Abstract
The default mode network (DMN) is particularly relevant to Alzheimer's disease (AD) since its structures are vulnerable to deposition of amyloid. Decreased levels of β-amyloid1-42 (Aβ42) and increased total tau protein (T-tau) and tau phosphorylated at position threonine 181 (P-tau181p) in cerebrospinal fluid (CSF) have been established as valid biomarkers for the diagnosis and prognosis of AD. However, the relationship between CSF biomarkers and change in the DMN is still unknown. In this study we investigated the correlation between the functional connectivity within the DMN and the ratio of Aβ42/P-tau181p in the CSF. We found that the ratio of Aβ42/P-tau181p was moderately positively correlated with the functional connectivity within the DMN in the left precuneus/cuneus. This finding implicates that the brain functional connectivity within DMN is affected by pathological changes at early stage in AD. This may provide a better understanding of AD pathology progression and improve AD diagnosis.
Alzheimer's disease (AD) is the most common dementia in elderly people. The pathological hallmarks of AD are amyloid plaques (AP) and neurofibrillary tangles (NFT). These proteins are made up of β-amyloid1-42 (Aβ42) and tau phosphorylated at position threonine 181 (P-tau181P), respectively1. Such brain changes occur decades before the onset of dementia, leading to progressive loss of functions, metabolic alterations and structural changes in the brain2. Immunocytochemical and biochemical analyses in AD biopsies and autopsies indicated that synaptic loss in the hippocampus and neocortex is another early event and currently the best neurobiological correlate of cognitive deficits in AD. There are growing evidences that still living neurons lose their synapses in AD and soluble assembly states of Aβ peptides can cause cognitive problems by disrupting synaptic function in the absence of significant neurodegeneration3.
The brain is in direct contact with the cerebrospinal fluid (CSF). Biochemical changes that reflect pathophysiologic processes in the brain are reflected in the CSF4,5. Both Aβ42 and tau proteins of CSF can be reliably measured6,7. The clinical and diagnostic usefulness and validity of these CSF biomarkers in AD patients have been supported by numerous studies8,9. In comparison with healthy elderly and patients with other dementia, AD patients have been found to have decreased levels of Aβ42 and increased levels of total tau protein (T-tau) and P-tau181P levels in CSF10,11. Mild cognitive impairment (MCI) is recognized as the prodromal stage of AD, representing a transitional period between normal aging and AD12. More than half of the MCI patients progress to dementia within 3 to 5 years13. There is evidence indicating that subjective cognitive impairment (SCI), also referred to as subjective memory complaints, is a stage prior to MCI in the eventual development of AD dementia14. A CSF AD profile is also common in patients with MCI and SCI. Levels of Aβ42, T-tau and P-tau181P in the CSF are strongly associated with future development of AD, which has been proven in many studies15,16.
During the past years, changes in resting-state functional MRI (rs-fMRI) have been used to study the pathophysiology of AD and MCI. As a biomarker of synaptic dysfunction, rs-fMRI may demonstrate abnormality very early in AD17. The rs-fMRI studies for AD have primarily focused on a characteristic set of brain regions, including the medial prefrontal cortex (mPFC), anterior cingulate cortex (ACC), posterior cingulate cortex (PCC)/precuneus and parietal cortex. Some studies have also investigated the sub-regions of the medial temporal lobe (MTL) including hippocampus (HC), parahippocampal gyrus (PHG) and middle temporal gyrus (MTG). This collection of brain regions that are deactivated during a broad range of cognitive tasks and believed to support a default mode activity of the human brain has been defined as the default mode network (DMN)18. Freethinking, remembering the past, envisioning future events, and considering the thoughts and perspectives of other people all activate multiple regions within the DMN19. The DMN is particularly relevant for AD and MCI since the DMN components, the mPFC and MTL, especially hippocampus, are responsible for episodic memory processing. Episodic memory loss is the earliest symptom of AD. The hippocampus also appears to play a prominent role in the DMN20. Altered connectivity within the DMN in AD and MCI has also been reported in many studies21,22. Moreover, Buckner et al.23 demonstrated a correlation between the DMN and the anatomical distributions of amyloid deposition, atrophy, glucose metabolism changes and blood flow abnormality in AD. In healthy older adults, Aβ42 accumulation had an adverse effect by decreasing neural connectivity in regions of the DMN24.
