Abstract
Background
Dementia is an important consequence of Parkinson’s disease (PD), with few known modifiable risk factors. Cumulative exposure to lead, at levels experienced in the community, may exacerbate PD-related neural dysfunction, resulting in impaired cognition.
Methods
Among 101 persons with PD (“cases”) and, separately, 50 persons without PD (“controls”), we evaluated cumulative lead exposure, gauged via tibia and patella bone lead concentrations, in relation to cognitive function, assessed using a telephone battery developed and validated in a separate sample of PD patients. We also assessed the interaction between lead and case-control status.
Results
After multivariable adjustment, higher tibia bone lead concentration among PD cases was associated with worse performance on all of the individual telephone tests. In particular, tibia lead levels corresponded to significantly worse performance on a telephone analogue of the Mini-Mental State Examination and tests of working memory and attention. Moreover, higher tibia bone lead concentration was associated with significantly worse global composite score encompassing all the cognitive tests (P=0.04). The magnitude of association per standard deviation increment in tibia bone lead level was equivalent to the difference in global scores among controls in our study who were about seven years apart in age. The tibia lead-cognition association was notably stronger within cases than within controls (Pdifference=0.06). Patella bone lead concentration was not consistently associated with performance on the tests.
Conclusions
These data provide evidence suggesting that cumulative exposure to lead may result in worsened cognition among persons with PD.
Keywords: lead exposure, cognitive function, Parkinson’s disease
INTRODUCTION
Dementia is a major health concern in individuals with Parkinson’s disease (PD), with a prevalence in PD patients estimated to be 2–6 times that in persons without PD.1 PD patients who develop dementia have higher risks of institutionalization and mortality, their health care is more costly, and their caregivers report more stress.2–5 Even PD patients without clinically evident intellectual deterioration exhibit subtle deficits in specific areas of cognitive function, such as verbal fluency, executive functions, visuospatial skills, and recall memory; these deficits, too, are associated with increased risk for disability and hospitalization.6–8 Nonetheless, few risk factors, particularly modifiable ones, have been identified. Whether environmental toxicants are involved in the development of PD-related dementia has not been addressed, although emerging evidence suggests that exposure to toxicants such as lead may affect risk of neurodegenerative conditions in adulthood.
Lead is a well-established neurotoxicant, and a growing body of data suggests that cumulative exposure to lead corresponds to poor cognitive function in older adulthood.9–11 Impaired cognitive function frequently portends the development of dementia in persons with PD.12–13 Recent data also indicate that lead exposure is associated with elevated risk of PD.14–16 Exposure to lead may have ramifications for cognition in PD, because lead, acting on several targets, may exacerbate damage that is already present in PD. One likely mechanism is oxidative stress: lead induces iron-dependent lipid peroxidation and also interferes with antioxidant capacity.17–20 Lead damages mitochondria,21 which can result in neural excitotoxicity and apoptosis, and interferes with neurotransmitter storage and release.22 In particular, lead appears to disrupt processes involved in cholinergic, glutamatergic and dopaminergic systems,22–23 pathways through which it may impair learning ability and executive function. Because lead accumulates throughout the brain,24 its toxic effects are not restricted to the substantia nigra, the anatomic region primarily affected in PD. Rather, lead exposure may magnify the burden of PD on cognition, reducing the available mechanisms for compensating for PD-related impairments.
PD is thought to involve oxidative damage to and mitochrondrial dysfunction in the dopaminergic cells of the substantia nigra.25–26 Given the overlap of PD and lead exposure pathologies, we hypothesized that higher cumulative exposure to lead corresponds to worse cognitive functioning in persons with PD. In addition, we explored a secondary hypothesis: that lead-induced dysfunction and damage on a background of PD-related damage and impaired neurotransmitter systems, may result in cognitive effects of lead that are more pronounced than among otherwise healthy older adults.
We evaluated these relationships in a subset of participants enrolled in an existing study in which participants’ cumulative exposure to lead was assessed by non-invasive in-vivo K-xray fluorescence (KXRF) spectrometry measurements of lead content in bone.
