Abstract
The complicity of centrosomes in carcinogenesis is unmistakable. Mounting evidence clearly implicates a robust correlation between centrosome amplification (CA) and malignant transformation in diverse tissue types. Furthermore, CA has been suggested as a marker of cancer aggressiveness, in particular the invasive phenotype, in breast and prostate cancers. One means by which CA promotes malignancy is through induction of transient spindle multipolarity during mitosis, which predisposes the cell to karyotypic changes arising from low-grade chromosome mis-segregation. It is well recognized that during cell migration in interphase, centrosome-mediated nucleation of a radial microtubule array is crucial for establishing a polarized Golgi apparatus, without which directionality is precluded. The question of how cancer cells maneuver their supernumerary centrosomes to achieve directionality during cell migration is virtually uncharted territory. Given CA is a hallmark of cancers and has been correlated with cancer aggressiveness, malignant cells are presumably competent in managing their centrosome surfeit during directional migration, although the cellular logistics of this process remain unexplored. Thus, another key angle worth pondering is whether an overabundance of centrosomes confers some advantage on cancer cells in terms of their migratory and invasive capabilities. Recent studies have uncovered a remarkable strategy that cancer cells employ to deal with the problem of excess centrosomes and ensure bipolar mitoses, viz., centrosome clustering. This review aims to change the narrative by exploring how an increased centrosome complement may, via aneuploidy-independent modulation of the microtubule cytoskeleton, enhance directional migration and invasion of malignant cells. We postulate that CA imbues cancer cells with cytoskeletal advantages that enhance cell polarization, Golgi-dependent vesicular trafficking, stromal invasion and other aspects of metastatic progression. We also propose that centrosome declustering may represent a novel, cancer cell-specific anti-metastatic strategy, as cancer cells may rely on centrosome clustering during migration as they do in mitosis. Elucidation of these details offers an exciting avenue for future research, as does investigating how CA may promote metastasis through enhanced directional migration.
“Coming together is a beginning; keeping together is progress; working together is success.”
-Henry Ford
Over the past decade, a tide of evidence implicating centrosomal aberrations in carcinogenesis has swelled to a nearly incontrovertible level. Perhaps the most striking and pervasive abnormality is a curious multiplication of the centrosome copy number, termed centrosome amplification (CA). This defect involves an increase in the normal centrosome endowment above one pre-S phase or two post-S phase. Additionally, amplified centrosomes are often structurally and functionally aberrant; for instance, they may exhibit increased pericentriolar material, enhanced microtubule nucleation capacity, excessive or too few centrioles, loss of centriole orthogonal orientation, and inappropriate localization in the cell (e.g., basal vs. apical), to name a few peculiarities [1, 2]. These anomalies in turn, spawn abnormalities in mitotic spindle assembly, fidelity of chromosome segregation during mitosis, and the maintenance of tissue architecture. While CA is a rarity in healthy adult human tissues, it afflicts a wide array of solid and hematological tumors to varying degrees, as detailed in a recent review [3]. Importantly, CA is frequently associated with various indices of tumor aggressiveness. For instance, CA correlates with increased tumor grade, size, metastasis, and/or recurrence in various types of cancer of the bladder, blood, bone and soft tissue, brain, breast, cervix, colorectum, head and neck, hepatobiliary tract, kidney, ovary, and prostate [4]. From these observations, the question naturally arises whether supernumerary centrosomes are simply innocent bystanders or whether they play a causative role in fueling tumor evolution. Incriminating studies have revealed that CA occurs in pre-cancerous and pre-invasive lesions, indicating CA is an early event in tumorigenesis [1]. Furthermore, one landmark study demonstrated that induction of CA can actually initiate tumor formation and metastasis in flies [5]. Thus it is becoming increasingly understood that rather than serving as a mere beacon of malignancy, supernumerary centrosomes actually drive malignant transformation. Indeed, several threads of evidence now suggest that cancer cells exploit the centrosome’s command over microtubule organization in order to evolve increasingly aggressive phenotypes.
Orchestrating a shape-shift: Role of the centrosome in cell polarization
Despite its diminutive size, the centrosome orchestrates a prodigious range of microtubule-dependent processes, such as polarized migration, morphogenesis, cell division, establishment of cellular polarity, intracellular trafficking, and cell signaling. These processes are typically disturbed in cancer cells. While the centrosome is not absolutely required for microtubule nucleation, it is often necessary to organize microtubules; hence, the centrosome is often termed the “microtubule organizing center” (MTOC) of the mammalian cell. Microtubules are inherently polarized structures because they are composed of repeating α, β-tubulin heterodimer subunits that are unidirectionally-oriented. Consequently, one terminus of the microtubule features α-tubulin (the “minus-end”) and the other β-tubulin (the “plus-end”), which microtubule motors can differentiate [6]. Anchoring microtubules at their minus-ends, the centrosome extends the filament’s plus-end towards the cell cortex. In an interphase cell with an approximately centrally-located centrosome (e.g., a non-migrating fibroblast), this radial configuration of polarized microtubules serves to distinguish the more peripheral reaches of the cytosol (which are near plus-ends) from the more central region (which is near minus-ends) [7].
Without any modification, such a microtubule array is radially symmetric and thus cannot designate apical-basal polarity. However, this symmetry can be broken by positioning the centrosome towards one or the other cellular pole, releasing microtubules from the centrosome, and/or selectively modifying microtubules in certain regions (e.g., stabilizing apical filaments vs. basal ones) [8]. Post-translational modification of microtubules may involve acetylation, detyrosination, polyglutamylation, and polyglycylation, which can demarcate subcellular localization and impact the binding of microtubule-associated proteins (MAPs) or plus-end-tracking proteins (+TIPs) such as EB1, CLIP-170, and APC [9]. Microtubule motors can distinguish between microtubules exhibiting certain post-translational modifications or MAP/+TIP-binding and appear to utilize these differences as guideposts. Moreover, the cell not only utilizes differentially-modified/modulated microtubules to designate cell polarity but also to drive cell polarization, in particular by reorganizing the cell cortex in a polarized fashion [10]. Microtubule plus-ends make contacts with the cell cortex, which permits delivery of cargo (e.g., PAR proteins) to distinct cortical regions. Furthermore, contact of microtubule plus-ends with factors embedded in the cortex (e.g., components of lipid rafts and focal adhesions or actin-regulating proteins) can alter the activity of these factors and/or the microtubules. Altogether, we envision that the cell modifies centrosome-nucleated microtubules to become specialized by location, becoming for instance “apical microtubules” or “basal microtubules,” which exhibit differences in structure, dynamicity, and affinity for MAPs, +TIPs, and motors.
Disparate cell lines differentially implement various mechanisms to break symmetry. For instance, fibroblasts establish front-rear polarity for migration by relocalizing the centrosome towards the leading edge (LE) and the nucleus towards the trailing edge [10]. Furthermore, fibroblasts use microtubules at the LE to promote actin polymerization and lamellipodium formation via stimulation of Rac1 and microtubules at the trailing edge to dissolve focal adhesions (FAs). A variety of other cells position the centrosome anteriorly and nucleus posteriorly during migration (such as macrophages, neurons, retinal pigment epithelial 1 (RPE1) cells, and U2OS osteosarcoma cells), whereas other cell lines establish an opposite orientation (such as lymphocytes, natural killer cells, and Potoroo kidney epithelial [4] cells) [11]. During development, epithelial cells, which rely on apical-basal polarity to execute their tissue functions, relocate the centrosome to the apical aspect of the cell and the nucleus at the basal aspect. Also, epithelial cells exhibit complex patterns of tubulin modification depending on their degree of polarization [12]. For example, spreading epithelial cells (i.e., ones that are polarized in two dimensions) feature a high proportion of detyrosinated microtubules, which point towards the spreading edge from the centrosomes. By contrast, epithelial cells undergoing morphogenesis (i.e, cells that are polarized in three dimensions) features microtubules with lesser degrees of tyrosination and substantially increased acetylation; in addition, these microtubules have been released from the centrosome in linear arrays [12]. In sum, the centrosome implements an extensive repertoire of molecular tools in different cell types to accomplish cell polarization for distinct purposes.
Ill-fated parity: How centrosome amplification drives tumorigenesis through disruption of cell polarity during mitosis
Polarity is acutely disturbed in cancer cells, especially in those derived from epithelia (i.e., carcinomas, which constitute the vast majority of cancers). It is hypothesized that epithelial cells oftentimes become metastatic by undergoing a physiological dedifferentiation and transitioning from an adherent, apically-basally-polarized, non-migratory phenotype to one more characteristic of mesenchymal cells, marked by a front-rear polarized, spindle-shaped morphology and high migratory capacity. We believe that the presence of too many centrosomes may trigger this loss of tissue architecture and power the epithelial-mesenchymal-transition (EMT) via at least two distinct mechanisms. Firstly, harboring a superabundant centrosome complement can predispose the cell to form more than two spindle poles during mitosis, a recently discovered cause of chromosomal instability (CIN). Cells with CA form a transient multipolar spindle intermediate during prophase, which results in incorrect microtubule-kinetochore attachments [13, 14]. In particular, there is a high frequency of merotelic attachments, in which one kinetochore is simultaneously attached to more than one pole. Merotelic attachments promote chromosome mis-segregation and thus CIN. These erroneous attachments frequently go undiagnosed by the cell, as the cell’s primary microtubule-kinetochore surveillance system, the spindle assembly checkpoint, poorly senses merotely. Numerical CA can therefore induce transient multipolarity during mitosis and, consequently, persistent karyotype rearrangement, which may allow cancer cells to evolve malignant phenotypes that resist checks on proliferation, migration, and invasion. A tantalizing possibility is that cells bearing amplified centrosomes may engender “metastasis stem cells” [15] that could harbor a genetic makeup and cytoskeletal architecture that together empower them to proliferate, migrate, and invade more aggressively, thus initiating tumor metastasis.
Secondly, centrosomes are often not only numerically but also functionally augmented, resulting in “empowered” centrosomes with amplified nucleation capacity. For instance, centrosomes derived from breast tumors exhibit markedly increased microtubule nucleation capacity that correlates with loss of tissue differentiation and p53 mutation [16]. In addition, breast tumors with numerically- and functionally-amplified centrosomes nucleate a microtubule array with significantly increased density [17]. Thus, cancer cells may be equipped with “super centrosomes” that possibly result in profoundly enhanced centrosome- and microtubule-dependent signaling. Studies of centrosomes in fly stem cells have shed much light on this paradigm. In asymmetric division of Drosophila larval neuroblasts, it is necessary for the spindle to be lopsided for normal development [18]. In this case, there is a precise division of labor between the mother and daughter centrosomes, which are structurally and functionally different. The mother centrosome is larger in size, exhibits robust microtubule-nucleating capacity, and localizes apically, whereas the smaller daughter centrosome nucleates a smaller aster and localizes to the basal aspect of the cell. Consequently, the cell can divide asymmetrically, partitioning specific cell fate determinants to one daughter (allowing it to differentiate) and thereby ensuring that the other daughter retains its stemness. When CA is present, it seems there are “too many cooks in the kitchen,” and the whole motley crew of centrosomes, clustered together at the two spindle poles, nucleates two robust asters. The result is an inappropriately symmetric spindle because there are “mother-like” centrosomes at both poles, resulting in equal partitioning of cell fate determinants to both progeny cells. The result of the symmetric division is production of two stem cells, which tips the scales in favor of hyperproliferation. When Drosophila neuroblasts were induced to exhibit CA (via overexpression of centrosome duplication factor, SAK) and then transplanted into the abdomens of wild-type hosts, these neuroblast cells formed tumors and even metastasized [5]. Altogether, the evidence that functionally amplified centrosomes can instigate or exacerbate cancer by perturbing the fine-tuned execution of mitosis in both stem- and non-stem cells is indeed compelling. It is worth pointing out that the centrosome is home to several oncogenic proteins and tumor suppressors [19] whose deregulation, owing to or in addition to CA, could clearly increase the risk for cellular transformation and cancer progression.
