Abstract
Rationale
Non-daily, or intermittent smokers (ITS), are increasingly prevalent. Their smoking may be more situational than that of daily smokers (DS), and thus is hypothesized to be more influenced by cues.
Objectives
To assess ITS’ response to cues, and compare it to that of DS.
Methods
Samples of 239 ITS and 207 DS (previously reported in (Shiffman et al., in press-a) were studied in 2,586 laboratory cue-reactivity sessions. Craving (Questionnaire of Smoking Urges) and smoking (probability, latency, puff parameters, and carbon monoxide increases) in response to cues was assessed following exposure to neutral cues and cues related to smoking, alcohol, negative affect, positive affect, and smoking prohibitions. Mixed effects models, GEE and random-effects survival analyses were used to assess response to cues and differences between DS and ITS.
Results
ITS’ craving increased following exposure to smoking and alcohol cues and decreased following positive affect cues, but cues had little effect on smoking behaviors. Cue reactivity was similar in ITS and DS. Among ITS, craving intensity predicted smoking probability, latency, and intensity, and the effects on latency were stronger among ITS than DS.
Conclusions
Contrary to hypotheses, ITS were not more responsive to laboratory cues than DS. Results show that ITS do experience craving and craving increases that are then associated with smoking.
Keywords: smoking, non-daily smoking, craving, cue reactivity, smoking topography
Most research on smoking behavior has focused on individuals who smoke daily and at relatively high rates. These smokers conform to the dominant model of smoking behavior, which posits that smoking regularly throughout the day maintains nicotine levels above the withdrawal threshold (Stolerman and Jarvis, 1995). However, recent population data indicate that 22%–33% of US adult smokers are intermittent (ITS) who do not smoke every day (Centers for Disease Control and Prevention, 2008a, b, 2011; Substance Abuse and Mental Health Services Administration, 2009). Little is known about the factors that drive ITS smoking.
We recently reported that ITS were less dependent, their smoking more variable, and more closely tied to particular situations or cues (Shiffman et al., 2012), consistent with the hypothesis that ITS smoke only in particular settings or situations (Shiffman and Paty, 2006). Indeed, in their self-reported smoking motives (Shiffman et al., in press-b) ITS give much greater weight to smoking in response to cues, which was the most highly ranked motive among ITS. This suggests that ITS’ smoking might be more cue-driven; that is, under greater stimulus control (Shiffman and Paty, 2006) – the tendency for a behavior to be linked to specific stimuli. If ITS’ smoking were under greater stimulus control, this might help explain their limited smoking patterns.
An established way to assess smokers’ responses to particular stimuli is the cue reactivity (CR) paradigm, in which smokers are exposed to cues in the laboratory that are thought to be associated with smoking (Carter and Tiffany, 1999; Drummond et al., 1995; Niaura et al., 1988). Dozens of studies have demonstrated that cues reliably elicit craving in DS (Carter and Tiffany, 1999), and differences in reactivity have been associated with dependence (Watson et al., 2010) and greater vulnerability to relapse (Abrams et al., 1988; Niaura et al., 1989), albeit inconsistently (Perkins, 2009). In this study, we compared cue reactivity among ITS and DS, hypothesizing that ITS would demonstrate greater cue reactivity, particularly to certain cues, not only with respect to craving, but with respect to actual smoking (see Perkins, 2009).
One previous CR study (Sayette et al., 2001) that examined differences between heavy smokers and chippers – smokers who smoke at very low levels, whether daily or not (Shiffman et al., 1994) – surprisingly found no differences in craving response to cigarette cues. However, the study may have lacked power, and did not examine smoking in response to cues (Sayette et al., 2000). Also, chippers, many of whom smoke every day (Shiffman et al., 1994), may respond differently than ITS.
The most commonly used stimuli in CR studies are ‘proximal’ cues (Conklin et al., 2008), such as cigarettes themselves, which are universally present during smoking (Carter et al., 2006; Carter and Tiffany, 1999). In a recent study (Shiffman et al., in press-a), we showed that cigarette cues increased craving among DS, though the cues did not increase smoking. Smoking cues should be especially relevant to ITS, particularly under the “social smoking” hypothesis, which suggests that they are cued to smoke by others’ smoking (Schane et al., 2009; Shiffman et al., 2012).
Smoking may also be linked to more ‘distal’ cues, such as alcohol, that become associated with smoking over time. Smoking is closely associated with drinking, (Shiffman and Balabanis, 1995), but may be particularly associated with ITS’ smoking given the potential social component of both drinking and smoking among ITS (Schane et al., 2009; Shiffman et al., 2009; Shiffman et al., 2012). Also, one study observed that ITS were more likely to be binge drinkers, and their smoking increased with greater alcohol consumption (Harrison, Desai, & McKee, 2008). We hypothesized that reactivity to alcohol cues would be greater among ITS than DS.
Another cue that is often considered important for smoking is negative affect. Despite the fact that Ecological Momentary Assessment (EMA) studies have generally shown no association between affect and ad-lib smoking (Shiffman et al., 2002; Shiffman et al., 2004), on global questionnaires, the majority of DS report negative-affect smoking (Ikard et al., 1969; Kassel et al., 2003; McKennell, 1970), which may reflect the influence of nascent nicotine withdrawal symptoms (Parrott, 1998), even during ad libitum smoking. Since it is unlikely that ITS would experience nicotine withdrawal (Shiffman et al., 1995), they may be less reactive to negative affect. On the other hand, if smoking can actually mitigate negative affect arising from stressors, rather than withdrawal (Parrott, 1998), this may provide negative reinforcement for smoking (Kassel et al., 2003), explaining why ITS report smoking when stressed (Shiffman et al., 2012). We thus expected ITS to demonstrate greater reactivity to negative affect cues.
We also hypothesize that positive affect may increase ITS’ craving to a greater extent than DS’, especially if ITS are particularly likely to smoke in positively-valenced situations such as social gatherings and parties. Indeed, smoking in positive affect situations has been observed in young, light smokers (Hedeker et al., 2009).
In addition, we tested cues associated with abstinence rather than smoking- that is, cues (such as “no-smoking” signs) associated with smoking prohibitions, which are increasingly prevalent. We hypothesize that ITS may show greater negative reactivity (e.g., decreased responding compared to neutral cues), as a prior study showed that chippers were unlikely to smoke when no one else was smoking (Shiffman and Paty, 2006).
In summary, we assessed ITS’ reactivity and compared it to DS’ in response to a panel of cues that included proximal smoking cues (cigarettes), distal cues such as alcohol and positive and negative affect, and an “anti-cue” - smoking prohibitions, evaluating response to each cue compared to a neutral control. To more generally assess the degree of stimulus control, above and beyond response to any one cue, we examined variability of response across cues to capture differential reactivity across the panel of cues, allowing for responses to particular cues to be idiosyncratic. Such idiographic analysis demonstrated that chippers’ smoking was under greater stimulus control, even where comparisons on specific stimuli did not show differences (Shiffman and Paty, 2006). Finally, we assessed craving and its relationship to smoking among ITS, who may either not experience craving at all or may experience craving that is unrelated to their smoking behavior, given their lower dependence. We analyzed cue reactivity, craving, and smoking among DS in a previous paper (Shiffman et al., in press-a); here we present analyses of these phenomena among ITS and comparisons of ITS and DS.
