Abstract
Previous studies have reported that T cell deficiency reduced infarct sizes after transient middle cerebral artery (MCA) suture occlusion in mice. However, how reperfusion and different models affect the detrimental effects of T cells have not been studied. We investigated the effects of T cell deficiency in nude rats using two stroke models and compared their infarct sizes with those in WT rats. In the distal MCA occlusion (MCAo) model, the distal MCA was permanently occluded and the bilateral common carotid arteries (CCAs) were transiently occluded for 60 min. In the suture MCAo model, the MCA was transiently occluded for 100 min by the insertion of a monofilament suture. Our results showed that T cell deficiency resulted in about a 50% reduction in infarct size in the suture MCAo model, whereas it had no effect in the distal MCAo model, suggesting the protective effects of T cell deficiency are dependent on the ischemic model used. We further found more total T cells, CD4 T cells and CD8 T cells in the ischemic brains of WT rats in the suture MCAo model than in the distal MCAo model. In addition, we detected more CD68-expressing macrophages in the ischemic brains of WT rats than in nude rats in the suture MCAo but not the distal MCAo model. Lymphocyte reconstitution in nude rats resulted in larger infarct sizes in the suture MCAo, but not in the distal MCAo stroke model. The results of regional CBF measurement indicated a total reperfusion in the MCAo model but only a partial reperfusion in the distal MCAo model. In conclusion, the protective effects of T cell deficiency on brain injury are dependent on the ischemic model used; likely associated with different degrees of reperfusion.
Keywords: Stroke, focal ischemia, nude rats T cells
1
T lymphocytes have been demonstrated to contribute to brain injury induced by stroke in mice (Yilmaz et al., 2006, Hurn et al., 2007, Liesz et al., 2009, Kleinschnitz et al., 2010). T cells, like macrophages and neutrophils, were found to infiltrate ischemic brains after stroke (Iadecola and Anrather, 2011), suggesting that T cells may form physical contact with brain tissues and directly destroy neurons. In addition, infarct sizes were robustly reduced in immunodeficient mice lacking both T cells and B cells and that the reconstitution of T cells, but not B cells, recovered infarct sizes(Hurn et al., 2007). More recent studies suggest that deficiency of either CD4 or CD8 T cells resulted in smaller infarct size (Yilmaz et al., 2006, Kleinschnitz et al., 2010), while deficiency of regulatory T cells (Treg) led to enlarged, delayed infarct sizes (Liesz et al., 2009). Our laboratory further showed the distinctive roles of T cell subtypes in mice, with reduced-infarction in Th1 deficient mice and increased-infarction in Th2 deficient mice (Gu et al., 2012). These studies that suggest a critical role of T cells in stroke-induced brain injury were performed mostly in the mouse suture MCAo model where withdrawal of the suture led to total reperfusion.
Like other neuroprotectants, the protective effects of T cell deficiency should be confirmed in multiple models using different species and strains to avoid potential bias, as stated by the guidelines of Good Laboratory Practice (Macleod et al., 2009). Here we investigated whether T cell deficiency also results in neuroprotection in nude rats and compared their infarct sizes to wild type (WT) Sprague Dawley (SD) rats. We examined in two stroke models infarct sizes, T cells and their subsets in the ischemic brains, CD68-expressing macrophages, and the effects of lymphocyte reconstitution on infarct sizes in nude rats. The two models are the MCA suture occlusion model and the distal MCA occlusion model. The suture MCA occlusion model is induced by transient MCA intraluminal suture occlusion where the suture withdrawal results in complete reperfusion (Zhao et al., 2004).
The distal MCAo model is induced by transient bilateral CCA occlusion combined with permanent distal MCA occlusion (Zhao et al., 2005, Zhao et al., 2006) where release of the bilateral CCA occlusion results in partial reperfusion. This model generally mimics frequent clinical cases in which partial reperfusion occurs, as previous studies report that most spontaneous recanalization after stroke results in partial reperfusion (Neumann-Haefelin et al., 2004), and further, that t-PA treatment leads to partial reperfusion in most stroke patients (Alexandrov et al., 2001).