Moving forward with a focus: The microtubule cytoskeleton collaborates with numerous accomplices to facilitate directional cell migration
As discussed above, a dramatic re-localization of the centrosome underlies the establishment of the nuclear-centrosomal axis, which defines the path along which the cell directs its movement [11]. Centrosomal microtubules are selectively stabilized (via posttranslational modifications) in the direction of cell migration [20]. Centrosome reorientation also plays a key, determining role in post-mitotic reassembly of the Golgi apparatus (discussed in a later section). Microtubule-mediated delivery of Golgi-derived vesicles to the LE provides membrane and associated proteins needed for forward protrusion [4]. Importantly, the centrosome plays a key role in the control of cell shape changes and orchestration of cell movement, in conjunction with the actomyosin cytoskeleton, focal adhesion complexes (FAs), Rho GTPases, and a multitude of signaling and effector pathways. The richness and intricacy of this interaction network has been the subject of several excellent review articles [21–24]. In this section, we will briefly summarize the involvement of the centrosome and its numerous accomplices in the process of directional cell migration, with the aim of spotlighting some aspects of this fascinating cellular process that could potentially be impacted by the presence of too many centrosomes within a cancer cell.
Directional cell migration consists of four basic sequential events: (1) protrusion at the LE, (2) adhesion to the extracellular matrix (ECM), (3) cell body translocation, and (4) retraction of the trailing edge [25]. It is widely accepted that the polymerization of actin to generate protrusive activity at the front (anterior aspect), coupled with actomyosin filaments generating contraction at the sides and rear (posterior aspect), provide the major thrust for migration. Lamellipodia, the main organelles for cell movement in 2D, are protrusions whose characteristic flat, sheet-like structure arises from the presence of long, unbranched actin filaments at the lamellipodial base that then progress into a highly laterally-branched actin network at the LE. Interestingly, actin filaments in the lamellipodium form a treadmilling dendritic array and are oriented with their growing barbed ends abutting the LE [26, 27]. Basically, upon reception of signals that stimulate cell motility, the localized activation of Rac1 GTPase is thought to promote actin polymerization to form a protrusive LE in the direction from which the signal is sensed. The constant forward-oriented actin filament polymerization both pushes the LE forward and also creates a retrograde flow of actin towards the cell center. Actin filament disassembly closer to the cell body and actin monomer diffusion, produces a stream of actin monomers recycling to the LE.
Studies have revealed that microtubules that extend in the direction of the LE exhibit net polymerization at their plus-ends. By contrast, microtubules suffer bending and breakage in the cell body owing to the strong tide of actin retrograde flow. A net concentration of localized microtubule growth near the LE activates Rac1 and lamellipodial protrusion, while microtubule turnover in the cell body locally activates RhoA-mediated stress fiber formation and FA assembly in the cell body [28, 29]. Rac1 activation at the LE sets in motion a self-perpetuating cycle of membrane protrusion and actin retrograde flow that stabilizes one particular LE to maintain directional movement of the cell [22]. In many cases, the lamellipodial LE is interspersed with chemosensory filopodia - bundles of actin filaments that are tightly packed together and protrude forward. Rac1 activation at the leading edge also promotes formation of smaller focal complexes in the LE. By contrast, RhoA activity in the cell body drives the organization of actin and myosin into contractile stress fibers thus enhancing cell contractility [22]. Stress fiber formation additionally induces integrin clustering, drives the assembly of larger focal complexes, and promotes their maturation into FAs - processes which are also facilitated by the targeting and capture of dynamic microtubules by FAs.
Lamellipodia interact with and attach to their environment via FAs which translate actin treadmilling into forward translocation of the cell. FAs possess a multilaminar architecture that allows them to directly couple to the ends of actin filaments [30]. ECM binding by integrins induces the maturation of small and short-lived focal complexes into mature and longer-lived FAs. This maturation process is associated with the tension developed by attached actin bundles; factors increasing the tension cause maturation of focal contacts and vice versa [31–33]. This cycle culminates in the activation of contraction by motor proteins that supply forward traction forces and thus power cell movements [34]. However, in order for cells to productively move forward, FAs also have to release and disassemble underneath the cell body and in the rear of the cell. Dynamic microtubules play an important role in controlling this localized FA turnover [35, 36]. Thus, cell movement involves sequential activation and binding of integrins followed by clustering, integrin association with cytoskeletal adaptor molecules that couple it with actin cytoskeleton, myosin-force-mediated maturation of focal contacts, and then detachment and recycling of FA components. In summary, the centrosome-nucleated microtubule system makes vital contributions not only to the assembly of the lamellipodial actin network at the front, but also to myosin contraction at the middle and adhesion site disassembly at the rear of the cell.
One of the main ways by which microtubules achieve so much is by serving as tracks for polarized, motor-mediated transport of cytoskeleton regulatory proteins. The microtubule cytoskeleton also plays a multitude of more subtle roles in regulating cell shape, polarity, and motility in different cell types. For example, it is believed that the ends of microtubules located in the center of the active edge near the cell’s nucleo-centrosomal axis maximally stimulate the polymerization of actin and extension of the lamellipodium there, resulting in longitudinal cell spreading in the intended direction of movement [37]. The elongation-promoting action of microtubules counteracts the contractile action of actin-myosin cortex [38], and it has been proposed that microtubule lengths and densities determine the lengths of some motile cells [39]. Microtubules exhibit both structural (static and dynamic) and regulatory interactions with the actin cytoskeleton. For instance, microtubules have been reported to serve as tracks on which motors deliver regulators of actomyosin dynamics to cell edges [40, 41], and some studies have uncovered that one of the normal functions of microtubules is also to exert some form of inhibitory control on the cells’ contractile strength and the organization of actin filaments in the cell body [42]. In certain cell types, microtubule plus-ends are also reeled into the cell’s LE by cortex-associated dynein motors, thus contributing to orienting the centrosome and Golgi toward the LE [43]. Based on the extensive involvement of the microtubule cytoskeleton in various facets of cell motility, it would not be surprising if the keen eye finds that supernumerary centrosomes in cancer cells cause alterations in many of the metrics of directional cell migration.
Horsepower found, outward bound: Role of the centrosome in powering the mechanics of directional migration
The revelation that CA promotes tumorigenesis by precipitating mitotic aberrancies produces a deluge of questions in the inquiring mind: what are the cellular consequences of harboring supernumerary centrosomes in interphase? How are interphase-specific processes such as directional cell migration and invasion impacted by centrosomal surplus? How do cancer cells organize and maneuver these extra centrosomes to successfully execute directional migration? It is particularly noteworthy that several clinical studies have demonstrated a strong correlation between CA and the aggressiveness and poor prognosis of cancers of the breast, prostate, bladder, and cervix as well as leukemia and myeloma [3], demonstrating the prognostic value of centrosome status in a wide range of cancers. These compelling (albeit circumstantial) lines of evidence, combined with data showing the presence of excess centrosomes in both pre-invasive lesions and in high-grade mature tumors, raise the crucial question of whether amplified centrosomes may perhaps play a causal, aneuploidy-independent, epigenetic role in promoting metastasis in cancer cells. Do the extra centrosomes perhaps facilitate acquisition of metastatic character by influencing cytoskeletal and organellar organization in a way that enhances cancer cell migration and invasion?
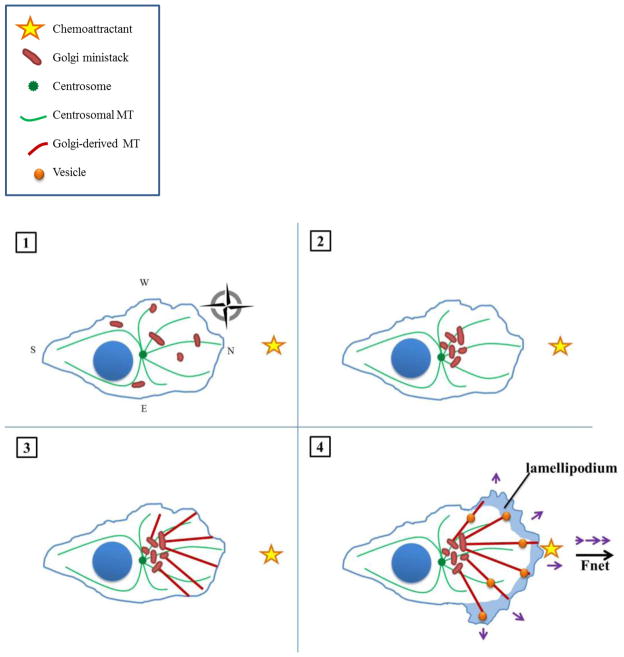
Exciting strides have recently been made in elucidating the surprising details of the centrosome’s role in migration, which may lend some answers. In the cell lines thus far analyzed, it seems the centrosome’s chief function in polarized migration is to promote proper organization of the Golgi apparatus, which itself functions as a centrosome-independent MTOC [44]. In order for the cell to directionally migrate (that is, achieve net displacement towards a stimulus), the Golgi apparatus must exhibit proper localization and morphology. This involves microtubule- and dynein-dependent peri-centrosomal assembly of the Golgi into a ribbon of stacked cisternae that feature cis-trans polarity and become continuous by connecting, lateral tubules [45, 46]. The Golgi apparatus mediates polarized cell migration by directing the traffic of vesicles laden with migration-promoting factors to the LE along Golgi-nucleated microtubules. Following mitosis, the Golgi is dispersed throughout the cytosol as ministacks, which cannot execute polarized secretion in their scattered state [47]. The centrosome orchestrates the collection of the Golgi ministacks into an intact apparatus by communicating the proper site for ministack coalescence (e.g., between centrosome and LE) [48]. Gathering of ministacks at this distinct peri-centrosomal focus is guided by a “centripetal signal” or “compass” generated by the radial array of microtubules originating from the centrosome, along with linear arrays of microtubules emanating from Golgi ministacks. This model is described in Figure 1, in which we illustrate the role of the centrosome, Golgi apparatus, and their respective microtubule arrays in executing polarized migration towards a chemoattractant in a cell that exhibits centrosome localization towards the LE.
Figure 1. Directional cell migration in the presence of a normal complement of centrosomes.
(1) In an interphasic pre-migratory cell with a numerically and functionally normal centrosome complement, Golgi ministacks are scattered throughout the cytosol. The centrosome nucleates a cell-wide, radially-symmetric array of microtubules, which serves as a cellular compass (here, illustrated as the cardinal directions north, east, south, and west [49]). A chemoattractant (yellow star) is situated arbitrarily due north. The compass may persist throughout migration but is omitted from subsequent frames for reasons of clarity. (2) Golgi ministacks are collected into a distinct, polarized mass between the centrosome and leading edge, as directed by the centripetal signal provided by the radial microtubule array. (3) The Golgi serves as an MTOC and nucleates an asymmetric array of microtubules directed towards the leading edge of the cell (here, assigned northward for directional migration towards a hypothetical Northern stimulus). (4) The Golgi-nucleated microtubule array serves as tracks to the LE for the transport of vesicles laden with factors necessary for migration (such as focal adhesion- and actin-regulating proteins) as well as invasion (such as matrix metalloproteinases). Vesicles are not directed solely due North; there is also some lateral delivery owing to the coarseness of the compass. As a result, some of the forces driving cell migration (purple arrows) cancel out, but the net force vector (bold black arrow) still points northward, bringing the cell closer to its chemoattracting stimulus.
