Abstract
Purpose
Chemoneuropathy remains a painful, burdensome complication of cancer treatment for patients receiving a range of chemotherapeutics, yet the cause and persistence of this condition are not fully documented. This study was designed to quantify the longevity of and contributions to neuropathy following treatment with the plant alkaloids paclitaxel and vincristine.
Methods
Quantitative sensory testing was conducted approximately 18 months apart on 14 patients, seven of which had been treated with paclitaxel and seven with vincristine and compared to data from 18 healthy control subjects. In addition, skin biopsies were obtained to investigate changes in the density of Meissner’s corpuscles and epidermal nerve fibers (ENFs), the loss of which is thought to contribute to multiple forms of neuropathy.
Results
Impairments in motor skills, as measured by a grooved peg-board, were found. Deficits in touch detection were observed using von Frey monofilaments, as were changes in sharpness detection using a weighted needle device. Using a Peltier device, warmth and heat detection were impaired. These deficits were consistent across time. Remarkably, the average length of time patients reported painful neuropathy was over four and a half years. Skin biopsies were found to be deficient in Meissner’s corpuscles and ENFs.
Conclusions
The combination of widespread deficits in sensory testing and decreases in skin innervation for cancer patients receiving paclitaxel or vincristine document a persistent polyneuropathy which severely impacts these patients. Decreases in Meissner’s corpuscles and ENFs indicate a possible mechanism for the neuropathy.
Keywords: Chemoneuropathy, Paclitaxel, Vincristine, Epidermal nerve fiber, Meissner’s corpuscle
Introduction
Chemotherapy-induced peripheral neuropathy remains a painful and burdensome complication of cancer treatment for patients receiving a range of chemotherapeutic agents including cisplatin [8], bortezomib [4], vincristine [22] and paclitaxel (Taxol) [11]. This neuropathy is characterized by paresthesia and dysesthesia that often presents in a stocking and glove distribution originating in the hands and feet [7]. Painful neuropathy is often severe, with patients reporting visual analog scores of 7 [11] or 8 [4] out of a possible 10, and is poorly responsive to pharmacological management [4].
Paclitaxel and vincristine, both of which are plant alkaloid derivatives, exert their antineoplastic effects by interfering with microtubule function. While both ultimately result in cell cycle arrest, paclitaxel works primarily by stabilizing microtubules [27], whereas vincristine inhibits microtubule assembly [16]. Both agents result in identical complaints of neuropathy, including pain and numbness, motor impairment, and decreased pinprick and vibration perceptions [9]. This neuropathy can occur as early as the first treatment and may last indefinitely. The relatively few studies that have examined the persistence of chemoneuropathy report that the pain continues for at least 1 year following bortezomib [4] or oxaliplatin [5], 6 months following cisplatin [13], and up to 48 months following paclitaxel [21].
While the cause of chemoneuropathy is not fully understood, new evidence indicates a loss of nerve fibers innervating the skin in an area of pain may greatly contribute. Decreased epidermal nerve fiber (ENF) densities have been reported in other diseases which result in neuropathy, such as diabetes [14] and postherpetic neuralgia [20]. Further, skin biopsies taken from animals treated with paclitaxel [3] and oxaliplatin [2] show decreased ENF density at a time when mechanical sensitivity is present. Most recently, a case report of a patient with chronic bortezomib-induced chemoneuropathy revealed a drastic loss of both ENFs and Meissner’s corpuscles that was most pronounced in the area of greatest neuropathy-related pain [4].
The current study investigated the persistence of paclitaxel and vincristine-induced painful neuropathy, both from a qualitative and quantitative perspective using psychophysical methods across a broad time span. To further understand the mechanisms of chemoneuropathy, a pilot study of patient skin biopsy was examined for changes in Meissner’s corpuscle and epidermal nerve fiber densities.
Methods
Patients
All procedures and the informed consent form were approved by the Institutional Review Board of the M.D. Anderson Cancer Center and were in accordance with the Helsinki Declaration of 1975. Study participants consisted of fourteen patients and eighteen control subjects. The control group consisted of subjects recruited from faculty, staff and volunteers from the institute which had no known risk factors for neuropathy. Patients included in this study were those who developed painful peripheral neuropathy that had persisted for at least 3 months following frontline treatment with either paclitaxel or vincristine and were seen by a physician at the Pain Management Center at the MD Anderson Cancer Center. Patients were re-tested at 6 months to 2 years following the first test.
Testing procedure
To begin each testing session, patients were given a list of twenty words (i.e., burning, tingling, numb, cold) and asked to identify those which described their pain [11]. Patients were also presented with a visual analog scale (VAS) which contains prompts of ‘no pain’ at the bottom and ‘most imaginable’ at the top and asked to specify both their on-going and daily maximum pain intensity [11].