Methods
Subjects
Subjects were 446 community volunteers from Pittsburgh, PA, recruited by advertisement and promotion. 207 were DS, and 239 were ITS (This overlaps with samples reported in Shiffman et al., in press-c; Shiffman et al., 2012). We oversampled African-American (AA) smokers. Participants had to be ≥ 21 years old, report smoking ≥ 3 years, smoking at their current rate for ≥ 3 months, and not planning to quit within the next month. DS had to report smoking every day, averaging 5–30 cigarettes per day (CPD; actual smoking: M = 16.0 ± 6.0 CPD). ITS had to report smoking 4–27 days per month (actual smoking: M = 4.4 ± 2.9 CPD; M = 16.8 ± 7.7 days per month). Subjects were paid $135 for the study (which included procedures not reported here). The DS data were previously reported in Shiffman et al. (in press-a), and are reported here for comparison with ITS.
Procedures
Procedures have been described in detail in Shiffman et al. (in press-a). Briefly, cue exposure occurred over six separate sessions, one cue per session, with at least one day between sessions (M = 5.2 ± 7.0 days). Order of cue presentation was randomized to ensure that each cue was equally likely to appear in each position, and to be preceded and followed by every other cue (see Shiffman et al., in press-a); randomization to cue order was identical for DS and ITS. After a 3-minute acclimation period and pre-cue craving and affect ratings, a 3-minute initial cue exposure period started, during which 30 cue images were displayed for 6 seconds each on a 20-inch monitor (see Shiffman et al., in press-a). After another assessment, subjects were told they could smoke, and had access to two cigarettes. The cues continued to be displayed during a 15-minute free-smoking period, which ended with a final assessment. (Pilot data had suggested that this maintained craving.)
Cues
Cues were static images. Cues were selected to represent constructs hypothesized to influence craving and smoking (i.e., smoking cues, alcohol cues, negative and positive affect, and smoking prohibitions), with particular attention that the cues be potentially relevant to both DS and ITS. Each was displayed once during the 3-minute primary cue exposure and redisplayed five times during the 15-minute smoking period. Cue images were drawn from Gilbert and Rabinovich (1999), the International Affective Picture System (IAPS; Center for the Study of Emotion and Attention, 1999), Warthen and Tiffany (2009), and original photographs, and were subject to pilot testing. Further detail about cue sets and images used can be found in Shiffman et al. (in press-a).
Measures
The brief 10-item QSU (Cox et al., 2001) was administered four times. Each response was marked on a segmented visual-analog scale with 49 segments; thus scores ranged from 1 to 49. The QSU yielded scores for appetitive craving and distress-relief craving. Difference scores (pre- to post-cue exposure) were used as an index of cue-induced craving. Due to extreme outliers, we applied a square root transform (preserving the sign of the difference) to reduce the scatter and improve model fits, which was used for all analyses unless otherwise indicated. To detect a uniform-response set, we inserted an item that was the opposite of an existing scale item. Sessions where subjects marked both items identically (i.e., in contradictory directions; 1 DS; 8 ITS) were removed as invalid.
The Affect Mood Form (Diener and Emmons, 1985) was administered at the same four time-points as the QSU. It yielded scores for positive and negative affect (PA and NA, respectively). In addition, participants’ time since last cigarette (TSLC) was recorded upon arrival to each session.
After the initial cue exposure and post-cue craving assessments, subjects began a 15-minute period in which they were allowed to smoke ad libitum. Smoking topography measures, including latency to smoke and number and duration of puffs, were coded from the video of the session by independent observers blind to cue condition smoking (Shiffman et al., in press-a). Prior research (Blank et al., 2009) has shown that observational data closely mirror those collected by topography instruments and were validated by CO measures (Shiffman et al., in press-a). Exhaled CO was assessed using a Vitalograph Breath CO monitor (Vitalograph Inc., Lenexa, KS) at three time-points: upon arrival, immediately prior to cue exposure, and immediately following the free-smoking period.
Analysis
Most subjects completed all six cues (201 DS; 97%; 211 ITS; 88%). We included subjects with partial data (22 DS; 28 ITS) whose data included the neutral stimulus and at least one other cue. The analysis comprised 2,586 cue-exposure sessions (DS: 1,201; ITS: 1,385).
Statistical Analysis
Mixed models (Brown and Prescott, 2006), using SAS PROC-MIXED (SAS Institute, Cary, NC), were used to examine continuous outcomes (affect, craving, CO, puffs, puff time). Generalized estimating equations (GEE), using a logit link, were used to examine probability of smoking and lighting a second cigarette after cue exposure. Survival analysis examined latency to smoke, using recurrent event models specifying a Gompertz survival function (using Stata streg; StataCorp, College Station, TX) with provisions for frailty (the equivalent of random intercept models) (Hosmer et al., 2008). All models except survival were weighted by race to account for oversampling of AA smokers. Stimulus effects (for all variables, including affect) were specified as contrasts to the neutral cue in all models. Analyses of alcohol cue effects were limited to those who reported drinking alcohol (DS: 150; ITS: 214). Finally, to capture whether ITS demonstrated more stimulus-specificity, we computed the between-cue variation in craving increase for each subject (i.e., the standard deviation of cue response, across cues) and compared these across groups. We observed order effects (the first session was different from subsequent sessions) and thus, session order was controlled for in all analyses. The response to a particular cue did not depend on which cue had been shown previously (i.e., there were no carry-over effects). A more detailed description of model selection and specification appears in Shiffman et al. (in press-a), and the majority of analytic plan for the current study is similar or identical to that approach, with a few exceptions. Specifically, the current paper reports race-weighted descriptive statistics for ITS and DS, as these two groups differed in racial composition (Trinidad et al., 2009). In addition, whereas Shiffman et al. (in press-a) used a log-transformation to examine post-cue craving effects on probability to smoke among DS, examination of the data suggested this was not a suitable approach for both groups; we therefore used untransformed data here. Because ITS and DS differed in latency to smoke, we adjusted for this in evaluating when subjects lit a second cigarette, as it affected the time available to light another cigarette. Because ITS and DS differed in pre-cue craving, we tested whether co-varying pre-cue craving affected analyses of cue reactivity. Among ITS, we also tested whether there were differences in reactivity based on heaviness of smoking, contrasting ITS who smoked less or more than the median cigarettes per day (n=2.37).
We estimated power to detect effects for various outcomes (see Shiffman et al., in press-a for assumptions). For cue effects on craving among ITS alone, there was 80% power to detect a standardized effect size of 0.24 (in SD units) for cue main effects and group by cue interactions. For comparing DS and ITS, there was 80% power to detect an effect size of 0.14 for between-group main effects and 0.18 for detecting group by cue interactions. Parallel values for the probability of smoking were 0.14 and 0.17 and for smoking parameters (number of puffs, puff duration, and CO; measured only among those who smoked), 0.17 and 0.21. These detectable effects are smaller than “small effect” sizes (Cohen, 1992). Finally, for survival analyses, detectable hazard ratios (HRs) were HR = 0.72 and 2.18, for main effects and interactions, respectively.
