Abstract
Attention-deficit/hyperactivity disorder (ADHD) is a prevalent psychiatric disorder that has poor long-term outcomes and remains a major public health concern. Recent theories have proposed that ADHD arises from alterations in multiple neural pathways. Alterations in reward circuits are hypothesized as one core dysfunction, leading to altered processing of anticipated rewards. The nucleus accumbens (NAcc) is particularly important for reward processes; task-based fMRI studies have found atypical activation of this region while the participants performed a reward task. Understanding how reward circuits are involved with ADHD may be further enhanced by considering how the NAcc interacts with other brain regions. Here we used the technique of resting-state functional connectivity MRI (rs-fcMRI) to examine the alterations in the NAcc interactions and how they relate to impulsive decision making in ADHD. Using rs-fcMRI, this study: examined differences in functional connectivity of the NAcc between children with ADHD and control children; correlated the functional connectivity of NAcc with impulsivity, as measured by a delay discounting task; and combined these two initial segments to identify the atypical NAcc connections that were associated with impulsive decision making in ADHD. We found that functional connectivity of NAcc was atypical in children with ADHD and the ADHD-related increased connectivity between NAcc and the prefrontal cortex was associated with greater impulsivity (steeper delayed-reward discounting). These findings are consistent with the hypothesis that atypical signaling of the NAcc to the prefrontal cortex in ADHD may lead to excessive approach and failure in estimating future consequences; thus, leading to impulsive behavior.
Keywords: Attention Deficit Hyperactivity Disorder, reward, nucleus accumbens, fMRI, delay discounting, functional connectivity
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a prevalent and persistent psychiatric disorder, which emerges early in childhood (American Psychiatric Association, 2000). The disorder is estimated to affect about 5% of the population world-wide (Polanczyk et al., 2007), is associated with several comorbid disorders, has poor long-term outcomes, and remains a major public health concern (Rommelse et al., 2009). A primary concern is to better characterize neural mechanisms related to ADHD so as to guide our understanding of pathophysiology.
ADHD heterogeneity
Until recently, causal and mechanistic models of ADHD have centered on identifying a single core dysfunction; in other words, many studies are designed with the premise of the existence of an individual major dysfunction. Thus, investigators typically compare a group of children with ADHD defined by core symptoms (e.g. via DSM criteria) to a group of control children without the disorder. Statistical group differences based on psychometrics, functional brain imaging, or genetics are then used to inform causal models of ADHD.
One necessary assumption of this approach is that ADHD (or even its clinical subtypes) represents a homogenous patient population. However, there is now considerable justification suggesting that multiple developmental and pathophysiological pathways inform ADHD symptomatology (Fair et al., 2012; Nigg et al., 2004; Sonuga-Barke et al., 2003). The implications of this premise are considerable. Including multiple etiologically distinct subgroups as a unitary sample in any study is likely to produce muted effects and could be why only modest effect sizes are often seen for large genetic and behavioral studies, and why neuroimaging studies yield difficult to replicate effects (Hyman, 2007). Of course, overcoming the limitations of conventional research in mental disorders is not straightforward and may require new approaches to understanding complex disease (Hyman, 2007). Nevertheless, understanding the neurobiology underlying the disease phenotypes is essential for improving treatment and consequently for the wellbeing of individuals with ADHD.
A two-step approach could be considered, that might include, first, a traditional group comparison of a group of children with ADHD to a group of control children to find brain connections that are atypical in the disorder. This approach may identify connections that are on average atypical in ADHD, even if the effects are not detectible or expected in all ADHD subjects (Nigg et al., 2005; Sonuga-Barke et al., 2003). However, this approach would also not clarify to what component of the disorder an atypical connection is related. Therefore, this conventional approach could then be followed, as a second step, by a dimensional method that would identify how those brain connections relate to specific endophenotypes even if they are not atypical in all subjects with the disease. In this sense, one might be able to identify neural circuits related to the etiology of relatively homogeneous component behaviors. In this report we apply this multi-level approach to elucidate the mechanisms of atypical reward processing in ADHD. The described approach does not rely on DSM-IV defined subgroups; instead, it focuses on a narrower behavioral domain central to ADHD – reward valuation (Sagvolden et al., 2005).
The role of impaired reward processing
Recent theories have proposed that ADHD arises from alterations in multiple neural pathways (Nigg and Casey, 2005; Sonuga-Barke, 2005). Nigg and Casey (2005) theorized that impairment in cognitive control circuits would contribute to executive dysfunctions, while impairment in affective and reward systems would lead to altered signaling of rewards and consequently to atypical approach and avoidance behaviors. They hypothesized that individuals with ADHD fail to estimate future consequences (future reward or non-reward), and thus exhibit behavior characterized by excessive approach (Nigg and Casey, 2005). Their idea is broadly similar to that of Sonuga-Barke (2005), who hypothesized that two pathways are involved in ADHD: (1) changes within the executive circuit, resulting in executive/inhibitory deficits, and (2) alterations in the reward circuit, resulting in delay aversion (Sonuga-Barke, 2005).
One region that seems to be particularly important for motivational/reward processes is the nucleus accumbens (NAcc), which has been relatively under-researched in ADHD, despite strong theoretical interest in the involvement of motivational/reward processes in the disorder. Functional MRI studies have concluded that the main brain regions recruited while subjects experienced appropriate rewarding stimuli (e.g., money or positive feedback) include the ventral striatum – or NAcc (McClure et al., 2004b). In addition, functional MRI studies in ADHD have found decreased NAcc activation during reward anticipation (Plichta et al., 2009; Scheres et al., 2007).
To examine the interactions between NAcc and other regions of the brain and how these interactions relate to reward processing, we used resting-state functional connectivity MRI (rs-fcMRI). Rs-fcMRI assesses spontaneous intrinsic correlated blood oxygen level dependent (BOLD) activity while subjects are not performing a specific task (Biswal et al., 1995; Fair et al., 2007; Fox and Raichle, 2007). It is based on the discovery that, at rest, functionally connected brain regions display correlated spontaneous low-frequency (<~0.1 Hz) BOLD signal fluctuations (Biswal et al., 1995). Using rs-fcMRI it is possible to identify interactions between regions by cross correlating the time series of a region of interest (or seed region) with all other voxels in the brain. This technique allows the assessment of the intrinsic functional organization of the brain, by determining which voxels are `functionally connected' to the seed region, without many issues inherent to task-related fMRI, such as variability in subject's performance/effort (Fair et al., 2007). Rs-fcMRI has been used to study brain functional connectivity in ADHD (Castellanos et al., 2008; Fair et al., 2010), but studies focusing on cortical-subcortical connections remain infrequent.