METHODS
Study participants
We developed a telephone-based assessment of cognitive functions that typically decline in PD, and we validated this assessment against an in-person assessment (see Supplemental Data, part 1). We recruited participants for our study of lead exposure and cognitive function from an existing case-control study of lead exposure and PD (see Supplemental Data, part 2).14 To ensure a broad representation of lead exposure and key covariates among participants in the cognitive study, we selected cases (persons with PD) and controls (persons without PD) within strata of previously measured tibia bone lead concentration (tertiles), age (<70; 70+) and sex. Of the 126 cases we invited for cognitive assessments, 4 had died, and of those remaining, 9 (7%) could not be located, 8 (7%) were too ill to participate, and 4 (3%) refused. Of the 60 controls invited, we were unable to locate 7 (12%), and 3 (<1%) refused. Altogether, we completed assessments of 101 cases and 50 controls. All cases were confirmed by movement disorder specialists using the U.K. Brain Bank criteria.27 This study was approved by the Human Research Committees of the Brigham and Women’s Hospital, and the Beth Israel Deaconess Medical Center. Written informed consent was obtained from participants in the validation study. Participants in the lead exposure study provided their written informed consent for the assessment of their lead exposure and oral consent for their cognitive assessment.
Development and validation of telephone cognitive battery
We developed our telephone cognitive assessment battery based on a validated telephone battery for assessing age-related cognitive decline.28 To this battery, we added tests of cognitive domains that typically decline in PD. To keep the battery at a length acceptable to participants, we removed tests from the original battery that likely would not provide additional useful information about PD patients’ cognitive status. Altogether, the telephone battery contained nine tests. The Telephone Interview for Cognitive Status (TICS)29 is a test of global cognition modelled on the Mini-Mental State Examination (MMSE);30 scores on the two tests are strongly correlated (Pearson correlation, 0.94).29 The battery also contained a test of delayed recall of the TICS 10-word list, as well as a test of delayed recognition of these words. For a test of category fluency, participants were asked to name as many animals as they could in 1 minute,31 and for a test of phonemic fluency, participants were asked to name as many words beginning with letter “f” as they could in 1 minute.32 Both of these tests also gauge executive function and psychomotor speed. The Digit Span Forward and Digit Span Backward tests measured working memory and attention.32 Finally, we administered an oral version of the Trail Making Test (TMT)32 of psychomotor speed and executive function in which we asked participants to count aloud from 1 to 52 as quickly and as clearly as possible (TMT Part A), and then to count aloud from 1 to 26 but interspersing between each number its coordinating ordinal letter (i.e., 1-A, 2-B, 3-C, etc.; TMT Part B). Both counting exercises were timed, and we subtracted the time to perform Part A from the time to perform Part B, in seconds, to obtain an orally based estimate of a “Trails B minus A” score. We also explored an alternative measure from this test: the difference between the number reached in Part A at 10 seconds and the number reached in Part B at 30 seconds. Because nearly all participants complete the test up to these time points even if they do not complete the entire oral TMT, this metric potentially minimizes missing data.
Assessment of cumulative exposure to lead
We assessed participants’ cumulative exposure to lead using KXRF spectrometric estimates of lead concentration in their tibia and patella bones. Tibia bone is primarily cortical bone, in which lead has a slow turnover rate—estimated at a half-life of over 40 years33—making it a good surrogate for lifetime exposure. Patella bone is primarily trabecular bone; lead in patella turns over at a faster rate, with a half-life of less than a decade. Together, these exposure measures can help determine the relevant exposure interval or duration.
Thirty-minute bone lead measurements were taken with a KXRF instrument of the left tibia and patella, after each region had been washed with a 50% solution of isopropyl alcohol.34 The tibia was measured at mid shaft—the mid-point between the tibial plateau and the medial malleolus. The KXRF beam collimator was sited perpendicular to the flat bony surface of the tibia and at 30° in the lateral direction for the patella. Tibia and patella bone lead measurements with estimated uncertainties greater than 10 and 15 μg/g bone, respectively, were excluded as these measurements usually reflect excessive subject movement during the measurement.
Statistical analysis
We evaluated the validity of the telephone cognitive assessment using Spearman correlations between the individual in-person/telephone test pairs and between in-person and telephone global scores. The global scores were computed as the average z score from each component score (where z scores were computed using the validation population means and standard deviations).
To describe our data from the lead exposure study, we computed mean patella and tibia bone lead concentrations across levels of key participant characteristics and tested differences across these levels using F tests. For this part of the study, we computed z scores for each cognitive test using the lead study participants’ means and standard deviations; global cognitive scores were the average z score from each component of the telephone test. Using data from the cases, we fit a series of linear regression models to evaluate the association of cumulative lead exposure with cognition in PD. We regressed the individual telephone cognitive test z score onto the cumulative lead exposure measure, with separate models for each cognitive test and each lead exposure measure (i.e., patella and tibia bone lead concentration), and report differences in standardized cognitive score per standard deviation (SD) increment in each bone lead measure (10-μg/g increment in bone lead concentration). All models were adjusted for age at cognitive assessment, sex, race (white, not white, missing), education (high school diploma or less, associate’s degree, bachelor’s degree, master’s or doctoral degree), and pack-years of cigarette smoking (never smoked, smoked for <20 pack-years, smoked for ≥20 pack-years). We conducted sensitivity analyses in which we: further adjusted for age of onset of PD symptoms (available for 66% of cases); changed the cut-point for pack-years to 10 (median among ever smokers); and in which we substituted the alternative trails difference scores for the original “B minus A” scores in computing the global cognitive score. To provide context for the magnitude of our findings, we estimated the sex-, race-, education-, and smoking-adjusted association between age and the global cognitive score among the controls; the beta coefficient for age served as a benchmark for interpreting the beta coefficients for bone lead.
