Abstract
Temperament differs among individuals both within and between species. Evidence suggests that differences in temperament of group members may parallel differences in social behavior among groups or between species. Here, we compared temperament between three closely related species of monkey - rhesus (Macaca mulatta), long-tailed (M. fascicularis), and pigtailed (M. nemestrina) macaques - using cage-front behavioral observations of individually housed monkeys at a National Primate Research Center. Frequencies of 12 behaviors in 899 subjects were analyzed using a PCA to identify temperament components. The analysis identified four components, which we interpreted as Sociability towards humans, Cautiousness, Aggressiveness, and Fearfulness. Species and sexes differed in their average scores on these components, even after controlling for differences in age and early-life experiences. Our results suggest that rhesus macaques are especially aggressive and unsociable towards humans, long-tailed macaques are more cautious and fearful, and pigtailed macaques are more sociable towards humans and less aggressive than the other species. Pigtailed males were notably more sociable than any other group. The differences observed are consistent with reported variation in these species’ social behaviors, as rhesus macaques generally engage in more social aggression and pigtailed macaques engage in more male-male affiliative behaviors. Differences in predation risks are among the socioecological factors that might make these species-typical behaviors adaptive. Our results suggest that adaptive species-level social differences may be encoded in individual-level temperaments, which are manifested even outside of a social context.
Keywords: temperament, macaque, Macaca, social behavior, personality, context
Animals from a broad range of taxa exhibit stable, biologically based individual patterns of behavior that can be quantified as multi-dimensional temperament traits [Gosling, 2001; Itoh, 2002; Sih et al 2004]. Animal models also allow us to test how various factors, like physiology and early life experience, might interact with temperament to produce differing social, reproductive, and survival outcomes [Dingemanse & Reale, 2005; Reale et al., 2007; Schuett, Tregenza & Dall, 2010; Bergmuller & Taborsky, 2010; Smith & Blumstein, 2010].
Temperament is especially well studied in nonhuman primates, where a variety of methods have been used to identify traits [Itoh, 2002; Uher, 2008; Freeman & Gosling, 2010: Weiss et al., 2011]. Nonhuman primates are particularly appealing to researchers interested in the relationship between temperament and social behavior. Many primates display complex social systems, and these systems vary widely, even among closely related species [e.g., Thierry, 2000, 2004]. Some studies have identified differences in average temperaments between closely related species with such differing social systems [Clarke & Mason, 1998; Uher & Asendorpf, 2008; Weiss et al., 2011; Konečná et al., 2012]. Several researchers have proposed that these underlying differences in temperament are responsible for group differences in social behavior – that is, that social dynamics may be an emergent phenomenon resulting from the mix of individuals in the group [e.g., Capitanio, 2004; Sih, 2011; Ha, Alloway & Sussman, 2011; Weinstein & Capitanio, 2008)].
Incorporating temperament enhances behavioral ecological models of social behavior, which explain social differences primarily in terms of environmental factors such as resource availability, habitat type, and predation risk, [e.g., Caldecott, 1986a; Hill & Lee, 1998]. These factors in turn regulate group size and kinship, which are strong predictors for many social behaviors [Call, Judge & de Waal. 1996; Lehmann, Korstjens & Dunbar, 2007]. However, these factors do not account for all differences in social behavior between or within species [e.g., Ha et al., 2011], and individual differences within a group are also an important explanatory factor. Both temperament and social structure are likely related to ecological selective pressures [Sih et al, 2004], and have evolved in concert. Thus, a true understanding of species differences requires incorporating information about temperament, social behavior, and ecological differences.
In this study, we quantified and compared temperament between three closely related species of monkey - rhesus (Macaca mulatta), long-tailed (M. fascicularis), and pigtailed (M. nemestrina) macaques - using rapid cage-front behavioral observations. We examined how temperament differences might relate to previously identified species differences in social behavior. We also propose how some ecological factors affecting these species in the wild might relate to both the reported group behaviors and the temperament components we identified.
Our three study species are ideal for this comparison because they differ in ecology and social behavior in the wild and in captivity. Like all macaques, they live in multi-male, multi-female groups with male dispersal [Melnick & Pearl, 1987]. Despite these basic similarities, the species vary in social dynamics. Species-typical social dynamics in macaques can range from rigidly hierarchical, despotic groups to more egalitarian groups with higher frequencies of affiliative behaviors [Matsumara, 1999; Thierry, 2000; Maestripieri, 2005]. Of our study species, rhesus macaques are considered to be among the most despotic, or rigidly hierarchical, while long-tailed and pigtailed macaques are considered somewhat more egalitarian [Thierry, 2000]. In captive social groups, rhesus macaques tend to display more frequent and intense aggression than other species, while long-tailed and pigtailed macaques show more frequent affiliative and appeasement behaviors [Bernstein, Williams & Ramsay, 1983; Ruehlmann et al., 1988; Thierry, 2000; Thierry, Iwaniuk & Pellis, 2000; Gouzoules & Gouzoules, 2000; Maestripieri, 2005]. Pigtailed macaques are reported to make use of a larger range of affiliative behaviors than the other species studied here [Oi, 1990a; Oi 1990b; Thierry, 2000; Maestripieri, 2005].
Much of the earliest work on nonhuman primate temperament was performed in rhesus macaques [Chamove, Eysenck & Harlow, 1972; Stevenson-Hinde & Zunz 1978]. Comparative studies found macaque species to differ with respect to traits like “hostility,” “fear” [Clarke & Mason, 1988], and “response to novelty” [Clarke & Lindburg, 1993; Montgomery, Bentson & Crockett, 2005]. Long-tailed macaques showed more fear and less hostility toward human observers than rhesus macaques [Clarke & Mason, 1988], and displayed greater aversion to novelty than pigtailed macaques [Montgomery et al., 2005]. No previous studies have assessed all three species simultaneously. Our large sample allows us to compare both interspecific and intraspecific sex differences in temperament in a manner not addressed in prior studies.