We used this analysis technique to assess alterations in functional connectivity of NAcc associated with ADHD and the relationship between these alterations and the degree to which larger, delayed rewards are discounted. Children with ADHD are hypothesized to have a steeper discounting gradient than typically developing children (Sagvolden et al., 2005), which was recently reported by our group (although in that study, modulated by IQ) (Wilson et al., 2011). Task fMRI studies have found the involvement of the NAcc on performing a delay discounting task (Ballard and Knutson, 2009; McClure et al., 2004a). We aimed to: (1) examine the differences between children with ADHD and typically developing children in functional connectivity of the NAcc; (2) define which NAcc connections are correlated with discounting of delayed rewards; and (3), finally, identify which of the delay gradient related-connections are altered in children with ADHD.
Considering the central role of NAcc on reward processing and the evidence of atypical reward-related behaviors in individuals with ADHD, it was hypothesized that functional connectivity of NAcc may be atypical in ADHD and that impulsive decision making (represented by steeper discounting gradient) may be related to the strength of specific connections from the NAcc.
To elucidate this hypothesis we subdivided this study into three sections: (1) we assessed the functional connectivity of the NAcc in children with and without ADHD and performed a group comparison; (2) we then correlated, in a sample of children who performed a delay discounting task, the functional connectivity of NAcc with impulsivity, as measured by the task; and finally, (3) we combined these 2 initial segments to identify the NAcc connections that were associated with impulsive decision making in ADHD.
2. Experimental procedures
Participants and measures
We recruited children 7 to 12 years old, with and without ADHD. Informed written consent or assent was obtained for all participants, and procedures complied with the Human Investigation Review Board at OHSU. Our analysis consisted of 3 sections, thus more details about the samples analyzed in each section are provided below.
Psychiatric diagnoses were based on evaluations with the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS-E; (Orvaschel, 1995)) administered to a parent; parent and teacher Conners' Rating Scale-3rd Edition (Conners, 2008), ADHD Rating Scale (DuPaul et al., 1998) and Strengths and Difficulties Questionnaire (Goodman, 1997); and a clinical review by a child psychiatrist and a neuropsychologist who had to independently agree on the diagnosis. Intelligence was estimated with a reliable and valid three-subtest short form (Block Design, Vocabulary, and Information) of the Wechsler Intelligence Scale for Children, Fourth Edition (Wechsler, 2003).
Children were excluded if they did not meet criteria for ADHD or non-ADHD groups (i.e. children deemed sub-threshold by the clinicians were excluded). Children were also excluded if parent report identified a history of neurological illness, chronic medical problems, sensorimotor handicap, autistic disorder, mental retardation, or significant head trauma (with loss of consciousness), or if they had evidence of psychotic disorder or bipolar disorder on the structured parent psychiatric interview. Children prescribed psychotropic medications other than stimulants were excluded and children prescribed stimulant medications were scanned after a minimum washout of five half-lives (i.e. 24-48 h depending on the preparation). Additional exclusion criteria for control children were: presence of conduct disorder, major depressive disorder, as well as presence of ADHD. Only right-handed children were included in the study.
The analysis was subdivided into 3 sections and slightly different groups of subjects participated in each type of analysis. The first section consisted of a comparison analysis between children with ADHD combined type (n=35, mean age = 9.57, % female = 22.9) and controls (n=64, mean age = 9.21, % female =39.1). See Table 1.A.
Table 1.
Participant characteristics. Table 1.A displays characteristics of participants involved in the comparison analysis (section 1). This section included 35 children with ADHD combined type (ADHD-C) and 64 control children. Table 1.B displays characteristics of participants involved in the correlation analysis (section 2), which included 52 children with ADHD and 70 children without ADHD.
| A. Comparison Analysis - Section 1 | |||||
|---|---|---|---|---|---|
|
| |||||
| Variable | Controls |
ADHD-C |
p | ||
| Mean | SD | Mean | SD | ||
| Age | 9.21 | 1.20 | 9.57 | 1.47 | 0.19 |
| IQ | 116.91 | 12.82 | 109.24 | 14.93 | 0.01 * |
| Movement (RMS) | 0.41 | 0.32 | 0.43 | 0.34 | 0.81 |
| Frame-to-frame displacement (FD) | 0.28 | 0.22 | 0.30 | 0.25 | 0.63 |
| ln(k) | −3.87 | 1.94 | −2.87 | 2.59 | 0.07 ** |
|
| |||||
| N | % | N | % | ||
|
| |||||
| Gender | |||||
| Male | 39 | 60.94 | 27 | 77.14 | |
| Female | 25 | 39.06 | 8 | 22.86 | |
| B. Correlation Analysis - Section 2 | |||||
|---|---|---|---|---|---|
|
| |||||
| Variable | Controls |
ADHD |
p | ||
| Mean | SD | Mean | SD | ||
| Age | 9.00 | 1.21 | 9.63 | 1.35 | 0.01 * |
| IQ | 117 | 13.08 | 110.80 | 15.51 | 0.02 * |
| Movement (RMS) | 0.40 | 0.33 | 0.47 | 0.32 | 0.27 |
| Frame-to-frame displacement (FD) | 0.27 | 0.23 | 0.32 | 0.21 | 0.22 |
| ln(k) | −3.77 | 2.06 | −2.97 | 2.83 | 0.09 *** |
|
| |||||
| N | % | N | % | ||
|
| |||||
| Gender | |||||
| Male | 34 | 48.57 | 38 | 73.08 | |
| Female | 36 | 51.43 | 14 | 26.92 | |
| ADHD subtype | |||||
| Combined | - | - | 30 | 57.69 | |
| Inattentive | - | - | 21 | 40.38 | |
| Hyperactive | - | - | 1 | 1.92 | |
SD, standard deviation; IQ, Full-Scale IQ; ln(k), natural log of the discounting gradient (k).
indicates p value < 0.05;
p value = 0.027, when adjusting for age;
p value = 0.015, when adjusting for age.
For the second section, we correlated the indices of discounting of delayed rewards with strength of NAcc connectivity using 122 participants who had performed a delay discounting task and had data considered systematic. The sample was comprised of typically developing children and children with ADHD (combined type, predominantly inattentive, or predominantly hyperactive type). We included all children (control children and all ADHD subtypes) in this analysis, as greater variation (more heterogeneity) in the delay-discounting indices is more appropriate for dimensional relationships (Hyman, 2007). See Table 1.B. Just as a note, 30 children with ADHD (all combined type) and 55 controls overlapped between sections 1 and 2.
Section 3 was a combination of sections 1 and 2; therefore no new participants were included in this section.
Delay discounting task
For the second section of the study, the strength of functional connectivity between NAcc and every other voxel in the brain was correlated with indices of the delay discounting task. The task measures impulsive choice by evaluating the intolerance to delay-of-gratification, or delay-discounting, proposed to be a core dysfunction in ADHD (Sagvolden et al., 1998; Sonuga-Barke, 2005). 133 children were presented a computerized task also utilized by Wilson and colleagues (Wilson et al., 2011) and based on the task described by Mitchell (1999). The task consisted of 91 questions for which children had to choose between smaller amounts of hypothetical money now and hypothetical $10.00 after a varying delay. Most of the participants completed a task with four hypothetical delays of 7, 30, 90, 180 days (126 subjects); a small number had four different delays of 1, 7, 30, 90 days (5 subjects); and two participants had five delays of 7, 30, 90, 180, 365 days delays. These variations did not affect results as explained later.