2. Experimental Procedures
2.1 Animals and stroke models
Experimental protocols were approved by the Stanford University Administrative Panel on Laboratory Animal Care (Protocol #: APLAC 12642), and experiments were conducted in accordance with the guidelines of Animal Use and Care of the National Institutes of Health and Stanford University. All efforts were made to minimize the number of animals used and their suffering. Male nude rats (NCI-Frederick, MD) and male Sprague Dawley (SD) rats (Charles River, MA) were used. Anesthesia was induced by 5% isoflurane and maintained at 1% to 2% isoflurane during surgery in male SD WT rats, and in T cell deficient nude rats (260–320g). In the distal MCAo model, focal cerebral ischemia was generated by occluding the bilateral CCA for 60 min combined with permanent occlusion of the left MCA above the rhinal fissure, as previously described (Zhao et al., 2005, Zhao et al., 2006, Zhao et al., 2007). In the suture MCAo model, the left MCA was occluded by inserting an intraluminal 4-0 nylon monofilament suture through the common carotid artery to the branch point of the MCA for 100 min (Zhao et al., 2004). Core body temperatures were maintained at 36.5–37.2°C throughout the experiments and arterial pO2, pCO2 and pH were controlled in normal ranges. Acute infarct size was measured as described (Zhao et al., 2005, Zhao et al., 2006, Zhao et al., 2007). Rats were euthanized with an overdose of isoflurane 48 h after stroke, perfused with phosphate buffered saline (PBS), and the brains removed. Brains were sectioned coronally at 2-mm intervals, generating a total of 5 sections, which were stained with a 2% solution of 2,3,4-triphenytetrazolium-chloride (TTC). Using a computerized image analysis system (NIH image, version 1.61), the area of infarction was measured at the sides of the inner section. Infarct volumes were integrated with the ischemic area timing the thickness of each slice, and adding all values together from 5 slices of each brain.
2.2. Cerebral blood flow measurement
Relative regional cerebral blood flow (rCBF) was monitored by using a laser Doppler probe (Dinapoli et al., 2006, Zhao et al., 2006, Gao et al., 2008). Briefly, two 1mm diameter holes was drilled to monitor CBF at an ischemic core (1.0mm posterior to the bregma, 4.5mm lateral to the midline) and ischemic margin (2mm posterior to the bregma, 2mm lateral to the midline) in animals subjected to suture middle cerebral occlusion (sMCAO) and distal middle cerebral occlusion (dMCAO). The laser probe with a diameter of 0.5mm was attached to the surface of the brain through the hole to detect CBF values at different time points. CBF values were expressed as percentages relative to the baseline (100%). For sMCAO, CBF was measured 15 min before ischemia onset (base line), during ischemia (every 15 min after stroke onset), and immediately, 15 min and 30 min after reperfusion. For dMCAO, CBF was measured at 15 min before CCA occlusion, immediately after CCA occlusion, immediately after MCA occlusion, during ischemia (every 15 min after stroke onset), and immediately, 15 and 30 min after CCA reperfusion.
2.3. Moderate hypothermia
The ischemic model of distal MCAo generates infarction only in the cortex, which is much smaller than that in the MCA suture occlusion model. There is a concern that T cell deficiency in nude rats does not reduce infarction because the infarction is too small to be reduced. Therefore, we used hypothermia as a positive control to show that infarction can still be robustly reduced by a neuroprotectant in this distal MCA occlusion model. Moderate hypothermia (30°C) was induced by spraying 100% alcohol on the rat body; temperature was controlled by a heating pad underneath the rat combined with an overhead light over the rat, as described previously (Zhao et al., 2005). Body temperature was adjusted to 30±0.5°C (at 10 min before ischemia onset) and maintained for 1 h during CCA occlusion. After CCA release, the wound was sutured and body temperature was increased to 37±0.5°C.
2.4. Brain cell isolation and FACS analysis
Brains were collected and cell isolations were conducted as described previously (Lim et al., 2011). In brief, rats were euthanized with an overdose of isoflurane 72 h after stroke onset and perfused with 200 ml cold PBS. The ischemic and contralateral cortex in the distal MCAo rats and ischemic and contralateral hemisphere in suture MCAo rats were harvested in FACS buffer (F-PBS, PBS with 1% FBS and 0.1% sodium azide) in a tissue culture plate, and homogenized by the plunger rubber of a 3 ml syringe on ice. The cells were resuspended in 7 ml FACS buffer, 3 ml 90% Percoll in PBS was added and completely mixed, and 1 ml 70% Percoll in PBS was underlaid to the suspension. The suspension was then centrifuged at 2470 rpm for 30 min at 4°C. Leukocytes at the interphase were isolated and washed 3 times in FACS buffer. Cells were filtered through a 70 μm strainer and re-suspended in 1ml FACS buffer and counted. The cells were stained with FITC-conjugated mouse anti-rat TCR (1:100, abD Serotec, Oxford, UK), PE- conjugated mouse anti-rat CD8 (1:100, BD Biosciences, Franklin Lakes, NJ) and Biotin-conjugated mouse anti-rat CD4 (1:100 abD Serotec, Oxford, UK) antibodies at 4°C for 20 min in the dark. To detect CD4, cells were then incubated with a secondary antibody (1 μl streptavidin (SA)-PE-Cy5, abD Serotec, Oxford, UK) on ice for 20 min in the dark. The fluorescence threshold between negative and positive cells was set on the basis of the reactivity of appropriate nonspecific fluorochrome- conjugated isotype controls. Stained samples were acquired on a BD FAC scan using CELLQuest software (Becton Dickinson, San Jose, CA). Data were analyzed using FlowJo version 7.6.2 (Tree Star, Ashland, OR) and the results were normalized by infarct volume.