We expected to find measurable, stable differences in temperament among the species. We hoped to better define the typical temperament of each species when housed in similar conditions. Our subjects were individually housed in visual, auditory, olfactory, and, for a subset, grooming contact with conspecifics, but were not housed in social groups. This allowed us to measure temperament in a context where behavior was minimally influenced by group dominance interactions. We considered the conditions of our subjects’ housing both a limitation and a strength of the study. As a limitation, individual housing is very different from a macaque’s natural social environment, and may alter the animals’ behavior, reducing the ecological validity of our observations. Individual behavior is shaped both by genes and by experiences, and given that our subjects had limited social interaction during this study and came from diverse developmental and social backgrounds, these gene-environment interactions were not constant within our sample. On the other hand, animal temperament measured in a social setting often differs from temperament measured in isolation [Krause, James, & Croft, 2010; Dingemanse et al., 2010], and dominance rank and third-party effects can influence individuals’ behavioral patterns within the group [e.g., Cheney, Seyfarth & Smuts, 1986]. These effects might be expected to differentially affect macaque species with stronger dominance hierarchies, or individuals with higher or lower rank in such groups [Cheney et al., 1986; Krause et al., 2010]. Our individually housed sample bypassed these effects, allowing us to better compare species differences.
The main goal of the study was to identify any temperament components that meaningfully describe individual differences in behaviors, and to compare the structure of these components across species. As in past work, we expected to find similar components present in closely related species [Uher & Asendorpf, 2008; Weiss et al., 2011; Konečná et al., 2012]. Our next goal was to compare temperament scores across species and sex, with the expectation that this comparison would mirror other reported differences. We had no a priori hypotheses regarding the directions of sex differences, but we expected to see some, given sex differences in hormones. We expected that rhesus and long-tailed macaques might be more similar to each other than to pigtailed macaques, given that they are more closely related phylogenetically [Hoelzer & Melnick, 1996]. Finally, we attempted to relate identified differences in temperament components to reports of species-typical social behavior in groups and differences in socioecology.
Methods
Subjects
Behavioral data were collected between 2003 and 2006 on monkeys housed at the National Primate Research Center, University of Washington (WaNPRC). The sample included 899 individuals: 556 pigtailed, 214 long-tailed, and 129 rhesus macaques. All tested subjects were at least one-year old. The distribution of ages varied somewhat by species (Table 1). The long-tailed macaque sample was skewed towards older animals and contained a smaller proportion of juveniles than other species. Most of the subjects were born in a captive social group in a breeding colony, but 14% had spent some of their first year of life nursery-reared at the WaNPRC’s Infant Primate Research Laboratory (IPRL). Most of these nursery-reared monkeys were pigtailed macaques. All animals had been housed at WaNPRC for at least one year prior to the test used in this analysis.
Table 1.
Comparison of sample characteristics for three species included. Age in months was calculated for the date of the test; ages for international animals were estimated.
| Species | Total N |
Juvenile (12-48 months) |
Young adult (48-96 months) |
Mature adult (>96 months) |
Nursery- reared monkeys |
Males: Females |
|---|---|---|---|---|---|---|
| Long- tailed |
214 | 1% | 42% | 57% | 13% | 0.88 |
| Rhesus | 129 | 36% | 50% | 15% | 1% | 1.58 |
| Pigtailed | 556 | 32% | 38% | 29% | 18% | 0.97 |
| Total N | 899 | 25% | 41% | 34% | 14% | 1.02 |
Housing and Approvals
Monkeys were individually housed indoors in single or grooming-contact [Crockett et al., 1997] stainless steel cages appropriate for their weight, as specified by USDA Animal Welfare Regulations [USDA, 1991]. Room sizes varied and typically housed 12 to 30 monkeys, generally all of the same species. Animal rooms were maintained on a 12:12 h light:dark cycle. The ambient temperature was 22.2° to 25.6° C with a relative humidity of 30% to 50%. The subjects received enrichment as specified in the Environmental Enhancement Plan such as a portable toy and a foraging device hung externally on the cage, and fresh produce or foraging opportunities seven days a week. Singly housed monkeys had visual contact with conspecifics. Commercial monkey biscuits were provided twice daily – once before 9:00 am and once after 2:00 pm, and water was provided ad libitum from water spigots (lixits). The University of Washington Institutional Animal Care and Use Committee approved the observational techniques of this study. Our research complied with legal requirements of the U.S.A., the state of Washington, and the AAALACi, as well as the American Society of Primatologists Principles for the Ethical Treatment of Non Human Primates.
The RATR Data Set
Temperament was assessed from behavioral measures recorded via the Rapid Assessment of Temperament and Reactivity (RATR) [Bentson, Crockett & Ha, 2003]. All observations were made by a single tester (the author KLB) in a 4-minute assessment. The observations were made at cage-front, with the tester standing directly in front of the subject’s cage. Prior to each 4-min cage-front observation, the tester stood in the middle of the room for 4 minutes. The observer was not known by the monkeys in any context besides testing. All tests were made between 2-5 p.m., and up to eight animals were tested each session, with testing order determined by a random number generator. If more than one test was available for a subject, the first test conducted when the monkey had been at WaNPRC for at least one year was used in the analysis.
Data were recorded on a PDA hand-held device (Event software programmed by JCH), with supplementary notes. Several behaviors, including posture/locomotion, facial/vocal expression, cage position, and responsiveness to the observer, were scored by instantaneous sampling every minute. Other behaviors were measured by whether or not they occurred during each minute of the observation period. At the end of each minute, the tester also made subjective assessments (i.e., ratings) of temperament, as a means of validating and interpreting the behavioral measures during analysis. The tester assessed which of seven labels– “confident,” “aggressive,” “tense,” “friendly,” “fearful,” “active,” and “inactive” – best described the subject’s behavior over the past minute. “Active” or “inactive” were used primarily when other temperament ratings did not apply. All behavioral measures and subjective assessments occurred once each minute, so all variables ranged from 0-4. Descriptions of 37 behavioral measures are included as on-line supplemental materials.
Statistical Analyses
Component Identification
Analyses were conducted using PASW Statistics 18.0 [IBM, 2008]. The behavioral measures were included in a principal components analysis (PCA), which was used to reduce the list of behaviors into uncorrelated components describing the majority of the variance. PCA was chosen over factor analysis because this was a purely exploratory analysis, and because the structure of the variance within the dataset was unknown. PCA is not appropriate for identifying latent “traits”, but, rather, identifies suites of inter-related behaviors [Dunteman, 1989; Joliffe, 2002].