Evidence for this hypothetical model stems from recent experiments employing laser ablation of the centrosome to uncover its role in directional migration among different cell lines [50]. In one study, the centrosome was ablated and its radial microtubule array depolymerized with nocodazole in RPE1 cells. Following this treatment, the Golgi ministacks disbanded throughout the cell. Upon recovery from nocodazole washout, the ministacks nucleated microtubules themselves, permitting collection of ministacks near the cell center; however, ribbon continuity was compromised, with membranes exhibiting decreased fusion, and the entire apparatus was also less compact and more circular. In conjuction with this aberrant morphology, cells exhibited inefficient post-Golgi exocytic vesicle trafficking (i.e., an increased proportion of vesicles directed laterally or backward instead of foreward), randomly-distributed cellular protrusions, decreased total cell migration distance, and compromised directional persistence (total distance/displacement). By contrast, ablation of the centrosome with preservation of the centrosomally-derived array of microtubules did not discernibly impact Golgi morphology, function, or directional migration. Furthermore, once the Golgi was properly assembled, ablation of the centrosome or depolymerization was inconsequential. Similar results were obtained in the U2OS osteosarcoma cell line in another study [51]. This inter-line analogy is unsurprising because the U2OS cell line exhibits a nuclear-centrosomal axis similar to that of RPE1 cells, as previously discussed. Thus, it can be concluded that the centrosome’s microtubule array, rather than the centrosome per se, is critical for the initiation but not continuation of polarized migration in these particular cell lines. Additionally, it can be hypothesized that other cell lines with similar centrosome-nuclear axis organization may exhibit comparable dependence on the centrosome. By contrast, the PtK2 cell line was found to rely on the centrosome not only for Golgi ribbon formation but also for its maintenance [51]. Intriguingly, this cell line, as mentioned, relegates the centrosome to the rear and positions the nucleus near the front. Thus, an interesting avenue for future research will be to determine whether there is a relationship between cell polarization axis (i.e., centrosome in front, back, or elsewhere) and the degree to which the cell relies on the centrosome in polarized migration. Also, cells that lack Golgi-mediated microtubule nucleation (e.g., HeLa, [52]) warrant further study in this light. In summary, while the centrosome is dispensable for migration once the Golgi has been properly organized in certain cell lines (e.g., RPE1 and U2OS cells), the centrosome is responsible for continued Golgi integrity and thus perpetuation of directional migration in others (e.g., PtK2 cells).
The more, the merrier: Amplified centrosomes may facilitate Golgi reassembly and directional cell migration
Cognizant of the pivotal role the centrosome assumes in polarized migration, we strongly suspect that CA influences this process in profound ways. We envision at least two potential mechanisms by which CA could enhance directional cell migration in cancer cells. Firstly, cancer cells with CA (whether numerical, functional, or both) nucleate a greater number of microtubules, which might provide an enriched centripetal signal for polarized Golgi assembly. As a result, perhaps even more precise Golgi cis-trans polarization is achieved permitting more focused post-Golgi trafficking of vesicles to the leading edge. These vesicles carry crucial factors for migration, such as FA proteins (to grasp the substratum ahead of the cell), actin-regulating proteins (for promotion of actin polymerization and lamellipodium protrusion), receptors for binding chemoattractants (permitting homing to particular tissues), and enzymes for ECM degradation (such as matrix metalloproteinases). As a result, more such vesicles could be expeditiously deposited at the proper location, providing the cell with enhanced “horsepower” to move cancer cells forward. Precise direction of migratory factors is vital for the cell to “make ground”; otherwise, the random movements result in a near-zero net displacement.
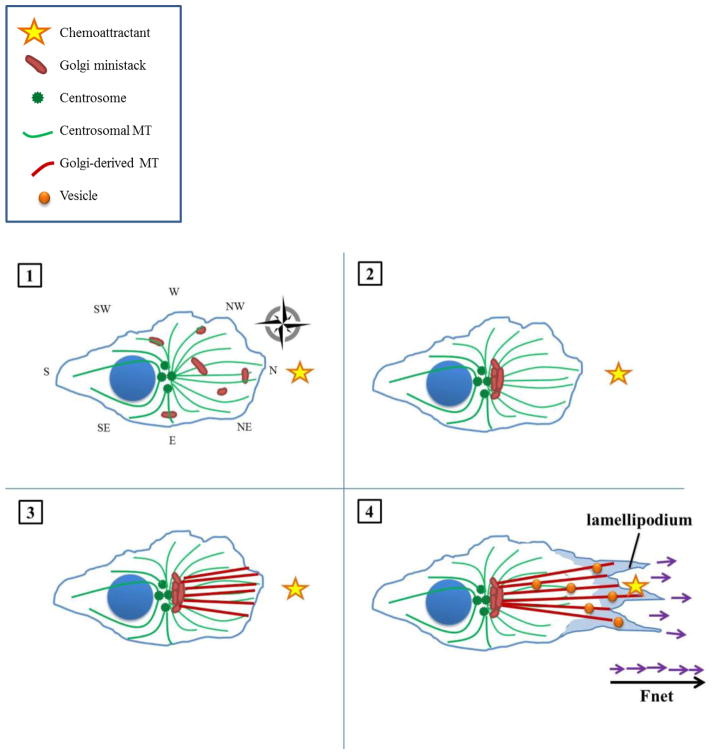
Furthermore, in cells that depend on the centrosome for continued Golgi maintenance like PtK2 cells, CA could promote migratory persistence. The more robustly polarized array of microtubules may be better able to maintain an integral Golgi apparatus for an extended period, as “many hands make light work.” We here propose a model depicting the advantage that CA may afford cancer cells in directional migration, as illustrated in Figure 2.
Figure 2. Enhanced directional cell migration in the presence of amplified centrosomes.
(1) In the interphase, pre-migratory cell exhibiting CA, Golgi ministacks are likewise scattered throughout the cytosol. The supernumerary centrosomes nucleate a similarly cell-wide, radially-symmetric array of microtubules; however, microtubule density is increased owing to the increased number of MTOCs as well as enhanced microtubule-nucleating capacity of individual MTOCs. The resultant amplified microtubule array constitutes an enhanced cellular compass (illustrated here as having, for instance, additional SW, NW, SE, and NE ordinal directions). A chemoattractant is arbitrarily situated due North. (2) Golgi ministacks are collected into a more compact, polarized, continuous mass in between the centrosomes and leading edge, as the cell is better able to gauge the precise location of the stimulus. (3) The Golgi directs its microtubules more precisely towards the stimulus, resulting in a more focused array. (4) Vesicles with migration- and invasion-promoting factors travel along microtubule tracks to a more well-defined point due North, allowing more forces (purple arrows) to be directed towards the stimulus and finally sum to a greater net force (bold black arrow). Thus, the cell can accelerate towards its stimulus more expeditiously.
Limited but exciting data suggest that CA does indeed improve migration. For instance, human wound cells undergo tetraploidization with consequent CA, which appears to boost their motility and capacity to invade [4]. Intriguingly, these cells are eliminated after the wound has healed, which is hypothesized to be necessary to mitigate the potentially oncogenic effects of CA. What is most fascinating about this finding is that amplified centrosomes may have stimulated enhanced migration long before enough time had passed for karyotype rearrangements, indicating that CA may provide an immediate boon to pre-cancerous or cancerous cells. This finding substantiates our intuition that CA does not spur carcinogenesis solely through mitotic events, such as chromosome mis-segregation or symmetric stem cell division, but that CA profoundly disrupts interphase activities as well, such as migration. In other words, low-grade chromosome missegregation is not solely responsible for engendering the particularly aggressive phenotypes correlated with CA; extra centrosomes may contribute to the acquisition of enhanced migratory abilities by endowing cells with altered cytoskeletal characteristics. It is noteworthy that aneuploidy is not necessarily a fail-safe means by which cells can become malignant. Indeed, aneuploidy is often described as a “wild card,” for it may actually prove tumorigenic or tumoristatic [53]. In general, loss or gain of a single chromosome is usually insufficient for cellular transformation; rather, generations of karyotypic rearrangement are necessary for procurement of a superlative genetic composition conducive to malignancy. Even then, compared to the staggering number of karyotypes possible, only a small proportion are cancer-promoting. Furthermore, while we also believe that CA encourages symmetric division and thus tumorigenesis in stem cells, it seems that a significant proportion of cancers arise from differentiated progenitors. Moreover, amplified centrosomes have also been occasionally detected in benign lesions of the breast [54], non-malignant tumors from soft tissue [55, 56] and pancreatic adenomas [57]. Importantly, although most cancers exhibit some degree of CA, this is not an invariant phenomenon [3], suggesting that CA promotes malignancy in certain cell types more than in others. Thus, CA alone is unlikely to be sufficient for the development of metastasis, although we believe that CA might, in conjunction with karyotypic changes accrued over generations of Darwinian clonal-selection, significantly augment migratory and invasive capabilities in certain types of cancer cells. Consequently, we conjecture that other repercussions flowing from CA (namely, migration enhancement) are involved in cancer progression in addition to chromosome missegregation and symmetric stem cell division. Certainly, more in-depth studies are needed to substantiate this paradigm, but the preliminary findings are quite enticing.
Illegal immigration: How centrosome amplification may facilitate various steps in cancer metastasis
Clinically, one of the most intractable aspects of the pathology of cancer has been its expansionist nature. In vivo, the metastatic process whereby cancer cells undergo dissemination throughout the body to seed secondary outcroppings of the disease at distant sites is a complex three-dimensional process involving the following steps: (i) detachment of tumor cells from the primary tumor, (ii) invasion into surrounding tissue, (iii) intravasation, (iv) dissemination via the blood stream or lymphatic system, and finally (v) extravasation and outgrowth to produce a secondary tumor. Each of these steps requires deployment of a specialized molecular program at the heart of which lies the modulation of the cytoskeletal properties of the disseminating tumor cell. In order to transcend the confines of the primary tumor and infiltrate surrounding tissue, tumor cells have to disrupt the architecture and boundaries of the original tissue, dissolve cell-cell contacts, remodel cell-matrix adhesion sites, and make their way through the treacherous ECM terrain, typically via pathways carved by secreted proteases. Attempts to crack the codes of metastasis have often been thwarted by the difficulty of reconciling data obtained from 2D motility assays of cells migrating across rigid substrates, with those obtained from assays involving cell movement within artificial 3D matrices. Currently available in vitro and in silico models developed for migration on 2D surfaces do not account for the full complexity of migration through a 3D matrix, such as the presence and distribution of cell-matrix attachments over the entire cell surface, the participation of stroma-supplied biochemical factors such as cytokines and growth factors, steric factors such as hindrance from matrix fibers, and mechanical factors such as matrix fiber stiffness. Hence there is a pressing need to develop methodologies that marry the prowess of 4D intravital microscopy and other sophisticated imaging technologies with the development of suitable animal models that can provide physiologically relevant insights into the cascade of events underlying metastatic progression in vivo. The following sections aim to capture a 3D view of the journey of metastatic cells to understand the challenges these cells encounter, the inventive strategies they adopt to sustain their colonialism, and how the supernumerary centrosomes they carry might be assisting them on their perilous odyssey.