We further explored whether the telephone battery could detect differences between persons with and without PD by incorporating data from controls in these models and comparing cases’ and controls’ cognitive test scores. To explore the possibility that PD and lead exposure have a synergistic effect on cognitive function, we also fit multivariable-adjusted models that incorporated controls. In these models, we added terms for case-control status as well as a cross-product between case-control status and the lead exposure measure. To ensure overlap of cases’ and controls’ ages, we restricted these analyses to participants who were aged 60–80 years at cognitive testing.
RESULTS
Validation of the telephone cognitive assessment
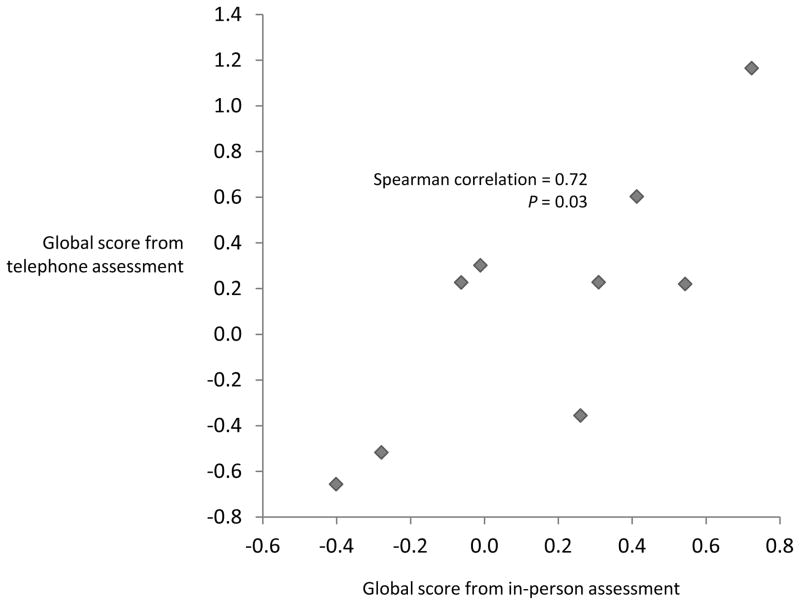
Among the 10 participants in our validation study, performance on the in-person cognitive tests corresponded well to performance on the telephone tests (Table 1, Supplemental data and Supplemental table 1). Importantly, global scores computed from the two modes of assessment were significantly correlated (Spearman correlation=0.72, P=0.03; Figure).
Table 1.
Spearman correlationsa between analogue cognitive test scores from the in-person assessment and telephone cognitive assessments.
| In-person cognitive test | Telephone cognitive test | Function(s) tested | Spearman correlation | P value |
|---|---|---|---|---|
| Global Cognition Tests | ||||
| Mattis Dementia Rating Scale (MDRS) | Telephone Interview for Cognitive Status (TICS) | Global | 0.60 | 0.07 |
|
| ||||
| Learning and Memory Tests | ||||
| Delayed word recall from CVLT, total correct | Delayed 10-word recall | Verbal learning and memory | 0.43 | 0.2 |
| Delayed word recall from CVLT, total correct | Delayed 10-word recognition | 0.29 | 0.4 | |
|
| ||||
| Fluency Tests | ||||
| Animal naming | Animal naming | Semantic fluency | 0.80 | 0.006 |
| “a” naming | “f” naming | Phonemic fluency | 0.60 | 0.07 |
| “f” naming | “f” naming | Phonemic fluency | 0.85 | 0.002 |
|
| ||||
| Trail Making Tests | ||||
| Trails A | Oral trails A | Psychomotor speed | 0.29 | 0.4 |
| Trails B | Oral trails B | Psychomotor speed, executive function | 0.75 | 0.02 |
| Trails B minus A | Oral trails B minus A | Executive function | 0.47 | 0.2 |
| Trails B minus A | Number reached by 10 seconds (A) minus letter-number pair reached by 30 seconds (B) | Executive function | 0.53 | 0.1 |
N=10 for all pairs, except for in-person and oral Trails B and in-person and oral trails B minus A.