Three variables (LEN to monkey, LEN to observer, and tooth grind) were removed because they did not occur in all species. LEN is a communicative gesture of pigtailed macaques [Oettinger, Crockett & Bellanca, 2007]. All other variables were included in an initial PCA, except for tester ratings of temperament, which were used in post hoc tests to help interpret the resulting principal components. Variables were removed in a stepwise process to obtain the final list of variables. Variables were removed if they did not load on any component at the 0.4 level, if they had extraction communalities < 0.5, or if they only contributed to one component which had no other variables contributing. “Sit” and “stand” were removed because they were the only variables contributing to a component, and such a component describing body position was not considered relevant for a discussion of temperament.
This process reduced the variable list to 12 out of the original 37 variables, which contributed to four components. Retention of four components was supported both by eigenvalues and by parallel analysis [Zwick and Velicer, 1986].
Components were rotated first with oblique (oblimin) rotation. Correlations between components were not large (largest r = −0.2, between components 1 and 4), so we chose to use orthogonal (varimax) rotations on subsequent analyses in keeping with widespread practice. We then repeated the PCA, specifying four components, and used the regression method to calculate individual component scores. We also used split-sample validation to confirm the reliability of these components. The sample was split into random halves, and the structure of the component matrices generated for the two samples were compared using Tucker’s congruence coefficients [Lorenzo-Sevo & ten Berge, 2006].
To interpret components, we examined both the past literature on macaque temperament and the tester-rating scores. We used the correlations between the tester scores and the component scores to help identify what temperament trait each component described, in keeping with widespread practice [e.g., Konečná et al., 2008].
Cross-species Validation
We also performed PCAs to separately analyze our sample set for each species to test for structural consistency between species. We compared the structure of the component matrices for each species using Tucker’s congruence coefficients [Lorenzo-Sevo & ten Berge, 2006].
Species and Sex temperament differences
Once the traits were identified, we used a general linear model (GLM) to identify differences in temperament among species. Component scores on the four traits were used as dependent variables, and species, sex, and a species-by-sex interaction variable were included as predictors. Since animal age and nursery-rearing experience (whether or not they had been raised in the infant nursery) varied by species and it is currently unknown whether the traits vary by those factors, we controlled for them as covariates. We interpreted significant differences with post-hoc Tukey-Kramer tests. Alpha was set at 0.05, and all analyses were two-tailed.
Results
Component Identification
PCA revealed four components, which together explained about 64% of the variance in the data after rotation (Table 2), and incorporated 12 of the 37 behavioral variables. The same measures were identified in a split-sample validation, and the Tucker’s congruence coefficients of the components on the two samples were all high (all > 0.95).
Table 2.
Results of PCA including all species, specifying 4 components and rotated with Varimax rotation. Variables that contribute with a loading of > |0.40| are shown in bold [Joliffe, 2002]. PC is principal component.Tucker’s congruence coefficients (□) for species similarities are shown at the bottom of the table.
| PC 1 | PC 2 | PC 3 | PC 4 | |
|---|---|---|---|---|
| Behaviors | Sociability | Cautiousness | Aggressiveness | Fearfulness |
| Front of cage | .82 | −.22 | .04 | −.04 |
| Back of cage | −.78 | .12 | −.14 | .03 |
| Reach | .64 | .28 | −.19 | −.05 |
| Lipsmack to tester | −.45 | .60 | −.20 | .11 |
| Quiet face | .41 | −.67 | −.33 | −.18 |
| Ignore tester | .08 | −.76 | −.19 | −.07 |
| Lean or approach tester | .36 | .69 | −.06 | −.14 |
| Open mouth | −.05 | .13 | .79 | −.05 |
| Lunge | .03 | −.02 | .72 | −.01 |
| Threat (tester /conspecific) |
.06 | .04 | .78 | −.03 |
| Shriek | −.06 | .03 | −.03 | .81 |
| Grimace | −.04 | .04 | −.04 | .82 |
|
| ||||
| Eigenvalue | 2.21 | 2.04 | 2.01 | 1.42 |
| Variance Explained | 18.44% | 16.98% | 16.72% | 11.84% |
| Total variance explained | 18.44% | 35.43% | 52.15% | 63.99% |
|
| ||||
| Long-tailed vs. Rhesus | 0.79 | 0.73 | 0.76 | 0.94 |
| Long-tailed vs. Pigtailed | 0.79 | 0.86 | 0.95 | 0.91 |
| Rhesus vs. Pigtailed | 0.80 | 0.71 | 0.64 | 0.98 |
We included a comparison with the tester temperament labels to help us interpret our PCA components. The results were consistent with our interpretation of the components based on their variable make-up, and led us to name the four components Sociability towards humans, Cautiousness, Aggressiveness, and Fearfulness (Table 3). These names were chosen, when possible, from terms used in past studies (see Discussion).
Table 3.
Correlations between tester-rated temperaments and PCA-derived temperament traits (N=899). Correlations significant at p<.05 and with a “medium” or greater effect size [Cohen, 1992] shown in bold.
| Tester Rating | Sociability | Cautiousness | Aggressiveness | Fearfulness |
|---|---|---|---|---|
|
|
||||
| Confident | .21 | −.25 | −.14 | −.16 |
| Aggressive | .06 | .10 | .78 | −.07 |
| Tense | −.31 | .11 | −.08 | −.02 |
| Friendly | .44 | .42 | −.22 | −.09 |
| Fearful | −.16 | .07 | −.06 | .77 |
| Active | −.02 | −.21 | −.08 | −.04 |
| Inactive | −.27 | −.13 | −.13 | −.08 |
Cross-species Validation
Species-specific PCAs demonstrated that the components existed in all three species, but that there were notable differences in structure (Table 2). Congruence coefficients were highest for Fearfulness. Long-tailed and pigtailed macaques were slightly more similar in their component structure, with structures for Cautiousness, Aggressiveness, and Fearfulness exceeding the suggested minimum congruence level of 0.85 [Lorenzo-Seva & ten Berge, 2006]. Rhesus and pigtailed macaques were least similar, with the lowest congruence on Aggressiveness (0.64). Differences in structure were mainly due to an apparent lack of importance of lipsmacks for rhesus macaques (this variable contributed to Cautiousness for long-tailed and pigtailed macaques). Also, aggressive behaviors contributed to all components for rhesus macaques, while long-tailed and pigtailed macaques had clearly defined “Aggressiveness” components. We proceeded with our cross-species comparison using the component scores obtained from the full sample PCA.