Subjects often report symmetrical pain and sensory disturbances that originate in the fingers and present in a stocking and glove type distribution, resulting in 3 distinct testing areas [6, 11]. Accordingly, these 3 areas were used for the current study:
The painful area: This was determined to be the zone of on-going pain and was predominately located on the tips of the fingers and/or toes. As has been done previously [6, 11], the tip of the index finger was the site studied here.
The border area: This area included body regions which were adjacent to but distinct from the painful area. Numbness and tingling were often present in this area, which often included the palms of the hands and/or soles of the feet. The thenar eminence was tested in this study, as in previous studies [6, 11].
The non-painful area: This area, which was adjacent to but distinct from the border area, was reported by the patient to be felt as ‘normal’ and was proximal to the wrists and/or ankles. As in previous studies [6,11], the volar surface of the arm was studied.
Touch detection thresholds and grooved peg-board test
To determine touch detection thresholds, von Frey monofilaments (Semmes–Weinstein) were applied in an up/down manner [11], beginning with a bending force of 0.02 g. Each von Frey monofilament was applied to the skin for approximately 1 s, and if the subject failed to detect the stimulus, the next higher force monofilament was applied to the same location. If a given monofilament was detected by the patient, the next lower von Frey was applied to the skin. Monofilaments were applied in this manner until the same filament was detected for three applications. This was then assigned as the touch detection threshold.
The grooved peg-board test was used to measure manual dexterity [26]. Patients filled a five-by-five slotted pegboard in an ordered fashion, either across rows or down columns. The number of seconds it took patients to complete the board for both the dominant and the non-dominant hand was measured.
Sharpness detection threshold
Sharpness detection was determined using a weighted needle device [12]. For this test, a blunted 30 gauge needle was attached to weights that were 8, 10, 16, 20, 32, 64 or 128 grams and applied to the testing area for 1 s in ascending order. Three trials were administered per testing site, each separated by an average intertrial interval of 30–90 s. The sharpness detection threshold was determined to be the average force of the stimuli deemed as “sharp” or “painful” by the patient.
Heat and cold detection thresholds
Patient thresholds for both hot and cold stimuli were assessed using a 36-mm by 36-mm Peltier probe (Medoc Inc.) applied to the skin at each of the testing sites [6, 12]. The threshold for any given site was calculated as the average of three trials. For heat ramps, the baseline temperature of the probe was set at 32 °C and a cutoff temperature of 52 °C was used. Once a trial began, the temperature of the probe increased at a rate of 0.30 °C/s. Subjects were instructed to first indicate when the probe was perceived as warm (warmth threshold) and then painful (heat threshold). The trial was immediately terminated when a patient reported that the heat threshold had been reached; however, in the situation where a subject failed to perceive warm or heat pain, the cutoff temperature was recorded. The threshold to detection of cooling (cool threshold) and then cold pain (cold threshold) was similarly determined, except that the cutoff temperature was 3 °C and the probe decreased at a rate of 0.50 °C/s. If a subject failed to perceive cold pain, the cutoff of 3 °C was recorded.
Meissner’s corpuscles and epidermal nerve fibers
One patient and seven healthy controls consented to having a 3-mm biopsy taken from each of the three testing sites in order to investigate Meissner’s corpuscle and epidermal nerve fiber densities. This tissue was investigated using immunohistochemistry as previously described (2). Briefly, 50-lm thick sections were exposed to antibodies to the pan-neuronal marker PGP 9.5 (AbD Serotec, Oxford, UK; 1/1000) and the basement membrane marker type IV collagen (Southern Biotech, Birmingham, AL; 1/25).
Confocal images were acquired with an Olympus DSU non-laser confocal microscope to determine ENF and Meissner’s corpuscle densities. Nerve fiber density was determined with Neurolucida Software (MicroBrightField, Colchester, VT) by tracing from the intersection of the nerve fiber (PGP-9.5-ir) with the dermal-epidermal junction basement membrane (type IV collagen-ir) to the ending within the epidermis. A counting unit was identified when a nerve crosses the basement membrane [14].
Statistical analysis
For all statistical analyses, p < .05 was used as the significance criterion. The analysis of touch detection thresholds was conducted using nonparametric methods (Kruskal–Wallis test). Peg-board completion times, sharpness detection thresholds and thermal thresholds were analyzed using analysis of variance and post hoc comparison of the means with Tukey HSD. Comparisons of mechanical and thermal thresholds were performed between healthy subjects and patients for the different areas of the tested skin.