Figure. Correlation between global scoresa from in-person and telephone cognitive assessments (N=9).
aGlobal scores were the average z score of all component tests. Component tests of the in-person global score: MDRS, the total correct delayed recall of words from the CVLT, animal naming, “a” naming, “f” naming, and Trails B minus A (reversed). Component tests of the telephone global score: TICS, delayed 10-word recall, delayed 10-word recognition, digit span forward, digit span backwards, animal naming, “f” naming, and Trails B minus A (reversed).
Association of cumulative exposure to lead with cognitive function in persons with PD
Among participants with PD, patella lead concentrations were significantly lower in those with less extensive smoking history, and tibia lead concentrations were significantly lower in those with more formal education (master’s degree or more)(Table 2).
Table 2.
Mean patella and tibia bone lead concentrations by key characteristics of participants with Parkinson’s disease in the study of lead exposure and cognition.
| Characteristic | Patella bone lead
|
Tibia bone lead
|
||||
|---|---|---|---|---|---|---|
| N (%)a | Mean concentration (sd), μg/g bone | P value | N (%)a | Mean concentration (sd), μg/g bone | P value | |
| Age at cognitive interview | 0.01 | 0.5 | ||||
| 54.0–64.9 | 20 (20%) | 6.3 (11.5) | 20 (20%) | 5.1 (11.5) | ||
| 65.0–69.9 | 35 (36%) | 11.1 (7.1) | 35 (35%) | 9.3 (10.9) | ||
| 70.0–74.9 | 24 (24%) | 6.6 (10.3) | 25 (25%) | 7.1 (8.4) | ||
| 75.0–80.9 | 19 (19%) | 14.7 (10.2) | 21 (21%) | 8.8 (11.8) | ||
| Sex | 0.2 | 0.9 | ||||
| Female | 44 (45%) | 8.4 (10.2) | 45 (45%) | 8.0 (10.5) | ||
| Male | 54 (55%) | 10.8 (9.7) | 56 (55%) | 7.7 (10.8) | ||
| Race | 0.7 | >0.9 | ||||
| White | 88 (87%) | 9.4 (10.1) | 88 (87%) | 7.9 (10.9) | ||
| Other | 5 (5%) | 9.4 (5.1) | 5 (5%) | 7.8 (9.4) | ||
| Missing | 8 (8%) | 12.7 (10.2) | 8 (8%) | 6.7 (8.5) | ||
| Educational attainment | 0.2 | 0.04 | ||||
| Up through high school | 19 (19%) | 11.6 (10.3) | 19 (19%) | 9.9 (9.1) | ||
| Associate’s degree | 12 (12%) | 10.2 (10.7) | 12 (12%) | 10.9 (11.5) | ||
| Bachelor’s degree | 28 (29%) | 11.8 (8.8) | 29 (29%) | 10.4 (12.5) | ||
| Master’s or doctoral degree | 39 (40%) | 7.1 (10.0) | 41 (41%) | 4.2 (8.7) | ||
| Smoking history | 0.004 | 0.07 | ||||
| Never smoked | 58 (59%) | 6.8 (9.0) | 59 (58%) | 6.2 (10.8) | ||
| Ever smoked | 40 (40%) | 13.8 (9.7) | 42 (42%) | 10.1 (10.1) | ||
| By doseb | 0.001 | 0.2 | ||||
| < 20 pack-years | 30 (75%) | 12.7 (9.5) | 32 (76%) | 9.7 (10.4) | ||
| 20+ pack-years | 10 (25%) | 17.3 (10.1) | 10 (24%) | 11.3 (9.3) | ||
| Age of PD symptom onset | 0.3 | 0.7 | ||||
| 35.0–54.9 | 12 (19%) | 7.5 (13.3) | 12 (18%) | 9.4 (12.0) | ||
| 55.0–64.9 | 30 (48%) | 7.9 (8.4) | 31 (47%) | 6.5 (11.1) | ||
| 65.0–78.9 | 21 (33%) | 12.1 (10.7) | 23 (35%) | 7.6 (10.7) | ||
Percentages are of the number of participants with valid data on the bone lead measure and characteristic.
Percentages shown are among ever smokers.
Higher tibia lead concentration was associated with worse performance on all of the telephone cognitive tests among these persons with PD (Table 3). In particular, in multivariable-adjusted analyses, tibia lead levels corresponded to significantly worse performance on the TICS (P=0.05), the digit span forward test (P=0.03), and the digit span backward test (P=0.05). Tibia lead’s association with worse performance on the “f” naming test was borderline significant, as well (P=0.06). Furthermore, higher tibia lead concentration was associated with significantly worse overall performance, as gauged by the global cognitive score (P=0.04). The difference in global scores per SD increment in tibia lead concentration was equivalent to the difference in scores among controls in our study who were about seven years apart in age. By contrast, patella bone lead concentration was not consistently associated with cognitive performance. Additional adjustment for age of PD symptom onset or alternative categorizations of smoking revealed similar findings. Findings were also similar when we substituted the alternative trails difference test score for the original oral “B minus A” score in computing the global score.