2.5. Tissue immunofluorescent staining
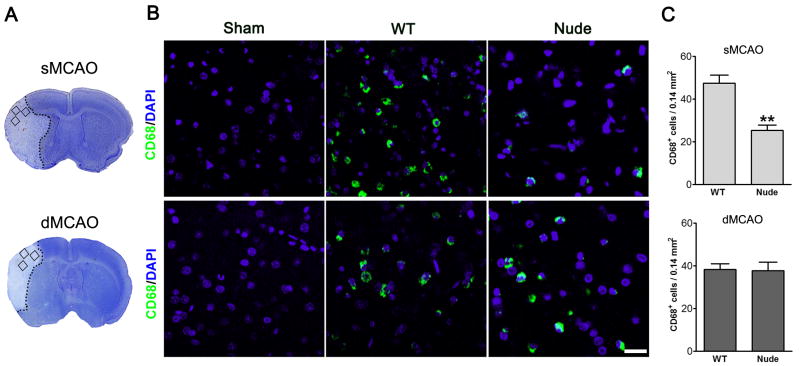
Rats were euthanized with overdose of isoflurane 72 h after stroke onset and perfused with cold PBS, followed by 4% paraformaldehyde in phosphate-buffered saline (PBS) (pH 7.4). The brains were taken and post-fixed for 48 h in 4% paraformaldehyde in PBS (pH 7.4) and cut into 50μm sections. Immunofluorescent staining was carried out on free-floating sections under moderate shaking. All washes and incubations were done in 0.1M PBS (pH 7.4) containing 0.3% triton X-100. Sections were incubated for 1 h with blocking solution (0.1M PBS, 0.3% Triton X-100 and 5% equine serum). After washes, sections were incubated overnight at 4°C with mouse anti-rat primary antibody for CD68 (diluted 1:200; MCA341GA, AbD Serotec, Oxford, UK), a marker for reactive macrophages/microglia, Sections were then rinsed and incubated for 2 h at room temperature with an Alexa 488-conjugated goat anti-mouse (diluted 1:200, Invitrogen, Carlsbad, CA). The sections were washed and mounted on glass slides using Vectashield mounting medium with 4′, 6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA). A negative control without primary antibodies was performed in parallel. CD68 expression was investigated with a Zeiss Axiovert inverted epifluorescence microscope (Zeiss LSM510, Germany) covering a total of 0.14 mm2. For each animal, 3 sections (0.20 to 0.70 mm rostral to the Bregma) were randomly chosen, from which the number of CD68-expressing cells in predefined areas (Figure 3) was counted using Image J software (NIH), and an average for each animal was calculated. All counts were performed blindly to the investigator on coded sections.
Fig. 3.
Macrophage activities differ between WT and nude rats treated with MCA suture occlusion model but not with distal MCA occlusion model. A. Representative infarction in the MCA suture occlusion model and the distal MCA occlusion model. The squares represent the areas where positive CD68 staining cells were counted. B. Representative immunostaining of CD68 in sham, WT and nude rats. Stroke brains were prepared 3 d post-stroke. C. The bar graphs show the statistic results of cell counting for CD 68 positive cells. ** P<0.01, vs WT. N=4–5 each group. Scale bar, 50 μM.
