Abstract
Background
Gentamicin reduces acetylcholine release and clindamycin causes end-plate ion channel blockade. Because of these reasons, two drugs show muscular relaxant effect and potentiate the action of nondepolarizing neuromuscular agents. This study was intended to evaluate the effect of gentamicin and clindamycin on rocuronium-induced neuromuscular blockade and the interaction between these drugs.
Methods
Male Sprague-Dawley rats' phrenic nerves and diaphragms were installed in a bath containing Krebs solution. They were divided into three study groups. The first group was pre-treated with 0.1 (n = 3), 0.2 (n = 4) or 0.5 (n = 3) mM gentamicin and the tension was measured as the concentration of rocuronium was increased. The second group was experimented by increasing gentamicin on 0.25 (n = 5), 0.5 (n = 6) or 1.0 (n = 6) mM clindamycin. The final group was pre-treated with various combinations of gentamicin and clindamycin. The drug concentration was gradually increased until single twitch tension decreased by around 80%. Effective concentration was calculated using a probit model and interaction indices derived the Loewe additivity.
Results
The administration of gentamicin and the combination of gentamicin and clindamycin enhanced rocuronium-induced neuromuscular blockade. At 0.2 and 0.5 mM gentamicin, synergistic interactions with rocuronium were observed. Likewise, at 0.5 and 1.0 mM clindamycin, synergistic interactions with gentamicin appeared. When all three drugs were combined, in the tetanic fade, all the groups except for those administered with 0.01 mM gentamicin and 0.25 mM clindamycin showed synergistic interactions.
Conclusions
This study demonstrate that gentamicin and clindamycin potentiated rocuronium induced neuromuscular blockade. Moreover, it was found that these drugs interacted synergistically.
Keywords: Clindamycin, Gentamicin, Interaction, Neuromuscular blockade, Rocuronium, Synergy
Introduction
Patients receiving antibiotic medications for the treatment or prophylaxis of infection are often encountered during anesthetic practice [1]. The main concern for anesthesiologists in this setting is the action of these antibiotics on the neuromuscular system [2]. Most antibiotics depress neuromuscular conduction because they have pre- and postsynaptic inhibitory effects at the neuromuscular junction [3,4].
Gentamicin is an aminoglycoside antibiotic used to treat many types of bacterial infections, particularly those caused by gram-negative bacteria [1]. At the neuromuscular junction, it enhances muscle relaxants' activity by decreasing the availability of calcium and by reducing the amount of acetylcholine released into the synaptic cleft [3,4]. In practice, it has been reported that gentamicin potentiates the neuromuscular blockade of vecuronium [5] and tubocurarine [6]. Although there have been many studies addressing the effects of gentamicin on various muscle relaxants, few studies report the effect of gentamicin on rocuronium-induced muscle relaxation.
Clindamycin is a lincosamide antibiotic indicated for patients with serious infections caused by susceptible anaerobic bacteria [1]. Because of its excellent coverage against anaerobes, gram-positive cocci, and Chlamydia trachomatis, clindamycin is a preferred antimicrobial agent for infections of the female genital tract [7]. It produces an acetylcholine receptor channel block at the end plate and decreases acetylcholine release at the motor nerve terminal [3,8,9]. It thereby blocks neuromuscular conduction and enhances the effects of non-depolarizing agents [10-12].
The combined use of gentamicin and clindamycin is known to be effective in treating pelvic inflammatory disease and postcesarean endometritis [7]. Because these two drugs have different mechanisms of neuromuscular blockade, they may synergistically interact to reduce the effective concentrations of each. It has been reported that the recovery of muscle relaxation was delayed in patients who were administered with vecuronium and then treated with two antibiotics [13]. However, analysis of the interaction between antibiotics and muscle relaxants is rare. Therefore, it is of clinical significance to know which interactions will occur following combined use.
The interactions between drugs were determined using interaction indices, which are based on the Loewe additivity model. Many researchers agree that this model is considered as the "gold standard" for defining drug interactions [14]. Chou and Hayball [15] suggested that a relationship is additive if the interaction index is between 0.9 and 1.1, while a relationship is synergistic if the interaction index is less than 0.9. If the interaction index is greater than 1.1, the relationship is antagonistic.
As a recent study has addressed the effect of clindamycin on rocuronium-induced neuromuscular blockade, the current study did not consider this relationship. It was found that clindamycin potentiated the effect of rocuronium, and an additive or synergistic interaction between the two drugs was observed [16].