Reinventing yourself inside out: Extra centrosomes may facilitate epithelial-to-mesenchymal transition (EMT) in cancer cells
A key early step in malignant progression of many epithelial cancer cells is a fundamental phenotypic switch (trans-differentiation) they manifest, referred to as the epithelial-to-mesenchymal transition (EMT) [58]. EMT involves a radical reprogramming of almost every facet of the cell’s biology and can be triggered by either intrinsic (e.g. gene mutations) or extrinsic signals (e.g. growth factor signaling or changes in the composition and architecture of the ECM). During EMT, non-motile, apicobasally-polarized epithelial cells dissolve their cell-cell junctions and morph into individual, non-polarized, motile, and invasive mesenchymal cells bearing an elongated morphology and cellular protrusions. Integrin-based adhesions and strong traction are the driving forces for such morphogenetic transformation in addition to ECM degradation by secreted and/or transmembrane proteases. Loss of cell–cell relations also correlate with compromised structural integrity of the barrier presented by the basement membrane (BM), another hallmark of malignant invasion [59]. Rho GTPases integrate and transmit signals from chemokine and growth factor receptors and from cellular adhesion receptors to a battery of effector proteins to direct actin cytoskeletal changes, protrusion formation and induction of both MMPs and tissue-specific inhibitors of MMPs, thus playing a pivotal role in EMT [60, 61]. Abundant evidence supports the idea that actin-microtubule crosstalk plays a key role in the sweeping cytoskeletal remodeling that EMT incites [61]. Interestingly, the presence of supernumerary centrosomes shows a striking correlation with a greater loss of tissue architecture and differentiation [17, 62, 63], suggesting a strong effect of extra centrosomes on EMT. It has been asserted that the destruction of tissue structure itself could be an oncogenic event, even in the absence of initial genetic mutation [64]. A logical extension of this idea is the proposition that extra centrosomes could play an epigenetic causal role in promoting EMT through modification of cytoskeletal architecture and function. Clearly, a closer examination of the repercussions of centrosomal excess on EMT would in all certainty provide insights that could have vast clinical relevance.
Sticking their necks out: Amplified centrosomes may promote leading edge protrusion
Initiation of metastasis involves the stepwise acquisition of migratory and invasive capabilities by cancer cells via a process regulated by myriad paracrine and autocrine cues. A subsequent dramatic reorganization of the actin cytoskeleton causes protrusion of the cell’s LE, a process primarily driven by polymerization of actin within two structures, filopodia, and pseudopodia [65]. Studies have revealed that the LE protruding into 3D tissue typically contains one or a few cylindrical pseudopodia with terminal filopodia that dynamically engage with ECM fibers [66]. It is also noteworthy that, while cells moving across 2D surfaces contain stress fibers that span between focal adhesions, cells migrating in 3D tissues generally lack stress fibers but form a cortical cytoskeleton [67]. Filopodia are rod-like extensions consisting of tightly-bundled actin fibers and carry out a sensory function by detecting and assimilating signals like chemoattractants or nutrients released from blood vessels. Interestingly, metastatic cells are rich in filopodia-like structures, which correlate with their invasiveness [68]. Pseudopodial extensions are initiated first in rather random locations along the cell’s edge, but eventually the cell reorientates and adapts to certain environmental cues, such as adhesiveness and rigidity of various areas of the ECM and chemotactic gradients of certain soluble factors in the plasma membrane’s immediate vicinity. Upon stimulation of chemokine- and adhesion receptor-mediated signaling, actin flow becomes polarized resulting in the protrusion of a leading pseudopod. Integrin engagement with the substrate and formation of focalized adhesion complexes allows the LE to become anchored to the ECM. The cell then undergoes contraction, which enables it to pull on the ECM. Focalized matrix proteolysis then ensues, allowing the cell body to expand and move forward through the newly generated remodeled ECM track. Actomyosin contraction along the cell’s long axis including the rear then leads to longitudinal tension. If the LE’s attachment to the matrix outweighs that of the trailing edge, then the rear end disassembles its matrix attachments and the cell moves forward. Actin-rich protrusions thus allow the cell to probe the ECM, locally attach to and pull on ECM fibrils, and detach from them dynamically during directional cell migration [67].
Recent studies have found that nucleo-cytoskeletal connections are crucial for protrusion formation and cell migration through 3D matrices. The nucleus is mechanically coupled to the cytoskeleton by LINC (linker of nucleoskeleton and cytoskeleton) complexes, which are comprised of SUN proteins and nesprins present in the inner and outer nuclear membranes, respectively. While SUN proteins bind the nuclear lamina, nesprin protein family members provide the connection to different components of the cytoskeleton. Nesprin-1 and -2 can establish a direct link with actin filaments, whereas nesprin-4 is capable of kinesin 1-dependent association with microtubules. Nesprin-3 can link the nuclear envelope to intermediate filaments and microtubules by its interactions with the cytoskeletal linker proteins BPAG1 (bullous pemphigoid antigen 1) and MACF (microtubule-actin cross-linking factor) that interconnect the microtubule and actin cytoskeletons. LINC complexes also play key roles in nuclear positioning and centrosome attachment to the nucleus [69]. By mediating physical connections between the nuclear lamina and the cytoskeleton, LINC complexes enable the formation of an integrated intracellular force transmission network and provide pivotal nuclear support to actin-based pushing and pulling. Studies have demonstrated that, for effective protrusion extension (with minimal dissipation) into the soft ECM, actin fibers need to be anchored to the “book-ending” nucleus so that these fibers can push within the cell against a stiff base (i.e. the nucleus). In particular, LINC-mediated connections between the nuclear lamina and the highly ordered array of thick actomyosin bundles that comprise the perinuclear actin cap provide robust mechanical support for formation of dynamic protrusions, matrix traction, and 3D cell migration [70]. Since microtubules impart rigidity and function as compression-resistant struts, we believe that intracellular actin-microtubule coupling provides valuable structural reinforcement to undergird actin polymerization-driven protrusive activity. In addition to the involvement of actin-based mechanisms in protrusion formation, several studies have previously established that intact microtubules are essential for restricting protrusion formation to one region of the leading edge [71], normal protrusion extension and orientation, and maximal unidirectional locomotion in several systems including migrating fibroblasts and neutrophils engaging in chemotaxis [72, 73]. High resolution imaging of real-time cytoskeletal behavior recently showed that EB1-mediated interactions with growing microtubules were crucial for coordinated protrusion, vesicular trafficking to the protrusion tip, and cell-matrix adhesion dynamics during directed migration into the ECM in Madin-Darby canine kidney cells undergoing epithelial remodeling in a 3D environment [74]. Based on this body of strong evidence, it is reasonable to conjecture that the presence of an increased centrosomal complement and greater microtubule density in cancer cells could, in concert with the action of the actin cytoskeleton, empower cells with an enhanced protrusive ability that could quicken the pace at which cells make their way through the ECM. Valuable insights could also be derived from closely examining the distribution of stabilized microtubules in such “super-centrosomal” cells, and studying the correlation between patterns of microtubule stabilization, protrusion formation, and various migration parameters to understand better how extra centrosomes alter the choreography of cell motility. Cells with supernumerary centrosomes would also bear the feature of enhanced centrosome-derived signaling, the consequences of which in the context of metastasis would be an exciting research avenue. Recent research in C. elegans has uncovered that the centrosome provides powerful, diffusible signals that induce cell polarization, independently of its function as an MTOC [75]. Clearly, we are only beginning to fathom the vast number of active roles centrosomes play in different cellular contexts, and by delving deeper into the biology of centrosomal excess, we will assuredly discover more about the colossal powers of this miniscule organelle.
Breaching barriers: Centrosome overabundance may facilitate invadopodia formation and matrix degradation
In addition to promoting the formation of cell protrusions for movement, we believe that cells with supernumerary centrosomes may possess an enhanced stroma-penetrating capacity. In order to breach the BM and move into the stromal compartment, cells develop on their ventral surface protrusions called invadopodia (literally “invading feet”) that are equipped with strong ECM-degrading activities. Invadopodia consist of an outer adhesive ring (comprised of integrins and their cytoplasmic interaction partners) and a central actin-rich core (containing the actin nucleation and assembly machinery) [76]. As key regulators of actin assembly and MMP exocytosis, the major Rho GTPases, RhoA, Rac1, and Cdc42, all play essential roles in invadopodia formation. Engaged integrins also play a role in promoting Rho GTPase activity within invadopodia [77]. BM dissolution by invadopodia represents the first step of the metastasis cascade, and it begins with a local enrichment of actin and cortactin at cell-ECM contact sites, which results in the recruitment and concentration of membrane type 1 matrix metalloprotease (MT1-MMP) at these sites for focused matrix degradation. Interaction of integrins with MT1-MMP also leads to the activation of other immature MMPs in the vicinity, thereby amplifying the cycle of localized ECM proteolysis. Invadopodial maturation occurs first by elongation of actin bundles, followed by the entry of microtubules and vimentin filaments, which penetrate mature invadopodia while linear actin bundles are being gradually replaced by an expanding branched actin network. Interestingly, dynamic microtubules are found to be restricted to the base of invadopodia, while microtubules in invadopodia shafts are stable. Late invadopodia are defined by dispersal of actin and cortactin leaving an MT1-MMP-enriched structure that maintains matrix degradation, allowing the cell to eventually penetrate into the stromal compartment [78].
Besides their important role in primary tumor invasion and tumor cell intravasation, invadopodia are also critical for extravasation at secondary sites. Growing circumstantial evidence supports the notion that microtubules may provide tracks for long-distance vesicular transport and the continuous and focused delivery to the cell surface of specific proteins essential for invadopodia elongation and deep stromal penetration [78, 79]. For example, exocytosis of MMP-2 and MMP-9 to the cell exterior is microtubule-dependent in melanoma cells, astrocytes, and neurons [80–82], and in activated macrophages, vesicles containing MMP-9 specifically traffic along stabilized microtubules [83]. In addition, MT1-MMP vesicles have been shown to be transported along microtubules and targeted to FAs. Microtubules stabilized at FAs may thus provide a localized exocytosis pathway for MMPs that cleave integrin interactions with the ECM and as a result initiate FA disassembly concomitantly with ECM degradation [84–86], allowing forward translocation of the cell. We postulate that the larger number of microtubules nucleated in cells with extra centrosomes thus not only assist with cell protrusion but also increase the ability of cancer cells to penetrate their underlying stroma through multiple mechanisms including the enhancement of microtubule-dependent MMP secretion.
We are what we surround ourselves with: Extra centrosomes may influence cancer cell-tumor microenvironment crosstalk to impact metastasis
Research over the last few decades has unearthed yet another monumental finding, viz., that the success of EMT and every step in the metastatic spread of cancer cells hinges critically upon the permissiveness of the tumor’s microenvironment, which is a complex system comprised of several cell types and non-cellular elements integral to the tumor’s anatomy and pathophysiology [87]. The concept that the tissue context profoundly influences malignant transformation and tumor progression was originally enshrined by Paget many years ago in his “seed and soil” hypothesis [88]. Essentially, tumors are heterogeneous organs that in addition to the malignant cells contain a retinue of cancer-associated tumor-companion cells including activated endothelial and lymphatic cells, altered fibroblasts, infiltrating immune cells, modified adipocytes, and even stimulated mesenchymal stem cells, all embedded in the ECM or “soil” [89]. Non-cellular aspects of the tumor microenvironment, such as hypoxia and an altered ECM, additionally contribute to tumor progression either directly by destabilizing tissue integrity and promoting tumor cell motility, invasion and survival, or indirectly by inducing tumor angiogenesis and enhancing tumor cell survival and selection [90].