Species and Sex temperament differences
The results of the GLM indicated that species and sex were both significant predictors of temperament, after controlling for age and infant rearing experience. Species was a significant predictor of all four components, and sex was a significant predictor of Cautiousness and Sociability towards humans. There was also a significant species-by-sex interaction effect for Aggressiveness and Fearfulness. All main and interaction effects are summarized in Table 4.
Table 4.
Main and Interaction effects of Species and Sex from GLM, after controlling for nursery rearing experience and age-in-months. Covariate effects for rearing experience and age are shown below. Significant differences (at the 0.05 level) are indicated in bold, and effect sizes (η2) are noted.
| Temperament Components |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sociability towards humans |
Cautiousness | Aggressiveness | Fearfulness | ||||||
| Predictors: | df, DF | F | η 2 | F | η 2 | F | η 2 | F | η 2 |
|
|
|||||||||
| Species | 2, 891 | 35.43 | .07 | 56.22 | .11 | 32.07 | .07 | 3.21 | .01 |
| Sex | 1, 891 | 47.86 | .05 | 20.57 | .02 | 0.73 | <.01 | 0.11 | <.01 |
| Species-by-sex | 2, 891 | 0.94 | <.01 | 1.00 | <.01 | 6.28 | .01 | 3.86 | .01 |
|
| |||||||||
| Covariates: | F | η 2 | F | η 2 | F | η 2 | F | η 2 | |
| Age-in-months | 1, 891 | 0.06 | <.01 | 161.31 | .15 | 4.27 | <.01 | .48 | <.01 |
| Nursery experience |
1, 891 | 47.77 | .05 | 3.29 | <.01 | 15.26 | .02 | .48 | <.01 |
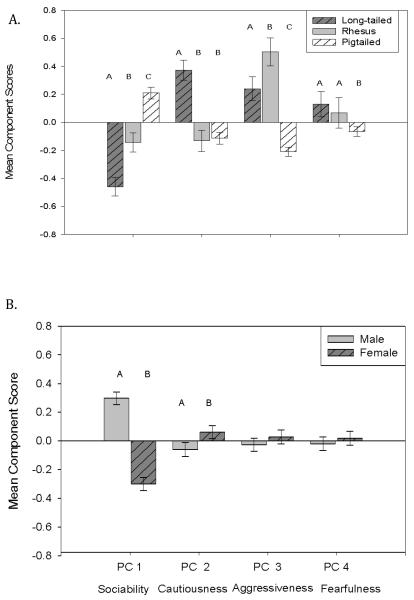
Post-hoc Tukey-Kramer tests demonstrated that long-tailed macaques were more cautious than other species (Tukey-Kramer 95% Confidence Interval rhesus: 0.27-0.74; pigtailed: 0.32-0.66), although rhesus and pigtailed macaques did not differ from each other (Table 4, Figure 1A). Pigtailed macaques were more sociable towards humans than other species (95% CI long-tailed: 0.50-0.84; rhesus: 0.15-0.56), and rhesus were more sociable than long-tailed macaques (95% CI: 0.08-0.55). Rhesus macaques were significantly more aggressive than either other species (95% CI long-tailed: 0.01-0.51; pigtailed: 0.49-0.93) and long-tailed macaques were significantly more aggressive than pigtailed macaques (95% CI: 0.27-0.63). Long-tailed macaques were significantly more fearful than pigtailed macaques (95% CI: 0.01-0.39), but rhesus macaques did not differ from other species on this component. For sex main effects, males were significantly less cautious than females (95% CI: 0.002-0.24) and were significantly more sociable towards humans (95% CI: 0.58-0.81, Figure 1B).
Figure 1.
Species and sex differences in z-standardized component scores, +/− SE. Letters represent significant differences. A. Species main effects; B. Sex main effects.
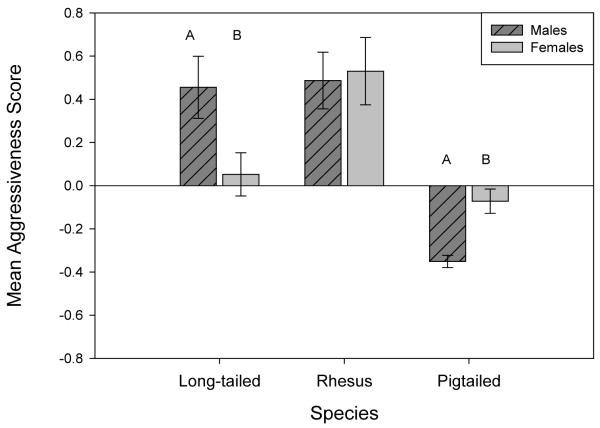
Examining interaction effects indicated that male pigtailed macaques tended to be less aggressive than other sex-species categories (Figure 2). Pigtailed males were significantly less aggressive than all other sex-species categories, including rhesus males (95% CI: 0.49-1.19), rhesus females (95% CI: 0.46-1.30), long-tailed males (95% CI: 0.49-1.12), long-tailed females (95% CI= 0.10-0.71), and pigtailed females (95% CI: 0.05-0.51). Pigtailed females were significantly less aggressive than rhesus males (95% CI= 0.21-0.91), rhesus females (95% CI=0.18-1.02), and long-tailed males (95% CI: 0.21-0.84), but did not differ from long-tailed females. Long-tailed females were significantly less aggressive than long-tailed males (95% CI: 0.03-0.78), rhesus males (95% CI: 0.03-0.83), and rhesus females (95% CI: 0.01-0.94). Long-tailed males, rhesus males, and rhesus females did not differ from each other (all p > 0.05).
Figure 2.
Species-by-sex interaction effects for z-standardized Aggressiveness, +/− SE. Letters represent significant differences.
For fearfulness interaction effects, only the two most extreme sex-species categories differed significantly. Long-tailed females were significantly more fearful than pigtailed males (95% CI: 0.01-0.65), but no other pairwise comparisons were significantly different.
While not directly tied to our hypotheses, we also noted that the covariates of our analyses – age and nursery experience – were also significant predictors of these temperament components (Table 4). Animals with nursery experience were significantly more sociable towards humans and less aggressive. Older animals tended to be more cautious and more aggressive. We explore these intriguing relationships in more detail in a separate paper [Sussman et al., in prep].