The aim of the current study was to determine whether gentamicin or the combined use of gentamicin and clindamycin potentiates rocuronium-induced muscle relaxation. In addition, the interactions between gentamicin and clindamycin and the interactions between rocuronium, gentamicin, and clindamycin were examined.
Materials and Methods
Our Institutional Animal Care and Use Committee approved the experimental protocol. Seventy-five male Sprague-Dawley rats (150-250 g in weight) were anesthetized by perivertebral injection of propofol (0.05 mg/g) at the lumbar level, and then killed. The phrenic nerve and diaphragm were excised en bloc and immersed in a 50 ml bowl containing oxygenated (95% O2, 5% CO2) Krebs solution (118 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, 30 mM NaHCO3, 1 mM KH2PO4, 1 mM MgCl2, and 11 mM glucose). Excess thoracic and abdominal wall bones and muscles were removed until only an 8-10 mm high costal wall remained. The left hemidiaphragm was separated from the rest of the diaphragm, preserving the middle diaphragmatic ligament from the vertebral bodies to the xiphoid process of the sternum.
Preparations of left phrenic nerve-hemidiaphragm were then mounted in a 20 ml organ bath filled with Krebs solution. The bath solution was maintained at 32℃; it was continuously aerated with a gas mixture of 95% O2 and 5% CO2 and maintained at a pH of 7.38-7.42. Spent Krebs solution was exchanged for fresh solution 10 min after the preparation was mounted. The preparation was attached to a force transducer (Model 1030, UFI, Morro Bay, CA, USA) with a stainless steel wire and allowed to stabilize for 20 min in the bath. The phrenic nerve was connected to a stimulating electrode and was stimulated with supramaximal square wave impulses of 0.2 ms duration using a stimulator (Model ML112, ADInstruments Pty Ltd., Bella Vista, NSW, Australia).
The preparation was stretched until the maximum output tension was recorded after stimulation; 10 min elapsed to allow stabilization before the experiment began. Three consecutive single twitch tensions at 0.1 Hz and a 1.9 s tetanic tension of 50 Hz were obtained at each concentration and then digitized and stored on a Power Macintosh 7100 (Apple Computer, Cupertino, CA, USA) using data acquisition software (MacLab, ADInstruments).
After the baseline tension was measured, the concentrations of gentamicin sulfate (n = 6), clindamycin phosphate (n = 7), and rocuronium (n = 6) in the bath were cumulatively increased until an 80-90% reduction in single twitch was attained. At least 20 min were allowed to elapse to establish a pseudo-steady state in the drug concentrations between the bath and the preparation; tension was measured during this state. Data were analyzed only if single twitch tension returned to more than 90% of the baseline recording in a drug-free solution.
To evaluate the effect of gentamicin on rocuronium-induced muscle relaxation, Krebs solution was pretreated using 0.1 (n = 3), 0.2 (n = 4), and 0.5 (n = 3) mM gentamicin, and the concentration of rocuronium was increased until an 80-90% reduction of single twitch was attained. The concentration of rocuronium was modified by adding booster doses after administration of the initial dose, and the dose was increased to obtain concentrations of 1, 3, 5, 7, 10, 12, 16, 18, and 20 µM.
To examine the interaction between gentamicin and clindamycin, Krebs solution was pretreated using 0.25 (n = 5), 0.5 (n = 6), or 1.0 (n = 6) mM of clindamycin, and the concentration of gentamicin was increased by 0.1 mM intervals from an initial concentration of 0.1 mM.
To evaluate the interactions between gentamicin, clindamycin, and rocuronium, Krebs solution was pretreated using various concentrations of gentamicin and clindamycin in combination. The concentration of rocuronium was then cumulatively increased by using the same method as mentioned previously. The combinations were as follows: gentamicin 0.01 mM + clindamycin 0.25 mM (n = 4); gentamicin 0.05 mM + clindamycin 0.1 (n = 6) or 0.25 (n = 5) mM and gentamicin 0.1 mM + clindamycin 0.1 (n = 5), 0.25 (n = 4), or 0.5 (n = 5) mM.