Although a growing body of evidence points to an association between CSF biomarkers and altered connectivity within the DMN in AD23,25,26, there has not been any study investigating the quantitative correlation between CSF biomarkers and functional connectivity change in the DMN. It is still an open question how pattern of DMN abnormality is related to CSF biomarkers. Therefore, we postulated the working hypothesis that the CSF ratio of Aβ42/P-tau181p reflecting AD pathology should be associated with change of functional connectivity in the DMN. To test the hypothesis, we measured the CSF biomarkers in an unselected cohort from a memory clinic and determined the ratio of Aβ42/P-tau181p, which has superior diagnostic usefulness than either measure alone10,27. We also performed whole-brain rs-fMRI measurements in these subjects and applied voxel-based analysis to characterize the relationship between the ratio Aβ42/P-tau181p and the functional connectivity in DMN.
Results
Ninety-seven subjects were included for the final data analyses including: 21 AD, 36 MCI, 23 SCI and 17 other dementias (OD) patients. The conventional MRI for all subjects showed no abnormality other than brain atrophy and age related white matter changes. The demographics and clinical data are shown in Table 1. As expected, AD subjects had the lowest mean MMSE score, concentration of CSF Aβ42 and ratio of Aβ42/P-tau181p, while the T-tau and P-tau181p were the highest among the four groups.
Table 1. Demographics of subjects.
| AD | MCI | OD | SCI | |
|---|---|---|---|---|
| Number | 21 | 36 | 17 | 23 |
| Age | 65.6 ± 7.1 | 60.4 ± 9.2 | 59.3 ± 8.1 | 57.6 ± 9.1 |
| Gender (M/F) | 8/13 | 17/19 | 12/5 | 9/14 |
| MMSE | 22.3 ± 5.2 | 26.1 ± 3.2 | 23.0 ± 4.0 | 27.7 ± 2.4 |
| Aβ42 (pg/mL) | 483.8 ± 130.8 | 841.7 ± 352.1 | 935.4 ± 258.8 | 1101.0 ± 304.3 |
| T-tau (pg/mL) | 634.7 ± 275.9 | 356.8 ± 197.8 | 248.5 ± 103.3 | 276.7 ± 122.4 |
| P-tau181p (pg/mL) | 88.9 ± 32.9 | 58.8 ± 24.2 | 43.8 ± 14.2 | 51.8 ± 19.4 |
| Aβ42/P-tau181p | 6.6 ± 4.5 | 18.0 ± 11.0 | 24.2 ± 10.4 | 22.9 ± 6.4 |
| GM | 406.6 ± 45.1 | 430.1 ± 38.1 | 423.9 ± 41.4 | 450.9 ± 35.4 |
| ICV | 1087.6 ± 48.1 | 1092.5 ± 98.4 | 1112.5 ± 78.3 | 1115.5 ± 95.8 |
Data are represented as mean ± standard deviation. Key: AD, Alzheimer's Disease; MCI, Mild Cognitive Impairment; OD, non-AD dementia; SCI, Subjective Cognitive Impairment; M, Male; F, Female. GM, grey matter volume; ICV, intracranial volume.
CSF biomarkers reference: Aβ42 < 450 pg/mL, T-tau > 400 pg/mL, P-tau181p >80 pg/mL.
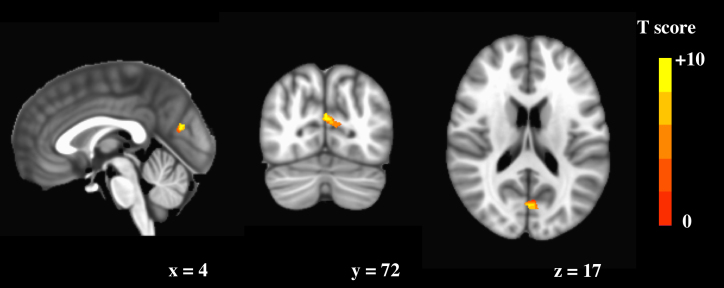
Voxel-wise correlation analysis result showed one statistical significance cluster with positive correlation between the ratio of Aβ42/P-tau181p and functional connectivity within the DMN, adjusted for age, gender and grey matter intensity map (Figure 1). The cluster consist of 17 voxels, peak T score is 3.44 and located in left precuneus. However, the most part of cluster is in the left cuneus.