Discussion
Three of the four temperament components we identified in individually housed rhesus, pigtailed, and long-tailed macaques were similar to other named traits identified in the literature [reviewed in Freeman & Gosling, 2010]. Other studies that also used large sample sizes and similar statistical techniques have identified 3 to 6 personality or temperament traits in macaques [Capitanio, 1999; Rouff, Sussman, & Stroube, 2005; Weiss et al., 2011]. Our four components are consistent with this range. The limited social context of our study (subject responses to a human tester) suggests that our components should describe just a subset of all possible temperament dimensions.
We interpreted and named our components based on similarity to other traits identified in the literature, and correlation with tester-rated temperament descriptors. Where appropriate, we used component names from the list of personality dimensions identified by Freeman and Gosling in a recent review [2010] that found fearfulness and aggressiveness to be among the most commonly identified traits in primates. Our first two components were less closely aligned to other dimensions described in the literature. Component 1 was interpreted as Sociability towards humans, because it describes tendencies to direct attention to the tester and reach toward the tester without threat or fear displays. Because of the context of our study, all interactive behaviors were primarily directed towards the human observer. However, as “sociability” is often used in the literature to describe the tendency to associate with conspecifics [e.g., Stevenson-Hinde & Zunz, 1978; Capitanio, 1999; Weiss et al., 2011], we added a contextual modifier for our term to differentiate it from this more common term. Other studies have identified similar components, such as “friendliness to humans” in great apes [Uher & Asendorpf, 2008]. The component that we call Cautiousness is similar, at the high end, to “reactivity” as used by Clarke and Mason [1988]. Monkeys scoring high on this component directed lipsmacks toward the tester while leaning or moving toward the tester. Monkeys scoring low on this component were in the front of the cage and did not change their behavior when the tester directed her gaze toward the monkey. These monkeys appear to be similar to ones described as equable or easy-going and passive [Capitanio, 1999].
Species-specific PCA comparisons show that behaviors contribute to similar components in all three species. However, congruence coefficients indicate that component structure is most similar between long-tailed and pigtailed macaques, and least similar between rhesus and pigtailed macaques. This is counter to our expectation that rhesus and long-tailed macaques might be more similar given their closer phylogenetic relationship [Hoelzer & Melnick, 1996]. However, these component structures do not account for differences in age and early-life experience between our samples. Further studies are necessary to fully refute the phylogenetic similarity hypothesis. We found that lipsmacks are an important variable for defining behavior in long-tailed and pigtailed, but not rhesus macaques. In addition, pigtailed and long-tailed macaques have clearly defined “Aggressiveness” components, while in rhesus macaques aggressive behaviors are also somewhat related to Cautiousness and Sociability. This importance of aggressive behaviors for rhesus macaques is consistent with the results of our GLM, as discussed below.
As we expected, species and sex were predictors of the temperament components examined, even after controlling for age and nursery experience. As elaborated below, our results are consistent with findings from the few past studies involving laboratory comparisons of these species. They are also consistent with reported differences in species’ social behavior and socioecology in the wild, although some important field observations are lacking. Our species comparisons demonstrated typical temperament profiles for each species (Figure 1A), after adjusting for age and early-life experience.
In our sample, long-tailed macaques were notable for being more cautious and less sociable towards humans than other species. They also tended to be more fearful than pigtailed macaques, and were intermediate in their aggressiveness. Rhesus macaques were more aggressive than other species (significantly more so than pigtailed macaques), and were intermediate in their sociability towards humans. Pigtailed macaques were much more sociable towards humans and less aggressive than both other species. Sociability towards humans appears to be an especially important trait for pigtailed macaques, and an identical component may not exist in other species, based on the low between-species coefficients for these traits. The facial expression LEN [Oettinger, et al., 2007] was not included in the PCA because it occurred only in pigtailed macaques. In an exploratory inclusion of LEN in the species-only PCA, LEN toward the observer loaded heavily with the sociability component and no others, suggesting that its exclusion understated the importance of the sociability component for this species.
The species categorizations and comparisons found in the present study are consistent with past work. In a similar laboratory environment, Clarke and Mason [1988] described long-tailed macaques as more “reactive” than other species and rhesus as more “hostile.” In captive social groups, rhesus macaques engage in more frequent and more severe intergroup aggression than other species [Bernstein et al., 1983; Thierry, 1985; Ruehlmann et al., 1988; Thierry, 2000;]. Both long-tailed and pigtailed macaques show comparatively less social aggression, lower influence of kinship in determining female rank, and a higher frequency of clasping and appeasing behaviors than rhesus [Bernstein et al., 1983; Thierry, 2000; Maestripieri, 2005; Thierry, 2006]. In captivity, for example, pigtailed macaques’ hourly rates of aggression are about half that of rhesus macaques’ [Bernstein et al., 1983], whereas their reconciliation rates are twice as frequent [Gouzoules & Gouzoules, 2000]. In captive groups, rhesus macaques received more than twice as many wounds as pigtailed macaques [Ruehlmann et al., 1988]. Some of these differences may be related to differences in captive conditions and in field study methodologies. However, the directions of these differences are consistent in most comparative studies [Thierry, 2000], and fit with our finding that rhesus macaques are more aggressive even when individually housed.
Some evidence suggests that pigtailed macaques are less aggressive and show higher rates of affiliative behavior than long-tailed macaques [Thierry, 2000]. Male-male affiliative behaviors in socially housed pigtailed macaques, lead to increased cooperation and intragroup tolerance relative to rhesus macaques [Maestripieri, 2005]. Pigtailed macaques also appear to have a larger affiliative vocabulary than some other species, including a species-specific affiliative greeting, the “LEN” [Oettinger et al, 2007], and extensive use of male-male affiliative eyebrow raises [Maestripieri, 1996]. Anecdotal reports also suggest that pigtailed macaques are “friendlier” than other macaque species, and more easily habituated to human presence in captivity. Oettinger et al. [2007] found that captive pigtailed macaques were likely to direct the LEN greeting towards human observers. In Sumatra, wild-caught pigtailed macaques were more easily trained to complete a task (picking coconuts) than long-tailed macaques [Crockett & Wilson, 1980]. Again, these differences are all consistent with our categorization of pigtailed macaques as more sociable towards humans and less aggressive than other species.