Single tension was determined from the mean of three consecutive single tension measurements. The single twitch at each concentration was compared with the control tension (percent reduction of control) using the following equation:
| Percent reduction = [1 - (tension in presence of agent/control tension)] × 100 |
The response of agents with regard to tetanic fade at each concentration (as percent increase of tetanic fade compared with peak tetanic tension in tetanic tension) was calculated using the following equation:
| Percent increase = [1 - (end tetanic tension/peak tetanic tension)] × 100 |
The effective concentrations (ECs) of rocuronium, gentamicin, and clindamycin were determined by the probit model, and concentration-response curves were obtained for each pretreatment.
The interactions between drugs were determined using interaction indices, which were based on the Loewe additivity model. For a combination of i drugs (i ≥ 2), the drug interactions can be characterized using the following equation:
Here di is the dose of each drug in the mixture of i drugs, which results in effect y; Dy,i is the dose of drug that results in effect y for each respective drug when administered alone.
The effective concentrations of 50% maximal effect (EC50) for rocuronium pretreated with gentamicin or with a combination of both gentamicin and clindamycin were compared with those of rocuronium alone. In addition, we compared the EC50 of gentamicin in combination with clindamycin to that of gentamicin alone. Statistical analysis was performed using Student's t-test with Bonferroni's correction. Differences were considered significant at P < 0.05.
Results
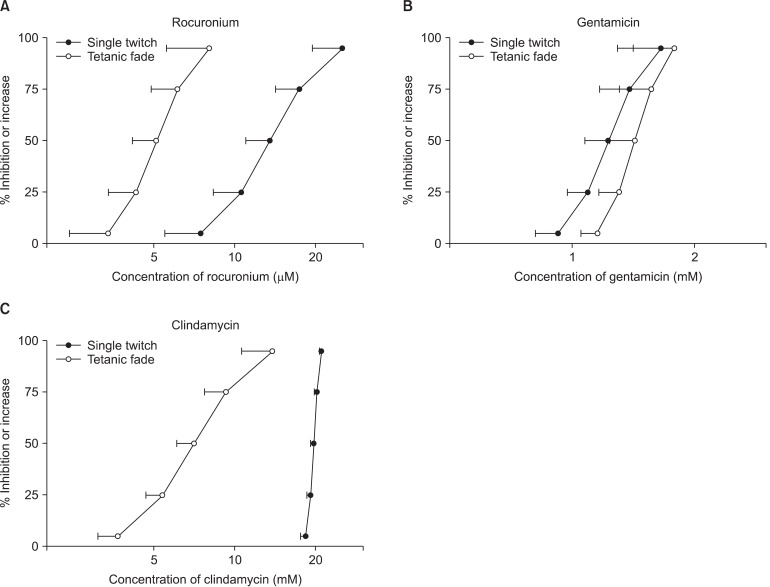
Rocuronium, gentamicin, and clindamycin each reduced the single twitches and increased the tetanic fade (Fig. 1). In single twitch, the values of EC50 for rocuonium, gentamcin, and clindamycin were 13.56 ± 2.56 µM, 1.23 ± 0.15 mM and 19.68 ± 0.50 mM, respectively. In tetanic fade, the values of EC50 were 5.11 ± 0.94 µM, 1.43 ± 0.19 mM and 8.82 ± 4.77 mM, respectively.
Fig. 1.
The cumulative concentration-response curves of rocuronium (A), gentamicin (B), and clindamycin (C) on single twitch at 0.1 Hz and tetanic fade at 50 Hz for 1.9 seconds. Rocuronium, gentamicin, and clindamycin reduced single twitch and increased tetanic fade. The indicated concentrations of rocuronium, gentamicin, and clindamycin are shown as the mean and the standard deviation (SD).
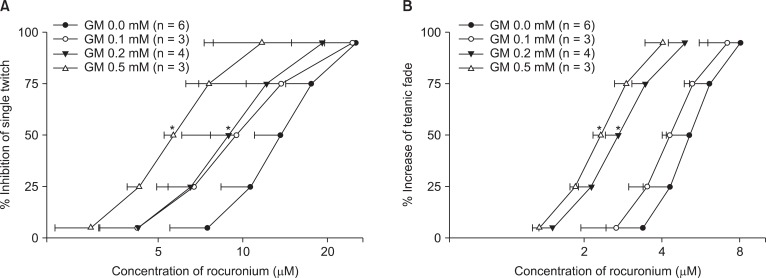
Gentamicin shifted the concentration-response curves of rocuronium to the left (Fig. 2). It increased the potency of rocuronium for single twitch and tetanic fade.