Figure 1. Voxel-wise correlation analysis result.
One cluster within DMN shows positive correlation with the ratio of Aβ42/P-tau181p, adjusted for age, gender and grey matter intensity map. The cluster consist of 17 voxels, peak T score is 3.44 and located in left precuneus. However, the most part of cluster is in the left cuneus.
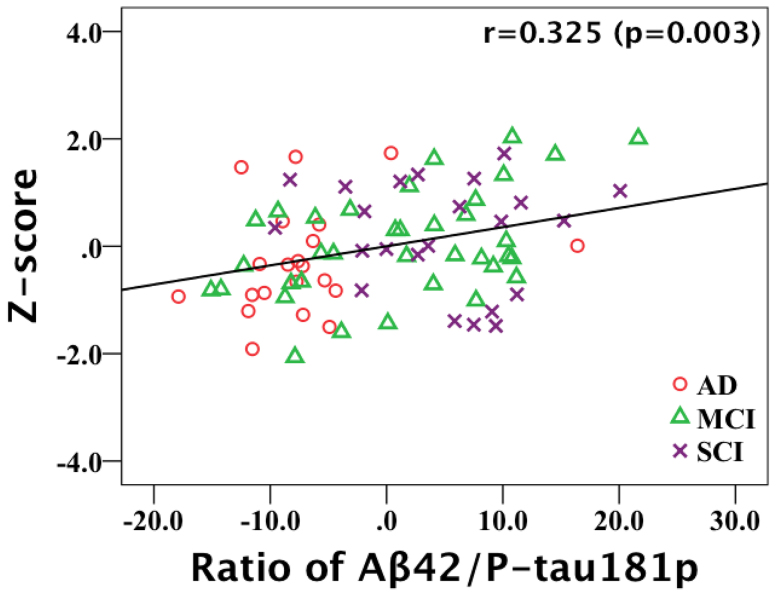
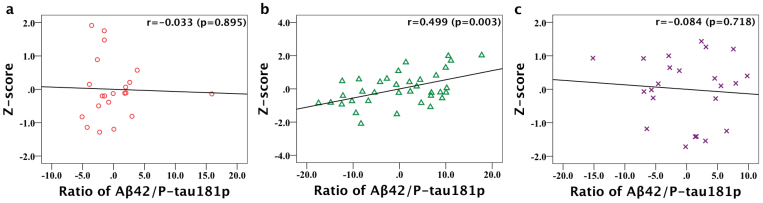
Having the voxel-wise result for the whole group, we extended the analysis to the individual diagnostic group using non-image partial correlation analyses. The individual average Z-score of precuneus/cuneus with statistical significant was calculated. The mean Z-scores and partial correlation test results with Aβ42/P-tau181p are shown in Table 2 for AD, MCI and SCI subjects in total and separately. There is no strong but a moderate correlation between Aβ42/P-tau181p and Z-scores of precuneus/cuneus (p = 0.003) for the AD, MCI and SCI subjects in total (Figure 2). When calculating partial correlations for the AD, MCI and SCI groups separately, only correlation coefficient in the MCI group showed moderate statistical significance (r = 0.499, p = 0.003). The scatterplots graphs of partial correlation results are shown in Figure 3. There were no statistically significant correlations for the AD and SCI group.
Table 2. Partial correlation test results between Z-score of left precuneus/cuneus and CSF ratio of Aβ42/P-tau181p for AD, MCI and SCI subjects, controlled by age and gender.
| Aβ42/P-tau181p | Z-score | r | p | |
|---|---|---|---|---|
| Total | 16.4 ± 10.5 | 1.93 ± 1.01 | 0.325 | 0.003 |
| AD | 6.6 ± 4.5 | 1.64 ± 1.03 | 0.033 | 0.895 |
| MCI | 18.0 ± 11.0 | 1.98 ± 0.99 | 0.499 | 0.003 |
| SCI | 22.9 ± 6.4 | 2.13 ± 0.99 | −0.084 | 0.718 |
Data are represented as mean ± standard deviation. Key: r, Pearson correlation coefficient; AD, Alzheimer's Disease; MCI, Mild Cognitive Impairment; SCI, Subjective Cognitive Impairment.