Table 3.
Adjusteda difference (95% confidence interval [CI]) in standardized cognitive score per 10-μg/g increment in bone lead concentration.
| Cognitive test | Patella bone lead
|
Tibia bone lead
|
||||
|---|---|---|---|---|---|---|
| N | Difference (95% CI) | P | N | Difference (95% CI) | P | |
| Telephone interview for cognitive status (TICS) | 98 | −0.08 (−0.32 to 0.15) | 0.5 | 101 | −0.20 (−0.40 to −0.00) | 0.05 |
| Delayed 10-word recall | 97 | 0.05 (−0.18 to 0.28) | 0.7 | 100 | −0.04 (−0.23 to 0.16) | 0.7 |
| Delayed 10-word recognition | 94 | 0.01 (−0.22 to 0.24) | 0.9 | 96 | −0.01 (−0.21 to 0.20) | >0.9 |
| Animal naming | 98 | −0.11 (−0.32 to 0.10) | 0.3 | 101 | −0.11 (−0.29 to 0.07) | 0.2 |
| “F” naming | 97 | −0.07 (−0.30 to 0.17) | 0.6 | 100 | −0.19 (−0.39 to 0.01) | 0.06 |
| Digit span forward | 97 | −0.02 (−0.27 to 0.22) | 0.9 | 100 | −0.23 (−0.43 to −0.03) | 0.03 |
| Digit span backward | 97 | 0.05 (−0.17 to 0.27) | 0.7 | 100 | −0.19 (−0.37 to −0.00) | 0.05 |
| Oral trails B minus Ab | 85 | 0.03 (−0.23 to 0.28) | 0.8 | 87 | −0.06 (−0.29 to 0.17) | 0.6 |
| Global score | 85 | −0.01 (−0.14 to 0.13) | 0.9 | 87 | −0.13 (−0.25 to −0.01) | 0.03 |
Analyses adjusted for age at cognitive assessment, sex, race, education, and smoking history.
Scores reversed so that lower score reflects worse performance.
Association of lead exposure with cognitive function, by PD status
Among all study participants, bone lead concentrations were lowest in the youngest age group; patella lead concentrations were significantly lower in those with less extensive smoking history and in those with more formal education (Supplemental table 2). On average, individuals with PD performed worse than controls on all of the cognitive tests. For example, in analyses adjusted for age, sex, race, smoking history, and tibia lead level, global cognitive scores of the cases were, on average, 0.32 standard units worse than scores of the controls (95% CI, −0.50 to −0.13). The inverse association of tibia lead level with global cognition was pronounced among the cases, but absent among the controls (Pdifference in tibia Pb associations=0.06). In a model that included both cases and controls, a SD increment in tibia lead level among the cases corresponded to a global cognitive score that was worse by 0.12 standard units (95% CI, −0.22 to −0.01), but among controls, tibia lead level was not significantly associated with global score (difference in score per 10-μg/g-unit increment in tibia lead, 0.06; 95% CI, −0.09 to 0.20). We did not find evidence of an interaction between patella lead and PD; however, the power to detect effects in controls was limited in this small sample.
DISCUSSION
We found that higher cumulative exposure to lead, gauged by tibia bone lead concentration, is associated with worse cognition in persons with PD, independent of age, sex, race education and smoking history. The decrement in global cognitive score per SD increment in tibia lead was similar to the decrement in scores we observed between persons without PD who were seven years apart in age. Our findings further suggest that lead exposure may specifically exacerbate the cognitive impairment caused by PD, although our power to detect effects in controls was lower. The significant associations in our study were confined to those pertaining to tibia lead concentration. Associations with patella bone lead concentration were inconsistent and not significant. This suggests that long-term exposure to lead may have greater influence than more recent exposure on cognition in PD, or, alternatively, that tibia lead, by integrating exposures over longer periods, is more likely than patella lead to capture the period of exposure relevant to cognition in PD. Our findings, in tandem with the previously observed association between higher tibia lead concentration and PD risk,14 are also consistent with the possibility that lead exposure influences the development of a particular phenotype of PD that entails more rapid onset of cognitive decline and dementia.13, 35
This study has several limitations and strengths. To assess participants’ cognition, we used a validated telephone-based rather than an in-person cognitive battery. Telephone-based cognitive assessments are receiving increasing use in large-scale research on aging-related cognitive decline (e.g., 28, 36–37) and they offer some practical advantages over in-person assessments, notably enhanced participation. This advantage is especially important for studies involving participants who have mobility difficulties, such as those with PD. By design, the telephone battery could not include tests of visuospatial function, which is adversely affected in PD13, 38 and by exposure to lead.9 Yet, it is clear that other cognitive functions which the battery measures well – attention, memory, and executive function – decline in PD.13, 38 Moreover, in our data, these functions, particularly attention and executive function, appeared to be deleteriously associated with lead exposure.