Fig. 2.
The cumulative concentration-response curves of rocuronium on single twitch at 0.1 Hz (A) and tetanic fade at 50 Hz for 1.9 s (B). Gentamicin shifts the concentration-response curves to the left. The concentrations of rocuronium are reported as the mean and the SD. GM represents gentamicin. *P < 0.05, compared with the EC50 for gentamicin 0.0 mM by t-test with Bonferroni's correction.
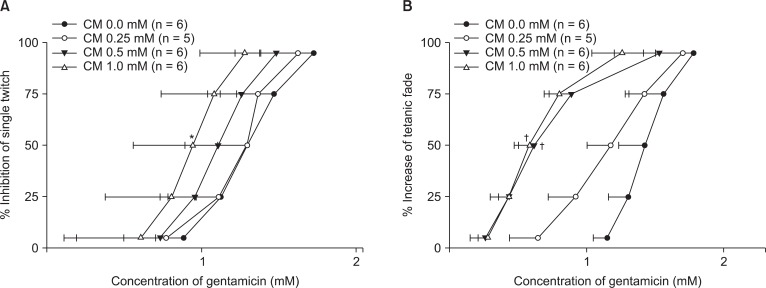
Clindamycin shifted the concentration-response curves of gentamicin to the left (Fig. 3). It was observed that higher concentrations of clindamycin more strongly affected gentamicin's effects on single twitch and tetanic fade.
Fig. 3.
The cumulative concentration-response curves of gentamicin on single twitch at 0.1 Hz (A) and tetanic fade at 50 Hz for 1.9 s (B). Clindamycin shifts the concentration-response curves to the left. The concentrations of gentamicin are reported as the mean and the SD. CM represents clindamycin. *P < 0.05 for single twitch and †P < 0.05 for tetanic fade, compared with the EC50 for clindamycin 0.0 mM by t-test with Bonferroni's correction.
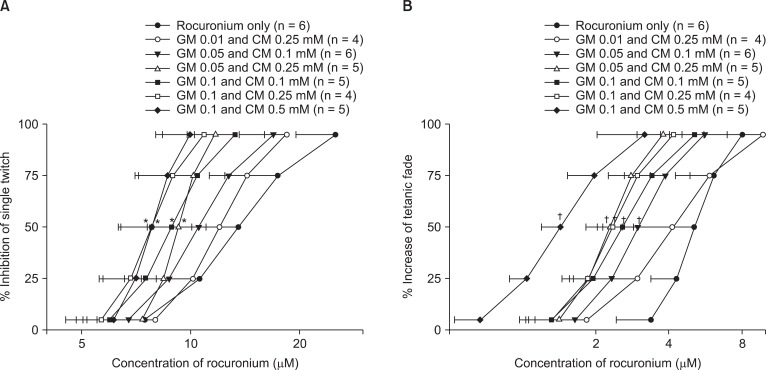
When gentamicin and clindamycin were combined, the concentration-response curves of rocuronium were shifted to the left in all concentration groups (Fig. 4).
Fig. 4.
The cumulative concentration-response curves of rocuronium on single twitch at 0.1 Hz (A) and tetanic fade at 50 Hz for 1.9 s (B). Combination of gentamicin and clindamycin shifts the concentration-response curves to the left. The concentrations of rocuronium are reported as the mean and the SD. GM and CM represents gentamicin and clindamycin, respectively. *P < 0.05 for single twitch and †P < 0.05 for tetanic fade, compared with the EC50 for rocuronium alone by t-test with Bonferroni's correction.
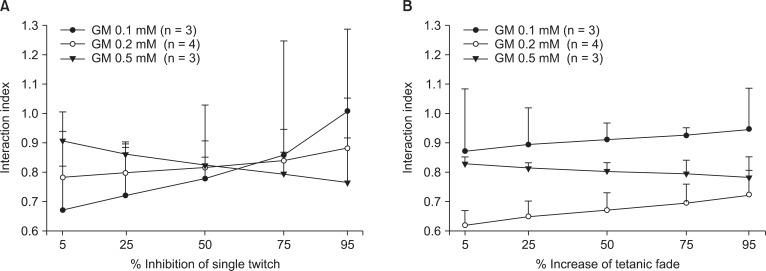
The interaction indices between rocuronium and gentamicin were less than 0.9 for single twitch and tetanic fade at gentamicin concentrations of 0.2 and 0.5 mM (Fig. 5). Because the interaction indices were less than 0.9, the two dugs interacted synergistically. In the case of gentamicin 0.1 mM, the interaction indices for single twitch and tetanic fade were between 0.67 and 1.02 and between 0.87 and 0.94, respectively.