Results
Subjects
The patient group was composed of 5 men and 9 women with an average age of 60.1 ± 2.30 years, and the control group consisted of 10 males and 8 females with an average age of 55.3 ± 2.38 years. A t test revealed no significant difference in age between the patient and the control groups (p = .21). Cancer diagnoses varied among patients (Table 1), but all patients were treated with either paclitaxel (n = 7) or vincristine (n = 7) as frontline agents. No significant differences were observed between these two groups in regard to quantitative sensory analyses. Three out of the fourteen patients (21 %) began to experience neuropathy symptoms after a single cycle of chemotherapy, while six patients (43 %) received four or more cycles before symptoms began. Overall, the average number of treatment cycles received prior to neuropathy onset was 3.4 ± .5. Patients were not excluded based on pre-existing or ongoing conditions which may predispose them to neuropathy, such as diabetes or tobacco use. Information regarding these potential risk factors is provided in Table 1.
Table 1.
Patient demographics, diagnosis, treatment and medication, and length of time with neuropathy are displayed
| Patient | Gender | Age | Cancer diagnosis |
Frontline treatment |
Possible risk factors |
Cycles to onset |
Total cycles received |
Time with neuropathy (months) |
MEDD |
|
|---|---|---|---|---|---|---|---|---|---|---|
| TEST | RETEST | |||||||||
| 1 | M | 66 | NSCLC | Taxol | None | 4 | 4 | 49 | ||
| 2 | F | 70 | Breast | Taxol | None | 5 | 5 | 60 | 19.3 | 19.3 |
| 3 | F | 71 | Breast | Taxol | Post polio syndrome |
1 | 4 | 140 | 43.4 | 43.4 |
| 4 | F | 62 | Breast | Taxol | Alcohol and tobacco use |
1 | 4 | 25 | 25.1 | 25.1 |
| 5 | F | 53 | Breast | Taxol | Previous radiation treatment |
5 | 6 | 28 | 51.7 | 46.7 |
| 6 | F | 60 | Breast | Taxol | None | 3 | 4 | 19 | 58.4 | 48.0 |
| 7a | F | 45 | Breast | Taxol | None | 3 | 6 | 68 | ||
| 8 | F | 62 | Lymphoma | Vincristine | Diabetes | 1 | 4 | 156 | 20.9 | 12.9 |
| 9 | M | 54 | Ewing’s sarcoma |
Vincristine | None | 6 | 6 | 49 | 111.6 | 96.0 |
| 10 | F | 62 | (ALL) Leukemia |
Vincristine | None | 3 | 4 | 78 | 19.3 | 19.3 |
| 11 | M | 45 | Lymphoma | Vincristine | None | 3 | 3 | 52 | ||
| 12 | M | 61 | Multiple myeloma |
Vincristine | None | 4 | 4 | 38 | 40.8 | 26.0 |
| 13 | F | 70 | Lymphoma | Vincristine | Alcohol and tobacco use |
6 | 6 | 35 | ||
| 14 | M | 61 | (ALL) Leukemia |
Vincristine | Diabetes | 2 | 5 | 15 | 21.7 | |
| Average ± SEM | 60.1 ± 2.3 | 3.4 ± .5 | 58.0 ± 11.3 | 41.2 ± 7. | 7 37.4 ± 6.8 | |||||
Of the fourteen patients, seven were treated with Taxol and seven with vincristine. From the time at which neuropathy began until the time of the retest (“time with neuropathy”), patients had neuropathy symptoms for an average of 58 months
NSCLC Non-small cell lung cancer, MEDD daily medications in morphine equivalent daily dosage
Patient biopsy shown in Fig. 5
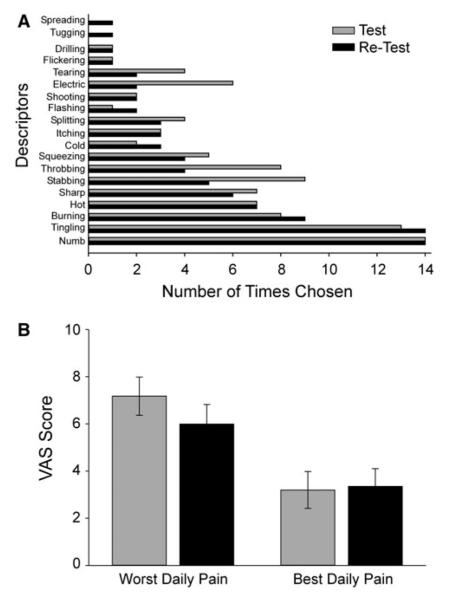
The average interval between the original test time point and the follow-up retest time point was 17.6 ± 3.76 months. At the time of the first test, patients primarily chose numbness and tingling to describe the quality of the neuropathy symptoms (Fig. 1a). Pain quality remained similar at the retest, with the notable exceptions that more patients described the pain as “dull”, “shooting” and “flickering” at that time. Pain intensity, both at its worst (VAS maximum) and its least (VAS minimum), also remained similar across time with maximum pain at 7.2 ± .81 at test and 5.9 ± .83 at retest. Daily minimum pain, reflecting the best effect of all pain medications received, was 3.2 ± .78 at test and 3.4 ± .75 at retest (Fig. 1b). The use of pain medications, calculated as the mean morphine equivalent daily dose (MEDD) also did not change across time, with MEDD of 41.2 ± 7.7 mg at test and 37.4 ± 6.8 mg at the retest (Table 1).