2.6. Isolation of lymphocyte from spleen and T cell reconstitution in rats
Naive SD rats were deeply anesthetized and euthanized with an overdose of isoflurane. Spleens were collected, chopped into small pieces, and homogenized by the plunger rubber of a 3-cc syringe on ice. The cells were filtered through a 70μm strainer. Erythrocytes were lysed by ACK Lysing buffer (GIBCO, Invitrogen, Carlsbad, CA). RPMI 1640 (Invitrogen, Carlsbad, CA) was added to stop the lyses reaction and the cells were centrifuged at 1200rpm for 5 min at room temperature. The cell pellet was re-suspended in 20ml RPMI 1640 and counted using a hemocytometer. The cell concentration was adjusted to 108/ml in RPMI 1640 and 100 μl was immediately administered to the nude rats intravenously via the external jugular vein. Stroke was induced 24 h after reconstitution.
2.7. Statistical Analyses
Data is expressed as mean ± SEM. Differences were considered statistically significant for p <0.05. Student’s t-tests were used when two groups were compared. Two-way ANOVAs were used for CBF measurement statistical analysis, followed by Bonferroni post-tests using Prism 5 (GraphPAD Software for Science, San Diego, CA).
3. Results
3.1. T cell deficiency resulted protection in MCA suture occlusion model but not in distal MCA occlusion model in rats
Stroke induced by distal MCAo resulted in a well-defined cortical infarction, whereas suture MCAo generated infarction in both the cortex and striatum (Fig. 1). In the distal MCAo model, infarct sizes measured at 2 d post-stroke did not differ among SD rats and nude rats. In the suture MCAo model, however, infarct sizes in nude rats were only half of those in SD rats (Fig. 1). Thus, T cell deficiency resulted in reduced-infarct sizes in the suture MCAo model, but not in the distal MCAo model.
Fig. 1.
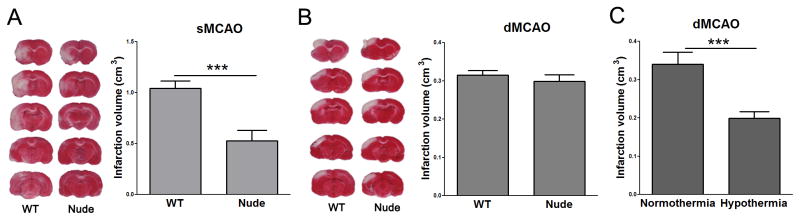
Infarct sizes in WT rats and T cell deficient nude rats. Stroke was generated in two models and animals were euthanized two days post-stroke for infarction measurement. A. Suture occlusion model. The representative staining of TTC indicates that infarction (the non-stained white region) was generated in both the cortex and striatum in the ischemic hemisphere in the WT rats, but the infracted cortex was largely abolished in the nude rats. The statistical results are presented by the bar graph in the bottom. * P<0.05; *** P<0.001. N=9–11 each group. B. Distal MCA occlusion model. The representative TTC staining indicates cortical infarction (above) and the bar graph stands for average infarct size (bottom). No difference was observed between groups. N=9–11 each group. C. Hypothermia reduced infarction in nude rats receiving distal MCA occlusion. Stroke was generated exactly the same as in B. Intra-ischemic hypothermia (30°C) reduced infarction compared with normothermia (37°C). * P<0.05; *** P<0.001, N= 10/group.
Infarction generated in the distal MCAo model is smaller than that in the MCA suture occlusion model, which leads to a concern that T cell deficiency in nude rats did not reduce infarction is because the infarction is too small to be reduced in the distal MCAo model. However, as a positive control, hypothermia could robustly reduce infarction in this distal MCA occlusion model, suggesting the relatively small infarction cannot be counted as the reason for the non-reduced infarction in distal MCA model.
3.2. More infiltrations of T cells in the ischemic brains receiving MCA suture occlusion than distal MCA occlusion in wild type rats
It is possible that the degree of reperfusion affects T cell infiltration, which accounts for the different roles of T cell deficiency in these 2 models. Indeed, FACS analyses showed that the total numbers of T cells, CD4 T cells and CD8 T cells per cm3 in the ischemic brains of WT rats were lower in the distal MCAo model than in the suture MCAo model (Fig. 2).
Fig. 2.