Results
We first present the results specific to ITS, followed by ITS-DS comparisons.
ITS
Time since last cigarette
The median TSLC was 5.50 hours, with broad variations. In 24.21% of sessions, ITS reported having smoked within 30 minutes of the session, with similar proportions reporting having smoked 30 minutes to 5 hours prior (25.58%), >5 hours to 1 day prior (28.32%), and >1 day (21.89%) prior to the session. ITS with TSLC ≤ 30 minutes showed significantly lower craving (both pre-cue and post-cue) than those with longer TSLC (data not shown). Those who smoked within 30 minutes had the greatest increase in craving following exposure, particularly compared to those who had not smoked for >1 day. Although those effects of TSLC were significant, they were very small, and there was no TSLC by cue interaction. TSLC was not significantly related to smoking parameters and did not interact with the effect of cues on smoking parameters. Covarying TSLC from craving and smoking (below) made no difference, so unadjusted data are presented.
Reactivity
Affect
Among ITS (Figure 1), NA ratings increased in response to negative affect cues (p < .0001) and decreased in response to positive affect cues (p < .001), compared to neutral cues. PA ratings increased in response to positive affect cues (p < .001) and alcohol cues (p < .01; among individuals who reported drinking alcohol), and decreased in response to negative affect cues (p < .001), compared to neutral cues. [For descriptive statistics of pre- and post-cue PA and NA ratings, see Online Resource 1].
Figure 1.

Change in positive affect (a) and negative affect (b) following exposure to cues, for ITS and DS. (Alcohol cue effects were limited to those who reported drinking alcohol.) Error bars are standard errors.
Craving
ITS reported modest levels of appetitive and distress-relief craving, both before and after cue exposure (see Online Resource 1). Both craving scores increased significantly after exposure to each cue, except for a non-significant decrease after the positive affect cues and a non-significant increase after the neutral cues. To evaluate reactivity, craving changes following each cue were compared to changes after the neutral cue. ITS experienced significant increases in appetitive craving after exposure to smoking cues and alcohol cues and significantly decreased appetitive craving after exposure to positive affect cues (compared to neutral cues; see Figure 2). Distress-relief craving also increased following negative affect cues, but otherwise showed a similar pattern, with increases following smoking and alcohol cues, and decreases following positive affect cues.
Figure 2.

Change in appetite craving (a) and distress-relief craving (b) following exposure to cues, for ITS and DS. (Alcohol cue effects were limited to those who reported drinking alcohol.) Error bars are standard errors.
Smoking
ITS smoked in 55% (n = 784) of all sessions, lighting up after a median of 44.5 seconds; in 12% of sessions where smoking occurred, a second cigarette was lit (Table 1). Cues had no effect on the probability of smoking, of smoking a second cigarette, or on latency to smoke. When smoking did occur, subjects took significantly more puffs (+1.10; p < .03) and puffed longer (+1.51 seconds; p < .03) when exposed to the smoking cue versus the neutral cue; the increased puff time was due to the increased number of puffs. CO increase was 0.54 ppm higher in the smoking prohibited condition compared to neutral (p < .05), although this was no longer significant when controlling for puff time.
Table 1.
Smoking Parameters by Cue
| ITS | DS | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutral | Smoking | Alcohol | Positive | Negative | Smoking prohibited | Neutral | Smoking | Alcohol | Positive | Negative | Smoking prohibited | ITSa (overall) | DSa,b (overall) | ITS-DSc difference | ||
| M (SD)/% | M (SD)/% | M (SD)/% | M (SD)/% | M (SD)/% | M (SD)/% | M (SD)/% | M (SD)/% | M (SD)/% | M (SD)/% | M (SD)/% | M (SD)/% | M (SE)/% | M (SE)/% | p | ||
| n | 239 | 229 | 229 | 227 | 234 | 229 | 207 | 201 | 194 | 205 | 196 | 198 | 239 | 207 | ||
| % who smoked | 54.57 | 56.36 | 56.33 | 53.08 | 54.77 | 53.47 | 82.99 | 85.48 | 85.18 | 84.40 | 82.59 | 83.47 | 54.89 | 84.14 | <.0001 | |
| % who lit second cigaretted | 12.46 | 17.92 | 15.47 | 16.69 | 12.09 | 13.37 | 20.62 | 27.83 | 24.24 | 25.92 | 26.10 | 19.81 | 12.14 | 21.68 | .0037e | |
| Median latency to smoke (sec)d | 42.00 | 36.00 | 43.00 | 48.00 | 41.00 | 43.00 | 37.00 | 39.00 | 38.00 | 39.00 | 39.00 | 41.00 | 44.5f | 40.5f | <.0001 | |
| # of puffsd | 13.88 (6.51) | 14.98 (8.85) | 14.48 (6.89) | 13.92 (8.40) | 14.18 (6.81) | 14.25 (7.67) | 14.83 (6.98) | 15.39 (7.40) | 14.58 (7.48) | 14.91 (7.41) | 15.03 (7.29) | 14.57 (6.29) | 13.32 (0.47) | 14.45 (0.44) | <.0001 | |
| Total puff time (sec)d | 19.81 (12.82) | 21.32 (13.52) | 20.09 (11.22) | 19.63 (13.19) | 21.00 (12.24) | 19.90 (12.23) | 22.78 (11.08) | 23.36 (11.66) | 22.90 (12.98) | 24.19 (13.91) | 23.99 (12.87) | 23.44 (12.64) | 18.99 (0.81) | 22.71 (0.79) | <.0001 | |
| CO change post-pre cue (ppm) | ||||||||||||||||
| Subjects who smoked | 2.71 (2.45) | 2.86 (2.42) | 2.99 (2.26) | 2.59 (2.64) | 3.06 (2.38) | 3.25 (2.36) | 3.22 (3.34) | 3.34 (3.69) | 3.21 (4.06) | 3.91 (3.66) | 3.40 (3.87) | 3.33 (4.40) | 2.76 (0.13) | 3.33 (0.18) | <.0001 | |
| Subjects who did not smoke | −0.49 (1.27) | −0.35 (1.53) | −0.61 (1.73) | −0.48 (1.10) | −0.29 (1.31) | −0.31 (1.08) | −1.70 (1.63) | −2.71 (3.05) | −2.38 (3.06) | −4.39 (6.10) | −2.44 (2.49) | −2.41 (2.63) | −0.49 (0.08) | −2.37 (0.27) | <.0001 | |
Note.
Mean of within-subject means
Among n = 207 DS. Details, including unweighted data by stimulus, have been presented in Shiffman et al. (in press-a).
Test of mean differences, controlling for order and stimulus.
Among those who smoked (DS: n = 1040 sessions, n = 198 participants; ITS: n = 784 sessions, n = 186 participants).