Fig. 5.
The interaction indices between rocuronium and gentamicin on single twitch and tetanic fade. They are lower than 0.9 for 0.2 and 0.5 mM gentamicin in single twitch. For 0.1 mM gentamicin, the indices are between 0.67 and 1.02; the interaction is synergistic or additive (A). The interaction indices are lower than 0.9 in 0.2 and 0.5 mM gentamicin for tetanic fade. In 0.1 mM gentamicin, the indices are between 0.87 and 0.94; the interaction is synergistic or additive (B). The values are reported as the mean and the SD. GM represents gentamicin.
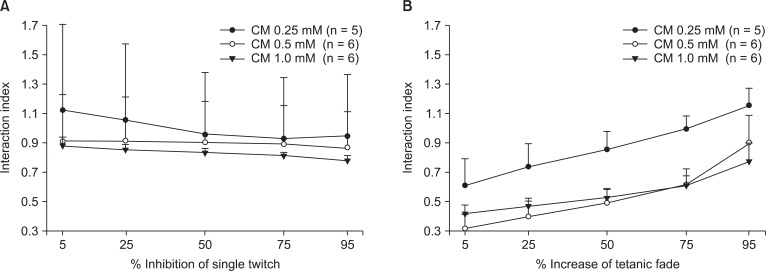
The interaction indices between gentamicin and clindamycin were less than 0.9 for single twitch and tetanic fade at clindamycin concentrations of 0.5 and 1.0 mM (Fig. 6). These indices also signify the synergistic interaction of the two drugs. In the case of the 0.25 mM clindamycin, the interaction indices for single twitch and tetanic fade were between 0.92 and 1.12 and between 0.60 and 1.11, respectively. Thus, both additive and synergistic interactions were observed.
Fig. 6.
The interaction indices between gentamicin and clindamycin on single twitch and tetanic fade. They are lower than 0.9 for 0.5 and 1.0 mM clindamycin in single twitch. The interaction indices at 0.25 mM clindamycin lie between 0.92 and 1.12; the interaction is synergistic or additive (A). The interaction indices are lower than 0.9 for 0.5, 1.0 mM clindamycin in tetanic fade. In 0.25 mM clindamycin, the indices are between 0.60 and 1.11; the interaction is additive or synergistic (B). The values are reported as the mean and the SD. CM represents clindamycin.
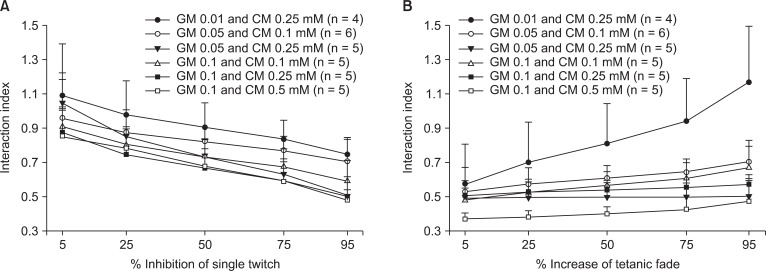
For single twitch inhibition, the interaction indices among rocuronium, gentamicin, and clindamycin were less than 0.9 when combination of 0.1 mM gentamicin with 0.1 0.25, and 0.5 mM clindamycin (Fig. 7) were prepared. At other concentration, the interaction indices were lower than 1.1. For increased tetanic fade, all indices were less than 0.9, the exception being the combination of 0.1 mM gentamicin with 0.25 mM clindamycin.
Fig. 7.
The interaction indices among rocuronium, gentamicin, clindamycin on single twitch and tetanic fade. They are lower than 0.9 for 0.1 mM gentamicin with 0.1, 0.25, and 0.5 mM clindamycin in single twitch; the interaction is synergistic. At other concentration combinations, the indices are lower than 1.1; the interaction is synergistic or additive (A). The interaction indices are lower than 0.9, except for the combination of 0.01 mM gentamicin with 0.25 mM clindamycin in tetanic fade; the interaction is synergistic (B). The values are reported as the mean and the SD. GM and CM represents gentamicin and clindamycin, respectively.