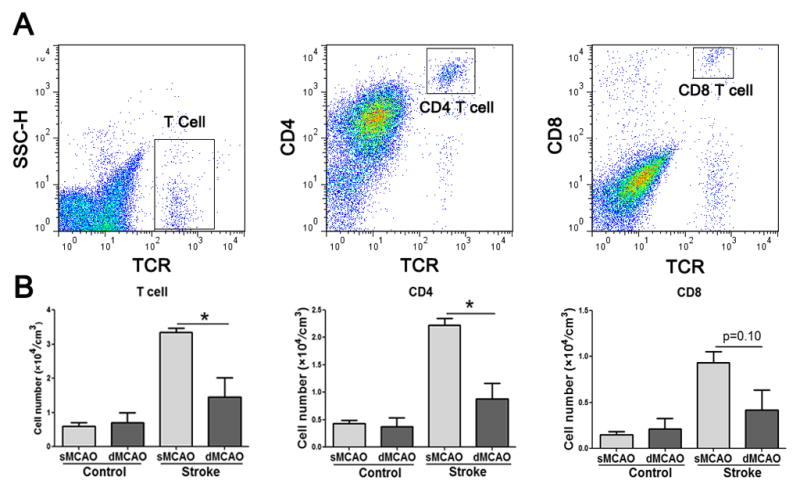
The number of T cells and T cell subsets in the ischemic brains. Ischemic and non-ischemic hemispheres of rat brains were collected 3 d after stroke onset for FACS analyses. A. Representative gating strategy for T cells, CD4 and CD8 T cells in MCA suture occlusion model. B. Average numbers of each cell type normalized by infarct size in the ischemic and non-ischemic hemispheres (MCA suture occlusion model) or cortex (distal MCA occlusion model). *, P<0.05 between indicated two groups. N=4–5 each group.
3.3. Macrophage activities differed between wild type rats and nude rats with MCA suture occlusion but not with distal MCA occlusion
We then examined protein expression of CD68, a molecular marker for macrophage activity (Fig. 3). Immunostaining results showed reduced numbers of CD 68 positive cells in the ischemic brains of nude rats compared with WT rats in the total reperfusion model but not in the partial reperfusion model.
3.4. T cell reconstitution resulted in larger infarct sizes in the MCA suture occlusion model but not in the distal MCA occlusion model
If T cells are responsible for the model-dependent effects of infarct sizes between WT and nude rats, T cell reconstitution in nude rats should result in different outcomes in each model. We used total spleen lymphocytes from WT rats to reconstitute T cells in nude rats and found that T cell reconstitution resulted in larger infarct sizes in thesuture MCAo model but not in the distal MCAo model (Fig. 4).
Fig. 4.
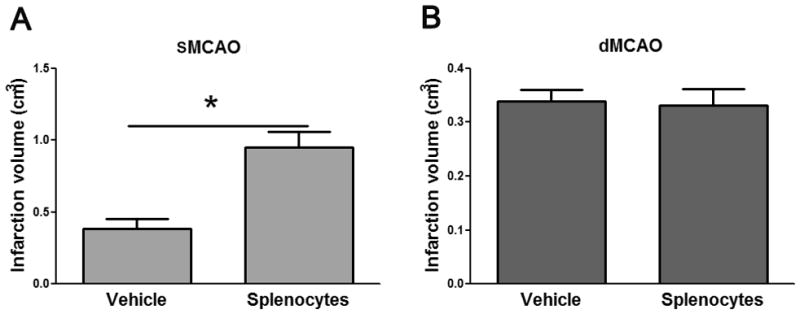
The effects of lymphocyte reconstitution on infarct sizes in nude rats. Splenocytes were harvested from WT rats, and 100 ul of splenocytes (108/ml) were injected via an external jugular vein to each nude rat. Stroke was induced 24h after lymphocyte reconstitution, and infarct sizes were measured 2 d after stroke. A. Infarct volumes in MCA suture occlusion model. B. Infarct sizes in distal MCA occlusion model. * P<0.05, vs vehicle. N=4–5 each group.
3.5. Different degrees of rCBF restoration may be associated with T cell infilatration and its detrimental effect
We hypothesize that the differential protective effects of T cell deficiency in the two models are associated with differences of rCBF restoration after stroke. We therefore measured and compared changes in CBF in these two stroke models (Fig 5). In the MCAO suture occlusion model, rCBF was abruptly decreased to 29±3.20% and 36±4.20 % of the baseline in ischemic core and margin, respectively. Suture withdrawal resulted in CBF restoration to 89±3.99% and 96±2.98% in the ischemic core and margin, respectively. In the dMCAO model, bilateral CCA occlusion reduced rCBF to 44±2.73% in the ischemic core, which is comparable with the levels of 48±5.40% in the ischemic margin. An additional distal MCA occlusion further reduced CBF to 22±1.61% and 29±2.20% in the ischemic core and margin, respectively. After CCA release while dMCA remained occluded, rCBF was increased to 90±5.90% of the baseline in the ischemic margin, but only a slight increase of CBF (42±3.31%) was observed in the ischemic core.