Whereas our study investigated captive macaques, some of the trends we identified show interesting parallels to ecological species differences in wild populations. One potential difference between the species in the wild, for example, is predation risk. Although no studies have directly compared predation rates among these species, it is likely that long-tailed macaques are under greater predation pressures, as they are smaller than the other two species and occupy habitats with many possible predators [Crockett & Wilson, 1980; Caldecott, 1986b]. Cheney and Wrangham [1987] presented estimated predation rates for numerous primate species gathered directly from field investigators. The only macaque species included was the long-tailed macaque, and it had one of the highest estimated suspected predation rates, second only to the vervet monkey (Cercopithecus aethiops). We propose, from the limited evidence available, that of the three species, long-tailed macaques are most vulnerable to predation ([most likely by felids, but crocodilian predation has also been reported [Galdikas and Yaeger, 1984]). Although primarily arboreal, long-tailed macaques are edge species and come to the ground along rivers and forest margins [Crockett & Wilson, 1980], where they are more likely to be vulnerable to predators. The larger-bodied pigtailed macaques, given their quiet behavior and smaller group size [Crockett & Wilson, 1980], may be less likely to attract predators. Pigtailed macaque groups also have much larger home ranges, usually in upland primary rainforest, making their location much less predictable to a predator [Crockett & Wilson, 1980; Caldecott, 1986b]. It is difficult to generalize predation rates on rhesus macaques across their large geographical distribution. Compared to long-tailed and pigtailed macaques, though, rhesus favor open habitats where they are likely to be terrestrial [Fooden, 2006], and thus may benefit from defending themselves by aggressive confrontation with some smaller predators.
Past work has suggested that reactivity, fearfulness, or novelty-aversion may be adaptive under conditions of greater predation pressure [Sih et al., 2004; Dingemanse & Reale, 2005; Reale et al., 2007; Smith & Blumstein, 2010]. This suggests that the temperament type most typical of long-tailed macaques in our sample – high Cautiousness and high Fearfulness scores – might be adaptive when predation risk is high. Similarly, the extreme aggressiveness we identified in rhesus macaques could be related to the adaptive benefit of aggressive troop defense strategies in the wild.
We also noted some sex differences. We found that females were significantly more cautious and less sociable towards humans than were males, which is consistent with past work: Female rhesus macaques are less “confident” and more “excitable” than males [Stevenson-Hinde, et al., 1980], and long-tailed and pigtailed macaque females are more novelty-averse than males [Montgomery et al., 2005]. Others have reported that the direction of sex differences in related traits varies by species [e.g., hyenas and humans, Gosling & John, 1999; rats and cichlids, Schuett et al., 2010]. This variability in findings is partially explained by the difference in the traits described, and partially related to species-specific differences. Males are the dispersing sex in macaques, [Melnick & Pearl, 1987], which might make relatively lower cautiousness and novelty aversion especially adaptive for males in leaving their home troops and integrating in new social groups.
Males in many species are more aggressive than females [Schuett et al, 2010]; however, in our sample the trend varied by species. Although long-tailed males were more aggressive than long-tailed females, rhesus macaques showed no significant sex difference, and pigtailed macaques showed a significant difference in the opposite direction. This lack of a main Aggressiveness effect in males may reflect a sex difference in response to the human observer overall. Males overall were also significantly more sociable towards humans, less cautious, and had a non-significant tendency to be less fearful. Together, these findings suggest that male macaques in these housing conditions are more confident and comfortable with the human observer than females.
Although many of our results support our initial hypotheses, it is important to note that species and sex only explained a small proportion of the variance in temperament. All significant relationships had small effect sizes (Table 4), suggesting that much of the individual variation in temperament remains to be explained. Within any species, individuals should vary on every trait, with some being more aggressive, some more sociable, etc. Thus, much of the variance in temperament is expected to exist on the individual level, not the species level. Considering that these species are closely related and have similar social systems (all are on the aggressive end of the despotic-egalitarian continuum [Thierry, 2000]), it is actually surprising to find as much temperament differentiation as we see here.
Overall, we found that between-species temperament differences are significant over-and-above the individual variations in temperament, and are evident even when animals are not housed in social group environments – although, they might be stronger in a social context. Our results also help distinguish between three species of macaques that are often considered similar in terms of social behavior [Thierry, 2000]. Rhesus macaques of both sexes tend to be more aggressive than other species, whereas long-tailed macaques are cautious and fearful of humans. Pigtailed macaques showed a unique behavioral style, and tended to be less aggressive, more sociable toward humans, and less fearful than other species. Such differences on the individual level may correspond to species-level differences in social style. While many of our connections to behaviors and ecological conditions in the wild are as yet untested owing to a scarcity of comparable field studies, we believe that these results may help direct future research. Together, our results provide support for the theory that differing social systems co-evolve with differences in temperament between species.
Supplementary Material
Acknowledgments
We thank Kim Wahl, Eric Runeson, and Heather Montgomery for maintaining the temperament testing database. We thank A. Weiss and two anonymous reviewers for helpful comments on the manuscript. This study was conducted in compliance with U.S. animal care regulations and applicable national laws. This research was supported by NIH RR00166 to the National Primate Research Center at the University of Washington and facilitated by grant P30 HD02274 from the National Institute of Child Health and Human Development.