Figure 2. Scatter plots graph of partial correlation analysis result for AD, MCI and SCI subjects in total.
There is a moderate correlation (r = 0.325, p = 0.003) between ratio of Aβ42/P-tau181p and Z-score of left precuneus/cuneus (adjusting for age and gender).
Figure 3. Scatter plots graphs of partial correlation analysis result for AD, MCI and SCI subjects separately.
Scatterplots graphs show partial correlation between ratio of Aβ42/P-tau181p and Z-score of left precuneus/cuneus in (a) AD, (b) MCI and (c) SCI respectively (adjusting for age and gender). There is a moderate significant positive correlation in the MCI group (b) and r is 0.499 (p = 0.003).
Discussion
To our knowledge, this study is the first one to investigate the relationship between CSF biomarkers and functional connectivity change within the DMN in AD. The main finding of this study is that the CSF ratio of Aβ42/P-tau181p is moderately positively correlated with the functional connectivity within the DMN in the left precuneus/cuneus.
Concentrations of Aβ42, T-tau and P-tau181p in CSF may be sensitive biomarkers of incipient NFT and AP formation in AD. Seppälä et al.28 demonstrated that amyloid plaques and hyperphosphorylated tau in cortical brain biopsies reflected low CSF Aβ42 and high CSF T-tau/P-tau181P levels, respectively. It has been reported that CSF Aβ42 concentrations are decreased, while T-tau and P-tau181p concentrations are increased in AD, even in MCI and SCI patients15,29,30. In line with this, we found 13 MCI and 3 SCI subjects with decreased CSF Aβ42 and/or increased T-tau and P-tau181p. Herukka et al.31 reported that the combination of Aβ42 and P-tau181p was the most predictive assay for AD among MCI patients and this maybe a sensitive marker of AD pathology. To build on this, we used the CSF ratio of Aβ42/P-tau181p as a pathology marker to investigate the relationship between AD pathology and functional connectivity changes.
Interestingly, the majority of regions with amyloid deposition in AD patients, assessed with positron emission tomography (PET), overlap with the DMN23. In AD and MCI patients, decreased functional connectivity within the DMN have been reported20,32,33,34, and progressed with disease severity22,35. Furthermore, the functional connectivity between brain regions of the DMN is disrupted in elderly normal adults with amyloid deposition25. There is evidence that soluble oligomers of Aβ can selectively impair synaptic plasticity to disrupt synaptic function both in mice model and in vitro3. In this study, a positive correlation in precuneus/cuneus between the CSF ratio of Aβ42/P-tau181p and functional connectivity was observed. This suggests that the disruption of the functional connectivity in DMN increases with disease progression. This is in line with previous reports showing abnormal changes within the DMN in AD and MCI compared to cognitively normal21,32,34,35. Functional connectivity mapping from rs-fMRI is thought to reflect the relationship between spontaneous neuronal activity and brain regions separated anatomically, and it is a biomarker of synaptic dysfunction17. The correlation between CSF biomarkers and DMN observed in this study supports that CSF Aβ42 and P-tau181p abnormalities are associated with synaptic dysfunction in AD patients. The coordinate in the cluster with the peak T-score is located in left precuneus, while most part of cluster is extended to left cuneus. The precuneus is known to have very early involvement of Aβ42 deposition23. Petrie et al.26 also reported that there is a negative correlation between CSF ratio of P-tau181p/Aβ42 and rates of cerebral glucose metabolism in precuneus in healthy individuals, which is consistent with the results of the present study. Involvement of cuneus has also been shown in several DMN studies33,36, it has been reported that cerebral blood flow in cuneus is decreased in AD patients37 and in cognitively impaired subjects with AD-like pathological changes38. Further, increased Aβ burden in precuneus/cuneus was associated with increased brain atrophy rate in MCI patients39. Although cuneus have not received considerable attention in previous reports, cuneus might offer an important structural support to the DMN.