The phenomenon of contact guidance described by Weiss and Garber more than 50 years ago suggested that the ECM that surrounds cells can itself orient and steer migrating cells. In mammals, the interstitial matrix, which comprises most of the ECM, is predominated by collagens, which together with fibronectin endow the tissue with mechanical strength. ECM components such as tenascin and proteoglycans provide tissue hydration, growth factor and cytokine binding functions, and crosslink the matrix to enhance its integrity [91, 92]. The ECM is not merely a passive scaffold enveloping cells within the tumor; instead, it provides valuable contextual information for tumor cells. Moreover, the composition and 3D organization of the ECM can undergo dynamic changes (affecting ligand density, matrix stiffness, and pore size) that influence many cellular processes by supplying physical and biochemical cues to the cancer cells embedded in it. The cornerstone of this regulatory interaction is a constant and rich communication between the adhesive adaptors of a cell, integrins, and the ECM. Integrins bind specific ECM components on the extracellular face and function as switchboards deftly integrating intracellular and extracellular signals to drive appropriate cellular responses to microenvironmental cues. Integrins also correctly localize proteases which may (i) aid in mining out a path for the cancer cell movement or (ii) liberate and/or activate ECM-bound growth factors (eg., TGF-β) and chemokines, all of which profoundly influence cell behavior during EMT. Many ECM components and remodeling enzymes are elevated in cancer patients and the architecture of tumor-associated ECM is fundamentally different from that of the normal tissue stroma [89]. It has also recently come to light that there exists a very dynamic and reciprocal interplay between the biophysical properties of the tumor cells and those of the ECM. As transformed cells proliferate extensively, they impose large compressional forces on their surrounding ECM. The ECM, in turn, resists deformation by inducing progressive matrix stiffening via enhanced collagen deposition and matrix crosslinking. Tumors cells, which continuously interrogate their mechanical microenvironment, respond to these force cues by exerting a reciprocal compensatory contractile force derived from Rho GTPase-driven actomyosin contractility and motor protein activity. This increased tractional force that tumor cells exert upon their surroundings, on one hand, enhances growth, survival, and invasion of malignant cells and, on the other, triggers further tension-dependent matrix remodeling to promote the linear reorientation of collagen fibrils surrounding the invasive tumor front [92, 93]. Finally, tensional stress can facilitate cell invasion by elevating MMP-9 release and activation [94]. We believe that malignant cells carrying amplified centrosomes would present altered cytoskeletal architecture and properties that are at present largely unknown. The impact of these cytoskeletal changes on malignant cell-ECM interactions and eventually, on the efficiency of metastatic dissemination, will undoubtedly be an exciting and fruitful domain for future research.
Charting new paths: Supernumerary centrosomes may facilitate amoeboid and collective cell migratory modes
While protease-dependent invasion programs rely on MMPs to cleave impeding ECM fibrils, tumor cell migration is highly plastic: several studies have shown that cells may employ alternative migratory modes while transitioning into the metastatic phase. One such mode is a less adhesive amoeboid mode of migration, in which an epithelial cell undergoes morphological deformation and displays very low-affinity and largely integrin-independent substrate binding [95]. This shape-driven migration is protease-independent and allows cells to physically displace matrix fibrils, circumnavigate, and squeeze through rather than degrade their ECM barriers. Owing to the lack of focal contacts, amoeboid cells move at 10- to 30-fold higher velocities than cells that use mesenchymal mechanisms. Although it is believed that rapid remodeling of the actin cortex and internal hydrostatic pressure predominantly mediate this migratory mode, regulated microtubule instability is emerging as an active co-contributor to the amoeboid phenotype [96, 97]. A recent study showed that the Diaphanous-related formin-3 (DIAPH3) protein, whose genomic locus is a consensus area of chromosomal deletion common to several carcinomas, functions as an important signaling node controlling the amoeboid phenotype. DIAPH3, a formin ortholog that nucleates, elongates, and bundles linear actin filaments, also regulates microtubule dynamics [98–100] and acts as a metastasis suppressor in normal epithelia by inhibiting conversion to an amoeboid phenotype. DIAPH3 silencing caused microtubule destabilization, deregulated endosomal trafficking, induced drastic signaling defects, evoked an amoeboid phenotype, and promoted metastasis, lending credence to the notion that modulation of microtubule dynamics is important for regulating the transition into amoeboid migration and accelerated metastasis. It is therefore vital to scrutinize carefully the impact of supernumerary centrosomes on the efficacy of this cancer cell escape mechanism. Current evidence also suggests that nuclear diameter acts as a bottleneck, restricting the ability of a cancer cell employing amoeboid movement to mechanically negotiate pores whose diameters are smaller than that of the cell’s rigid nucleus [101]. It is also noteworthy that rapid cancer cell migration occurs within sites where there are pre-cleared matrix tunnels carved out by infiltrating fibroblasts or endothelial cells [102–106] or in tumor zones with damaged blood vessels and necrosis [107]. Moreover, cancer cells exhibit remarkable and highly opportunistic switches in migration from a “path-generating” proteolysis-dependent mesenchymal mode to a “pathfinding” amoeboid mode (i.e., mesenchymal-to-amoeboid transition) depending on the demands of their milieu. It is reasonable to speculate that cancer cells with more centrosomes and microtubules and a reinforced nucleo-cytoskeletal connection might possess enough “horsepower” not only to digest their way through the ECM more effectively, but also to propel themselves through narrow ECM pores with greater ease.
Augmentation of metastatic potency could be particularly important in an alternative mode of cellular migration, viz., collective cell migration, wherein aggregated cells that retain cell-cell junctions can move as one functional unit. Supernumerary centrosomes could engender the genesis of either a single or a small group of highly motile “path-generating” cells in the front of the cell sheet or cluster that generate migratory traction via protrusive activity and passively drag adhesive neighboring cells along [108–110]. Such collective migration is prevalent in certain normal cellular process like wound healing and morphogenesis, but it is also a common migratory strategy in epithelial cancers (i.e., carcinomas). This migratory mode has the potential to confer the following important advantages on the cell group [95]. Firstly, cell groups can produce higher autocrine concentrations of pro-migratory factors and matrix proteases, thus facilitating invasion of other nearby cells from the primary tumor. Secondly, outer cells in the cell cluster can protect inner cells from immunological attack. Thirdly, the highly motile “lead cells” can facilitate the metastasis of cells of different clonal origin which may possess different biological capabilities (e.g., they could facilitate the metastasis of less migratory, yet possibly apoptosis-resistant, cells), thus promoting their survival and dissemination to distant locations in the body. Therefore, the role of CA in individual cell (mesenchymal and amoeboid) as well as collective cell migration represents an untapped wellspring of important insights that urgently requires the development of “test-beds” and multi-dimensional model systems to recapitulate the physiological complexity of ECM architecture and cell movement more accurately.
Making breathless haste: Hypoxia promotes metastasis by many means, including stimulating centrosome amplification
Perhaps the most central non-cellular regulator of tumor pathophysiology is intratumoral hypoxia, which is present in all solid tumors over 1 cm3 in volume [111]. Studies suggest that hypoxia actually drives a stepwise selection process that spurs the expeditious acquisition of a metastatic phenotype [112]. By facilitating the switch from E- to N-Cadherin expression, hypoxia not only leads to a disruption of tissue integrity but concomitantly grants cancer cells an escape route from anoikis. Through upregulation of urokinase-type plasminogen activator receptor (uPAR) expression, hypoxia boosts stromal penetration by malignant cells. Cell migration towards the blood or lymphatic microcirculation is increased through hypoxia-induced hepatocyte growth factor-MET receptor signaling. Hypoxia-induced vascular endothelial growth factor (VEGF) activity facilitates metastatic spread of malignant cells in at least three ways. Firstly, by promoting angiogenesis and lymphangiogenesis within the primary tumor, VEGF activity brings the highways for dissemination right to the tumor cells’ doorsteps. Secondly, by heightening vascular permeability, VEGF fosters both intravasation and extravasation. Thirdly, VEGF-induced angiogenesis in the secondary tissue is essential for establishment of metastatic lesions. Thus, hypoxia promotes the metastatic cascade through transcriptional regulation of multiple molecular targets. A new set of floodlights were turned on recently when research showed that hypoxia leads to an upregulation of Aurora A kinase expression [112]. Aurora A has been implicated in triggering CA [113] and it is tantalizing to suggest that hypoxia, by exacerbating the “extra centrosome phenotype”, may also accelerate the acquisition of metastatic character in cancer cells.
Talking a neighbor into becoming an accomplice: Extra centrosomes may affect the malignant cell-stromal cell interaction in cancer metastasis
Cellular components of the stroma include activated endothelial and lymphatic cells, altered fibroblasts, infiltrating immune cells, modified adipocytes, and even stimulated mesenchymal stem cells, whose individual and combined activities modulate various stages of tumor progression [89]. Tumors enlist stromal cells for pro-metastatic ECM remodeling and increased angiogenesis. Cancer-associated fibroblasts produce copious amounts of collagen transforming ECM biochemistry and structure to facilitate tumor progression. This desmoplastic response is frequently seen in breast, pancreatic, prostate, colon, and lung cancers and has been correlated with the recruitment of inflammatory cells and induction of angiogenesis. Tumor-associated immune cells are potent facilitators of cancer progression because they release several soluble factors that stimulate malignant cell growth and motility and tumor angiogenesis. Importantly, invadopodia-inducing growth factors are not only produced by cancer cells themselves but are frequently supplied by tumor-associated macrophages (TAMs), which themselves are attracted to the tumor microenvironment by tumor- released chemokines. TAMs also enable intravasation by secreting growth factors that steer tumor cells towards blood vessels [114]. Cancer biologists, bedazzled by the feverishly evolving and undeniably riveting characteristics of malignant cells, have oftentimes overlooked the events in the penumbra of these cells. Given that the behavior and fate of malignant cells are inextricably guided by their interaction with stromal elements, it is clear that evaluation of the metastatic characteristics of cells harboring an overabundance of centrosomes needs to be carried out in in 3D systems that closely mimic the stromal environment.
Float safely and grab new shores: Extra centrosomes may promote microtentacle formation in disseminating cancer cells
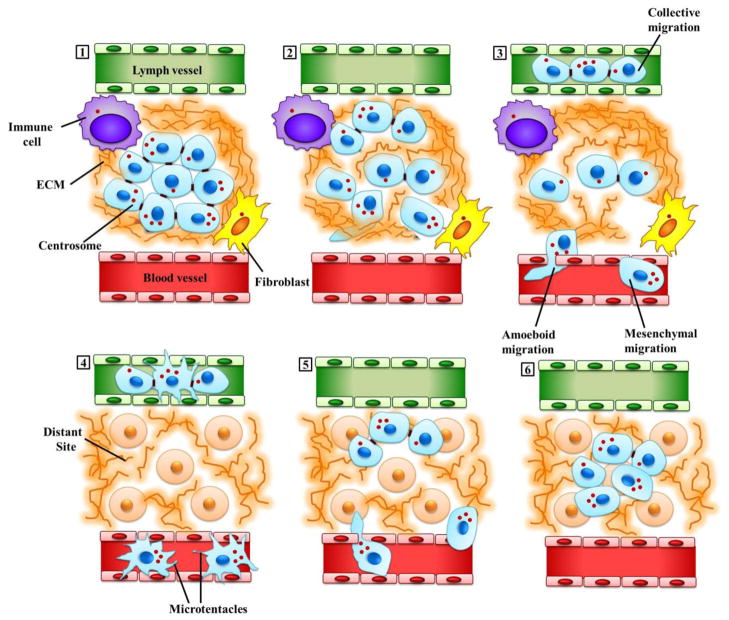
The challenges encountered by cancer cells take a new turn once these cells enter the blood stream or lymphatic fluid. Circulating Tumor Cells (CTCs) could perish either due to immune-mediated destruction or collision- and shear-induced fragmentation as they negotiate the narrow dimensions of the microvasculature. Some protections from these hazards can be derived from homotypic and heterotypic aggregation of CTCs, and cell lines selected in vitro for efficient homotypic clustering display better metastatic efficiency in vivo [115]. In vitro CTC modeling revealed that detached cells form microtentacles (McTNs), which are dynamic and persistent microtubule-enriched plasma membrane extensions whose formation is normally counteracted by tension in the actin cytoskeleton that attempts to stabilize cell shape [115]. Studies show that McTNs enhance homotypic cell aggregation[116] and activate CTC reattachment to the endothelium through heterotypic aggregation of CTCs with platelets [115]. As a result, CTCs equipped with McTNs survive better in the microvasculature [117]. Moreover, stabilized (viz., glutamylated) microtubules, which are known to normally orient in the direction of cell migration [20], concentrate within McTNs [115]. Interestingly, McTN formation and tumor cell reattachment were enhanced upon weakening of the actin cortex [118], illustrating how the balance in cytoskeletal forces impacts metastatic success of CTCs. Based on these observations, we propose that an overabundance of centrosomes in CTCs can tip the cytoskeletal force balance in favor of enhanced McTN formation, which could strongly promote the survival and dissemination of metastatic tumor cells and increase the efficiency with which they seed and colonize distant locations. This exciting hypothesis could be experimentally validated relatively easily, given the technological advances that have spurred the easy isolation and characterization of CTCs in recent years. We illustrate some of the ways in which extra centrosomes may facilitate directional migration and metastasis in Figure 3.