The main variable used was the discounting gradient (k). k value represents the rate of discounting of the delayed outcome and was calculated for each subject as described by Mitchell (1999) and Wilson et al. (2011). Greater k values indicate greater preference for immediate rewards. As k values are not normally distributed, a natural log-transformation was applied and the transformed values (ln(k)) were used for the analyses. Although some subjects took a different version of the delay-discounting task, as outlined above, k values (and ln(k)) are not influenced by this variation, so all the individuals were included wherever possible.
Out of the 133 participants who performed the delay discounting task, 11 had to be excluded. Four individuals were excluded because their k values were negative and the log-transformation could not be performed; seven other individuals were excluded because their data were deemed unsystematic (i.e., the data pattern indicated the action of processes other than reward/delay evaluation). Identification of unsystematic data was performed as described in Johnson and Bickel (2008). In summary, a total of 122 participants were included in the correlation analysis (section 2; see Table 1.B).
Analyses were also performed using the area under the empirical discounting curve (AUC), another popular index of discounting closely related to ln(k) (Myerson et al., 2001). Lower AUC values indicate steeper discounting of delayed rewards. The additional analyses yielded the same results. Results using AUC are displayed in the Supplementary Material (Tables S.3 and S.4, Figures S.2, and S.5–S.9).
MRI acquisition
Participants were scanned using a 3.0 Tesla Siemens Magnetom Tim Trio scanner with a twelve-channel head-coil at the OHSU Advanced Imaging Research Center. One high resolution T1-weighted MPRAGE sequence lasting 9 minutes and 14 seconds (TR=2300ms, TE=3.58ms, orientation=sagittal, 256×256 matrix, resolution=1mm3) was collected. BOLD-weighted functional imaging data were collected in an oblique plane (parallel to the ACPC) using T2*-weighted echo-planar imaging (TR=2500ms, TE=30ms, flip angle=90°, FOV=240mm, 36 slices covering the whole brain, slice thickness = 3.8mm, in-plane resolution=3.8×3.8mm). Steady state magnetization was assumed after 5 frames (~10 s). Three runs of 3.5 minutes each were obtained. During rest periods subjects were instructed to stay still, and fixate on a standard fixation-cross in the center of the display.
Imaging preprocessing and movement attenuation
All functional images were preprocessed in the same manner to reduce artifacts (Miezin et al., 2000). These steps included: (i) removal of a central spike caused by MR signal offset, (ii) correction of odd vs. even slice intensity differences attributable to interleaved acquisition without gaps, (iii) correction for head movement within and across runs, and (iv) within-run intensity normalization to a whole brain mode value of 1,000. Atlas transformation of the functional data was computed for each individual via the MPRAGE scan. Each run was then resampled in atlas space (Talairach and Tournoux, 1988), combining movement correction and atlas transformation in one interpolation. All subsequent operations were performed on the atlas-transformed volumetric time series.
Connectivity preprocessing followed prior methods, well described and widely used in the imaging literature (Fair et al., 2007; Fair et al., 2009). These steps included: (i) a temporal band-pass filter (0.009 Hz < f <0.08 Hz), (ii) regression of six parameters obtained by rigid body head motion correction, (iii) regression of the whole brain signal averaged over the whole brain, (iv) regression of ventricular signal averaged from ventricular region of interest (ROI), and (v) regression of white matter signal averaged from white matter ROI. Regression of first order derivative terms for the whole brain, ventricular, and white matter signals were also included in the correlation preprocessing. These preprocessing steps aim to reduce spurious variance unlikely to reflect neuronal activity (Fox and Raichle, 2007).
Participant movement was corrected/quantified using an analysis of head position based on rigid body translation and rotation. The data derived from the adjustments needed to realign head movement on a frame-by-frame basis were calculated as root mean square (RMS) values for translation and rotation in the x, y, and z planes in millimeters. Total RMS values were calculated on a run-by-run basis for each participant. Participants' BOLD runs with movement exceeding 1.5 mm root mean square were not included in the analysis. Movement was low across all subjects (Table 1).
Because our findings could be particularly sensitive to potential movement confounds, additional corrections for movement were applied. Movement generally results in changes in MRI signal. Thus, we also evaluated MRI signal change by comparing each BOLD volume and the preceding volume to exclude volumes with excessive movement (Shannon et al., 2011). An algorithm was used to measure the variance in BOLD signal and exclude the volumes (or frames) whose variation was more than 3 standard deviations above the mean (for details see Shannon et al. (2011)).
Also, to ensure that framewise displacement was not related to our outcome measures, we calculated the mean frame-to-frame displacement (FD) (Power et al., 2012) for the remaining frames and matched our participants, such that there was no significant difference in this variable between controls and children with ADHD. FD was calculated as a scalar quantity using a formula that sums the values for framewise displacement in the six rigid body parameters (FDi=|Δdix|+|Δdiy|+|Δdiz|+|Δαi|+|Δβi|+|Δγi|, where Δdix =d(i−1)x −dix, and similarly for the other five rigid body parameters) (Power et al., 2012). There was no relationship between mean FD and measures of delay discounting of rewards (n=122; r=−0.13; p=0.14), and for the comparison analysis, the groups were matched by mean FD for the remaining frames.
Region of interest definition
Left and right NAcc regions of interest (ROIs) were obtained using FMRIB's Integrated Registration and Segmentation Tool (FIRST), distributed with FMRIB Software Library (FSL). FIRST is a method to do automatic segmentation of subcortical structures, including NAcc, in human brain MRI (Patenaude et al., 2011). Left and right NAcc were combined in one ROI, which was used for our analyses. The ROIs were created in MNI atlas space and then converted to Talairach atlas space. All subsequent operations were performed on the Talairach atlas-transformed NAcc ROI.
Section 1: Direct comparison between children with ADHD and control children
In this section, 64 typically developing children (controls) were compared to 35 participants with ADHD-C. To control for the effects of motion, the groups were matched by mean FD, as explained above. Only combined-type ADHD (ADHD-C) participants were included in the direct comparison, because, given the theorized association between reward processing impairment and impulsive symptoms, the ADHD-C group is expected to present greater impairment in reward processing.
For both ADHD-C group and control group the resting BOLD time series for the NAcc was correlated with all other voxels in the brain, generating voxelwise functional connectivity maps. We performed two-sample two-tailed t-tests on all potential connections (Fisher transformed r-values) to compare ADHD-C children and controls (assuming unequal variance; p ≤ 0.05). The voxelwise approach provides three different maps for the seed region: an ADHD-C map, a control map, and a map representing the direct comparison between the groups. To account for multiple comparisons, thresholding based on Monte Carlo simulation was implemented (Forman et al., 1995). To obtain multiple comparisons corrected, p < 0.05 voxel clusters, a threshold of 53 contiguous voxels with a Z-value > 2.25 was used.