ICA provided a measure of the magnitude of the DMN co-activation. In this study, the Z-score change of the left precuneus/cuneus is similar to the trend of CSF ratio of Aβ42/P-tau181p with AD progression. The Z-score is correlated with CSF Aβ42/P-tau181p for all AD, MCI and SCI subjects. There was only a moderate significant correlation in the MCI group when calculating the correlation for individual diagnostic groups. These results indicate that both the CSF Aβ42/P-tau181p and the DMN activity changes in AD are parallel to each other only at some stage. In Sperling's17 hypothetical model of dynamic biomarkers, rates of change in each biomarker vary over time, representing a sigmoid shaped time course. At a given point, the slopes (rate of change) of biomarkers might be different although they have the same shape over the course of disease progression. This is in line with what our results shows, a relationship between CSF and synaptic dysfunction biomarkers. The rate of change in Aβ42/P-tau181p is similar to the DMN activity change in the MCI stage, but different rates in AD and SCI stages. This temporal lag maybe altered by factors such as brain reserve, cognitive reserve and the added contributions of coexisting pathologies17.
This study has several limitations. First, the sample size is limited. Second, some selected variables were controlled, but there might still be factors confounding the association such as APOE gene. Third, the correlations between the CSF ratio of Aβ42/P-tau181p and functional connectivity in the DMN was assessed at only one time point, additional longitudinal work is warranted. Fourth, the presence of occult AD-related neurodegenerative processes based on the presence of altered CSF Aβ42 and P-tau181p concentrations without autopsy confirmation of NFTs and APs was inferred.
In summary, the results implicate a connection between biochemical AD pathology and functional connectivity within the DMN. Although the correlation is weak between functional connectivity and the CSF ratio of Aβ42/P-tau181p, aberrant DMN may reflect pathology changes in AD. This investigation is beneficial to further understanding of the relationship of CSF Aβ42, P-tau181p and synaptic dysfunction at different stages of AD. Moreover, it may help us to better understand the AD pathology progression and diagnose AD at an early stage.
Methods
Participants
A total of 110 subjects (97 included in the present study, 13 excluded due to image quality issues) were consecutively recruited from the Memory Clinic at the University Hospital of Karolinska Huddinge, in Stockholm, Sweden, from November 2010 to February 2012. The study was approved by Regional ethics committee in Stockholm (Dnr 2011/1987-31/4) for human studies prior to the start of the data collection. All participants underwent a clinical examination in a comprehensive manner, including physical and psychiatric evaluations, MRI scans, lumbar puncture and blood analyses, as well as neuropsychiatric, linguistic and occupational therapeutic examinations. Informed consent was obtained from all subjects. For those patients who were unable to give informed consent, informed consent was obtained from their legal guardian.
Clinical diagnoses were made according to established international criteria. AD and OD were diagnosed according to DSMIV/ICD-10 criteria. MCI was defined using Winblad et al. criteria40. Patients categorized as SCI had cognitive complaints but without impairment on objective cognitive tasks.
Twenty-one patients fulfilled the criteria for probably having AD, 36 for MCI and 23 for SCI. Seventeen patients were diagnosed as OD, including 1 Parkinson's disease, 1 Alcohol dependence, 1 organic personality disorder, 2 other symptoms and signs involving cognitive functions and awareness subjects, 3 other amnesia, 3 Pick's disease and 6 unspecified dementia.
All participants underwent lumbar puncture in the L3–4 or L4–5 interspace. CSF Aβ42 was analyzed using a sandwich ELISA, constructed to specifically measure β-amyloid1-4241. CSF T-tau was determined using a sandwich enzyme-linked immunoabsorbent assay (ELISA) constructed to measure total tau, both normal tau and hyperphosphorylated tau6. CSF P-tau181p was determined using a sandwich ELISA, constructed to specifically measure tau phosphorylated at position threonine 1817. All CSF samples were analysed at the University Hospital of Karolinska Huddinge, Stockholm, Sweden.
MRI imaging
All MRI image data sets were acquired on a Siemens whole-body clinical MRI 3T scanner (Magnetom Trio, Erlangen, Germany) equipped with 32-channel phase-array head coil. The MRI protocol included a high-resolution sagittal 3D T1-weighted image acquired with MPRAGE sequence, TR/TE = 1900/2.57 ms, 176 sagittal slices, voxel size 1 × 1 × 1 mm3, and flip angle = 9.