Figure 3. Extra centrosomes may promote directional migration and metastasis in malignant cells.
(1) Malignant cells (depicted in light blue) are shown here in a cross-section through a primary tumor in situ. Some of these cells may have acquired extra centrosomes due to genetic mutations and other aberrant processes, while others, which may be distant from blood vessels and experience acute hypoxia, may have undergone hypoxia-induced CA. Tumor cells are connected to each other through cell-cell contacts, and are embedded within and interact extensively with the tumor microenvironment. Signals emanating from both the acellular components (including the ECM, depicted here as a brown fibrous matrix, and intratumoral conditions such as hypoxia) as well as the cellular components (such as fibroblasts, depicted here in yellow and immune cells, depicted in purple) of the tumor microenvironment, can potently influence various aspects of malignant cell behavior, including migration. (2) Initiation of cell migration from the primary tumor and invasion of the surrounding stroma involve the formation of actin polymerization-driven cellular protrusions. Supernumerary centrosomes may endow the cells bearing them with an enhanced radial signal to organize a compact, polarized Golgi apparatus which can efficiently direct vesicular traffic to the protrusions allowing more efficient delivery of enzymes for digesting the basement membrane, degrading ECM and carving paths for cell movement through the ECM. In addition, migration of such “super-centrosomal cells” may be enhanced by an “augmented” microtubule cytoskeleton characterized by increased microtubule density, more intense centrosome-dependent signaling and more robust nuclear-cytoskeletal connections. (3) Malignant cells exhibit great plasticity in the migratory modes they employ: they are capable of moving through interstitial spaces and intravasating into blood vessels (shown in red) or the lymphatic system (shown in green) either individually (amoeboid or mesenchymal) or collectively (the migration of cohesive multicellular units). ECM pore dimensions, ligand density, fibril stiffness and orientation together with cellular determinants such as cell-cell and cell-matrix adhesion, signals from tumor microenvironment and pericellular proteolysis, all influence the choice of migration mode. (4) The tumor cells circulate in the patient’s vasculature. Extra microtubules may help these cells to resist the mechanical stress of circulation. Circulating tumor cells protrude microtentacles, a process which may be facilitated by extra microtubules and/or centrosomes. Microtentacles enhance circulating tumor cell survival by facilitating their aggregation, and also allow them to grasp the vessel endothelium to ease and expedite extravasation. (5) Tumor cells exit the circulation (extravasate) and colonize distant niches. (6) Tumor cells produce a secondary tumor.
By highlighting the involvement of centrosomes and the microtubule cytoskeleton in various steps of metastasis, we have merely brought into focus key “touch-points” and potential investigative avenues, the pursuit of which could shed light on the contribution of amplified centrosomes to metastatic progression. It must nevertheless be acknowledged that the presence of extra centrosomes may not incite an enhancement of all microtubule-dependent processes. Furthermore, effects of centrosomal excess on metastasis may hinge critically upon subtle and highly localized subcellular changes in the organization and dynamic properties of microtubules nucleated by supernumerary centrosomes (rather than the number or density of microtubules nucleated per se). Even so, we do believe that extra centrosomes confer new cytoskeletal characteristics upon malignant cells, the study of which merits attention. Since supernumerary centrosomes are a cancer cell-specific trait, in-depth evaluation of how this feature could contribute to the metastatic potency of malignant cells through aneuploidy-independent cytoskeletal modulation could unmask new cancer cell-selective interventional targets.
Clustering for a cause: Supernumerary centrosomes come together to turn problems into opportunities
Recently, centrosome research in oncology has been shaken out of its torpor and several studies have explored how cancer cells maneuver and manage excess centrosomes during the process of mitosis. These studies have uncovered the intricacies of the fascinating phenomenon of centrosome clustering [119, 120] that deploys microtubules, microtubule-binding proteins, actin, focal adhesions, cortical cues, and various motor proteins to herd the centrosomes together to two spindle poles. The clustered configuration permits “pseudo-bipolar” mitosis, allowing cancer cells to avoid the potentially devastating consequences of multipolarity. By contrast, if centrosomes remain strewn throughout the cell, then a multipolar spindle is erected and the cell either arrests in mitosis (which may result in death by mitotic catastrophe) or divides in multipolar fashion. Multipolar mitosis may produce daughters with a mortally-high grade of aneuploidy. Thus, by constructing a pseudo-bipolar spindle, the cancer cell cleverly evades its demise. Moreover, the cancer cell also benefits from merotelic attachments that accrued during the transiently multipolar state, allowing it to experience low-grade chromosome mis-segregation (e.g., loss or gain of a single chromosome) that may be survivable and confer malignant traits on the cell.
In moving the spotlight out of mitosis, we are entering largely uncharted waters by asking how cancer cells may successfully migrate in interphase, despite the presence of too many centrosomes that may provide confusing signage for cellular polarization. We hypothesize that in order for centrosomes to provide the requisite centripetal signal for properly polarized reorganization of a single Golgi apparatus as the cell exits mitosis, it seems likely that centrosomes must exhibit a highly limited spread within the cell. Otherwise, a single radially symmetric array of microtubules could not be organized. Rather, a disorganized web of microtubules might result, which would falsely indicate a multitude of cell “centers,” and Golgi ministacks would not be collected into a central focus. As a result, Golgi ministacks would remain scattered throughout the cytosol, and their microtubule tracks would be directed to the various cellular poles. Post-Golgi vesicular trafficking would then not be directed towards the desired trajectory (e.g., towards a stimulus) but rather at assorted points circumferentially, hopelessly stymying polarized cell migration. We posit that cancer cells employ centrosome clustering not only during mitosis but also during directional migration in interphase to manage their “excess baggage.” This strategy would not only serve as ongoing problem management but could represent “creative opportunity finding” wherein cells with “super-centrosomes” derive the side benefits of enhanced metastasis. To achieve this end, cancer cells with CA would need to muster the necessary intracellular organizational support and infrastructure. For example, these cells might need to upregulate their centrosome clustering machinery and ensure its availability throughout the cell cycle. In fact, it has been demonstrated that levels of an important centrosome-clustering protein, HSET (KiFC1), are strongly correlated with metastasis of non-small cell lung cancer to brain [121]. Thus, centrosomal clustering throughout the cell cycle perhaps manifests the wisdom of Ben Franklin’s words, “We must all hang together, or assuredly we shall all hang separately.” If our proposed paradigm is experimentally validated, then centrosome clustering represents a promising new target for the development of anti-metastatic drugs. Importantly, very few protein mediators of metastasis have thus far been identified [122], so empirical validation of a clustering mediator as a metastatic facilitator would constitute a groundbreaking finding.
Future travels in anticancer therapy: Using declustering agents to decelerate cancer cell migration
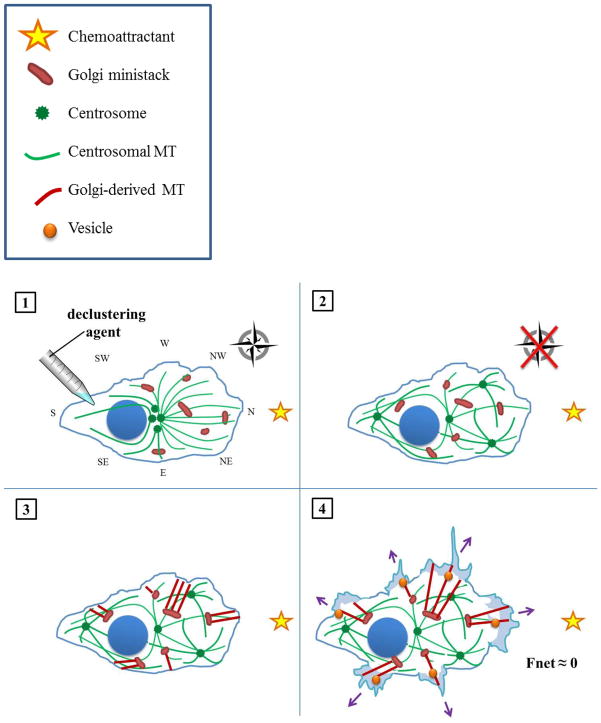
Metastasis (which literally means “beyond stillness”) is an issue of utmost gravity and urgency in cancer research because, unlike localized lesions, metastatic tumors staunchly resist therapies of all ilk–pharmacological, radiological, surgical. In general, detailed studies to examine how cancer cells organize amplified centrosomes during migration, as well as the tantalizing involvement of centrosome clustering mechanisms therein, are urgently called for. Considering the persuasive, we envision the next frontier in the development of drugs that inhibit centrosome clustering is to explore their anti-metastatic efficacy. Such declustering drugs represent a promising, novel class of minimally toxic agents that force cancer cells with supernumerary centrosomes to assume a multipolar configuration during mitosis. Declustering drugs, such as griseofulvin, brominated noscapines, and PJ34, all appear to selectively eradicate tumor cells, sparing healthy ones, since only cancer cells harbor excess centrosomes that can be declustered [119, 120]. By inhibiting centrosome clustering, we predict cancer cells should be rendered immobile by virtue of Golgi ministack scattering. We have constructed a model of the mechanism by which centrosome declustering drugs may thwart polarized cell migration, portrayed in Figure 4. Application of a declustering drug is hypothesized to act as a novel kind of “Cluster Bomb” preventing corralling of centrosomes into a distinct focus, resulting in a disorganized web of microtubules throughout the cell. Without its compass, the cell should fail to coalesce Golgi ministacks into a continuous apparatus; instead, the trans faces of the dispersed ministacks may point in various directions, resulting in non-polarized exocytic vesicular trafficking and obstruction of migration. We hope this model can serve as a springboard for the development of testable hypotheses regarding this fascinating new line of inquiry, the surface of which has only just been scratched.
Figure 4. How declustering agents may hinder directional migration in cancer cells with amplified centrosomes.
(1) A cell harboring supernumerary centrosomes is treated with a declustering agent. (2) Following scattering of centrosomes throughout the cell, the once highly organized, radial array of microtubules devolves into disarray. Hence, the cell literally loses its bearings. As a result, the cell lacks the centripetal signal necessary to organize the Golgi apparatus into a central, polarized mass. (3) Owing to their lack of polarization, the scattered Golgi ministacks nucleate microtubule arrays in various directions. (4) The cell directs trans-Golgi trafficking of vesicles to multiple cellular poles. Consequently, the exerts forces without respect to direction (purple arrows), which sum to a negligible net force (bold black arrow). The cell does not move appreciably towards its stimulus.