References
- Bentson KL, Crockett CM, Ha JC. A rapid home cage procedure for assessing individual and group differences in behavioral reactivity of monkeys. American Journal of Primatology. 2003;60(Suppl.):77. [Google Scholar]
- Bergmuller R, Taborsky M. Animal personality due to social niche specialization. Trends in Ecology and Evolution. 2010;25(9):504–511. doi: 10.1016/j.tree.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Bernstein I, Williams L, Ramsay M. The expression of aggression in old world monkeys. International Journal of Primatology. 1983;4(2):113–125. [Google Scholar]
- Caldecott JO. Mating patterns, societies, and the ecogeography of macaques. Animal Behaviour. 1986a;34:208–220. [Google Scholar]
- Caldecott JO. An ecological and behavioural study of the pig-tailed macaque. Karger; New York: 1986b. [Google Scholar]
- Call J, Judge PG, de Waal FBM. Influence of kinship and spatial density on reconciliation and grooming in rhesus monkeys. American Journal of Primatology. 1996;39(1):35–45. doi: 10.1002/(SICI)1098-2345(1996)39:1<35::AID-AJP3>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Capitanio JP. Personality dimensions in adult male rhesus macaques: Prediction of behaviors across time and situation. American Journal of Primatology. 1999;47(4):299–320. doi: 10.1002/(SICI)1098-2345(1999)47:4<299::AID-AJP3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Capitanio JP. Personality factors between and within species. In: Thierry B, Singh M, Kaumanns W, editors. Macaque societies: A model for the study of social organization. Cambridge University Press; Cambridge, UK: 2004. pp. 13–33. [Google Scholar]
- Chamove AS, Eysenck HJ, Harlow HF. Personality in monkeys: Factor analyses of rhesus social behavior. Quarterly Journal of Experimental Psychology. 1972;24:496–504. doi: 10.1080/14640747208400309. [DOI] [PubMed] [Google Scholar]
- Cheney DL, Wrangham RW. Predation. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate Societies. University of Chicago Press; Chicago: 1987. pp. 227–239. [Google Scholar]
- Cheney D, Seyfarth R, Smuts B. Social relationships and social cognition in nonhuman primates. Science. 1986;234(4782):1361–1366. doi: 10.1126/science.3538419. [DOI] [PubMed] [Google Scholar]
- Clarke AS, Mason WA. Differences among three macaque species in responsiveness to an observer. International Journal of Primatology. 1988;9(4):347–364. [Google Scholar]
- Clarke AS, Lindburg DG. Behavioral contrasts between male cynomolgus and lion-tailed macaques. American Journal of Primatology. 1993;29:49–59. doi: 10.1002/ajp.1350290106. [DOI] [PubMed] [Google Scholar]
- Cohen J. A Power Primer. Psychological Bulletin. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Crockett CM, Wilson WL. The ecological separation of Macaca nemestrina and M. fascicularis in Sumatra. In: Lindburg DG, editor. The Macaques: Studies in Ecology, Behavior and Evolution. Van Nostrand Reinhold; New York: 1980. pp. 148–181. [Google Scholar]
- Crockett CM, Bellanca RU, Bowers CL, Bowden DM. Grooming-contact bars provide social contact for individually caged laboratory macaques. Contemporary Topics in Laboratory Animal Science. 1997;36:53–60. [PubMed] [Google Scholar]
- Dingemanse NJ, Reale D. Natural selection and animal personality. Behaviour. 2005;142(9-10):1159–1184. [Google Scholar]
- Dingemanse NJ, Kazem AJN, Reale D, Wright J. Behavioural reaction norms: Animal personality meets individual plasticity. Trends in Ecology and Evolution. 2010;25(2):81–89. doi: 10.1016/j.tree.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Dunteman GH. Principal Component Analysis. Sage University Paper series on Quantitative Applications in the Social Sciences. Sage; Newbury Park, California: 1989. p. 69. [Google Scholar]
- Fooden J. Comparative review of fascicularis group species of macaques (primates: Macaca) Fieldiana Zoology. 2006;107:1–43. [Google Scholar]
- Freeman HD, Gosling SD. Personality in nonhuman primates: a review and evaluation of past research. American Journal of Primatology. 2010;72(8):653–671. doi: 10.1002/ajp.20833. [DOI] [PubMed] [Google Scholar]
- Galdikas BMF, Yeager CP. Crocodile predation on a crab-eating macaque in Borneo. American Journal of Primatology. 1984;6:49–51. doi: 10.1002/ajp.1350060106. [DOI] [PubMed] [Google Scholar]
- Gosling SD, John OP. Personality dimensions in nonhuman animals: a cross species review. Current Directions in Psychological Science. 1999;8:69–75. [Google Scholar]
- Gosling SD. From mice to men: What can we learn about personality from animal research? Psychological Bulletin. 2001;127(1):45–86. doi: 10.1037/0033-2909.127.1.45. [DOI] [PubMed] [Google Scholar]
- Gouzoules H, Gouzoules S. Agonistic screams differ among four species of macaques: the significance of motivation-structural rules. Animal Behaviour. 2000;59:501–512. doi: 10.1006/anbe.1999.1318. [DOI] [PubMed] [Google Scholar]
- Ha JC, Alloway H, Sussman A. The Effect of aggression on reproductive outcome in pigtailed macaques (Macaca nemestrina) American Journal of Primatology. 2011;73(11):L1169–1175. doi: 10.1002/ajp.20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Lee PC. Predation risk as an influence on group size in cercopithecoid primates: implications for social structure. Journal of Zoology (London) 1998;245:447–456. [Google Scholar]
- Hoelzer GA, Melnick DJ. Evolutionary relationships of the macaques. In: Fa JE, Lindburg DG, editors. Evolution and ecology of macaque societies. Cambridge University Press; Cambridge, UK: 1996. pp. 3–19. [Google Scholar]
- Itoh K. Personality research with nonhuman primates: Theoretical formulation and methods. Primates. 2002;43(3):249–261. doi: 10.1007/BF02629652. [DOI] [PubMed] [Google Scholar]
- Joliffe IT. Springer Series in Statistics. 2nd Ed Springer-Verlag; New York, NY: Principal Component Analysis. [Google Scholar]
- Konečná M, Lhota S, Weiss A, Urbánek T, Adamová T, Pluháček J. Personality in free-ranging Hanuman langur (Semnopithecus entellus) males: Subjective ratings and recorded behavior. Journal of Comparative Psychology. 2008;122:379–389. doi: 10.1037/a0012625. [DOI] [PubMed] [Google Scholar]
- Konečná M, Weiss A, Lhota S, Wallner B. Personality in Barbary macaques (Macaca sylvanus): Temporal stability and social rank. Journal of Research in Personality. 2012;46:581–590. [Google Scholar]