Fig. 5.
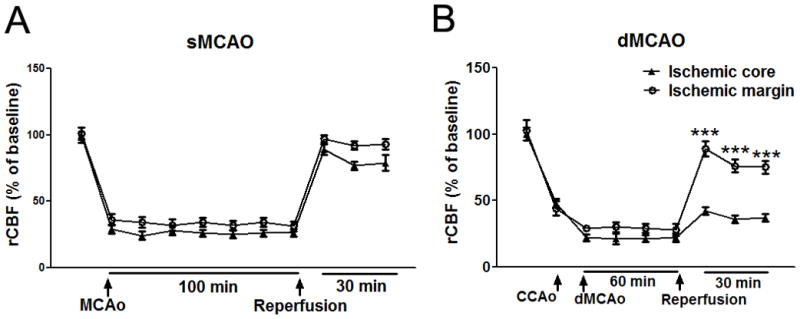
Changes in rCBF in animals subjected to sMCAO and dMCAO. (A) Laser Doppler measurements revealed that MCA suture occlusion reduced rCBF to 29±3.20% in the ischemic core (circle line) and to 36±4.20 % of the baseline in the ischemic margin (triangle line) in rats subjected to sMCAO. After reperfusion, rCBF was sharply restored to 89±3.99% and 96±2.98% of the baseline in the ischemic core and margin, respectively. No significant difference in rCBF was found between the ischemic core and the ischemic margin. (B) In dMCAO model animals, bilateral CCA occlusion resulted in rCBF reduction to 44±2.73% of the baseline in the ischemic core and to 48±5.40% in the ischemic margin. An additional MCA occlusion further induced a secondary reduction in CBF both in the core and margin. With releases of the bilateral CCAs, rCBF was sharply increased to 90±5.90% in the ischemic margin while it was only increased to 42±3.31% of the baseline in the ischemic core. No significant difference was found before and during ischemia, but significant differences were found after reperfusion between ischemic core and ischemic margin. N=5/group; *** P<0.001 vs ischemic core).
4. Discussion
Our study provides the first evidence that the protective effects of T cell deficiency may depend on the ischemic model used. First, infarct sizes induced by distal MCAo in nude rats are comparable to those in WT rats. Infarct sizes induced by suture MCAo in T cell deficient nude rats, however, were half of those in WT rats. Second, greater numbers of total T cells, CD4 T cells and CD8 T cells were in the ischemic brains of WT rats receiving suture MCAo than those receiving distal MCAo. Third, more CD68-expressing macrophages were observed in WT rats undegoing suture MCAo than in nude rats; however, no differences were found in rats undergoing distal MCAo. Fourth, T cell reconstitution in nude rats resulted in larger infarctions in the suture MCAo but not the distal MCAo model. Lastly, we confirm that partial reperfusion occurred in the distal MCAo model while total reperfusion occurred in the suture MCAo model, suggesting that different degrees of CBF restoration may be a factor in determining the differential protective effects of T cell deficiency.
We speculate there are at least 3 mechanisms responsbile for the distinctive effects of T cell deficiency in these two models. First are their different degrees of reperfusion. Suture withdrawal in the suture MCAo model results in complete reperfusion, while permanent occlusion of the MCA in the distal MCAo model results in partial reperfusion (Chen et al., 1986, Gao et al., 2008). Total reperfusion may result in a stronger inflammatory response with increased recruitment of immune cells, including T cells, into the brain (Chan, 1996, Ren et al., 2011). We confirmed that total reperfusion occurred in the suture MCAo model while partial reperfusion occurred in the distal MCAo model. Our results show that fewer T cells infilatrated into the ischemic brains of the partial reperfusion model than in the total reperfusion model, and T cell deficiency resulted in less inflammation in the total reperfusion model but not in the partial reperfusion model. As a result, T cell deficiency may avoid the detrimental effects of the inflammatory response and thus lead to smaller infarctions. In the case of partial reperfusion, the contribution of T cells to the formation of the infarction may be minor, therefore, their absence does not significantly alter infarction size. Second, the location of infarction differs in each model. The area of infarct in the distal MCAo model exclusively affected the cortex, while the suture MCAo model affected both the cortex and striatum. Other studies have suggested that brain lesion sizes correlate with different immune responses (Jankovic et al., 1991, Kang et al., 1991, Kriegsfeld et al., 2003). Lymphocyte response may be dependent on where the infarct occurs. Third, infarct volumes differ. Suture MCAo resulted in a much larger infarct in the whole hemisphere but the distal MCAo infarct is restricted to the cortex. The larger ischemic region may recruit more immune cells after reperfusion, and the infiltrated immune cells further worsen the infarct. Therefore, larger infarct sizes are usually accompanied by a stronger inflammatory response, and the detrimental effects of T cells might be less significant in the distal MCAo model. Taken together, the protective effects of T cell deficiency are dependent on the ischemic model used. Nevertheless, we cannot completely exclude other contributing factors to the model-dependent effects as the genetic background of nude rats, exclusive of T cells, may differ from SD WT rats. Given that no difference in infarction was observed between SD and nude rats in the distal MCAo model, however, we believe the protective effects of T cell deficiency after suture MCAo are largely due to model-dependent rather than genetic factors.