References
- 1.D’Assoro AB, Lingle WL, Salisbury JL. Centrosome amplification and the development of cancer. Oncogene. 2002;21(40):6146–53. doi: 10.1038/sj.onc.1205772. [DOI] [PubMed] [Google Scholar]
- 2.Lingle WL, Salisbury JL. Altered centrosome structure is associated with abnormal mitoses in human breast tumors. Am J Pathol. 1999;155(6):1941–51. doi: 10.1016/S0002-9440(10)65513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan JY. A clinical overview of centrosome amplification in human cancers. Int J Biol Sci. 2011;7(8):1122–44. doi: 10.7150/ijbs.7.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prigozhina NL, Waterman-Storer CM. Decreased polarity and increased random motility in PtK1 epithelial cells correlate with inhibition of endosomal recycling. J Cell Sci. 2006;119(Pt 17):3571–82. doi: 10.1242/jcs.03066. [DOI] [PubMed] [Google Scholar]
- 5.Basto R, et al. Centrosome amplification can initiate tumorigenesis in flies. Cell. 2008;133(6):1032–42. doi: 10.1016/j.cell.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bornens M. Organelle positioning and cell polarity. Nature reviews. Molecular cell biology. 2008;9(11):874–86. doi: 10.1038/nrm2524. [DOI] [PubMed] [Google Scholar]
- 7.de Forges H, Bouissou A, Perez F. Interplay between microtubule dynamics and intracellular organization. The international journal of biochemistry & cell biology. 2012;44(2):266–74. doi: 10.1016/j.biocel.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Vinogradova T, Miller PM, Kaverina I. Microtubule network asymmetry in motile cells: role of Golgi-derived array. Cell cycle. 2009;8(14):2168–74. doi: 10.4161/cc.8.14.9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verhey KJ, Hammond JW. Traffic control: regulation of kinesin motors. Nature reviews. Molecular cell biology. 2009;10(11):765–77. doi: 10.1038/nrm2782. [DOI] [PubMed] [Google Scholar]
- 10.Siegrist SE, Doe CQ. Microtubule-induced cortical cell polarity. Genes & development. 2007;21(5):483–96. doi: 10.1101/gad.1511207. [DOI] [PubMed] [Google Scholar]
- 11.Luxton GW, Gundersen GG. Orientation and function of the nuclear-centrosomal axis during cell migration. Current opinion in cell biology. 2011;23(5):579–88. doi: 10.1016/j.ceb.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quinones GB, et al. The posttranslational modification of tubulin undergoes a switch from detyrosination to acetylation as epithelial cells become polarized. Molecular biology of the cell. 2011;22(7):1045–57. doi: 10.1091/mbc.E10-06-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silkworth WT, et al. Multipolar spindle pole coalescence is a major source of kinetochore mis-attachment and chromosome mis-segregation in cancer cells. PloS one. 2009;4(8):e6564. doi: 10.1371/journal.pone.0006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganem NJ, Godinho SA, Pellman D. A mechanism linking extra centrosomes to chromosomal instability. Nature. 2009;460(7252):278–82. doi: 10.1038/nature08136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baccelli I, Trumpp A. The evolving concept of cancer and metastasis stem cells. J Cell Biol. 2012;198(3):281–93. doi: 10.1083/jcb.201202014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lingle WL, et al. Centrosome amplification drives chromosomal instability in breast tumor development. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(4):1978–83. doi: 10.1073/pnas.032479999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lingle WL, et al. Centrosome hypertrophy in human breast tumors: implications for genomic stability and cell polarity. Proc Natl Acad Sci U S A. 1998;95(6):2950–5. doi: 10.1073/pnas.95.6.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamashita YM, et al. Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science. 2007;315(5811):518–21. doi: 10.1126/science.1134910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukasawa K. Oncogenes and tumour suppressors take on centrosomes. Nature reviews. Cancer. 2007;7(12):911–24. doi: 10.1038/nrc2249. [DOI] [PubMed] [Google Scholar]
- 20.Gundersen GG, Bulinski JC. Selective stabilization of microtubules oriented toward the direction of cell migration. Proc Natl Acad Sci U S A. 1988;85(16):5946–50. doi: 10.1073/pnas.85.16.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84(3):359–69. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 22.Waterman-Storer CM, Salmon E. Positive feedback interactions between microtubule and actin dynamics during cell motility. Curr Opin Cell Biol. 1999;11(1):61–7. doi: 10.1016/s0955-0674(99)80008-8. [DOI] [PubMed] [Google Scholar]
- 23.Wittmann T, Waterman-Storer CM. Cell motility: can Rho GTPases and microtubules point the way? J Cell Sci. 2001;114(Pt 21):3795–803. doi: 10.1242/jcs.114.21.3795. [DOI] [PubMed] [Google Scholar]
- 24.Mogilner A, Keren K. The shape of motile cells. Curr Biol. 2009;19(17):R762–71. doi: 10.1016/j.cub.2009.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ridley AJ, et al. Cell migration: integrating signals from front to back. Science. 2003;302(5651):1704–9. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 26.Borisy GG, Svitkina TM. Actin machinery: pushing the envelope. Curr Opin Cell Biol. 2000;12(1):104–12. doi: 10.1016/s0955-0674(99)00063-0. [DOI] [PubMed] [Google Scholar]
- 27.Mullins RD, Heuser JA, Pollard TD. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc Natl Acad Sci U S A. 1998;95(11):6181–6. doi: 10.1073/pnas.95.11.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson WJ. Tube morphogenesis: closure, but many openings remain. Trends Cell Biol. 2003;13(12):615–21. doi: 10.1016/j.tcb.2003.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vasiliev JM. Cytoskeletal mechanisms responsible for invasive migration of neoplastic cells. Int J Dev Biol. 2004;48(5–6):425–39. doi: 10.1387/ijdb.041806jv. [DOI] [PubMed] [Google Scholar]
- 30.Kanchanawong P, et al. Nanoscale architecture of integrin-based cell adhesions. Nature. 2010;468(7323):580–4. doi: 10.1038/nature09621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133(6):1403–15. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dugina V, et al. Focal adhesion features during myofibroblastic differentiation are controlled by intracellular and extracellular factors. J Cell Sci. 2001;114(Pt 18):3285–96. doi: 10.1242/jcs.114.18.3285. [DOI] [PubMed] [Google Scholar]
- 33.Geiger B, Bershadsky A. Exploring the neighborhood: adhesion-coupled cell mechanosensors. Cell. 2002;110(2):139–42. doi: 10.1016/s0092-8674(02)00831-0. [DOI] [PubMed] [Google Scholar]
- 34.Defilippi P, et al. Actin cytoskeleton organization in response to integrin-mediated adhesion. Microsc Res Tech. 1999;47(1):67–78. doi: 10.1002/(SICI)1097-0029(19991001)47:1<67::AID-JEMT7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 35.Kaverina I, Krylyshkina O, Small JV. Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J Cell Biol. 1999;146(5):1033–44. doi: 10.1083/jcb.146.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rid R, et al. The last but not the least: the origin and significance of trailing adhesions in fibroblastic cells. Cell Motil Cytoskeleton. 2005;61(3):161–71. doi: 10.1002/cm.20076. [DOI] [PubMed] [Google Scholar]
- 37.Vasiliev JM, et al. Rho overexpression leads to mitosis-associated detachment of cells from epithelial sheets: a link to the mechanism of cancer dissemination. Proc Natl Acad Sci U S A. 2004;101(34):12526–30. doi: 10.1073/pnas.0404723101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levina EM, et al. Cytoskeletal control of fibroblast length: experiments with linear strips of substrate. J Cell Sci. 2001;114(Pt 23):4335–41. doi: 10.1242/jcs.114.23.4335. [DOI] [PubMed] [Google Scholar]
- 39.Kharitonova MA, Vasiliev JM. Controlling cell length. Semin Cell Dev Biol. 2008;19(6):480–4. doi: 10.1016/j.semcdb.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez OC, et al. Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat Cell Biol. 2003;5(7):599–609. doi: 10.1038/ncb0703-599. [DOI] [PubMed] [Google Scholar]
- 41.Chang YC, et al. GEF-H1 couples nocodazole-induced microtubule disassembly to cell contractility via RhoA. Mol Biol Cell. 2008;19(5):2147–53. doi: 10.1091/mbc.E07-12-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Danowski BA. Fibroblast contractility and actin organization are stimulated by microtubule inhibitors. J Cell Sci. 1989;93(Pt 2):255–66. doi: 10.1242/jcs.93.2.255. [DOI] [PubMed] [Google Scholar]
- 43.Dujardin DL, et al. A role for cytoplasmic dynein and LIS1 in directed cell movement. J Cell Biol. 2003;163(6):1205–11. doi: 10.1083/jcb.200310097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sutterlin C, Colanzi A. The Golgi and the centrosome: building a functional partnership. J Cell Biol. 2010;188(5):621–8. doi: 10.1083/jcb.200910001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vinke FP, Grieve AG, Rabouille C. The multiple facets of the Golgi reassembly stacking proteins. The Biochemical journal. 2011;433(3):423–33. doi: 10.1042/BJ20101540. [DOI] [PubMed] [Google Scholar]
- 46.Yadav S, Linstedt AD. Golgi positioning. Cold Spring Harbor perspectives in biology. 2011;3(5) doi: 10.1101/cshperspect.a005322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yadav S, Puri S, Linstedt AD. A primary role for Golgi positioning in directed secretion, cell polarity, and wound healing. Molecular biology of the cell. 2009;20(6):1728–36. doi: 10.1091/mbc.E08-10-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller PM, et al. Golgi-derived CLASP-dependent microtubules control Golgi organization and polarized trafficking in motile cells. Nature cell biology. 2009;11(9):1069–80. doi: 10.1038/ncb1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurokawa K, et al. Mechanism and role of localized activation of Rho-family GTPases in growth factor-stimulated fibroblasts and neuronal cells. Biochem Soc Trans. 2005;33(Pt 4):631–4. doi: 10.1042/BST0330631. [DOI] [PubMed] [Google Scholar]
- 50.Vinogradova T, et al. Concerted effort of centrosomal and Golgi-derived microtubules is required for proper Golgi complex assembly but not for maintenance. Molecular biology of the cell. 2012;23(5):820–33. doi: 10.1091/mbc.E11-06-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wakida NM, et al. An intact centrosome is required for the maintenance of polarization during directional cell migration. PloS one. 2010;5(12):e15462. doi: 10.1371/journal.pone.0015462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hurtado L, et al. Disconnecting the Golgi ribbon from the centrosome prevents directional cell migration and ciliogenesis. The Journal of cell biology. 2011;193(5):917–33. doi: 10.1083/jcb.201011014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weaver BA, Cleveland DW. Aneuploidy: instigator and inhibitor of tumorigenesis. Cancer research. 2007;67(21):10103–5. doi: 10.1158/0008-5472.CAN-07-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo HQ, et al. Analysis of the cellular centrosome in fine-needle aspirations of the breast. Breast cancer research : BCR. 2007;9(4):R48. doi: 10.1186/bcr1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perucca-Lostanlen D, et al. Distinct MDM2 and P14ARF expression and centrosome amplification in well-differentiated liposarcomas. Genes, chromosomes & cancer. 2004;39(2):99–109. doi: 10.1002/gcc.10303. [DOI] [PubMed] [Google Scholar]
- 56.Gong Y, et al. Localization of TEIF in the centrosome and its functional association with centrosome amplification in DNA damage, telomere dysfunction and human cancers. Oncogene. 2009;28(12):1549–60. doi: 10.1038/onc.2008.503. [DOI] [PubMed] [Google Scholar]
- 57.Sato N, et al. Centrosome abnormalities in pancreatic ductal carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 1999;5(5):963–70. [PubMed] [Google Scholar]
- 58.Yang J, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117(7):927–39. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 59.Ingber DE, Madri JA, Jamieson JD. Role of basal lamina in neoplastic disorganization of tissue architecture. Proc Natl Acad Sci U S A. 1981;78(6):3901–5. doi: 10.1073/pnas.78.6.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lozano E, Betson M, Braga VM. Tumor progression: Small GTPases and loss of cell-cell adhesion. Bioessays. 2003;25(5):452–63. doi: 10.1002/bies.10262. [DOI] [PubMed] [Google Scholar]