Analysis controlled for log latency to light the first cigarette, which affected the time available to light a second.
Median of within-subject medians across cues.
Effect of craving on smoking
ITS’ craving intensity robustly predicted smoking. When subjects reported higher craving intensity just before the smoking period (i.e., post-cue), they were more likely to smoke, and latency to smoking was reduced (Table 2). In both cases, the effects were non-linear, being greatest at lower levels of craving intensity. For the likelihood of smoking (Figure 3), the probability initially rises steeply with increased craving; the rate of increase diminishes beyond mid-level craving intensity. For latency to smoke (Figure 4), ITS smoked more rapidly at higher levels of craving, but differences in magnitude of this effect were significantly greater at the lower end of craving intensity. Craving increases had a linear effect on smoking parameters (number of puffs, puff duration, and CO increase; see Table 2).
Table 2.
Effects of Craving on Smoking Parameters
| Outcome Variable | |||||||
|---|---|---|---|---|---|---|---|
| Predictor Variable | ITS | DS | Interaction: ITS v. DS | ||||
|
| |||||||
| Likelihood of Smoking (Logistic Regression using GEE) | OR | 95% CI | p | OR | 95% CI | p | p |
| Appetitive Craving | |||||||
| Post-cue craving effects | 1.11 | 1.09 – 1.13 | <.0001 | 1.06 | 1.04 – 1.08 | <.0001 | <.02 |
| Quadratic | 0.998 | 0.998 – 0.999 | <.0001 | 0.9992 | 0.998 – 1.0002 | <.07 | — |
| Decomposition of post-cue craving | |||||||
| Pre-Cue Craving | 1.10 | 1.09 – 1.12 | <.0001 | 1.06 | 1.04 – 1.08 | <.0001 | <.09 |
| Change in Cravinga | 1.37 | 1.26 – 1.48 | <.0001 | 1.13 | 1.03 – 1.23 | <.01 | <.07 |
| Distress-Relief Craving | |||||||
| Post-cue craving effects | 1.13 | 1.09 – 1.17 | <.0001 | 1.07 | 1.05 – 1.10 | <.0001 | <.08 |
| Quadratic | 0.997 | 0.996 – 0.998 | <.0001 | 0.999 | 0.997 – 0.999 | <.04 | <.08 |
| Decomposition of post-cue craving | |||||||
| Pre-Cue Craving | 1.11 | 1.07 – 1.15 | <.0001 | 1.08 | 1.06 – 1.10 | <.0001 | — |
| Change in Cravinga | 1.31 | 1.20 – 1.43 | <.0001 | 1.11 | 1.03 – 1.20 | <.01 | <.06 |
|
| |||||||
| Likelihood of Smoking 2 Cigarettes vs. 1 (Logistic Regression)b, c | OR | 95% CI | p | OR | 95% CI | p | p |
|
| |||||||
| Appetitive Craving | |||||||
| Post-cue craving effects | 1.03 | 1.01 – 1.05 | <.0008 | 1.03 | 1.01 – 1.04 | <.003 | — |
| Quadratic | 1.00 | 0.998 – 1.00 | — | 1.001 | 1.00 – 1.002 | — | — |
| Decomposition of post-cue craving | |||||||
| Pre-Cue Craving | 1.03 | 1.01 – 1.04 | <.002 | 1.04 | 1.02 – 1.06 | <.0001 | — |
| Change in Cravinga | 1.11 | 0.99 – 1.25 | <.08 | 1.08 | 0.98 – 1.20 | — | — |
| Distress-Relief Craving | |||||||
| Post-cue craving effects | 1.13 | 1.09 – 1.17 | <.0001 | 1.02 | 1.00 – 1.04 | <.04 | — |
| Quadratic | 0.997 | 0.996 – 0.998 | <.0001 | 1.002 | 1.00 – 1.003 | <.02 | — |
| Decomposition of post-cue craving | |||||||
| Pre-Cue Craving | 1.03 | 1.01 – 1.06 | <.002 | 1.03 | 1.01 – 1.05 | <.001 | — |
| Change in Cravinga | 1.24 | 1.09 – 1.41 | <.002 | 1.04 | 0.96 – 1.12 | — | <.05 |
|
| |||||||
| Latency to Smoke (Shared Frailty Survival Analysis) | HR | 95% CI | p | HR | 95% CI | p | p |
|
| |||||||
| Appetitive Craving | |||||||
| Post-cue craving effects | 1.06 | 1.06 – 1.07 | <.0001 | 1.04 | 1.03 – 1.04 | <.0001 | <.0001 |
| Quadratic | 0.998 | 0.998 – 0.999 | <.0001 | 0.99 | 0.999 – 1.00 | — | <.0001 |
| Decomposition of post-cue craving | |||||||
| Pre-Cue Craving | 1.06 | 1.05 – 1.07 | <.0001 | 1.03 | 1.03 – 1.04 | <.0001 | <.0001 |
| Change in Craving a | 1.19 | 1.14 – 1.24 | <.0001 | 1.09 | 1.04 – 1.13 | <.0001 | <.003 |
| Distress-Relief Craving | |||||||
| Post-cue craving effects | 1.91 | 1.74 – 2.11 | <.0001 | 1.03 | 1.02 – 1.04 | <.0001 | <.0001 |
| Quadratic | 0.98 | 0.91 – 1.06 | — | 0.999 | 0.998 – 0.999 | <.0001 | <.006 |
| Decomposition of post-cue craving | |||||||
| Pre-Cue Craving | 1.06 | 1.04 – 1.07 | <.0001 | 1.03 | 1.02 – 1.04 | <.0001 | <.0001 |
| Change in Cravinga | 1.17 | 1.11 – 1.24 | <.0001 | 1.05 | 1.01 – 1.10 | <.0001 | <.001 |
|
| |||||||
| Number of puffsc | B | 95% CI | p | B | 95% CI | p | p |
|
| |||||||
| Appetitive Craving | |||||||
| Post-cue craving effects | 0.07 | 0.04 – 0.10 | <.0001 | 0.09 | 0.06 – 0.12 | <.0001 | — |
| Quadratic | 0.0003 | −0.002 – 0.002 | — | 0.003 | 0.001 – 0.005 | <.01 | <.08 |
| Decomposition of post-cue craving | |||||||
| Pre-Cue Craving | 0.08 | 0.04 – 0.11 | <.0001 | 0.08 | 0.05 – 0.11 | <.0001 | — |
| Change in Cravinga | 0.12 | −0.06 – 0.29 | — | 0.28 | 0.12–0.43 | <.001 | — |
| Distress-Relief Craving | |||||||
| Post-cue craving effects | 0.12 | 0.07 – 0.16 | <.0001 | 0.11 | 0.07 – 0.15 | <.0001 | — |
| Quadratic | 0.002 | −0.001 – 0.004 | — | 0.004 | 0.002 – 0.006 | <.001 | — |
| Decomposition of post-cue craving | |||||||
| Pre-Cue Craving | 0.11 | 0.05 – 0.16 | <.0001 | 0.1 | 0.06 – 0.14 | <.0001 | — |
| Change in Cravinga | 0.34 | 0.13–0.55 | <.002 | 0.29 | 0.13 – 0.44 | <.001 | — |
|
| |||||||
| Puff Duration c | B | 95% CI | p | B | 95% CI | p | p |
|
| |||||||
| Appetitive Craving | |||||||
| Post-cue craving effects | 0.10 | 0.05 – 0.14 | <.0001 | 0.15 | 0.11 – 0.20 | <.0001 | — |
| Quadratic | 0.003 | −0.001 – 0.01 | — | 0.01 | 0.003 – 0.01 | <.0002 | — |
| Decomposition of post-cue craving | |||||||
| Pre-Cue Craving | 0.11 | 0.06 – 0.16 | <.0001 | 0.13 | 0.08 – 0.18 | <.0001 | — |
| Change in Cravinga | 0.20 | −0.07 – 0.46 | — | 0.51 | 0.25 – 0.77 | <.0002 | — |
| Distress-Relief Craving | |||||||
| Post-cue craving effects | 0.16 | 0.09 – 0.23 | <.0001 | 0.17 | 0.11 – 0.23 | <.0001 | — |
| Quadratic | 0.003 | −0.002 – 0.007 | — | 0.01 | 0.004 – 0.01 | <.0001 | <.08 |
| Decomposition of post-cue craving | |||||||