Fig. 1.
Description of pain (a) and average pain ratings (±SEM) (b) in patients with paclitaxel and vincristine-induced neuropathy pain at an initial test (gray bars) and at a retest time point (black bars). VAS visual analog scale (range: 0–10)
Persistence of neuropathy symptoms
Patient histories were investigated to determine the length of time patients complained of neuropathy symptoms (Table 1). On average, patients complained of neuropathy for 40.4 ± 11.17 months prior to the first test. By the time of the retest session, patients had chemotherapy-induced neuropathy for an average of 58.0 ± 11.32 months, or almost 5 years.
Threshold for touch detection and peg-board performance
Within the area of pain (fingertips) and in the border zone (thenar eminence) of sensory disturbance, touch detection was severely impaired at both testing sessions for patients (Fig. 2). In the volar forearm (non-painful area), deficits in touch detection were detected at the time of the original test but not at the retest. Further, impairments in this area were overall not as pronounced as in the other two areas tested. While there was a trend for increased grooved peg-board completion times at the time of the original test when comparing patients to control subjects, this trend was not significant. By the time of the retest, peg-board completion times had significantly increased (Fig. 2).
Fig. 2.
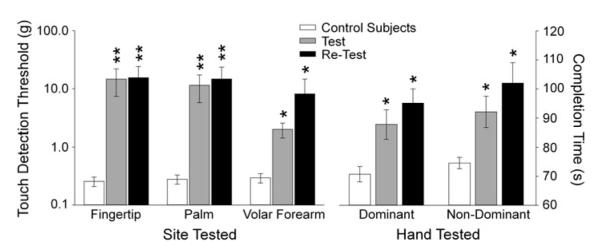
Touch detection thresholds and peg-board completion times in patients at the test and the retest. The left side of the graph depicts mean (±SEM) von Frey touch detection thresholds obtained from the three areas of the body tested (Fingertip, Palm, and Volar Forearm) for control subjects (open bars) and from patients with chemoneuropathy at the test (gray bars) and retest (black bars) examinations. The right side of the graph shows the mean (±SEM) slotted peg-board completion times for the dominant and non-dominant hands in control subjects (open bars) and patients at the test (gray bars) and retest (black bars) examinations. *p < .05; **p < .01
Threshold for detection of sharpness
A non-significant trend for sharpness detection deficits was observed in both the border area and the non-painful area at the test. This remained unchanged at the retest. On the contrary, within the area of pain, sharpness detection was impaired (Fig. 3), and these deficits were still present at the time of the retest.
Fig. 3.
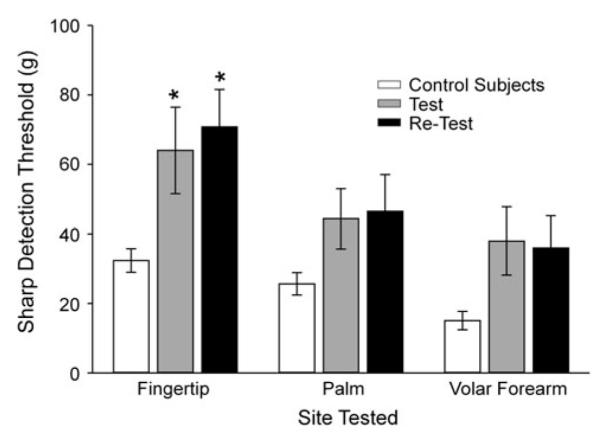
Sharpness detection thresholds (±SEM) for control subjects (open bars) and from patients with chemoneuropathy at the test (gray bars) and retest examination (black bars). *p < .05
Thermal threshold
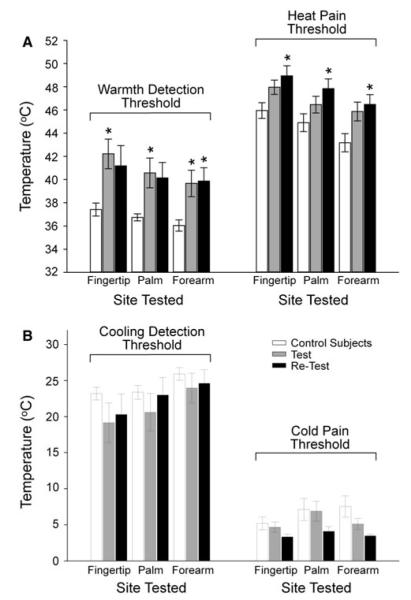
The threshold to detect warmth was significantly elevated in all three areas tested at the time of the initial test, while the threshold for pain was significantly increased in all areas at the time of the retest (Fig. 4a). The thresholds to detect cool and cold pain were not found to be different from controls, either at the original test or at the retest (Fig. 4b).