- Krause J, James R, Croft DP. Personality in the context of social networks. Philosophical Transaactions of the Royal Society: Biological Sciences. 2010;365(1560):4099–4106. doi: 10.1098/rstb.2010.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J, Korstjens AH, Dunbar RIM. Group size, grooming, and social cohesion in primates. Animal Behaviour. 2007;74(6):1617–1629. [Google Scholar]
- Lorenzo-Seva U, ten Berge JMF. Tucker’s congruence coefficient as a meaningful index of similarity. Methodology: European Journal of Research Methods for the Behavioral and Social Sciences. 2006;2(2):57–64. [Google Scholar]
- Maestripieri D. Gestural communication and its cognitive implications in pigtail macaques (Macaca nemestrina) Behaviour. 1996;133(13/14):997–1022. [Google Scholar]
- Maestripieri D. Gestural communication in three species of macaques (Macaca mulatta, M. nemestrina, M. arctoides): Use of signals in relation to dominance and social context. Gesture. 2005;5(1-2):57–73. [Google Scholar]
- Matsumara S. The evolution of “egalitarian” and “despotic” social systems among macaques. Primates. 1999;40(1):23–31. doi: 10.1007/BF02557699. [DOI] [PubMed] [Google Scholar]
- Melnick DJ, Pearl MJ. Cercopithecines in multimale groups: Genetic diversity and population structure. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate Societies. University of Chicago Press; Chicago: 1987. pp. 121–134. [Google Scholar]
- Montgomery HB, Bentson KL, Crockett CM. Responses to novelty in Macaca nemestrina and Macaca fascicularis varies by species-sex and time in facility. American Journal of Primatology. 2005;66(1):146–147. [Google Scholar]
- Oettinger BC, Crockett CM, Bellanca RU. Communicative contexts of the LEN facial expression of pigtailed macaques (Macaca nemestrina) Primates. 2007;48:293–302. doi: 10.1007/s10329-007-0046-1. [DOI] [PubMed] [Google Scholar]
- Oi T. Patterns of dominance and affiliation in wild pig-tailed macaques (Macaca nemestrina nemestrina) in West Sumatra. International Journal of Primatology. 1990a;11(4):339–356. [Google Scholar]
- Oi T. Population organization of wild pig-tailed macaques (Macaca nemestrina nemestrina) in West Sumatra. Primates. 1990b;31:15–31. [Google Scholar]
- Reale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. Integrating animal temperament within ecology and evolution. Biological Reviews. 2007;82(2):291–318. doi: 10.1111/j.1469-185X.2007.00010.x. [DOI] [PubMed] [Google Scholar]
- Rouff JH, Sussman RW, Strube MJ. Personality traits in captive lion-tailed macaques (Macaca silenus) American Journal of Primatology. 2005;67(2):177–198. doi: 10.1002/ajp.20176. [DOI] [PubMed] [Google Scholar]
- Ruehlmann TE, Bernstein IS, Gordon TP, Balcaen P. Wounding patterns in three species of captive macaques. American Journal of Primatology. 1988;14:125–134. doi: 10.1002/ajp.1350140203. [DOI] [PubMed] [Google Scholar]
- Schuett W, Tregenza T, Dall SRX. Sexual selection and animal personality. Biological Reviews. 2010;85:217–246. doi: 10.1111/j.1469-185X.2009.00101.x. [DOI] [PubMed] [Google Scholar]
- Sih A, Bell AM, Johnson JC, Ziemba RE. Behavioral syndromes: an ecological and evolutionary overview. Trends in Ecology and Evolution. 2004;19(7):372–378. doi: 10.1016/j.tree.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Sih A. Behavioral syndromes: A behavioral ecologist’s view on the evolutionary and ecological implications of animal personalities. In: Weiss A, King JE, Murray L, editors. Personality and Temperament in Nonhuman Primates, Developments in Primatology: Progress and Prospects, Part 3. Springer; NY: 2011. pp. 313–336. [Google Scholar]
- Smith BR, Blumstein DT. Behavioral types as predictors of survival in Trinidadian guppies (Poecilia reticulate) Behavioral Ecology. 2010;21(5):919–926. [Google Scholar]
- Stevenson-Hinde J, Zunz M. Subjective assessment of individual rhesus monkeys. Primates. 1978;19:473–482. [Google Scholar]
- Stevenson-Hinde J, Stillwell-Barnes R, Zunz M. Subjective assessment of rhesus monkeys over four successive years. Primates. 1980;21:66–82. [Google Scholar]
- Thierry B. Patterns of agonistic interactions in three species of macaque (Macaca mulatta, M fascicularis, M tonkeana) Aggressive Behavior. 1985;11(3):223–233. [Google Scholar]
- Thierry B. Covariation of conflict management patterns across macaque species. In: Aureli F, de Waal F, editors. Natural conflict resolution. University of California Press; Berkeley, CA: 2000. pp. 106–128. [Google Scholar]
- Thierry B, Iwaniuk AN, Pellis SM. The influence of phylogeny on the social behaviour of macaques (Primates: Cercopithecidae, genus Macaca) Ethology. 2000;106:713–728. [Google Scholar]
- Thierry B, Singh M, Kaumanns W. Why macaque societies? In: Thierry B, Singh M, Kaumanns W, editors. Macaque societies: A model for the study of social organization. Cambridge University Press; Cambridge, UK: 2004. pp. 3–10. [Google Scholar]
- Uher J. Comparative personality research: Methodological approaches. European Journal of Personality. 2008;22:427–455. [Google Scholar]
- Uher J, Asendorpf JB. Personality assessment in the Great Apes: Comparing ecologically valid behavior measures, behavior ratings, and adjective ratings. Journal of Research in Personality. 2008;42:821–838. [Google Scholar]
- U. S. Department of Agriculture Animal Welfare, Standards, Final Rule (Part 3, Subpart D: Specifications for the humane handling, care, treatment, and transportation of nonhuman primates) Fed Register. 1991;56(32):6495–6505. [PubMed] [Google Scholar]
- Weinstein TAR, Capitanio JP. Individual differences in infant temperament predict social relationships of yearling rhesus monkeys, Macaca mulatta. Animal Behavior. 2008;76:455–465. doi: 10.1016/j.anbehav.2008.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A, Adams MJ, Widdig A, Gerald MS. Rhesus macaques (Macaca mulatta) as living fossils of hominoid personality and subjective well-being. Journal of Comparative Psychology. 2011;125:72–83. doi: 10.1037/a0021187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwick WR, Velicer WF. Comparison of Five Rules for Determining the Number of Components to Retain. Psychological Bulletin. 1986;99(3):432–442. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.