Section 2: Correlation between delay discounting and NAcc functional connectivity
To assess the relationship between functional connectivity of NAcc and delayed-reward discounting gradient, we performed a voxel-wise correlation of the Fisher Z transformed correlation coefficients with ln(k) using an in-house software maintained by Neuro-Imaging Laboratory (Washington University). Monte Carlo simulation was implemented to account for multiple comparisons (threshold of 53 contiguous voxels with a Z-value > 2.25).
Section 3: Identification of regions associated with ADHD and related to delay discounting (combination of sections 1 and 2)
We identified cortical regions from the comparison map that were also correlated with delay discounting performance. In order to do that, we overlapped the comparison map (section 1) and the correlation map (section 2) to identify the NAcc connections that were atypical in ADHD (i.e. regions from the comparison map) and were also related to delay discounting (ln(k)).
This process consisted of two steps. First, peak ROIs were generated from the Monte Carlo corrected comparison maps (section 1). The produced ROIs were pre-blurred 4mm FWHM, with peaks at least 10 mm apart (peaks within 10 mm were consolidated). Second, the peaks generated from the comparison analysis were masked with the results of the correlation analysis (section 2); this way, “overlap” regions were generated. ROIs were created for each region identified (Figure 3).
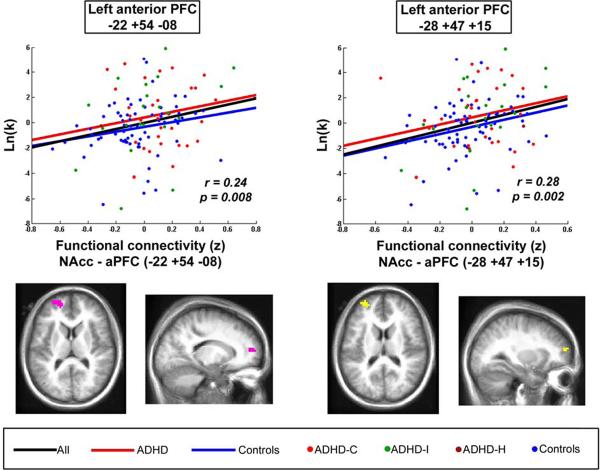
Figure 3. Identification of regions associated with ADHD and related to impulsive decision making (as measured by the delay discounting task).
Volume maps show connections with NAcc that were both, associated with ADHD (from the comparison map – section 1), and correlated with delay discounting gradient (section 2). Results are adjusted for the effects of age and gender. Scatter plots display the correlation. Lines represent the best fitted lines for: all children (black), control children (blue), children with ADHD (red). Dots represent: control subjects (blue), children with ADHD combined type (red), inattentive type (green) and hyperactive/impulsive type (dark red). Results suggest that atypical connection between NAcc and anterior PFC is associated with impulsive decision making in children with ADHD.
Additionally, for each subject, resting-state BOLD time series for the ROIs created were extracted and correlated with the time series for the NAcc. We then performed a partial correlation to characterize the relationship between ln(k) and the connectivity strength between NAcc and the newly generated ROIs, adjusting for the effects of age and gender. Graphs were created to display the partial correlation results.
3 Results
Sample overview
Participant characteristics are summarized in Table 1. Table 1.A summarizes the characteristics of participants involved in the comparison analysis (section 1), where children with ADHD-C and controls were included. Age did not vary across groups, but the proportion of male and female participants was different, as expected, since ADHD is more common among males. Estimated IQ was in the normal range for all the participants, but as it is commonly found, the ADHD group had a lower estimated full-scale IQ than the control group. Additional analysis was performed to verify whether IQ differences might have accounted for the findings (see Section 1 below). For the comparison analysis, as mentioned before, the controls and children with ADHD-C were matched for FD. The groups did not differ significantly on ln(k); however, when controlling for age, controls and ADHD-C were significantly different in ln(k) (p value = 0.027).
Participant characteristics for the correlation analysis (section 2) are summarized in Table 1.B. Children with and without ADHD were included in this section. Age and estimated IQ were again significantly different between control and ADHD groups, and proportion of male and female participants was different between groups. ln(k) was not different when comparing controls and children with ADHD, but when adjusting for age, the difference was statistically significant (p value = 0.015). To examine the effect of age, gender and IQ on the correlations, we performed additional analysis using these variables as covariates; results are discussed in Section 2 below. In this section analysis, the correlations between functional connectivity and delay-discounting were adjusted for the effects of age and gender.
Section 1: Direct comparison between children with ADHD and control children
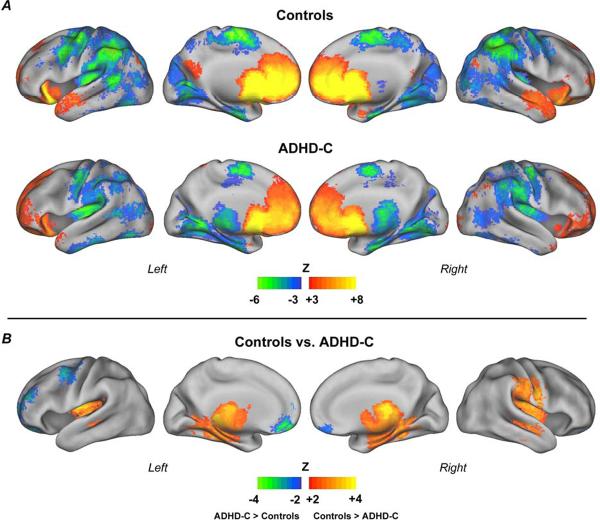
Voxelwise functional connectivity mapping was employed to examine differences in resting-state functional connectivity between children with ADHD combined type (ADHD-C) and typically developing children (controls). The voxelwise maps resulted from the correlation of the BOLD signal time series for the NAcc (segmented using FSL-FIRST) with every other voxel in the brain. This method produced three statistical maps: ADHD-C, controls and direct statistical comparison between groups. The results of the voxel-wise approach are displayed in Figure 1.
Figure 1. Voxelwise resting state functional connectivity maps for the nucleus accumbens (NAcc).
Results for control children and children with combined type ADHD (ADHD-C) (A); and direct comparison between groups (B). Results show atypical functional connectivity of the NAcc in children with ADHD-C. For all analyses Monte Carlo simulation was applied to correct for multiple comparisons (minimum Z>2.25, p<0.05, corrected).
In both groups, a significant positive functional connectivity was observed between NAcc and: prefrontal cortex (e.g., ventromedial prefrontal cortex [vmPFC]) and thalamus, as previously reported in adults (Di Martino et al., 2008). In ADHD-C, the connection between NAcc and portions of the prefrontal cortex was stronger than in controls (i.e. left anterior prefrontal cortex and vmPFC), as evidenced on the between-group difference map (Figure 1).