Fig. 4.
Thermal detection thresholds in patients at the test and the retest examinations. The bar graph in (a) shows the mean (±SEM) temperature for detection of warming and heat pain at the three bodily test areas (Fingertip, Palm, Volar Forearm) for control subjects (open bars) and patients with chemoneuropathy at the time of the initial test (Test, gray bars) and the long-term follow-up examination (Retest, black bars). The bar graph in (b) shows the mean (±SEM) temperature for the detection of skin cooling and cold pain in the same manner depicted in (a). *p < 0.05
Meissner’s corpuscles and epidermal nerve fibers
To investigate possible changes in Meissner’s corpuscles and epidermal nerve fibers as a result of chemotherapy, consent was obtained from a 45 year of female to collect three 3-mm biopsies, one from each testing site, at the time of the retest. These samples were compared to those obtained from seven healthy control subjects, 4 males and 3 females with an average age of 44.4 ± 2.19 years. The patient was diagnosed with breast cancer and received paclitaxel as frontline treatment. Pain for this patient was described at the initial test as “sharp”, “itching”, “numb” and “cold”, and as “sharp”, “squeezing”, “tingling”, “numb” and “flickering” at the retest. Maximum and minimum pain ratings were 6.9 and 4.8, respectively, at the initial test. At the retest, pain was consistently rated as 4.5 for both maximum and minimum pain levels. Touch detection thresholds at the first test were .95 for the area of pain, .95 for the border area and 1.81 for the non-painful area. Thresholds remained at these same elevated levels at the time of the retest. Deficits were also found for peg-board completion and sharpness; these deficits remained relatively stable across time.
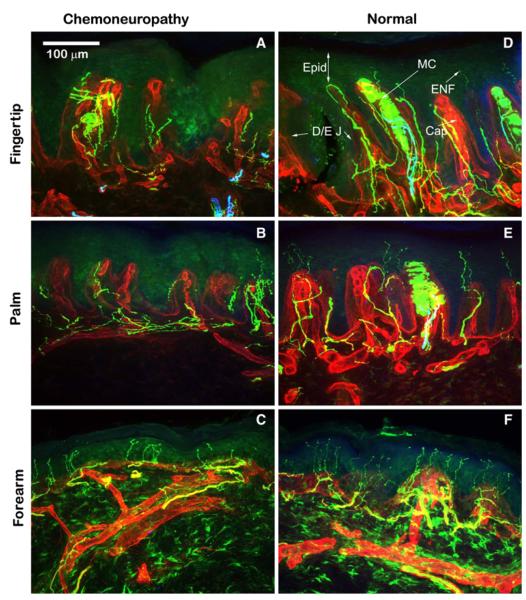
There was a clear decrease in both Meissner’s corpuscle and ENF densities in the patient biopsies as compared to biopsies obtained from healthy control subjects (Fig. 5). Whereas control subjects had an average of 34 Meissner’s corpuscles/mm2 in the fingertip, this patient’s fingertip biopsy contained only 10, indicating a 71 % difference between the control subjects and the chemotherapy-treated patient. Regarding ENF densities, the patient biopsy contained 92 ENFs/mm2 within the 3-mm biopsy from the area of pain (finger tip), 71 ENFs/mm2 within the border area (palm), and 504 ENFs/mm2 within the non-painful area (forearm) compared to average ENF densities from the control biopsies of 127 ENFs/mm2 in the fingertip, 236 ENFs/mm2 in the palm, and 633 ENFs/mm2 in the forearm.
Fig. 5.