- 61.Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16(10):522–9. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 62.Pihan GA, et al. Centrosome defects can account for cellular and genetic changes that characterize prostate cancer progression. Cancer Res. 2001;61(5):2212–9. [PubMed] [Google Scholar]
- 63.Lingle WL, et al. Centrosome amplification drives chromosomal instability in breast tumor development. Proc Natl Acad Sci U S A. 2002;99(4):1978–83. doi: 10.1073/pnas.032479999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bissell MJ. Modelling molecular mechanisms of breast cancer and invasion: lessons from the normal gland. Biochem Soc Trans. 2007;35(Pt 1):18–22. doi: 10.1042/BST0350018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28(1–2):15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 66.Petroll WM, Ma L. Direct, dynamic assessment of cell-matrix interactions inside fibrillar collagen lattices. Cell Motil Cytoskeleton. 2003;55(4):254–64. doi: 10.1002/cm.10126. [DOI] [PubMed] [Google Scholar]
- 67.Friedl P, Wolf K. Proteolytic interstitial cell migration: a five-step process. Cancer Metastasis Rev. 2009;28(1–2):129–35. doi: 10.1007/s10555-008-9174-3. [DOI] [PubMed] [Google Scholar]
- 68.Wang W, et al. Single cell behavior in metastatic primary mammary tumors correlated with gene expression patterns revealed by molecular profiling. Cancer Res. 2002;62(21):6278–88. [PubMed] [Google Scholar]
- 69.Ketema M, Sonnenberg A. Nesprin-3: a versatile connector between the nucleus and the cytoskeleton. Biochem Soc Trans. 2011;39(6):1719–24. doi: 10.1042/BST20110669. [DOI] [PubMed] [Google Scholar]
- 70.Khatau SB, et al. The differential formation of the LINC-mediated perinuclear actin cap in pluripotent and somatic cells. PLoS One. 2012;7(5):e36689. doi: 10.1371/journal.pone.0036689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vasiliev JM, et al. Effect of colcemid on the locomotory behaviour of fibroblasts. J Embryol Exp Morphol. 1970;24(3):625–40. [PubMed] [Google Scholar]
- 72.Bershadsky AD, Vaisberg EA, Vasiliev JM. Pseudopodial activity at the active edge of migrating fibroblast is decreased after drug-induced microtubule depolymerization. Cell Motil Cytoskeleton. 1991;19(3):152–8. doi: 10.1002/cm.970190303. [DOI] [PubMed] [Google Scholar]
- 73.Malech HL, Root RK, Gallin JI. Structural analysis of human neutrophil migration. Centriole, microtubule, and microfilament orientation and function during chemotaxis. J Cell Biol. 1977;75(3):666–93. doi: 10.1083/jcb.75.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gierke S, Wittmann T. EB1-recruited microtubule +TIP complexes coordinate protrusion dynamics during 3D epithelial remodeling. Curr Biol. 2012;22(9):753–62. doi: 10.1016/j.cub.2012.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sumiyoshi E, Sugimoto A. Cell polarity: centrosomes release signals for polarization. Curr Biol. 2012;22(8):R281–3. doi: 10.1016/j.cub.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 76.Block MR, et al. Podosome-type adhesions and focal adhesions, so alike yet so different. Eur J Cell Biol. 2008;87(8–9):491–506. doi: 10.1016/j.ejcb.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 77.Nakahara H, et al. Activation of beta1 integrin signaling stimulates tyrosine phosphorylation of p190RhoGAP and membrane-protrusive activities at invadopodia. J Biol Chem. 1998;273(1):9–12. doi: 10.1074/jbc.273.1.9. [DOI] [PubMed] [Google Scholar]
- 78.Schoumacher M, et al. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J Cell Biol. 2010;189(3):541–56. doi: 10.1083/jcb.200909113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kikuchi K, Takahashi K. WAVE2- and microtubule-dependent formation of long protrusions and invasion of cancer cells cultured on three-dimensional extracellular matrices. Cancer Sci. 2008;99(11):2252–9. doi: 10.1111/j.1349-7006.2008.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schnaeker EM, et al. Microtubule-dependent matrix metalloproteinase-2/matrix metalloproteinase-9 exocytosis: prerequisite in human melanoma cell invasion. Cancer Res. 2004;64(24):8924–31. doi: 10.1158/0008-5472.CAN-04-0324. [DOI] [PubMed] [Google Scholar]
- 81.Sbai O, et al. Vesicular trafficking and secretion of matrix metalloproteinases-2, -9 and tissue inhibitor of metalloproteinases-1 in neuronal cells. Mol Cell Neurosci. 2008;39(4):549–68. doi: 10.1016/j.mcn.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 82.Sbai O, et al. Differential vesicular distribution and trafficking of MMP-2, MMP-9, and their inhibitors in astrocytes. Glia. 2010;58(3):344–66. doi: 10.1002/glia.20927. [DOI] [PubMed] [Google Scholar]
- 83.Hanania R, et al. Classically activated macrophages use stable microtubules for matrix metalloproteinase-9 (MMP-9) secretion. J Biol Chem. 2012;287(11):8468–83. doi: 10.1074/jbc.M111.290676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takino T, et al. Membrane-type matrix metalloproteinase modulates focal adhesion stability and cell migration. Exp Cell Res. 2006;312(8):1381–9. doi: 10.1016/j.yexcr.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 85.Takino T, et al. Inhibition of membrane-type matrix metalloproteinase at cell-matrix adhesions. Cancer Res. 2007;67(24):11621–9. doi: 10.1158/0008-5472.CAN-07-5251. [DOI] [PubMed] [Google Scholar]
- 86.Wang Y, McNiven MA. Invasive matrix degradation at focal adhesions occurs via protease recruitment by a FAK-p130Cas complex. J Cell Biol. 2012;196(3):375–85. doi: 10.1083/jcb.201105153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1(1):46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8(2):98–101. [PubMed] [Google Scholar]
- 89.Erler JT, V, Weaver M. Three-dimensional context regulation of metastasis. Clin Exp Metastasis. 2009;26(1):35–49. doi: 10.1007/s10585-008-9209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bhowmick NA, Moses HL. Tumor-stroma interactions. Curr Opin Genet Dev. 2005;15(1):97–101. doi: 10.1016/j.gde.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bosman FT, Stamenkovic I. Functional structure and composition of the extracellular matrix. J Pathol. 2003;200(4):423–8. doi: 10.1002/path.1437. [DOI] [PubMed] [Google Scholar]
- 92.Kumar S, V, Weaver M. Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev. 2009;28(1–2):113–27. doi: 10.1007/s10555-008-9173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Paszek MJ, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–54. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 94.Reno F, et al. Release and activation of matrix metalloproteinase-9 during in vitro mechanical compression in hypertrophic scars. Arch Dermatol. 2002;138(4):475–8. doi: 10.1001/archderm.138.4.475. [DOI] [PubMed] [Google Scholar]
- 95.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nature reviews. Cancer. 2003;3(5):362–74. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 96.Belletti B, et al. p27kip1 controls cell morphology and motility by regulating microtubule-dependent lipid raft recycling. Mol Cell Biol. 2010;30(9):2229–40. doi: 10.1128/MCB.00723-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Berton S, et al. The tumor suppressor functions of p27(kip1) include control of the mesenchymal/amoeboid transition. Mol Cell Biol. 2009;29(18):5031–45. doi: 10.1128/MCB.00144-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bartolini F, Gundersen GG. Formins and microtubules. Biochim Biophys Acta. 2010;1803(2):164–73. doi: 10.1016/j.bbamcr.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gaillard J, et al. Differential interactions of the formins INF2, mDia1, and mDia2 with microtubules. Mol Biol Cell. 2011;22(23):4575–87. doi: 10.1091/mbc.E11-07-0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- 101.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23(1):47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 102.Hiraoka N, et al. Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell. 1998;95(3):365–77. doi: 10.1016/s0092-8674(00)81768-7. [DOI] [PubMed] [Google Scholar]
- 103.Chun J, et al. Cultures of ligament fibroblasts in fibrin matrix gel. Connect Tissue Res. 2003;44(2):81–7. [PubMed] [Google Scholar]
- 104.Sabeh F, et al. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167(4):769–81. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Filippov S, et al. MT1-matrix metalloproteinase directs arterial wall invasion and neointima formation by vascular smooth muscle cells. J Exp Med. 2005;202(5):663–71. doi: 10.1084/jem.20050607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gaggioli C, et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9(12):1392–400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 107.Nagano S, et al. Cancer cell death enhances the penetration and efficacy of oncolytic herpes simplex virus in tumors. Cancer Res. 2008;68(10):3795–802. doi: 10.1158/0008-5472.CAN-07-6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Trepat X, Fredberg JJ. Plithotaxis and emergent dynamics in collective cellular migration. Trends Cell Biol. 2011;21(11):638–46. doi: 10.1016/j.tcb.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hegerfeldt Y, et al. Collective cell movement in primary melanoma explants: plasticity of cell-cell interaction, beta1-integrin function, and migration strategies. Cancer research. 2002;62(7):2125–30. [PubMed] [Google Scholar]
- 110.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nature reviews. Molecular cell biology. 2009;10(7):445–57. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 111.Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93(4):266–76. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 112.Klein A, Flugel D, Kietzmann T. Transcriptional regulation of serine/threonine kinase-15 (STK15) expression by hypoxia and HIF-1. Mol Biol Cell. 2008;19(9):3667–75. doi: 10.1091/mbc.E08-01-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Katayama H, et al. Interaction and feedback regulation between STK15/BTAK/Aurora-A kinase and protein phosphatase through mitotic cell division cycle. J Biol Chem. 2001;276(49):46219–24. doi: 10.1074/jbc.M107540200. [DOI] [PubMed] [Google Scholar]
- 114.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–6. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 115.Glinskii AB, et al. Viable circulating metastatic cells produced in orthotopic but not ectopic prostate cancer models. Cancer Res. 2003;63(14):4239–43. [PubMed] [Google Scholar]
- 116.Whipple RA, et al. Vimentin filaments support extension of tubulin-based microtentacles in detached breast tumor cells. Cancer Res. 2008;68(14):5678–88. doi: 10.1158/0008-5472.CAN-07-6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Matrone MA, et al. Microtentacles tip the balance of cytoskeletal forces in circulating tumor cells. Cancer Res. 2010;70(20):7737–41. doi: 10.1158/0008-5472.CAN-10-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Whipple RA, Cheung AM, Martin SS. Detyrosinated microtubule protrusions in suspended mammary epithelial cells promote reattachment. Exp Cell Res. 2007;313(7):1326–36. doi: 10.1016/j.yexcr.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kramer A, Maier B, Bartek J. Centrosome clustering and chromosomal (in)stability: a matter of life and death. Mol Oncol. 2011;5(4):324–35. doi: 10.1016/j.molonc.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ogden A, Rida PC, Aneja R. Let’s huddle to prevent a muddle: centrosome declustering as an attractive anticancer strategy. Cell Death Differ. 2012 doi: 10.1038/cdd.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Grinberg-Rashi H, et al. The expression of three genes in primary non-small cell lung cancer is associated with metastatic spread to the brain. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(5):1755–61. doi: 10.1158/1078-0432.CCR-08-2124. [DOI] [PubMed] [Google Scholar]
- 122.Kraljevic Pavelic S, et al. Metastasis: new perspectives on an old problem. Molecular cancer. 2011;10:22. doi: 10.1186/1476-4598-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]