;117:1469–1480. doi: 10.1242/jcs.00988. [DOI] [PubMed] [Google Scholar]
- Quittnat F, Nishikawa Y, Stedman TT, Voelker DR, Choi JY, et al. On the biogenesis of lipid bodies in ancient eukaryotes: synthesis of triacylglycerols by a Toxoplasma DGAT1-related enzyme. Mol Biochem Parasitol. 2004;138:107–122. doi: 10.1016/j.molbiopara.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan S, Docampo MD, Macrae JI, Pujol FM, Brooks CF, van Dooren, et al. Apicoplast and endoplasmic reticulum cooperate in fatty acid biosynthesis in apicomplexan parasite Toxoplasma gondii. J Biol Chem. 2012;287:4957–4971. doi: 10.1074/jbc.M111.310144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos DS, Donald RGK, Morissette NS, Moulton ALC. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 1994;45:27–63. doi: 10.1016/s0091-679x(08)61845-2. [DOI] [PubMed] [Google Scholar]
- Sandager L, Gustavsson MH, Stahl U, Dahlqvist A, Wiberg E, Banas A, et al. Storage lipid biosynthesis is non-essential in yeast. J Biol Chem. 2002;277:6478–6482. doi: 10.1074/jbc.M109109200. [DOI] [PubMed] [Google Scholar]
- Sibley LD, Niesman IR, Parmley SF, Cesbron-Delauw MF. Regulated secretion of multi-lamellar vesicles leads to formation of a tubulo-vesicular network in host-cell vacuoles occupied by Toxoplasma gondii. J Cell Sci. 1995;108:1669–1677. doi: 10.1242/jcs.108.4.1669. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartne FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Soldati D, Boothroyd JC. Transient transfection and expression in the obligate intracellular parasite Toxoplasma gondii. Science. 1997;260:349–352. doi: 10.1126/science.8469986. [DOI] [PubMed] [Google Scholar]
- Sonda S, Ting LM, Novak S, Kim K, Maher JJ, Farese RV, Jr, et al. Cholesterol esterification by host and parasite is essential for optimal proliferation of Toxoplasma gondii. J Biol Chem. 2001;276:34434–34440. doi: 10.1074/jbc.M105025200. [DOI] [PubMed] [Google Scholar]
- Vielemeyer O, McIntosh MT, Joiner KA, Coppens I. Neutral lipid synthesis and storage in the intraerythrocytic stages of Plasmodium falciparum. Mol Biochem Parasitol. 2004;135:197–209. doi: 10.1016/j.molbiopara.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Wilcox LJ, Balderes DA, Wharton B, Tinkelenberg AH, Rao G, Sturley SL. Transcriptional profiling identifies two members of the ATP-binding cassette transporter superfamily required for sterol uptake in yeast. J Biol Chem. 2002;277:32466–32472. doi: 10.1074/jbc.M204707200. [DOI] [PubMed] [Google Scholar]
- Zweytick D, Leitner E, Kohlwein SD, Yu C, Rothblatt J, Daum G. Contribution of Are1p and Are2p to steryl ester synthesis in the yeast Saccharomyces cerevisiae. Eur J Biochem. 2000;267:1075–1082. doi: 10.1046/j.1432-1327.2000.01103.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.