Meissner’s corpuscle and epidermal nerve fiber densities in biopsies obtained from a healthy control subject and a patient with chemoneuropathy. This figure shows immunohistochemistry staining with the pan-neuronal marker PGP9.5 (green) in the biopsies of a patient with bortezomib-related chemoneuropathy in the area of pain (finger tip) (a), the border zone (palm) (b) and the non-painful area (forearm) (c). These biopsies can be compared to similar biopsies from a control subject (d, e, f). Epidermal Nerve fibers (ENF) can be seen in the control subject crossing the dermalepidermal junction (D/E J) into the epidermis (Epid) in all regions. Note the extensive decrease in epidermal nerve fibers in the patient biopsies. Also within the control subject biopsies from the fingertip and palm, Meissner’s corpuscles (MC) can be clearly seen within the epidermis. These structures are notably absent in the patient biopsies. (scale: 100 μm)
Discussion
The results of the present experiment indicate that if painful neuropathy resulting from treatment with either paclitaxel or vincristine becomes persistent, that is, lasting more than 3 months after therapy has ended, it may become very refractory to improvement. It is important to emphasize that not all patients who develop pain following chemotherapy transition into this chronic condition, but may improve. Yet, the specific numbers of patients in each cohort remain unknown. Biopsies taken from a patient signify that this condition may be attributable to a loss of nerve fibers innervating the skin (ENFs). Patients were assessed an average of 40 months, or over 3 years, after neuropathy first developed. At that time, patients complained of severe and unrelenting pain, in addition to marked impairments in touch and sharpness detection, motor impairments and decreased ability to discern thermal stimuli. These issues remained stable at a later test time, which occurred approximately 18 months after the initial examination. Ab function assessed using touch detection with von Frey filaments, revealed compromise of large myelinated fibers in the areas of reported sensory dysfunction as well as on the volar surface of the forearm which is an area outside of symptom complaint [4, 6, 10, 11]. Compromise in Aδ fiber function, revealed by impaired sharpness detection, was found specifically in areas of chemotherapy-induced pain. Finally, compromise in C fiber function as shown by change in heat detection threshold was mild in painful areas of skin in these patients.
At the time of the retest, all patients were continuing to experience neuropathy. Five patients had neuropathy lasting longer than 5 years. Two of these five patients have neuropathy for over 11 years. At the time of the original test, these patients were taking morphine equivalent doses in excess of 20 mg per day, with little change by the time of the retest assessment. The long-term effects of chronic opiate treatment are well documented and include addiction and abuse, tolerance, opiate-induced hyperalgesia, hypogonadism and immune suppression, among others [23]. Taken together, these results highlight a need for better understanding and prevention of chemoneuropathy. Unfortunately, the primary cause of this condition is not yet fully understood.
What is understood is that treatment with chemotherapeutic agents such as paclitaxel and vincristine result in many factors that can influence pain, including activation of glial cells within the spinal cord [19], changes in the expression of glutamate transporters [29] and increased substance P release in the spinal cord [17]. These results do not, however, explain why patients consistently complain of pain in the more distal regions of the body, such as the fingertips and toes. One possible explanation is that these drugs interfere with microtubule function and axonal transport, and ultimately disrupt normal nerve physiology. Considering that both paclitaxel and vincristine do interrupt microtubule function [16, 27], this seems to be a plausible explanation; however, other commonly used chemotherapies such as bortezomib and oxaliplatin, which do not hinder microtubules, still produce robust neuropathies. For example, oxaliplatin effectively treats cancer by interfering with DNA synthesis [24], yet this drug results in a painful and complex neuropathy [1].
One possible explanation for the genesis or persistence of neuropathy, as well as the regional bodily location of this condition, is a recent and growing finding of decreased ENFs within the area of pain. Significant losses in ENF density has been found in animal models of paclitaxel [3, 28], oxaliplatin [2] and cisplatin [15]–induced hypersensitivity. In humans, bortezomib-induced chemoneuropathy also appears to be associated with ENF loss, and this loss is most pronounced in the area of pain (fingertip) [4]. This finding of decreased ENF density extends to other conditions that result in neuropathy. Diabetes produces similar symptoms of pain and sensory disturbances as is seen in chemoneuropathy, and biopsies from patients with diabetic-related peripheral neuropathy also show decreased ENF density [14]. In patients with post-herpetic neuralgia, ENF loss is greatest in areas specific to the neuropathic pain [18, 20, 25]. If ENF loss is a major contributing factor to either the genesis or the persistence of neuropathy, it remains to be determined why these fibers are lost.
In summary, the results of this study clearly show a severe and persistent neuropathy resulting from paclitaxel or vincristine treatment in patients. On average, patients reported painful chemoneuropathy lasting over four and a half years. The resulting impairments in motor skills, touch, sharpness and thermal detection were pronounced and consistent across time. Skin biopsies taken from a patient were found to be deficient in Meissner’s corpuscles and nerve fibers, indicating a possible mechanism for the neuropathy.
Acknowledgments
The work was supported by grants NS046606 and CA124787 and by a gift from the Peggy and Avinash Ahuja Center of Excellence in Pain Research and Treatment. The authors would like to thank Dr. Allen Burton and Dr. Sergio Giralt for their assistance with data collection.