The NAcc also displayed strong negative connectivity with insula and control regions, such as: anterior cingulate cortex, middle temporal cortex (region MT+) and parietal regions. The ADHD-C group displayed stronger negative connections to the thalamus, to the right inferior parietal lobule (precentral gyrus) and to the posterior insula bilaterally. Controls displayed stronger negative connectivity to a region on the left middle frontal gyrus, near the precentral sulcus. The between-group difference was confirmed by the direct comparison. For a list of all regions found in the comparison analysis, see Supplementary Material, Table S.1.
It is important to note that average IQ score is significantly lower in the ADHD group; therefore one may argue that IQ differences might account for the findings. As poorer performance in IQ test may be interpreted as an inherent characteristic of ADHD (and of other neurodevelopmental disorders), covarying IQ when studying ADHD might be inappropriate as one would be controlling for a component of the disorder (Dennis et al., 2009). However, we recognize that this is not a universal view and, as such, decided to examine the effect of IQ differences on our findings by repeating the comparison analysis after matching for IQ scores. The table with the demographics for the new group is shown in the Supplementary Material, Table S.2. Supplementary Figure S.1 displays the results and shows that lower IQ scores in the ADHD group does not account for our findings.
Section 2: Correlation between delay discounting and NAcc functional connectivity
The second analysis examined the relationship between functional connectivity of NAcc and the delayed reward discounting gradient. Using the NAcc as a seed region, a voxel-wise correlation map was generated as follows: Fisher Z transformed correlation coefficients between the seed region and every other voxel in the brain was correlated with ln(k). The results of this approach were corrected for multiple comparisons and statistically significant correlations (p<0.05) are displayed in Figure 2.
Figure 2. Correlation between functional connectivity of NAcc and delayed-reward discounting gradient.
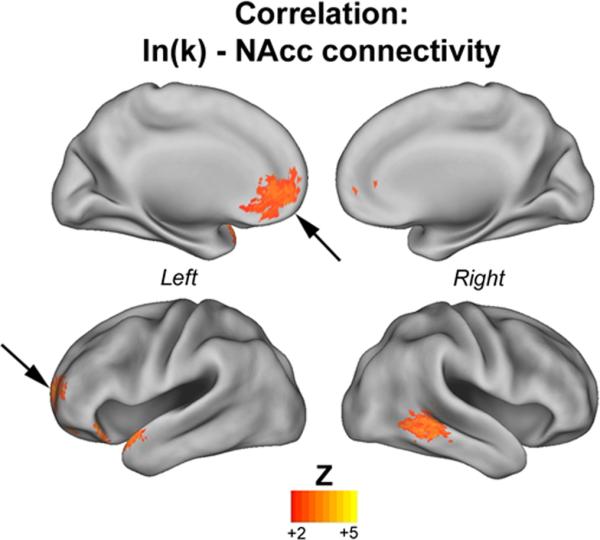
The map displays the correlation between NAcc connectivity and ln(k) – natural log of the discounting gradient (k). Warm colors represent NAcc connections that were positively correlated with ln(k). There were no connections negatively correlated with ln(k). Results show strong correlation between delay discounting gradient and connectivity from the NAcc to the prefrontal cortex (PFC; indicated by the arrows), including the ventromedial PFC and the left anterior PFC. For all analyses Monte Carlo simulation was applied to correct for multiple comparisons (minimum Z>2.25, p<0.05, corrected).
The results showed positive correlation between ln(k) and: (a) NAcc-vmPFC connectivity, (b) NAcc-left anterior PFC connectivity (pointed by the arrow) and (c) NAcc-right middle temporal gyrus connectivity. In other words, an increase in ln(k) (greater impulsivity) was correlated with stronger connectivity between the NAcc and vmPFC and left anterior PFC, and reduced negative connectivity with the right middle temporal gyrus. Table S.3 (Supplementary Material) displays a list of all coordinates.Results for the correlation between area under the discounting curve (AUC) and NAcc functional connectivity are displayed in Figure S.2
In order to investigate the effects of age, gender and IQ on the correlation between NAcc connectivity and delay discounting, we repeated the correlation analysis adjusting for these variables. We first repeated the correlation analysis adjusting for age and sex. The new correlation maps (for lnk: Supplementary Figure S.3; for AUC: Figures S.5 and S.7) also show positive correlation between delay discounting and (or negative correlation between AUC and): (a) NAcc-vmPFC connectivity, (b) NAcc-left anterior PFC connectivity (pointed by the arrow) and (c) NAcc-right middle temporal gyrus connectivity, among other regions. We note that after adding the additional covariates the statistical significance of these findings decreased, and only the correlation with AUC remained after correcting for multiple comparisons (Supplementary Figure S.7); however, the general picture was unchanged suggesting that age and gender have minimal effects on the overall patterns observed.
We also repeated the correlation analysis adjusting for age and IQ scores. The results were very similar to the ones described above, suggesting that IQ scores also have minimal effects on the findings (Supplementary Figures S.4, S.6 and S.8). Results from the correlation without covariates were used for further analyses.
Section 3: Identification of regions associated with ADHD and related to delay discounting (combination of sections 1 and 2)
To evaluate how the changes in functional connectivity of the NAcc in ADHD relate to impulsivity, we combined the comparison map generated in section 1 to the correlation map generated in section 2, identifying only the connections that overlapped and were present in both independent analyses. This procedure enabled us to identify connections associated with ADHD (from the comparison map – section 1) that were also correlated with delay discounting gradient. We found that atypical functional connectivity between the left anterior PFC (aPFC/ Talairach: −22 +54 +08 and −28 +47 +15) and the NAcc in ADHD was associated with ln(k). More specifically, stronger correlation between NAcc and left aPFC was associated with greater ln(k) (i.e. greater impulsivity). It was also found that atypical NAcc connectivity to the vmPFC was correlated to ln(k) (Talairach: −10 +41 −13), but, because the overlap region was very small, it was not displayed in the figure. Figure 3 displays the main results. See Table 2 for all the coordinates.
Table 2.
Peak coordinates for the overlap analysis (section 3). Peak coordinates for regions whose connectivity to the nucleus accumbens was atypical in ADHD-C and, at the same time, related to delay discounting gradient (ln(k)). Structure details were generated with Talairach Client (Lancaster et al, 2000). Peak coordinates are in Talairach space. Regions represented in Figure 3 are in bold.
| Overlap |
|||||
|---|---|---|---|---|---|
| Structure | Peak coordinates | # of voxels | B.A. | ||
| x | y | z | |||
| ln(k) | |||||
| Left Hemisphere | |||||
| Left Medial Frontal Gyrus | −10 | 41 | −13 | 7 | 10 |
| Left anterior PFC (Left Superior Frontal Gyrus) | −22 | 54 | 8 | 34 | 10 |
| Left anterior PFC (Left Middle Frontal Gyrus) | −28 | 47 | 15 | 17 | 10 |
B.A., Brodmann Area; ln(k), natural log of the discounting gradient (k).