| Pre-Cue Craving | 0.13 | 0.05 – 0.21 | <.002 | 0.15 | 0.08 – 0.21 | <.0001 | — |
| Change in Cravinga | 0.52 | 0.20 – 0.84 | <.002 | 0.44 | 0.18 – 0.70 | <.001 | — |
|
| |||||||
| CO Increase (ppm)c | B | 95% CI | p | B | 95% CI | p | p |
|
| |||||||
| Appetitive Craving | |||||||
| Post-cue craving effects | 0.03 | 0.02 – 0.04 | <.0001 | 0.03 | 0.01 – 0.05 | <.001 | — |
| Quadratic | 0.0005 | −0.0005 – 0.001 | — | 0.001 | 0.00 – 0.003 | — | — |
| Decomposition of post-cue craving | |||||||
| Pre-Cue Craving | 0.04 | 0.02 – 0.05 | <.0001 | 0.03 | 0.01 – 0.05 | <.01 | — |
| Change in Cravinga | 0.01 | −0.06 – 0.09 | — | 0.12 | −0.001 – 0.25 | <.06 | — |
| Distress-Relief Craving | |||||||
| Post-cue craving effects | 0.03 | 0.02–0.05 | <.0003 | 0.04 | 0.02 – 0.06 | <.001 | — |
| Quadratic | 0.0001 | −0.001 – 0.001 | — | 0.00 | −0.00 0.00 | — | — |
| Decomposition of post-cue craving | |||||||
| Pre-Cue Craving | 0.03 | 0.01 – 0.05 | <.003 | 0.05 | 0.02 – 0.07 | <.001 | — |
| Change in Cravinga | 0.07 | −0.02 – 0.17 | — | 0.03 | −0.09 – 0.15 | — | — |
Note. – denotes p ≥.10. All analyses controlled for session number and stimulus and were weighted by race. Effect on the first line represents linear effects.
Analysis also controlled for pre-cue craving and uses square-root transformed QSU change scores to adjust for skewness.
Analysis controlled for log latency to light the first cigarette, which affected the time available to light a second cigarette.
Among those who smoked (DS: n = 1040 sessions, n = 198 participants; ITS: n = 784 sessions, n = 186 participants).
Figure 3.

Relationship between post-cue (just before the free-smoking period) appetitive craving (a) and distress-relief craving (b), and the probability of smoking, for DS and ITS. The group × craving interaction was significant for appetitive craving but not significant for distress-relief craving (see Table 2 for p-values). The figure shows the relationship based on raw data smoothed by the LOESS function (SAS PROC LOESS; smoothing parameter = .7).
Figure 4.

Model-based survival curves of latency to smoke among ITS for appetitive craving (a) and distress-relief craving (b) post cue exposure, just before the free smoking period. The effect of craving decreases at higher levels of craving, illustrating the nonlinear effects of craving on smoking. In comparison with DS, significant group × craving interactions in the quadratic effects indicate that this effect is much stronger among ITS. (For comparison, see DS survival curves in Shiffman et al., in press-a).
Craving reported after cue exposure, just before smoking, was decomposed into two additive components: craving before cue exposure and the change during initial exposure (Table 2). Both pre-cue craving and the post-cue increment in appetitive and distress-relief craving predicted latency to smoking and the probability of smoking. Distress-relief craving also predicted the probability of lighting a second cigarette, and number of puffs and puff duration.
Heaviness of smoking
We also examined whether heaviness of smoking moderated (interacted with) cue effects. Mostly, it did not. There were no moderating effects on craving, but there was an effect on the number of puffs, such that lighter-smoking ITS took significantly more puffs in response to the negative affect cues (+2.4 puffs; p= .03) and smoking cues (+3.9 puffs; p< .001), compared to neutral; a similar trend in response to alcohol cues among alcohol drinkers (+2.6 puffs; p= .06). Heavier ITS demonstrated no increases. No other smoking parameters were affected.
COMPARISON OF ITS AND DS
Affect
ITS and DS did not differ in pre-cue, post-cue, or change in PA or NA ratings (Online Resource 1). However, compared to DS, ITS showed a greater increase in PA ratings in response to the alcohol cue (p = .05) and 2.63-point greater decrease in PA ratings in response to the negative affect cue (p = .01; Figure 1), compared to the neutral cue. There was no stimulus by group interaction for NA ratings.
Craving
Prior to cue exposure, ITS reported far less appetitive and distress-relief craving than DS (Online Resource 1); ITS’ craving intensity was roughly half that of DS’. ITS and DS also differed in response to the neutral cue, with DS reporting greater increases in appetitive craving (p < .0001), but only a non-significant trend for distress-relief craving (p > .25; Figure 2).
There were significant group main effects, indicating that DS showed greater overall increases in craving, regardless of cue (i.e., including the neutral cue). There were also main effects of cues: as previously observed for each group separately, smoking cues and alcohol cues increased appetitive craving, and positive affect cues decreased appetitive craving. Smoking, alcohol, and negative affect cues increased distress-relief craving, and positive affect cues decreased it, for both groups combined. Because DS and ITS differed substantially in their pre-cue craving, we re-analyzed the cue-related increases in craving while controlling for pre-cue craving. This did not affect the pattern of stimulus effects on craving. The key question of whether DS and ITS differ in reactivity was addressed by the cue × group interaction, which were not significant: DS and ITS reacted similarly to the active cues.
Stimulus control
We assessed whether ITS were more stimulus-specific in craving, by comparing the between-cue standard deviation in response to cues. For appetitive craving, ITS averaged 5.01 ± 4.22 and DS averaged 4.77 ± 3.94; the two were not significantly different. For distress-relief craving, contrary to our hypothesis, ITS demonstrated significantly lower between-cue variation in distress-relief craving (p = 0.002), at 2.79 ± 3.45, versus DS at 3.74 ± 3.00.