Discussion
Antibiotics are administered in advance of an operation to prevent wound infection and postoperative infection. Moreover, anesthesiologists can administer antibiotics in the pre- and intra-operative periods even though other physicians might have already prescribed them [1]. Anesthesiologists should thus be fully aware of the actions and side effects of antibiotics, and should have thorough knowledge of their interaction with anesthetics in particular.
Previous studies have reported the effects of gentamicin on various neuromuscular relaxants, such as d-tubocurarine [17], pancuronium [18], and vecuronium [5]. However, few studies have considered the effect of gentamicin and clindamycin on neuromuscular blockade caused by rocuronium, which is a drug that has been increasingly used because of its advantages, including rapid onset and intermediate duration. This study contributes to the literature by reporting on the effects of gentamicin or clindamycin on rocuronium-induced neuromuscular blockade.
In this experiment, single twitch and tetanic tension were applied and single twitch depression and tetanic fade were recorded at various concentration levels. Twitch blockade and tetanic fade are known as separative and independent actions. Twitch tension depression is known to appear due to competitive blockades of postsynaptic nicotinic acetylcholine receptors (nAChRs), and tetanic fade is known to occur due to the inhibition of presynaptic acetylcholine autoreceptors at the motor nerve ending [19,20]. Because gentamicin, clindamycin, and rocuronium are known to have an influence on both presynaptic and postsynaptic sites [17,21], the measurement of single twitch and tetanic fade is appropriate.
This study demonstrated that gentamicin potentiated rocuronium-induced muscle relaxation, and clindamycin potentiated the neuromuscular blockade produced by gentamicin. The higher the gentamicin concentration, the greater the rocuronium potency. Likewise, the higher the clindamycin concentration, the greater the gentamicin potency. In a test combining both gentamicin and clindamycin in various concentrations, all of the conditions appear to have potentiated the relaxant effect of rocuronium.
Magnesium-like depression has been proposed as the mechanism of muscular paralysis caused by gentamicin. Magnesium causes muscular paralysis by reducing the release of acetylcholine, an action that is antagonized by calcium. Similarly, gentamicin blocks calcium channels at the motor nerve terminal and decreases the release of acetylcholine [3,4,18]. However, magnesium produces tetanic ascent instead of tetanic fade, as induced by gentamicin [22]. Therefore, there are differences in the Ca2+ channel blockade mechanisms between these two drugs.
Clindamycin triggers muscular relaxation [11] and potentiates the action of non-depolarizing neuromuscular blocking agents [10,23]. In practice, there have been clinical reports indicating that neuromuscular blockade has been delayed because of the administration of clindamycin [11,12]. Various explanations are available for the mechanism of clindamycin-induced neuromuscular blockade. Several researchers have argued that single twitch decreases when clindamycin reduces the MEPP (miniature endplate potential) amplitude: this phenomenon reflects the postsynaptic blocking action of clindamycin [24]. Other researchers have reported that clindamycin appears to influence both pre- and post-junctional sites [9].
The concentrations of gentamicin and clindamycin used in this test were higher than those used for therapeutic purposes. The maximal therapeutic levels of gentamicin and clindamycin are 12 µg/ml (≒ 0.021 mM) and 17 µg/ml (≒ 0.034 mM), respectively [25]. Many studies have examined the influence of antibiotics on neuromuscular transmission at therapeutic concentrations. Caputy et al. [25] examined the neuromuscular blocking effects of antibiotics in normal rat skeletal muscle at the maximum therapeutic level of antibiotics. The maximum therapeutic levels of kanamycin, lincomycin, gentamicin, streptomycin, tobramycin, amikacin, and neomycin interrupted neuromuscular transmission, which is acknowledged as a realistic measure of possible clinical neuromuscular blockade. Moreover, Schlesinger et al. [26] reported that the half-maximal blocking concentration is within a clinically relevant concentration or close to it. Potter et al. [17] also reported that gentamicin increased the blocking effect of d-tubocurarine when administered to cats at a therapeutic dose of 1 mg/kg. The 0.05 mM gentamicin and 0.1 mM clindamycin used in this study are concentrations close to the clinical plasma concentration. When these two drugs were used in combination, the action of rocuronium was significantly potentiated.