Scatter plots were generated to display the correlation between connectivity and delay discounting gradient after controlling for age and gender (Figure 3). The procedure was as follows: BOLD time series for left aPFC regions were correlated with BOLD time series for the NAcc, and the Fisher Z correlation coefficients were then correlated with ln(k). Functional connectivity between the NAcc and aPFC was significantly correlated with ln(k) (left aPFC: −22 +54 +08, R= 0.24, p= 0.008; and −28 +47 +15, R= 0.28, p= 0.002).
2. Discussion
The nucleus accumbens has been recognized as a key neural region involved in reward processes. Because alterations in reward functioning are a major candidate for one of the pathophysiolgical mechanisms in ADHD, NAcc-related connectivity is of central importance to examine in this population. Because the NAcc is part of the reward circuit, the atypical interaction between this region and other regions of the circuit was hypothesized to be implicated in atypical reward processing. In trying to understand the role of the NAcc in the pathophysiology of ADHD, a major question arises: which atypical interactions of the NAcc are associated with reward-related processes in ADHD?
This study was divided into three parts to address that basic question of phenotype versus component phenotype. We started with a broader approach to better illustrate the importance of the NAcc and its connectivity in the neurobiology of ADHD. This was followed by a narrower approach to assess the association between specific NAcc connections and a particular behavioral domain: impulsive decision making, or reward discounting.
The findings of each section lend their own contribution to further understand the neurobiology underlying the ADHD symptomatology and each can stand alone, so we consider them one by one, but we also conclude by considering them together. As an overview, in the first section we found that NAcc is atypically connected to several cortical regions in children with ADHD, including regions previously shown to present atypical correlated spontaneous activity in adults and children with the disorder (Castellanos et al., 2008; Fair et al., 2010; Mills et al., 2012). This is a new finding important in its own right. Then, in sections two and three, we showed that many cortico-NAcc connections play a role in reward valuation, consistent with other studies on the role of this circuit. However, the impairment of only specific connections may be related to pathophysiology of specific behavioral dysfunctions in ADHD. All these findings are consistent with atypical functioning of the NAcc and suggest that it may be linked to a specific behavioral domain (impulsive decision making) as a component phenotype of ADHD.
Traditional approach: comparison between controls and children with ADHD
Our first analysis followed the traditional approach to investigate the pathophysiology of mental disorders, including ADHD. This approach selects a specific dysfunction hypothesized to be important for the disorder and performs a comparison between subjects with and without the disorder, aiming to identify characteristics (e.g. genetic traits, changes in brain connectivity) that are, on average, more common in the group affected with the disorder than in the group of controls. Another important attribute of this approach is that the groups are usually defined based on symptoms that tend to cluster together; as a result, sometimes individuals who have different behavioral traits can be included in the same group if the criteria for the disorder (or lack of disorder) contain their behavioral traits (Hyman, 2007).
We first applied this traditional approach and evaluated how the NAcc spontaneous activity correlates with the rest of the brain in children with ADHD-C compared to children without the disorder. Differences were prominent in the connectivity to the vmPFC. The vmPFC is commonly implicated with reward/motivational processes and was also shown to display atypical functional connectivity in ADHD in previous studies that looked at the integration of the default network in ADHD (Castellanos et al., 2008; Fair et al., 2010). The results, thus, suggest an atypical interaction between the reward circuit and the default network in ADHD. The default network is a set of brain regions proposed to be related to future planning and other self-referential processes (Buckner et al., 2008). These findings suggest an association between internal processes and NAcc functioning, consistent with the idea that altered signaling of rewards from the NAcc to the prefrontal cortex in ADHD would lead to impairment in estimating future consequences and impulsivity (Nigg and Casey, 2005).
The NAcc was also found to be atypically connected to the left anterior PFC (aPFC), which appears to be associated with task-control functions and important for the implementation of plans and strategies for complex tasks, as well as decision making (Dosenbach et al., 2007; Dosenbach et al., 2008). We also found that in the ADHD group, the NAcc was atypically connected to other regions involved with control processes, such as middle frontal gyrus and precentral sulcus (Dosenbach et al., 2007). This may indicate involvement of NAcc with cognitive and attention processes and is consistent with studies that found correlation between NAcc alterations and inattentive symptoms (Depue et al., 2010; Volkow et al., 2009). Furthermore, the altered connectivity between NAcc and regions involved in control processes is indicative of the importance of interplay between control and reward circuits in ADHD, and suggests that an unbalanced interaction between reward circuit and cognitive control circuits might be related to ADHD etiology, consistent with the model proposed by Nigg and Casey (2005).
Altered connectivity was also found in the ADHD group between NAcc and posterior insula, a region implicated in various functions, including emotional processing and sensory-motor processing (Chang et al., 2012), and previously shown to present atypical functioning in ADHD (Valera et al., 2010). Interestingly, an increased connectivity between NAcc and insula was found by Di Martino et al. (2011) in children with autism, a disorder that shares several behavioral characteristics with ADHD.
Another important difference found between ADHD and typically developing children was the connectivity between NAcc and thalamus. As an important hub in the cortico-basal ganglia system, the thalamus is involved with mediating key behaviors – such as reward/motivation, planning and cognition – significant for goal-directed behaviors (Haber and Calzavara, 2009). Atypical patterns of thalamic functional connectivity were identified in children with ADHD and related to dysfunctions in working-memory performance (Mills et al., 2012). The findings of atypical connectivity of this region with the NAcc in the ADHD group might indicate the involvement of the thalamus with atypical reward processes as well, and also suggest an integration between cognitive and reward processes.
In summary, this traditional between-group approach does provide extensive, valuable information about potential ADHD pathophysiology related to the NAcc. However, this approach and its findings also have some inherent limitations. First, since ADHD is a heterogeneous disorder, it is likely that not all the children in the ADHD group present the alterations found here. Second, and most important, we do not know how any given difference relates to specific behavioral components that accompany the ADHD phenotype.
Dealing with heterogeneity of ADHD and other disorders
In sections two and three, we considered the issue of specific behavioral components. Recently, it has been proposed that research in mental disorders, including ADHD, begin to focus on defining the underlying neurobiology of behavior dimensions instead of relying on a classification based on cluster of symptoms (which is currently used) (Hyman, 2007). This new strategy would permit the identification of pathways leading to specific behavioral phenotypes and, consequently, would contribute to better characterize the spectrum of variation in behavioral traits of each disorder (Hyman, 2007). Following this idea, we used a larger sample, for which we had available delay discounting data, to assess how discounting of delayed rewards relates to functional connectivity of NAcc; we then evaluated which atypical connections were associated with impulsive decision making in ADHD.