Relatively small infarction is generated only in the cortex in the distal MCAo model while a larger infarction is induced in both the cortex and striatum in the suture MCAo model. Thus there is a concern that the infarction might be too small to be reduced in the distal MCAo model by the deficiency of T cells. Nevertheless, we have demonstrated that induced-hypothermia was able to robustly reduce infarct sizes in the distal MCAo model, suggesting the smaller infarct sizes should not be a major reason for the absence of neuroprotection for the lack of T cells in nude rats receiving distal MCA occlusion.
Our finding has high clinical relevence as strategies to inhibit T cell activities are developed for stroke treatment. A large portion of stroke patients may have no reperfusion, and in patients who have spontaneous recanalization after stroke, partial reperfusion occurs in most cases (Neumann-Haefelin et al., 2004). Furthermore, t-PA treatment may also lead to partial reperfusion in most stroke patients (Alexandrov et al., 2001). Given that T cell deficiency in this study did not provide protection in the partial reperfusion model, development of anti-inflammation strategies must consider the reperfusion status in stroke patients.
5. Conclusion
T cell deficiency resulted in protection against stroke in nude rats undergoing total reperfusion but not partial reperfusion, suggeting the detrimental role of T cells is ischemic-model dependent.
Highlights.
We used two stroke models with total reperfusion or with partial reperfusion.
T cell deficiency reduced infarction with total, but not partial reperfusion
Total reperfusion resulted in more. T cell infilatration than partial reperfusion
T cell reconstitution resulted in larger infarct sizes in total reperfusion
T protective effects of T cell deficiency are ischemic model dependent
Acknowledgments
The authors thank Ms. Cindy H. Samos and Felicia Beppu for manuscript assistance. This project is partly supported by AHA grant in aid and 1R01NS064136-01 (HZ).
Footnotes
Conflicts of interest: None declared
Authorship credit: Lijuan Gu, Xiaoxing Xiong and Hongfei Zhang performed the experiments, analyzed and presented the data; Baohui Xu and Shengmei Zhu contributed to experimental designs and critically revised the final manuscript; Heng Zhao designed and drafted the manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexandrov AV, Burgin WS, Demchuk AM, El-Mitwalli A, Grotta JC. Speed of intracranial clot lysis with intravenous tissue plasminogen activator therapy: sonographic classification and short-term improvement. Circulation. 2001;103:2897–2902. doi: 10.1161/01.cir.103.24.2897. [DOI] [PubMed] [Google Scholar]
- Chan PH. Role of oxidants in ischemic brain damage. Stroke. 1996;27:1124–1129. doi: 10.1161/01.str.27.6.1124. [DOI] [PubMed] [Google Scholar]
- Chen ST, Hsu CY, Hogan EL, Maricq H, Balentine JD. A model of focal ischemic stroke in the rat: reproducible extensive cortical infarction. Stroke. 1986;17:738–743. doi: 10.1161/01.str.17.4.738. [DOI] [PubMed] [Google Scholar]
- Dinapoli VA, Rosen CL, Nagamine T, Crocco T. Selective MCA occlusion: a precise embolic stroke model. J Neurosci Methods. 2006;154:233–238. doi: 10.1016/j.jneumeth.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Gao X, Ren C, Zhao H. Protective effects of ischemic postconditioning compared with gradual reperfusion or preconditioning. J Neurosci Res. 2008;86:2505–2511. doi: 10.1002/jnr.21703. [DOI] [PubMed] [Google Scholar]