There have been reports indicating that these drugs did not affect neuromuscular blockades at the doses commonly used in the clinic [27,28]. However, in recent years diverse drugs have been used simultaneously in many cases. Some of those drugs affect neuromuscular blockades. Therefore, more studies at clinically therapeutic concentrations are necessary.
This study determined that gentamicin and rocuronium are synergistic or additive in depressing neuromuscular transmission according to Chou and Hayball's criteria. Furthermore, gentamicin and clindamycin have been proven to be synergistic or additive. In addition, the combination of gentamicin and clindamycin is synergistic, except for the combination of 0.1 mM gentamicin with 0.25 mM clindamycin. As the results of this study demonstrate, neuromuscular blocking effects may be induced even at commonly used doses because of the synergistic interactions. In point of case, there have been reports indicating that the effect of vecuronium was prolonged in patients treated with gentamicin and clindamycin at clinical doses [13].
In conclusion, this report determined that gentamicin and a combination of gentamicin and clindamycin enhanced rocuronium-induced neuromuscular blockade. It was also found that these drugs also interact synergistically. This information indicates that careful attention is needed when using antibiotics together with muscle relaxants, because the synergy effect triggered by the combination could cause deeper muscle relaxation and delay recovery.
Acknowledgments
This study was supported by the Dong-A University Research Fund.
Footnotes
This article is a Doctor's Thesis by Ji Hyeon Lee.
References
- 1.Cheng EY, Nimphius N, Hennen CR. Antibiotic therapy and the anesthesiologist. J Clin Anesth. 1995;7:425–439. doi: 10.1016/0952-8180(95)00034-f. [DOI] [PubMed] [Google Scholar]
- 2.Sokoll MD, Gergis SD. Antibiotics and neuromuscular function. Anesthesiology. 1981;55:148–159. doi: 10.1097/00000542-198108000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Singh YN, Marshall IG, Harvey AL. Pre- and postjunctional blocking effects of aminoglycoside, polymyxin, tetracycline and lincosamide antibiotics. Br J Anaesth. 1982;54:1295–1306. doi: 10.1093/bja/54.12.1295. [DOI] [PubMed] [Google Scholar]
- 4.Singh YN, Marshall IG, Harvey AL. Depression of transmitter release and postjunctional sensitivity during neuromuscular block produced by antibiotics. Br J Anaesth. 1979;51:1027–1033. doi: 10.1093/bja/51.11.1027. [DOI] [PubMed] [Google Scholar]
- 5.Dotan ZA, Hana R, Simon D, Geva D, Pfeffermann RA, Ezri T. The effect of vecuronium is enhanced by a large rather than a modest dose of gentamicin as compared with no preoperative gentamicin. Anesth Analg. 2003;96:750–754. doi: 10.1213/01.ANE.0000050280.59508.70. [DOI] [PubMed] [Google Scholar]
- 6.Hall DR, McGibbon DH, Evans CC, Meadows GA. Gentamicin, tubocurarine, lignocaine and neuromuscular blockade. A case report. Br J Anaesth. 1972;44:1329–1332. doi: 10.1093/bja/44.12.1329. [DOI] [PubMed] [Google Scholar]
- 7.Zambrano D. Clindamycin in the treatment of obstetric and gynecologic infections: a review. Clin Ther. 1991;13:58–80. [PubMed] [Google Scholar]
- 8.Wright JM, Collier B. Characterization of the neuromuscular block produced by clindamycin and lincomycin. Can J Physiol Pharmacol. 1976;54:937–944. doi: 10.1139/y76-130. [DOI] [PubMed] [Google Scholar]
- 9.Fiekers JF, Henderson F, Marshall IG, Parsons RL. Comparative effects of clindamycin and lincomycin on end-plate currents and quantal content at the neuromuscular junction. J Pharmacol Exp Ther. 1983;227:308–315. [PubMed] [Google Scholar]
- 10.Becker LD, Miller RD. Clindamycin enhances a nondepolarizing neuromuscular blockade. Anesthesiology. 1976;45:84–87. doi: 10.1097/00000542-197607000-00015. [DOI] [PubMed] [Google Scholar]