The rs-fMRI measurements lasted 10 min and 30 s. The main acquisition parameters included: TR/TE = 1600/35 ms, 400 time frames of gradient recalled echo EPI, and 42 contiguous oblique slices of 4 mm thick, FOV = 240 mm, matrix = 64 × 64, parallel data acquisition with an acceleration factor of 2. The slices were all parallel to the plane of the anterior and posterior commissure line. During the acquisition of the resting-state the subjects were instructed to close their eyes, not to think anything in particular and not to fall asleep.
Data analysis
Structural MRI data analysis was performed using a VBM protocol with FSL (FMRIB Software Library, http://www.fmrib.ox.ac.uk/fsl/). The images were segmented into grey matter, white matter and CSF, and co-registered to the MNI template.
All rs-fMRI data were carried out using AFNI (http://afni.nimh.nih.gov/afni). The following pre-statistics processing was applied: exclusion of the first 10 time frames in each data set to ensure that the rs-fMRI signal reached the steady state; correction for slice-dependent time shifts; head motion correction by using 3dvolreg based on 6-parameter rigid body image registration (subjects who had more than 3 mm movements were excluded from further analysis); spatial smoothing using a Gaussian kernel of 4-mm full width at half maximum (FWHM); and transformed to MNI152 standard space to yield a volumetric time series resampled at 4 mm isotropic voxels. A temporal band-pass filter was performed within the frequency range of 0.01–0.1 Hz. After pre-processing, data were entered into a spatial Independent component analysis (ICA) by using the Group ICA of fMRI Toolbox (GIFT) version 1.3i (http://mialab.mrn.org/software/gift), which was implemented in MATLAB (MathWorks, Massachusette, U.S.A) and established for the analysis of fMRI data. Afterward, 20 meaningful components were extracted after the ICA group analysis. For comparison, individual data set was scaled to Z-score to compensate for inter-individual differences in measured signal levels as previous study42. DMN component was selected by visual inspection based on the anatomy reported previously18.
The individual DMN component map was entered into one-sample t-test using 3dttest++ program in AFNI to determine correlation with the ratio of Aβ42/P-tau181p controlled by age, gender and grey matter intensity map. Grey matter intensity map is a voxel-level covariate. A threshold at t > 2.9 and a minimum spatially connected cluster size > 11voxels were employed. The Monte Carlo inference using the AlphaSim program from AFNI indicated that the statistical significance is at least p < 0.05 when the uncorrected voxel threshold was set at 0.005. Region of interest (ROI) masks were created using cluster with statistical significance. The individual average Z-scores of ROIs were calculated using 3dmaskave program in AFNI. Controlled by age and gender, the partial correlation between CSF ratio of Aβ42/P-tau181p and ROI Z-score was tested both for AD, MCI, SCI subjects in total and separately, which were performed with SPSS software package version 20.0 (SPSS, Chicago, IL, U.S.A.).
Author Contributions
T.-Q.L. and L.-O.W. designed the study and oversight the execution of the research program. N.A. and L.-O.W. recruited and clinically evaluated the subjects. M.K.W. was responsible for the execution of the MRI data acquisition protocol. X.L. is the dedicated Ph.D. student for the project and was mainly responsible for the data collection and analysis. X.L. prepared also the manuscript with supervisions from E.W., T.-Q.L. and L.-O.W.
Acknowledgments
We would like to thank the NIH-Karolinska Graduate Program for international Ph.D., Swedish Brain Power, the Strategic Research Programme in Neuroscience at Karolinska Institutet (StratNeuro), Swedish Research Council (VR), the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet, Stockholm Medical Image Laboratory and Education (SMILE), the foundation for age related diseases at Karolinska Institutet and Loo and Hans Ostermans foundation for medical research.