- Gu L, Xiong X, Zhang H, Xu B, Steinberg GK, Zhao H. Distinctive effects of T cell subsets in neuronal injury induced by cocultured splenocytes in vitro and by in vivo stroke in mice. Stroke. 2012;43:1941–1946. doi: 10.1161/STROKEAHA.112.656611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurn PD, Subramanian S, Parker SM, Afentoulis ME, Kaler LJ, Vandenbark AA, Offner H. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb Blood Flow Metab. 2007;27:1798–1805. doi: 10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic BD, Maric D, Ranin J, Veljic J. Magnetic fields, brain and immunity: effect on humoral and cell-mediated immune responses. Int J Neurosci. 1991;59:25–43. doi: 10.3109/00207459108985447. [DOI] [PubMed] [Google Scholar]
- Kang DH, Davidson RJ, Coe CL, Wheeler RE, Tomarken AJ, Ershler WB. Frontal brain asymmetry and immune function. Behav Neurosci. 1991;105:860–869. doi: 10.1037//0735-7044.105.6.860. [DOI] [PubMed] [Google Scholar]
- Kleinschnitz C, Schwab N, Kraft P, Hagedorn I, Dreykluft A, Schwarz T, Austinat M, Nieswandt B, Wiendl H, Stoll G. Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood. 2010;115:3835–3842. doi: 10.1182/blood-2009-10-249078. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Hotchkiss AK, Demas GE, Silverman AJ, Silver R, Nelson RJ. Brain mast cells are influenced by chemosensory cues associated with estrus induction in female prairie voles (Microtus ochrogaster) Horm Behav. 2003;44:377–384. doi: 10.1016/j.yhbeh.2003.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, Giese T, Veltkamp R. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- Lim JK, Obara CJ, Rivollier A, Pletnev AG, Kelsall BL, Murphy PM. Chemokine receptor Ccr2 is critical for monocyte accumulation and survival in West Nile virus encephalitis. J Immunol. 2011;186:471–478. doi: 10.4049/jimmunol.1003003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod MR, Fisher M, O’Collins V, Sena ES, Dirnagl U, Bath PM, Buchan A, van der Worp HB, Traystman R, Minematsu K, Donnan GA, Howells DW. Good laboratory practice: preventing introduction of bias at the bench. Stroke. 2009;40:e50–52. doi: 10.1161/STROKEAHA.108.525386. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin T, du Mesnil de Rochemont R, Fiebach JB, Gass A, Nolte C, Kucinski T, Rother J, Siebler M, Singer OC, Szabo K, Villringer A, Schellinger PD. Effect of incomplete (spontaneous and postthrombolytic) recanalization after middle cerebral artery occlusion: a magnetic resonance imaging study. Stroke. 2004;35:109–114. doi: 10.1161/01.STR.0000106482.31425.D1. [DOI] [PubMed] [Google Scholar]
- Ren X, Akiyoshi K, Dziennis S, Vandenbark AA, Herson PS, Hurn PD, Offner H. Regulatory B cells limit CNS inflammation and neurologic deficits in murine experimental stroke. J Neurosci. 2011;31:8556–8563. doi: 10.1523/JNEUROSCI.1623-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- Zhao H, Sapolsky RM, Steinberg GK. Interrupting reperfusion as a stroke therapy: ischemic postconditioning reduces infarct size after focal ischemia in rats. J Cereb Blood Flow Metab. 2006;26:1114–1121. doi: 10.1038/sj.jcbfm.9600348. [DOI] [PubMed] [Google Scholar]
- Zhao H, Shimohata T, Wang JQ, Sun G, Schaal DW, Sapolsky RM, Steinberg GK. Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J Neurosci. 2005;25:9794–9806. doi: 10.1523/JNEUROSCI.3163-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Wang JQ, Shimohata T, Sun G, Yenari MA, Sapolsky RM, Steinberg GK. Conditions of protection by hypothermia and effects on apoptotic pathways in a rat model of permanent middle cerebral artery occlusion. J Neurosurg. 2007;107:636–641. doi: 10.3171/JNS-07/09/0636. [DOI] [PubMed] [Google Scholar]
- Zhao H, Yenari MA, Sapolsky RM, Steinberg GK. Mild postischemic hypothermia prolongs the time window for gene therapy by inhibiting cytochrome C release. Stroke. 2004;35:572–577. doi: 10.1161/01.STR.0000110787.42083.58. [DOI] [PubMed] [Google Scholar]