- 11.al Ahdal O, Bevan DR. Clindamycin-induced neuromuscular blockade. Can J Anaesth. 1995;42:614–617. doi: 10.1007/BF03011880. [DOI] [PubMed] [Google Scholar]
- 12.Best JA, Marashi AH, Pollan LD. Neuromuscular blockade after clindamycin administration: a case report. J Oral Maxillofac Surg. 1999;57:600–603. doi: 10.1016/s0278-2391(99)90083-6. [DOI] [PubMed] [Google Scholar]
- 13.Jedeikin R, Dolgunski E, Kaplan R, Hoffman S. Prolongation of neuromuscular blocking effect of vecuronium by antibiotics. Anaesthesia. 1987;42:858–860. doi: 10.1111/j.1365-2044.1987.tb04111.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee JJ, Kong M. Confidence intervals of interaction index for assessing multiple drug interaction. Stat Biopharm Res. 2009;1:4–17. doi: 10.1198/sbr.2009.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chou TC, Hayball M. CalcuSyn for windows: Multiple-drug dose-effect analyzer and manual. Cambridge Place, Cambridge, United Kingdom: Biosoft; 1996. [Google Scholar]
- 16.Kim SS, Lee SI, Chung CJ, Lee SC. The antagonistic effect of neostigmine on rocuronium-, clindamycin-, or both-induced neuromuscular blocking in the rat phrenic nerve-hemidiaphragm. Korean J Anesthesiol. 2011;61:320–326. doi: 10.4097/kjae.2011.61.4.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potter JM, Edeson RO, Campbell RJ, Forbes AM. Potentiation by gentamicin of non-depolarizing neuromuscular block in the cat. Anaesth Intensive Care. 1980;8:20–25. doi: 10.1177/0310057X8000800105. [DOI] [PubMed] [Google Scholar]
- 18.Chinyanga HM, Stoyka WW. The effect of colymycin M, gentamycin and kanamycin on depression of neuromuscular transmission induced by pancuronium bromide. Can Anaesth Soc J. 1974;21:569–579. doi: 10.1007/BF03006018. [DOI] [PubMed] [Google Scholar]
- 19.Bowman WC. Prejunctional and postjunctional cholinoceptors at the neuromuscular junction. Anesth Analg. 1980;59:935–943. [PubMed] [Google Scholar]
- 20.Martyn JA, Fagerlund MJ, Eriksson LI. Basic principles of neuromuscular transmission. Anaesthesia. 2009;64(Suppl 1):1–9. doi: 10.1111/j.1365-2044.2008.05865.x. [DOI] [PubMed] [Google Scholar]
- 21.Prior C, Fiekers JF, Henderson F, Dempster J, Marshall IG, Parsons RL. End-plate ion channel block produced by lincosamide antibiotics and their chemical analogs. J Pharmacol Exp Ther. 1990;255:1170–1176. [PubMed] [Google Scholar]
- 22.Lee C, Zhang X, Kwan WF. Electromyographic and mechanomyographic characteristics of neuromuscular block by magnesium sulphate in the pig. Br J Anaesth. 1996;76:278–283. doi: 10.1093/bja/76.2.278. [DOI] [PubMed] [Google Scholar]
- 23.Fogdall RP, Miller RD. Prolongation of a pancuronium-induced neuromuscular blockade by clindamycin. Anesthesiology. 1974;41:407–408. doi: 10.1097/00000542-197410000-00023. [DOI] [PubMed] [Google Scholar]
- 24.Rubbo JT, Gergis SD, Sokoll MD. Comparative neuromuscular effects of lincomycin and clindamycin. Anesth Analg. 1977;56:329–332. doi: 10.1213/00000539-197705000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Caputy AJ, Kim YI, Sanders DB. The neuromuscular blocking effects of therapeutic concentrations of various antibiotics on normal rat skeletal muscle: a quantitative comparison. J Pharmacol Exp Ther. 1981;217:369–378. [PubMed] [Google Scholar]
- 26.Schlesinger F, Krampfl K, Haeseler G, Dengler R, Bufler J. Competitive and open channel block of recombinant nAChR channels by different antibiotics. Neuromuscul Disord. 2004;14:307–312. doi: 10.1016/j.nmd.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Cooper R, Maddineni VR, Mirakhur RK. Clinical study of interaction between rocuronium and some commonly used antimicrobial agents. Eur J Anaesthesiol. 1993;10:331–335. [PubMed] [Google Scholar]
- 28.Wong J, Brown G. Does once-daily dosing of aminoglycosides affect neuromuscular function? J Clin Pharm Ther. 1996;21:407–411. doi: 10.1111/j.1365-2710.1996.tb00039.x. [DOI] [PubMed] [Google Scholar]