Before discussing the findings regarding the atypical connections that are associated with impulsive decision in ADHD, it is important to highlight the findings of the correlation analysis examining reward discounting and NAcc connectivity (section 2), because this analysis is novel. Greater ln(k) (i.e. impulsive decision making) was found to be related to the connectivity between NAcc and: vmPFC, aPFC and middle temporal gyrus. Again, the association between increased NAcc-vmPFC connectivity can be linked to the idea that impairment in planning the future and estimating future consequences may lead to impulsive decision making, as the vmPFC is part of a network associated with internal processes such as past recollection and future planning – the default network (Buckner et al., 2008). The middle temporal gyrus and aPFC are regions usually associated with control functions (Dosenbach et al., 2007). As such, their relationship with a key reward region (NAcc) and association with impulsive decision making suggest that a balanced interaction between reward network and cognitive control regions may be important for impulsive control.
Integrating our findings with models of ADHD
Our findings, in general, are in accordance with previous studies that showed the involvement of NAcc in ADHD and the association of impairments in this region with reward processes and impulsivity. Functional MRI studies have determined that some of the main brain regions recruited while subjects experienced appropriate rewarding stimuli (e.g., money or positive feedback) include the ventral striatum (i.e., NAcc), orbitofrontal cortex (OFC) and other areas of PFC (McClure et al., 2004b). In our sample we found that spontaneous correlated activity between NAcc and left aPFC, and NAcc and vmPFC are correlated with indices of delay discounting, that is, the strength of correlation between these regions is associated with greater impulsivity as measured by the delay discounting task.
The findings from the third section, that NAcc-aPFC and NAcc-vmPFC overconnectivity in ADHD is associated with impulsive decision, can be linked to one ADHD theory. That theory hypothesized that overactivity of NAcc and its increased signaling to the prefrontal cortex would lead to excessive approach and failure in estimating future consequences, and as a result, impulsivity (Nigg and Casey, 2005). Functional MRI studies in ADHD have found atypical activation of NAcc and OFC (usally overlapping with vmPFC) during reward anticipation and outcome gain, respectively (Plichta et al., 2009; Scheres et al., 2007). The left aPFC cited above was shown to be part of the cingulo-opercular network and is involved in control functions and decision making (Dosenbach et al., 2007). From this point of view, these results suggest that an atypical interaction between a control network and the reward circuit leads to impulsive decision in ADHD.
The multi-level approach we used in this study enabled us to evaluate a central brain region proposed to be involved in ADHD pathophysiology and also to identify how the atypical functioning of this region in individuals with ADHD may be related to a core, but component, behavioral dysfunction. Because reward discounting is a trans-diagnostic phenotype (related to ADHD but not specifically so), these results can inform ADHD as well as the relation of ADHD to other psychopathologies that also involve reward processes as one of their components.
Our study was premised on the view that ADHD is complex, with multiple behavioral domains being part of its characterization (impulsivity, inattention, hyperactivity, emotional regulation, and executive dysfunctions). Presumably, alterations in these domains can happen alone or in complex combinations, and in various magnitudes. Thus, it is likely that different brain circuits are involved in their neurobiology, and alterations in these circuits may, to some extent, relate to specific behavioral impairments. Therefore, it has become essential to understand how the different involved brain regions or circuits are associated with specific behavioral domains.
One limitation to be discussed is that the ADHD and control groups both had average or higher IQ scores. There are two possible explanations for this finding. The most obvious is some degree of sample self-selection in our University setting (higher-IQ children or higher-IQ parents interested in participating in the study) or selection in who was lost due to motion artifact. We checked this last possibility by correlating IQ scores with movement (measured by mean frame-to-frame displacement), and found that IQ scores and movement were not correlated (r=−0.006, p value=0.95). While the relatively higher-than-average IQ scores of the sample and the supplementary analyses provide reassurance that low IQ scores do not account for the findings, it leaves unclear the extent to which the findings would generalize to a broader or lower functioning ADHD group.
Conclusion and potential future studies
This study demonstrated that resting-state fMRI data can be correlated with behavioral data to better characterize ADHD and, by extension, other mental disorders. Further, we identified NAcc connections that may be involved in the pathophysiology of impulsive decision making in ADHD.
Subsequent connectivity investigations of NAcc and other brain regions should enable the evaluation of other behavioral problems and their underlying neurobiology in ADHD. Future studies might also be able to investigate what behavioral impairments (e.g. executive dysfunctions, emotional regulation) are related to the other atypical connections of NAcc.
The approach presented in this study proved to be a helpful instrument to overcome the limitation of heterogeneity, however, it remains unable to identify subgroups of children based on connectivity or behavioral profile. Therefore, future studies should focus on further characterizing the heterogeneity in ADHD and control populations and identify subgroups of individuals that might present specific patterns of connectivity or behavior that, in turn, may be linked to specific patterns of symptoms, outcomes, or response to treatment.
Supplementary Material
Acknowledgments
We thank Michelle C. Fenesy and Michael S. Blythe for prolific discussions and help with data collection and preparation; and also Colleen Schmitt for coordinating participant scheduling and data collection. We also thank all the participants of the study.
Role of Funding Source: This research was supported by K99/R00 MH091238 (Fair), R01 MH096773 (Fair), R01 MH086654 (Nigg), Oregon and Translational Research Institute (Fair), Medical Research Foundation (Fair, Costa Dias). Support from the National Center for Research Resources (NCRR) through Oregon and Translational Research Institute (Costa Dias), grant number UL1 RR024140, also made this research possible. The funding sources had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Contributors: Author T.C.D. performed the statistical analysis and prepared the manuscript. Authors V.W. and S.M. provided the delay discounting task protocol and helped with the analysis and interpretation of delay discounting data. Authors S.I. and D.B. designed the computational tools for analyzing behavioral and neuroimaging data in combination. Authors K.M., B.T., C.S., S.C. and D.G. helped with neuroimaging data collection, processing and analysis. Author E.M. helped with behavioral data collection, data management and statistical analysis. Author J.N. designed and supervised the behavioral and diagnostic protocol and also supervised data analysis and helped with interpretation. Author D.F. designed the neuroimaging protocol, supervised neuroimaging processing and analysis, supervised all statistical analysis and manuscript preparation, read and revised the manuscript. Author D.F. is also a corresponding author. All authors contributed to and have approved the final manuscript.