Our study was small and cross-sectional in design. In spite of the study’s size, we detected several strong and significant associations between tibia lead concentrations and cognitive function. A larger study would have provided greater statistical power to detect more subtle effects, including potentially, those corresponding to patella bone lead concentration. Likewise, a larger sample of persons without PD would have improved our capacity to detect and precisely estimate the tibia lead-cognition association in this group. While studies of community-exposed adults that included more than 10 times as many participants were able to detect significant adverse associations between tibia lead level and cognition, these associations were comparably small (about one-third the association in our participants with PD).10, 39
Although the cross-sectional design precluded us from evaluating longitudinal changes in cognition, it is unlikely that reverse causation is a concern in our study. The extended exposure windows captured by the bone lead measures meant that lead exposure preceded the cognitive outcomes and, especially in the case of tibia lead, the onset of PD itself.
In this observational study, it remains possible that our findings could be explained by confounding. One potential source of confounding is duration of PD. As described previously,14 in the recent environment of relatively low lead levels, the normal process of bone formation could dilute bone lead concentrations. PD-related osteopenia could slow this process;40 as a result, for a given history of exogenous lead exposure, shorter duration of PD at the time of lead exposure assessment could result in lower bone lead concentrations. Longer duration of PD is also associated with adverse cognitive sequelae.2 Yet disease duration is unlikely to be an important source of confounding in our data, because further adjustment for age at PD symptom onset did not change our findings. Moreover, because vascular perfusion is greater in trabecular than in cortical bone, the link between disease duration and osteopenia would be more likely to influence association estimates for patella lead than for tibia lead. However, findings from patella lead analyses were null. Unmeasured dimensions of socioeconomic status also may be a potential source of confounding. Via the study enrollment process, our participants demonstrated their similar access to health care, and we adjusted all analyses for age, sex, race, education and smoking history. Moreover, in alternative analyses restricted to the 41 PD cases with a masters or doctoral degree, tibia bone lead concentration remained associated with significantly worse global cognitive score.
Findings from several studies of adults with occupational and community-level exposures to lead have identified associations of cumulative lead exposure with both poor cognition9–11 and PD.14–16 The present study is the first of which we are aware to identify an association between lead exposure and cognition among persons with PD. If this represents a causal relationship, then, in addition to being a primary risk factor for PD, lead exposure would be among the few known modifiable risk factors for cognitive decline and, potentially, PD-related dementia. Lead’s involvement could also reveal mechanisms of cognitive decline in PD. By inducing oxidative stress17–18 impairing the brain’s ability to respond to oxidative stress,19, 41 damaging mitochondria, interfering with calcium-dependent enzymes, chronic exposure to lead may increase the vulnerability of cortical and subcortical regions of a PD-affected brain to inflammation, impaired neurotrophic capacity and apoptosis,2 all of which may adversely affect cognitive functioning, particularly working memory.
In summary, this study of persons with PD provides evidence that higher levels of cumulative exposure to lead are associated with worse cognitive function, suggesting yet another way in which community-level exposure to lead manifests itself in chronic diseases of older age.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Kathy Gimbel, Hongshu Guan, Neechi Mosha, Kim Newton, Brooke Schuemann, Sanjin Tunovic for their contributions to the cognitive assessments, coordination of this study, and review of the data.
Funding sources for the study: Support for this research was provided in part by the National Institute of Environmental Health Sciences, National Institutes of Health grants P30ES000002, R01ES010798, and P30ES017885.
Footnotes
Financial disclosure/Conflicts of Interest: The authors report no conflict of interest related to the research described in this manuscript.
Author Roles
Dr. Weuve: research project conception, organization and execution; statistical analysis design, execution and interpretation; manuscript writing and revision study concept and design, data analysis and interpretation, drafting or revising the manuscript
Dr. Press: research project organization and execution; manuscript revision
Dr. Grodstein: manuscript revision
Dr. Wright: statistical analysis interpretation; manuscript revision
Dr. Hu: research project conception; statistical analysis interpretation; manuscript revision
Dr. Weisskopf: research project conception, organization and execution; statistical analysis interpretation; manuscript revision
Full Financial Disclosures of all Authors for the Past Year
Over the past year, Dr. Weuve received support from the National Institutes of Health (NIH)-National Institute on Environmental Health Sciences (NIEHS). She also was a consultant for the Alzheimer’s Association and an anonymous foundation. Dr. Press has no disclosures to report. Drs. Grodstein, Wright, Hu and Weisskopf all received support from the NIH.