Contributor Information
Jessica A. Boyette-Davis, Department of Psychology, York College of Pennsylvania, Appell Life Sciences Building, York, PA 17403, USA
Juan P. Cata, Department of Anesthesia and Pain Medicine, The University of Texas MD Anderson Cancer Center, 1400 Holcombe, Houston, TX 77030, USA
Larry C. Driver, Department of Anesthesia and Pain Medicine, The University of Texas MD Anderson Cancer Center, 1400 Holcombe, Houston, TX 77030, USA
Diane M. Novy, Department of Anesthesia and Pain Medicine, The University of Texas MD Anderson Cancer Center, 1400 Holcombe, Houston, TX 77030, USA
Brian M. Bruel, Department of Anesthesia and Pain Medicine, The University of Texas MD Anderson Cancer Center, 1400 Holcombe, Houston, TX 77030, USA
Deidre L. Mooring, Department of Anesthesia and Pain Medicine, The University of Texas MD Anderson Cancer Center, 1400 Holcombe, Houston, TX 77030, USA
Gwen Wendelschafer-Crabb, Department of Neurology, University of Minnesota, 515 Delaware SE, Minneapolis, MN 55455, USA.
William R. Kennedy, Department of Neurology, University of Minnesota, 515 Delaware SE, Minneapolis, MN 55455, USA
Patrick M. Dougherty, Department of Anesthesia and Pain Medicine, The University of Texas MD Anderson Cancer Center, 1400 Holcombe, Houston, TX 77030, USA;1515 Holcombe BLVD, Unit 0409, Houston, TX 77030, USA
References
- 1.Argyriou AA, Polychronopoulos P, Iconomou G, Koutras A, Makatsoris T, Gerolymos MK, Gourzis P, Assimakopoulos K, Kalofonos HP, Chroni E. Incidence and characteristics of peripheral neuropathy during oxaliplatin-based chemotherapy for metastatic colon cancer. Acta Oncol. 2007;46:1131–1137. doi: 10.1080/02841860701355055. [DOI] [PubMed] [Google Scholar]
- 2.Boyette-Davis J, Dougherty PM. Protection against oxaliplatin-induced mechanical hyperalgesia and intraepidermal nerve fiber loss by minocycline. Exp Neurol. 2011;229:353–357. doi: 10.1016/j.expneurol.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyette-Davis J, Xin W, Zhang H, Dougherty PM. Intraepidermal nerve fiber loss corresponds to the development of Taxol-induced hyperalgesia and can be prevented by treatment with minocycline. Pain. 2011;152:308–313. doi: 10.1016/j.pain.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyette-Davis JA, Cata JP, Zhang H, Driver LC, Wendelschafer-Crabb G, Kennedy WR, Dougherty PM. Follow-up psychophysical studies in bortezomib-related chemoneuropathy patients. J Pain. 2011 doi: 10.1016/j.jpain.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burakgazi AZ, Messersmith W, Vaidya D, Hauer P, Hoke A, Polydefkis M. Longitudinal assessment of oxaliplatin-induced neuropathy. Neurology. 2011;77:980–986. doi: 10.1212/WNL.0b013e31822cfc59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cata JP, Weng H-R, Burton AW, Villareal H, Giralt S, Dougherty PM. Quantitative sensory findings in patients with bortezomib-induced pain. J Pain. 2007;8:296–306. doi: 10.1016/j.jpain.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Cata JP, Weng H-R, Dougherty PM. Clinical and experimental findings in humans and animals with chemotherapy-induced peripheral neuropathy. Minerva Anes. 2006;72:151–169. [PubMed] [Google Scholar]
- 8.Cavaletti G, Bogliun G, Marzorati L, Zincone A, Marzola M, Colombo N, Tredici G. Peripheral neurotoxicity of taxol in patients previously treated with cisplatin. Cancer. 1995;75:1141–1150. doi: 10.1002/1097-0142(19950301)75:5<1141::aid-cncr2820750514>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhry V, Chaudhry M, Crawford TO, Simmons-O’Brien E, Griffin JW. Toxic neuropathy in patients with pre-existing neuropathy. Neurology. 2003;60:337–340. doi: 10.1212/01.wnl.0000043691.53710.53. [DOI] [PubMed] [Google Scholar]
- 10.Dougherty PM, Cata JP, Burton AW, Vu K, Weng HR. Dysfunction in multiple primary afferent fiber subtypes revealed by quantitative sensory testing in patients with chronic vincristine-induced pain. J Pain Symptom Manage. 2007;33:166–179. doi: 10.1016/j.jpainsymman.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Dougherty PM, Cata JP, Cordella JV, Burton A, Weng H-R. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain. 2004;109:132–142. doi: 10.1016/j.pain.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Fuchs PN, Campbell JN, Meyer RA. Secondary hyperalgesia persists in capsaicin desensitized skin. Pain. 2000;84:141–149. doi: 10.1016/s0304-3959(99)00194-3. [DOI] [PubMed] [Google Scholar]