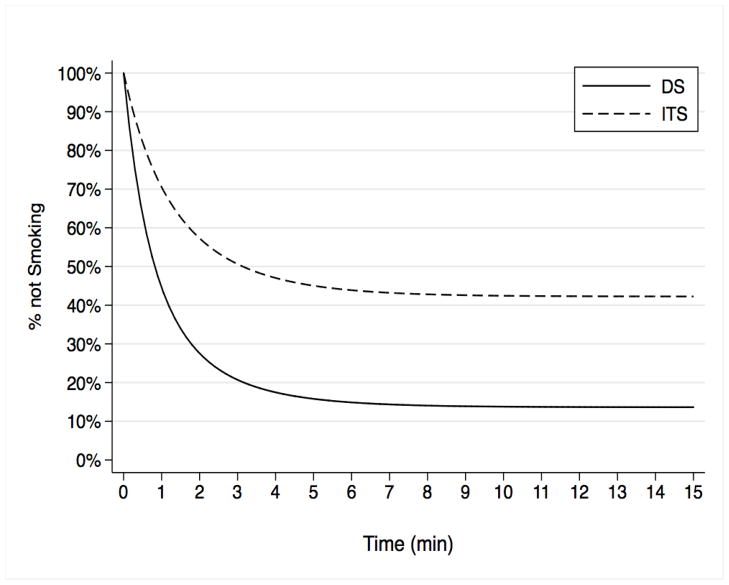
Smoking
ITS smoked significantly less often than did DS (Table 1) and waited longer to smoke (Figure 5). When they did smoke, ITS were about half as likely as DS to light a second cigarette. When they smoked, ITS and DS smoked a similar number of puffs, but ITS puffed for 3.72 seconds less (p < .01), even when covarying number of puffs. ITS’ CO also increased by 0.58 ppm less (p < .02), which was explained by their shorter puff times. To assess differences in reactivity, we computed group × cue interactions on smoking parameters (Online Resource 2). There were no interactions; cues did not differentially affect DS and ITS smoking.
Figure 5.
Model-based survival curves of latency to smoke among ITS and DS in the free smoking period following cue exposure (across all cues).
Effect of craving on smoking
We examined whether the relationship between craving intensity and subsequent smoking differed between ITS and DS. In survival models, post-cue craving had a greater influence on progression to smoking among ITS (Figure 4): both the linear and quadratic effects were stronger among ITS (Table 2). As can be seen in Figure 3, ITS demonstrated a steep relationship between post-cue craving and likelihood of smoking, especially at the lower end of craving intensity (see Shiffman et al., in press-a for comparison to DS). Stated another way, when craving is very intense, DS and ITS are about equally as likely to smoke and progress about equally rapidly to smoking. But when craving intensity is low, DS are more likely to smoke (appetitive craving, p < .02; ns for distress-relief craving; Table 2) and progress more rapidly to smoking than ITS. For likelihood of smoking a second cigarette, puff parameters (i.e., number of puffs and total puff time), and CO increase among those who did smoke, the relationship between craving and these variables was similar for both ITS and DS; there were no group × craving interactions (Table 2).
Discussion
The aim of this study was to explore hypothesized differences between ITS and DS in reactivity to both proximal and distal cues for smoking. The data revealed no differences in cue reactivity – either in craving or in smoking behavior – across a battery of five diverse cues. Although their absolute levels of craving and smoking were consistently lower, ITS’ reactions to cue exposures were similar to those of DS.
Moreover, while we had hypothesized that ITS would demonstrate more differentiation among cues (i.e., a more variable reaction), this was not seen; indeed, ITS demonstrated lower differentiation among cues for distress-relief craving. Thus, these data from laboratory cue presentations provide little evidence supporting the hypothesis that ITS’ craving or smoking are under greater stimulus control. This stands in contrast to a previous finding (Shiffman and Paty, 2006) in which real-time monitoring of smoking using EMA (Shiffman, 2009) showed that chippers’ smoking was under greater stimulus control. It may be that behavior of current ITS may be materially different from that of chippers 25 years ago, when light smoking was quite rare. It could also be that data from real-world EMA could differ from lab cue reactivity data, since the latter represent passive exposure to isolated stimuli, whereas EMA captures responses to holistic and complex situations that smokers voluntarily encounter in the real world. It would be useful to understand the relationship between lab cue reactivity responses and real-world smoking patterns.
Our findings also seem at odds with data collected by questionnaire: whereas ITS were more likely than DS to cite drinking and stress among their top-three smoking situations (Shiffman et al., 2012) and to cite response to cues as their most important smoking motive (Shiffman et al., in press-b), the laboratory data showed no differences in craving or smoking when subjects were shown alcohol-related or emotionally-distressing cues. This discrepancy may be explained by the fact that questionnaire reports of smoking patterns do not appear to accurately capture real-world patterns of smoking or craving (Shiffman, 1993).
Although differences in cue reactivity were not seen, there were notable differences between ITS and DS. Consistent with their less frequent smoking and low dependence (Shiffman et al., 2012), ITS reported less craving, both before and after cue exposure. ITS were also less likely to smoke when allowed. If they did smoke, they waited almost 5 times longer to light up, were less likely to light a second cigarette, and spent less time puffing.
Although craving is sometimes regarded as pathognomonic of addiction, the data clearly show that ITS not only experience craving, but that their craving is associated with subsequent smoking: among ITS, higher craving intensity was associated with greater likelihood of smoking and more intense smoking. Indeed, the relationship between craving and smoking was actually stronger among ITS than DS. EMA studies similarly found that craving better predicts smoking among chippers than heavy smokers (Shiffman and Paty, 2006). Thus, craving is not limited to dependent smokers and may actually play a greater role in smoking among less-dependent smokers.
Since DS and ITS did not differ in their response to cues, the combined data addresses cue reactivity of smokers in general. As already seen among DS in this sample (Shiffman et al., in press-a), exposure to proximal smoking cues or to alcohol cues increases craving, while exposure to positive affect cues reduces craving. The only additional effect seen in this larger combined sample was that distress-relief craving (but not appetitive craving) was increased by exposure to negative affect cues. Both DS and ITS may look to smoking to help relieve emotional distress, consistent with the finding that ITS designate being under stress as a situation conducive to smoking (Shiffman et al., 2012).
The combined data also confirmed the conclusion previously seen with DS alone (Shiffman et al., in press-a): that specific cues had no effects on smoking. Even in a sample of 446 individuals and 2,586 sessions, with within-subject controls, we observed no influence of cues on the probability of smoking, latency to smoking, amount of smoking, or biochemical exposure during smoking. This raises significant questions about the relevance of laboratory cue reactivity assessments to actual smoking behavior, rather than just to craving (Perkins, 2009).