References
- 1.Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson disease. J Neurol Sci. 2010;289(1–2):18–22. doi: 10.1016/j.jns.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 2.Caviness JN, Lue L, Adler CH, Walker DG. Parkinson’s disease dementia and potential therapeutic strategies. CNS Neurosci Ther. 2011;17(1):32–44. doi: 10.1111/j.1755-5949.2010.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aarsland D, Larsen JP, Karlsen K, Lim NG, Tandberg E. Mental symptoms in Parkinson’s disease are important contributors to caregiver distress. Int J Geriatr Psychiatry. 1999;14(10):866–874. [PubMed] [Google Scholar]
- 4.Aarsland D, Larsen JP, Tandberg E, Laake K. Predictors of nursing home placement in Parkinson’s disease: a population-based, prospective study. J Am Geriatr Soc. 2000;48(8):938–942. doi: 10.1111/j.1532-5415.2000.tb06891.x. [DOI] [PubMed] [Google Scholar]
- 5.Louis ED, Marder K, Cote L, Tang M, Mayeux R. Mortality from Parkinson disease. Arch Neurol. 1997;54(3):260–264. doi: 10.1001/archneur.1997.00550150024011. [DOI] [PubMed] [Google Scholar]
- 6.Buchman AS, Leurgans SE, Boyle PA, Schneider JA, Arnold SE, Bennett DA. Combinations of motor measures more strongly predict adverse health outcomes in old age: the rush memory and aging project, a community-based cohort study. BMC Med. 2011;9:42. doi: 10.1186/1741-7015-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray AM, Bennett DA, Mendes de Leon CF, Beckett LA, Evans DA. A longitudinal study of parkinsonism and disability in a community population of older people. J Gerontol A Biol Sci Med Sci. 2004;59(8):864–870. doi: 10.1093/gerona/59.8.m864. [DOI] [PubMed] [Google Scholar]
- 8.Guttman M, Slaughter PM, Theriault ME, DeBoer DP, Naylor CD. Burden of parkinsonism: a population-based study. Mov Disord. 2003;18(3):313–319. doi: 10.1002/mds.10333. [DOI] [PubMed] [Google Scholar]
- 9.Shih RA, Hu H, Weisskopf MG, Schwartz BS. Cumulative lead dose and cognitive function in adults: a review of studies that measured both blood lead and bone lead. Environ Health Perspect. 2007;115(3):483–492. doi: 10.1289/ehp.9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weuve J, Korrick SA, Weisskopf MA, et al. Cumulative exposure to lead in relation to cognitive function in older women. Environ Health Perspect. 2009;117(4):574–580. doi: 10.1289/ehp.11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bandeen-Roche K, Glass TA, Bolla KI, Todd AC, Schwartz BS. Cumulative lead dose and cognitive function in older adults. Epidemiology. 2009;20(6):831–839. doi: 10.1097/EDE.0b013e3181b5f100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hobson P, Meara J. Risk and incidence of dementia in a cohort of older subjects with Parkinson’s disease in the United Kingdom. Mov Disord. 2004;19(9):1043–1049. doi: 10.1002/mds.20216. [DOI] [PubMed] [Google Scholar]
- 13.Williams-Gray CH, Evans JR, Goris A, et al. The distinct cognitive syndromes of Parkinson’s disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132(Pt 11):2958–2969. doi: 10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- 14.Weisskopf MG, Weuve J, Nie H, et al. Association of cumulative lead exposure with Parkinson’s disease. Environ Health Perspect. 2010;118(11):1609–1613. doi: 10.1289/ehp.1002339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coon S, Stark A, Peterson E, et al. Whole-body lifetime occupational lead exposure and risk of Parkinson’s disease. Environ Health Perspect. 2006;114(12):1872–1876. doi: 10.1289/ehp.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorell JM, Johnson CC, Rybicki BA, et al. Occupational exposures to metals as risk factors for Parkinson’s disease. Neurology. 1997;48(3):650–658. doi: 10.1212/wnl.48.3.650. [DOI] [PubMed] [Google Scholar]
- 17.Adonaylo VN, Oteiza PI. Pb2+ promotes lipid oxidation and alterations in membrane physical properties. Toxicology. 1999;132(1):19–32. doi: 10.1016/s0300-483x(98)00134-6. [DOI] [PubMed] [Google Scholar]
- 18.Acharya S, Acharya UR. In vivo lipid peroxidation responses of tissues in lead-treated Swiss mice. Ind Health. 1997;35(4):542–544. doi: 10.2486/indhealth.35.542. [DOI] [PubMed] [Google Scholar]