- 13.Grunberg SM, Sonka S, Stevenson LL, Muggia FM. Pro- gressive paresthesias after cessation of therapy with very highdose cisplatin. Cancer Chemother Pharmacol. 1989;25:62–64. doi: 10.1007/BF00694340. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy WR, Wendelschafer-Crabb G, Johnson T. Quantitation of epidermal nerves in diabetic neuropathy. Neurology. 1996;47:1042–1048. doi: 10.1212/wnl.47.4.1042. [DOI] [PubMed] [Google Scholar]
- 15.Lauria G, Lombardi R, Borgna M, Penza P, Bianchi R, Savino C, Canta A, Nicolini G, Marmiroli P, Cavaletti G. Intraepidermal nerve fiber density in rat foot pad: neuropathologic-neurophysiologic correlation. J Peripher Nerv Syst. 2005;10:202–208. doi: 10.1111/j.1085-9489.2005.0010210.x. [DOI] [PubMed] [Google Scholar]
- 16.Lobert S, Vulevic B, Correia JJ. Interaction of vinca alkaloids with tubulin: a comparison of vinblastine, vincristine and vinorelbine. Biochemistry. 1996;35:6806–6814. doi: 10.1021/bi953037i. [DOI] [PubMed] [Google Scholar]
- 17.Miyano K, Tang HB, Nakamura Y, Morioka N, Inoue A, Nakata Y. Paclitaxel and vinorelbine, evoked the release of substance P from cultured rat dorsal root ganglion cells through different PKC isoform-sensitive ion channels. Neuropharmacology. 2009;57:25–32. doi: 10.1016/j.neuropharm.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Oaklander AL. The density of remaining nerve endings in human skin with and without postherpetic neuralgia after shingles. Pain. 2001;92:139–145. doi: 10.1016/s0304-3959(00)00481-4. [DOI] [PubMed] [Google Scholar]
- 19.Peters CM, Jimenez-Andrade JM, Kuskowski MA, Ghilardi JR, Mantyh PW. An evolving cellular pathology occurs in dorsal root ganglia, peripheral nerve and spinal cord following intravenous administration of paclitaxel in the rat. Brain Res. 2007;1168:46–59. doi: 10.1016/j.brainres.2007.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen KL, Rice FL, Farhadi M, Reda H, Rowbotham MC. Natural history of cutaneous innervation following herpes zoster. Pain. 2010;150:75–82. doi: 10.1016/j.pain.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Pignata S, De PS, Biamonte R, Scambia G, Di VG, Colucci G, Febbraro A, Marinaccio M, Lombardi AV, Manzione L, Carteni G, Nardi M, Danese S, Valerio MR, de MA, Massidda B, Gasparini G, Di MM, Pisano C, Perrone F. Residual neurotoxicity in ovarian cancer patients in clinical remission after first-line chemotherapy with carboplatin and paclitaxel: the multicenter Italian trial in ovarian cancer (MITO-4) retrospective study. BMC Cancer. 2006;6:5. doi: 10.1186/1471-2407-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Postma TJ, Benard BA, Huijgens PC, Ossenkoppele GJ, Heimans JJ. Long term effects of vincristine on the peripheral nervous system. J Neuro-Onocol. 1993;15:23–27. doi: 10.1007/BF01050259. [DOI] [PubMed] [Google Scholar]
- 23.Raghavan S, Harvey AD, Humble SR. New opioid side effects and implications for long-term therapy. Trends Anaesth Crit Care. 2011;1:18–21. [Google Scholar]
- 24.Raymond E, Faivre S, Woynarowski JM, Chaney SG. Oxaliplatin: mechanism of action and antineoplastic activity. Semin Oncol. 1998;25:4–12. [PubMed] [Google Scholar]
- 25.Rowbotham MC, Yosipovitch G, Connoly MK, Finlay D, Forde G, Fields HL. Cutaneoous innervation density in the allodynic form of postherpetic neuralgia. Neurobiol Dis. 1996;3:204–214. doi: 10.1006/nbdi.1996.0021. [DOI] [PubMed] [Google Scholar]
- 26.Ruff RM, Parker SB. Gender and age-specific changes in motor speed and eye-hand coordination in adults: normative values for finger tapping and grooved pegboard tests. Percept Motor Skills. 1993;76:1219–1230. doi: 10.2466/pms.1993.76.3c.1219. [DOI] [PubMed] [Google Scholar]
- 27.Schiff PB, Horwitz SB. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci USA. 1980;77:1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siau C, Xiao W, Bennett GJ. Paclitaxel- and vincristineevoked painful peripheral neuropathies: loss of epidermal innervation and activation of Langerhans cells. Exp Neurol. 2006;201:507–514. doi: 10.1016/j.expneurol.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weng HR, Aravindan N, Cata JP, Chen JH, Shaw AD, Dougherty PM. Spinal glial glutamate transporters downregulate in rats with taxol-induced hyperalgesia. Neurosci Lett. 2005;386:18–22. doi: 10.1016/j.neulet.2005.05.049. [DOI] [PubMed] [Google Scholar]