The study further found no relationship between ITS’ exposure to particular cues and subsequent smoking. Among ITS, neither the likelihood of smoking nor the latency to smoking were associated with any of the five particular cue exposures tested in this laboratory-based paradigm. ITS did smoke slightly more intensely when exposed to a proximal smoking cue, but no effects were seen for alcohol or affect cues, and ITS’ smoking responses to cues did not differ from the pattern observed among DS. Importantly, our findings of no cue effects on smoking, and no difference in reactivity between DS and ITS, cannot be attributed to lack of power; the study had adequate power to detect even small effects.
The data present a seeming paradox: cues did affect craving levels, but did not affect smoking, even though smoking was correlated with craving. One possible explanation is that the cue-specific effects on craving were not strong enough to translate into effects on smoking behavior. If different subjects reacted to different cues, this would weaken cue effects while still showing effects of craving, including increased craving across cues. Subjects’ craving may have been driven by non-specific factors, including their reactions to the laboratory setting (see Sayette et al., 2000) and non-specific or idiosyncratic responses to the cues, including the neutral cue.
Of course, real-world responses may differ from what is observed in the laboratory. Otherwise, these findings may require a reconceptualization of ITS’ smoking. While ITS do not smoke in order to maintain nicotine levels, their smoking does not appear, in this laboratory assessment, to be triggered by particular situational stimuli, challenging the suggestion that their smoking can be explained by stimulus control (Shiffman and Paty, 2006).
Interpretation of this study must consider methodological limitations. Although the cue exposure manipulations were experimentally assigned and thus allow causal inferences, the analyses relating craving to smoking were correlational, so causal inferences cannot be made. The absolute magnitude of reactivity observed here was modest; perhaps other cues (e.g., personalized cues; Conklin et al., 2010) or study procedures would have elicited stronger responses. The modest effects observed may be a function of the experimental conditions. Perhaps larger differences between DS and ITS would be observed under conditions of social influence (likely to affect ITS > DS) or greater deprivation (likely to affect DS > ITS). Notably, though, Sayette et al. (2001) saw no differences in reactivity between chippers and heavy smokers even under conditions of deprivation. Importantly, if ITS smoke only in specific settings, they may have found the laboratory setting (a bare room in the middle of the day) not conducive to craving or smoking. The cues used in the study may have been of limited potency. However, modest mean effects, reflecting wide variation in response to cues, may actually be more conducive to observing individual or group differences in response, which could be overwhelmed by uniformly potent cues. It is also worth noting that the cues and context were potent enough to yield increases in craving among ITS, which predicted smoking, and to evoke smoking in most ITS (77% smoked in at least one session, suggesting that the approach in this study did elicit meaningful increases in craving and smoking behavior. In any case, the study, based on 446 subjects and 2,586 reactivity sessions, was well-powered to detect even small differences between DS and ITS.
There are also some issues of interest that are not covered by the present analyses. For example, these analyses did not compare the responses of ITS who previously engaged in daily smoking to those of ITS who had never smoked daily. The analyses also did not address the relationship of smoking to affect and affective reactivity, or to variations in nicotine dependence. These questions await further detailed analyses.
Within these limitations, the data challenge the idea that ITS are more responsive to particular cues, or to cues overall, while underscoring the relevance of craving to ITS’ smoking. ITS showed meaningful variations in craving that predicted the probability and intensity of smoking. Along with data showing that there are meaningful variations in dependence among ITS (Shiffman et al., in press-c), the data suggest that ITS are not entirely free of dependence, and that their smoking appears to be driven to some degree by the same factors that affect DS smoking (i.e., cigarette craving). This may also help explain why ITS seem to have unexpected difficulty quitting, achieving quit rates not much higher than DS (Tindle and Shiffman, 2011). Understanding ITS’ smoking may help shed light more broadly on the role of external cues as well as internal motivations that drive and maintain smoking and impede cessation.
Supplementary Material
Acknowledgments
This work was supported by grant R01-DA020742 (Shiffman) from the National Institutes of Health, National Institute on Drug Abuse. Additional support for authors was provided by National Science Foundation Graduate Research Fellowship (Dunbar), National Center for Research Resources (KL2-RR024154-03; Tindle), National Cancer Institute grants R25-CA057703-15 (Dunbar) and R01-CA141596-02 (Tindle) and Cancer Council Tasmania (Ferguson). The authors are grateful to Ken Perkins and Steve Tiffany for input on study design; to Anna Tsivina, Joe Stafura, Rachelle Gish, and Aileen Butera for their work conducting research sessions; to Chantele Mitchell-Miland for data management and preparation; to Laura Homonnay-Demilio for editorial assistance; and to Ellen Beckjord for providing critique of a draft of this article..
References
- Abrams DB, Monti PM, Carey KB, Pinto RP, Jacobus SI. Reactivity to smoking cues and relapse: Two studies of discriminant validity. Behav Res Ther. 1988;26:225–233. doi: 10.1016/0005-7967(88)90003-4. [DOI] [PubMed] [Google Scholar]
- Blank MD, Disharoon S, Eissenberg T. Comparison of methods for measurement of smoking behavior: Mouthpiece-based computerized devices versus direct observation. Nicotine Tob Res. 2009;11:896–903. doi: 10.1093/ntr/ntp083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H, Prescott R. Applied Mixed Models in Medicine. John Wiley & Sons; New York: 2006. [Google Scholar]
- Carter BL, Robinson JD, Lam CY, Wetter DW, Tsan JY, Day SX. A psychometric evaluation of cigarette stimuli used in a cue reactivity study. Nictotine Tob Res. 2006;8:361–369. doi: 10.1080/14622200600670215. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Center for the Study of Emotion and Attention. International Affective Pictures System: Digitized photographs. The Center for Research in Psychophysiology, University of Florida; Gainesville: 1999. [Google Scholar]
- Centers for Disease Control and Prevention; U.S. Department of Health and Human Services. Behavioral Risk Factor Surveillance System Survey Data. Centers for Disease Control and Prevention; Atlanta: 2008. [Google Scholar]
- Centers for Disease Control and Prevention . Cigarette smoking among adults -- United States, 2007. MMWR Surveill Summ. 2008;57:1221–1226. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . Vital signs: Current cigarette smoking among adults aged >18 years - United States, 2005–2010. MMWR Surveill Summ. 2011;60:1207–1212. [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis. Curr Dir Psychol Sci. 1992;1:98–101. [Google Scholar]