- 19.Ercal N, Treeratphan P, Hammond TC, Matthews RH, Grannemann NH, Spitz DR. In vivo indices of oxidative stress in lead-exposed C57BL/6 mice are reduced by treatment with meso-2,3-dimercaptosuccinic acid or N-acetylcysteine. Free Radic Biol Med. 1996;21(2):157–161. doi: 10.1016/0891-5849(96)00020-2. [DOI] [PubMed] [Google Scholar]
- 20.Ercal N, Gurer-Orhan H, Aykin-Burns N. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem. 2001;1(6):529–539. doi: 10.2174/1568026013394831. [DOI] [PubMed] [Google Scholar]
- 21.White LD, Cory-Slechta DA, Gilbert ME, et al. New and evolving concepts in the neurotoxicology of lead. Toxicol Appl Pharmacol. 2007;225(1):1–27. doi: 10.1016/j.taap.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Agency for Toxic Substances and Disease Registry. Toxicological profile for lead. Altanta, GA: Public Health Service, U.S. Department of Health and Human Services; 2007. [Google Scholar]
- 23.Neal AP, Guilarte TR. Molecular neurobiology of lead (Pb(2+)): effects on synaptic function. Mol Neurobiol. 2010;42(3):151–160. doi: 10.1007/s12035-010-8146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Widzowski DV, Cory-Slechta DA. Homogeneity of regional brain lead concentrations. Neurotoxicology. 1994;15(2):295–307. [PubMed] [Google Scholar]
- 25.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 2003;302(5646):819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 26.Keane PC, Kurzawa M, Blain PG, Morris CM. Mitochondrial dysfunction in Parkinson’s disease. Parkinsons Dis. 2011;2011:716871. doi: 10.4061/2011/716871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. Jama. 2004;292(12):1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 29.Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsych, Neuropsychol, Behav Neurol. 1988;1:111–117. [Google Scholar]
- 30.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A practical method for grading the state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 31.Goodglass H, Kaplan E. The Assessment of Aphasia. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- 32.Lezak MD. Neuropsychological Assessment. 3. New York, NY: Oxford; 1995. Chapter 9: Orientiation and Attention; pp. 335–384. [Google Scholar]
- 33.Wilker E, Korrick S, Nie LH, et al. Longitudinal changes in bone lead levels: the VA Normative Aging Study. J Occup Environ Med. 2011;53(8):850–855. doi: 10.1097/JOM.0b013e31822589a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aro AC, Todd AC, Amarasiriwardena C, Hu H. Improvements in the calibration of 109Cd K x-ray fluorescence systems for measuring bone lead in vivo. Phys Med Biol. 1994;39(12):2263–2271. doi: 10.1088/0031-9155/39/12/009. [DOI] [PubMed] [Google Scholar]
- 35.Halliday GM, McCann H. The progression of pathology in Parkinson’s disease. Ann N Y Acad Sci. 2010;1184:188–195. doi: 10.1111/j.1749-6632.2009.05118.x. [DOI] [PubMed] [Google Scholar]
- 36.DeFries T, Avendano M, Glymour MM. Level and change in cognitive test scores predict risk of first stroke. J Am Geriatr Soc. 2009;57(3):499–505. doi: 10.1111/j.1532-5415.2008.02132.x. [DOI] [PubMed] [Google Scholar]
- 37.Wadley VG, Unverzagt FW, McGuire LC, et al. Incident cognitive impairment is elevated in the stroke belt: the REGARDS study. Ann Neurol. 2011;70(2):229–236. doi: 10.1002/ana.22432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22(12):1689–1707. doi: 10.1002/mds.21507. quiz 1837. [DOI] [PubMed] [Google Scholar]
- 39.Shih RA, Glass TA, Bandeen-Roche K, et al. Environmental lead exposure and cognitive function in community-dwelling older adults. Neurology. 2006;67(9):1556–1562. doi: 10.1212/01.wnl.0000239836.26142.c5. [DOI] [PubMed] [Google Scholar]
- 40.Invernizzi M, Carda S, Viscontini GS, Cisari C. Osteoporosis in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(5):339–346. doi: 10.1016/j.parkreldis.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 41.Adonaylo VN, Oteiza PI. Lead intoxication: antioxidant defenses and oxidative damage in rat brain. Toxicology. 1999;135(2–3):77–85. doi: 10.1016/s0300-483x(99)00051-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.