Conflict of Interest: All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 2.Ballard K, Knutson B. Dissociable neural representations of future reward magnitude and delay during temporal discounting. Neuroimage. 2009;45:143–150. doi: 10.1016/j.neuroimage.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn.Reson.Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 4.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann.N.Y.Acad.Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 5.Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal B, Sonuga-Barke EJ, Rotrosen J, Adler LA, Milham MP. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol.Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the Role of the Insula in Human Cognition: Functional Parcellation and Large-Scale Reverse Inference. Cereb.Cortex. 2012 doi: 10.1093/cercor/bhs065. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conners CK. Conners 3rd Edition Manual. Multi-Health Systems Inc; Toronto: 2008. [Google Scholar]
- 8.Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. J.Int.Neuropsychol.Soc. 2009;15:331–343. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Depue BE, Burgess GC, Willcutt EG, Bidwell LC, Ruzic L, Banich MT. Symptom-correlated brain regions in young adults with combined-type ADHD: Their organization, variability, and relation to behavioral performance. Psychiatry Res. 2010;182:96–102. doi: 10.1016/j.pscychresns.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Martino A, Kelly C, Grzadzinski R, Zuo XN, Mennes M, Mairena MA, Lord C, Castellanos FX, Milham MP. Aberrant striatal functional connectivity in children with autism. Biol.Psychiatry. 2011;69:847–856. doi: 10.1016/j.biopsych.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: a resting state FMRI study. Cereb.Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- 12.Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn.Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc.Natl.Acad.Sci.U.S.A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DuPaul G, Power T, Anastopoulos A, Reid R. ADHD rating scales-IV: Checklists, norms and clinical interpretation. Guilford Press; New York: 1998. [Google Scholar]
- 15.Fair DA, Bathula D, Nikolas MA, Nigg JT. Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proc.Natl.Acad.Sci.U.S.A. 2012;109:6769–6774. doi: 10.1073/pnas.1115365109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a “local to distributed” organization. PLoS Comput.Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proc.Natl.Acad.Sci.U.S.A. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fair DA, Posner J, Nagel BJ, Bathula D, Dias TG, Mills KL, Blythe MS, Giwa A, Schmitt CF, Nigg JT. Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biol.Psychiatry. 2010;68:1084–1091. doi: 10.1016/j.biopsych.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn.Reson.Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 20.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat.Rev.Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 21.Goodman R. The Strengths and Difficulties Questionnaire: a research note. J.Child Psychol.Psychiatry. 1997;38:581–586. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 22.Haber SN, Calzavara R. The cortico-basal ganglia integrative network: the role of the thalamus. Brain Res.Bull. 2009;78:69–74. doi: 10.1016/j.brainresbull.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyman SE. Can neuroscience be integrated into the DSM-V? Nat.Rev.Neurosci. 2007;8:725–732. doi: 10.1038/nrn2218. [DOI] [PubMed] [Google Scholar]
- 24.Johnson MW, Bickel WK. An algorithm for identifying nonsystematic delay-discounting data. Exp.Clin.Psychopharmacol. 2008;16:264–274. doi: 10.1037/1064-1297.16.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum.Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004a;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 27.McClure SM, York MK, Montague PR. The neural substrates of reward processing in humans: the modern role of FMRI. Neuroscientist. 2004b;10:260–268. doi: 10.1177/1073858404263526. [DOI] [PubMed] [Google Scholar]
- 28.Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- 29.Mills KL, Bathula D, Dias TG, Iyer SP, Fenesy MC, Musser ED, Stevens CA, Thurlow BL, Carpenter SD, Nagel BJ, Nigg JT, Fair DA. Altered cortico-striatal-thalamic connectivity in relation to spatial working memory capacity in children with ADHD. Front.Psychiatry. 2012;3:2. doi: 10.3389/fpsyt.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology (Berl) 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- 31.Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. J.Exp.Anal.Behav. 2001;76:235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nigg JT, Casey BJ. An integrative theory of attention-deficit/ hyperactivity disorder based on the cognitive and affective neurosciences. Dev.Psychopathol. 2005;17:785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- 33.Nigg JT, Goldsmith HH, Sachek J. Temperament and attention deficit hyperactivity disorder: the development of a multiple pathway model. J.Clin.Child.Adolesc.Psychol. 2004;33:42–53. doi: 10.1207/S15374424JCCP3301_5. [DOI] [PubMed] [Google Scholar]
- 34.Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJ. Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes? Biol.Psychiatry. 2005;57:1224–1230. doi: 10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 35.Orvaschel H. Schedule for Affective Disorders and Schizophrenia for School-Aged Children - Epidemiologic Version 5 (K-SADS-E) Nova Southeastern University; Ft. Lauderdale, FL: 1995. [Google Scholar]
- 36.Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plichta MM, Vasic N, Wolf RC, Lesch KP, Brummer D, Jacob C, Fallgatter AJ, Gron G. Neural hyporesponsiveness and hyperresponsiveness during immediate and delayed reward processing in adult attention-deficit/hyperactivity disorder. Biol.Psychiatry. 2009;65:7–14. doi: 10.1016/j.biopsych.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 38.Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am.J.Psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 39.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rommelse NN, Altink ME, Fliers EA, Martin NC, Buschgens CJ, Hartman CA, Buitelaar JK, Faraone SV, Sergeant JA, Oosterlaan J. Comorbid problems in ADHD: degree of association, shared endophenotypes, and formation of distinct subtypes. Implications for a future DSM. J.Abnorm.Child Psychol. 2009;37:793–804. doi: 10.1007/s10802-009-9312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sagvolden T, Aase H, Zeiner P, Berger D. Altered reinforcement mechanisms in attention-deficit/hyperactivity disorder. Behav.Brain Res. 1998;94:61–71. [PubMed] [Google Scholar]
- 42.Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav.Brain Sci. 2005;28:397–419. doi: 10.1017/S0140525X05000075. discussion 419–68. [DOI] [PubMed] [Google Scholar]
- 43.Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biol.Psychiatry. 2007;61:720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 44.Shannon BJ, Raichle ME, Snyder AZ, Fair DA, Mills KL, Zhang D, Bache K, Calhoun VD, Nigg JT, Nagel BJ, Stevens AA, Kiehl KA. Premotor functional connectivity predicts impulsivity in juvenile offenders. Proc.Natl.Acad.Sci.U.S.A. 2011;108:11241–11245. doi: 10.1073/pnas.1108241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sonuga-Barke EJ. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol.Psychiatry. 2005;57:1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 46.Sonuga-Barke EJ, Dalen L, Remington B. Do executive deficits and delay aversion make independent contributions to preschool attention-deficit/hyperactivity disorder symptoms? J.Am.Acad.Child Adolesc.Psychiatry. 2003;42:1335–1342. doi: 10.1097/01.chi.0000087564.34977.21. [DOI] [PubMed] [Google Scholar]
- 47.Talairach J, Tournoux P. Thieme Medical Publishers. New York: 1988. Co-Planar Stereotaxic Atlas of the Human Brain. [Google Scholar]
- 48.Valera EM, Spencer RM, Zeffiro TA, Makris N, Spencer TJ, Faraone SV, Biederman J, Seidman LJ. Neural substrates of impaired sensorimotor timing in adult attention-deficit/hyperactivity disorder. Biol.Psychiatry. 2010;68:359–367. doi: 10.1016/j.biopsych.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, Fowler JS, Zhu W, Logan J, Ma Y, Pradhan K, Wong C, Swanson JM. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA. 2009;302:1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wechsler D. Wechsler Intelligence Scale for Children. 4th Edition (WISC-IV®) Harcourt Assessment; San Antonio, TX: 2003. [Google Scholar]
- 51.Wilson VB, Mitchell SH, Musser ED, Schmitt CF, Nigg JT. Delay discounting of reward in ADHD: application in young children. J.Child Psychol.Psychiatry. 2011;52:256–264. doi: 10.1111/j.1469-7610.2010.02347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.