- Conklin CA, Perkins KA, Robin N, McClernon FJ, Salkeld RP. Bringing the real world into the laboratory: Personal smoking and nonsmoking environments. Drug Alcohol Depend. 2010;111:58–63. doi: 10.1016/j.drugalcdep.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Robin N, Perkins KA, Salkeld RP, McClernon FJ. Proximal versus distal cues to smoke: The effects of environment on smokers’ cue-reactivity. Exp Clin Psychopharmacol. 2008;16:207–214. doi: 10.1037/1064-1297.16.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christensen A. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Diener E, Emmons RA. The independence of positive and negative affect. J Pers Soc Psychol. 1985;47:1105–1117. doi: 10.1037//0022-3514.47.5.1105. [DOI] [PubMed] [Google Scholar]
- Drummond DC, Tiffany S, Glautier SP, Remington B. Cue exposure in understanding and treating addictive behaviours. In: Drummon DCSTT, Glautier SP, Remington B, editors. Addictive Behaviour: Cue Exposure Theory And Practice. John Wiley and Sons; Chichester: 1995. [Google Scholar]
- Gilbert DG, Rabinovich NE. International smoking images series with neutral counterparts. Southern Illinois University, Intagrative Neuroscience Laboratory, Department of Psychology; 1999. [Google Scholar]
- Harrison ELR, Desai RA, McKee SA. Nondaily smoking and alcohol use, hazardous drinking, and alcohol diagnosis among young adults: Findings from the NESARC. Alcohol Clin Exp Res. 2008;32:2081–2087. doi: 10.1111/j.1530-0277.2008.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedeker D, Mermelstein RJ, Berbaum ML, Cambell RT. Modeling mood variation associated with smoking: An application of a heterogeneous mixed-effects model for analysis of ecological momentary assessment (EMA) data. Addiction. 2009;104:297–307. doi: 10.1111/j.1360-0443.2008.02435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmer JE, Lemeshow S, May S. Applied survival analysis: Regression modeling to time to event data. Wiley; New York: 2008. [Google Scholar]
- Ikard FF, Green D, Horn D. A scale to differentiate between types of smoking as related to the management of affect. Int J Addict. 1969;4:649–659. [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- McKennell AC. Smoking motivation factors. Br J Soc Clin Psychol. 1970;9:8–22. doi: 10.1111/j.2044-8260.1970.tb00632.x. [DOI] [PubMed] [Google Scholar]
- Niaura RS, Abrams DB, Demuth B, Pinto R, Monti PM. Responses to smoking-related stimuli and early relapses to smoking. Addict Behav. 1989;14:419–428. doi: 10.1016/0306-4603(89)90029-4. [DOI] [PubMed] [Google Scholar]
- Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. J Abnorm Psychol. 1988;97:133–152. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- Parrott A. Nesbitt’s Paradox resolved? Stress and arousal modulation during cigarette smoking. Addiction. 1998;93:27–39. doi: 10.1046/j.1360-0443.1998.931274.x. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Does cigarette smoking cause stress? Am Psychol. 1999;54:817–820. doi: 10.1037//0003-066x.54.10.817. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Wertz JM, Shiffman S, Perrott MA. A multi-dimensional analysis of cue-elicited craving in heavy smokers and tobacco chippers. Addiction. 2001;96:1419–1432. doi: 10.1080/09652140120075152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Shiffman S, Tiffany ST, Niaura R, Martin CS, Shadel WG. The measurement of drug craving. Addiction. 2000;95:S189–S210. doi: 10.1080/09652140050111762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schane RE, Glantz SA, Ling PM. Nondaily and social smoking: An increasingly prevelent pattern. AMA Arch Intern Med. 2009;169:1742–1744. doi: 10.1001/archinternmed.2009.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S. Assessing smoking patterns and motives. J Consult Clin Psychol. 1993;61:732–742. doi: 10.1037//0022-006x.61.5.732. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Ecological Momentary Assessment (EMA) in studies of substance abuse. Psychol Assessment. 2009;21:486–497. doi: 10.1037/a0017074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Balabanis M. Association between alcohol and tobacco. In: Ferting JB, Allen JP, editors. Alcohol and Tobacco: From Basic Science to Clinical Practice, NIH Publication. United States Department of Health and Human Services; Washington, D.C: 1995. p. 397. [Google Scholar]
- Shiffman S, Dunbar M, Kirchner T, Li X, Tindle H, Anderson S, Scholl S. Smoker reactivity to cues: Effects on craving and on smoking behavior. J Abnorm Psychol. doi: 10.1037/a0028339. (in press-a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, Scholl SM, Tindle HA. Smoking motives of daily and non-daily smokers: A profile analysis. Drug Alcohol Depend. doi: 10.1016/j.drugalcdep.2012.05.037. (in press-b) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Ferguson SG, Dunbar MS, Scholl SM. Tobacco dependence among intermittent smokers. Nicotine Tob Res. doi: 10.1093/ntr/nts097. (in press-c) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Gwaltney CJ, Balabanis M, Liu KS, Paty JA, Kassel JD, Hickcox M, Gnys M. Immediate antecedents of cigarette smoking: An analysis from ecological momentary assessment. J Abnorm Psychol. 2002;111:531–545. doi: 10.1037//0021-843x.111.4.531. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Kirchner TR, Ferguson SG, Scharf DM. Patterns of intermittent smoking: An analysis using Ecological Momentary Assessment. Addict Behav. 2009;34:514–519. doi: 10.1016/j.addbeh.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Paty JA. Smoking patterns and non-dependent smokers: Contrasting chippers and dependent smokers. J Abnorm Psychol. 2006;115:509–523. doi: 10.1037/0021-843X.115.3.509. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JD, Elash C. Nicotine withdrawal in chippers and regular smokers: Subjective and cognitive effects. Health Psychol. 1995;14:301–309. doi: 10.1037//0278-6133.14.4.301. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gwaltney CJ, Dang Q. Immediate antecedents of cigarette smoking: An analysis of unrestricted smoking patterns. J Abnorm Psychol. 2004;113:116–171. doi: 10.1037/0021-843X.113.1.166. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Kassel JD, Gnys M, Zettler-Segal M. Smoking behavior and smoking history of tobacco chippers. Exp Clin Psychopharmacol. 1994;2:126–142. [Google Scholar]
- Shiffman S, Tindle H, Li X, Scholl S, Dunbar M, Mitchell-Miland C. Characteristics and smoking patterns of intermittent smokers. Exp Clin Psychopharmacol. 2012 doi: 10.1037/a0027546. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacol (Berl) 1995;117:2–10. doi: 10.1007/BF02245088. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA); Substance Abuse and Mental Health Services Administration. Results from the national survey on drug use and health: National findings. NSDUH Series Office of Applied Studies; Rockville, MD: 2009. [Google Scholar]
- Tindle HA, Shiffman S. Smoking cessation behavior among intermittent smokers versus daily smokers. Am J Public Health. 2011;101:e1–3. doi: 10.2105/AJPH.2011.300186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinidad DR, Perez-Stable EJ, Emery SL, White MM, Grana RA, Messer KS. Intermittent and light daily smoking across racial/ethnic groups in the United States. Nicotine Tob Res. 2009;11:203–210. doi: 10.1093/ntr/ntn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warthen M, Tiffany ST. Evaluation of cue reactivity in the natural environment of smokers using Ecological Momentary Assessment. Exp Clin Psychopharmacol. 2009;17:70–77. doi: 10.1037/a0015617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson N, Carpenter M, Saladin M, Gray K, Upadhyaya H. Evidence for greater cue reactivity among low-dependent vs. high-dependent smokers. Addict Behav. 2010;35:673–677. doi: 10.1016/j.addbeh.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.