References
- Blennow K., De Leon M. J. & Zetterberg H. Alzheimer's disease. Lancet 368, 387–403 (2006). [DOI] [PubMed] [Google Scholar]
- Morris J. C. & Price J. L. Pathologic Correlates of Nondemented Aging, Mild Cognitive Impairment, and Early-Stage Alzheimer's Disease. J Mol Neurosci 17, 101–118 (2001). [DOI] [PubMed] [Google Scholar]
- Arendt T. Synaptic degeneration in Alzheimer's disease. Acta Neuropathol 118, 167–79 (2009). [DOI] [PubMed] [Google Scholar]
- Tapiola T. et al. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol 66, 382–9 (2009). [DOI] [PubMed] [Google Scholar]
- Engelborghs S. & Bastard N. Le The Impact of Cerebrospinal Fluid Biomarkers on the Diagnosis of Alzheimer's Disease. Mol Diagn Ther 16, 135–141 (2012). [DOI] [PubMed] [Google Scholar]
- Vandermeeren M. et al. Detection of Proteins in Normal and Alzheimer's Disease Cerebrospinal Fluid with a Sensitive Sandwich Enzyme-Linked Immunosorbent Assay. J Neurochem 61, 1828–1834 (1993). [DOI] [PubMed] [Google Scholar]
- Vanmechelen E. et al. Quantification of tau phosphorylated at threonine 181 in human cerebrospinal fluid: a sandwich ELISA with a synthetic phosphopeptide for standardization. Neurosci Lett 285, 49–52 (2000). [DOI] [PubMed] [Google Scholar]
- Andreasen N. et al. Evaluation of CSF-tau and CSF-Abeta42 as diagnostic markers for Alzheimer disease in clinical practice. Arch Neurol 58, 373–9 (2001). [DOI] [PubMed] [Google Scholar]
- Schoonenboom N. S. M. et al. Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology 78, 47–54 (2012). [DOI] [PubMed] [Google Scholar]
- Ibach B. et al. Cerebrospinal fluid tau and beta-amyloid in Alzheimer patients, disease controls and an age-matched random sample. Neurobiol Aging 27, 1202–11 (2006). [DOI] [PubMed] [Google Scholar]
- Sunderland T. et al. Tau Levels in Cerebrospinal Fluid of Patients With Alzheimer Disease. JAMA 289, 2094–2103 (2003). [DOI] [PubMed] [Google Scholar]
- Petersen R. C. et al. Aging, Memory, and Mild Cognitive Impairment. Int Psychogeriatr 9, 65–69 (1997). [DOI] [PubMed] [Google Scholar]
- Petersen R. C. et al. Current concepts in mild cognitive impairment. Arch Neurol 58, 1985–92 (2001). [DOI] [PubMed] [Google Scholar]
- Reisberg B. & Gauthier S. Current evidence for subjective cognitive impairment (SCI) as the pre-mild cognitive impairment (MCI) stage of subsequently manifest Alzheimer's disease. Int Psychogeriatr 20, 1–16 (2008). [DOI] [PubMed] [Google Scholar]
- Hansson O. et al. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol 5, 228–34 (2006). [DOI] [PubMed] [Google Scholar]
- Mattsson N. et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 302, 385–393 (2009). [DOI] [PubMed] [Google Scholar]
- Sperling R. A. et al. Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging and the Alzheimer's Association workgroup. Alzheimers Dement 7, 280–292 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M. E. et al. A default mode of brain function. Proc Natl Acad Sci U S A 98, 676–82 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R. L., Andrews-Hanna J. R. & Schacter D. L. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124, 1–38 (2008). [DOI] [PubMed] [Google Scholar]
- Greicius M. D., Srivastava G., Reiss A. L. & Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: Evidence from functional MRI. Proc Natl Acad Sci U S A 101, 4637–4642 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T.-Q. & Wahlund L.-O. The search for neuroimaging biomarkers of Alzheimer's disease with advanced MRI techniques. Acta Radiol 52, 211–22 (2011). [DOI] [PubMed] [Google Scholar]
- Petrella J. R., Sheldon F. C., Prince S. E., Calhoun V. D. & Doraiswamy P. M. Default mode network connectivity in stable vs progressive mild cognitive impairment. Neurology 76, 511–7 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R. L. et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 25, 7709–17 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy K. M. et al. Effects of beta-amyloid accumulation on neural function during encoding across the adult lifespan. Neuroimage 62, 1–8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Y. I. et al. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry 67, 584–7 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie E. C. et al. Preclinical evidence of Alzheimer changes: convergent cerebrospinal fluid biomarker and fluorodeoxyglucose positron emission tomography findings. Arch Neurol 66, 632–637 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddalena A. et al. Biochemical Diagnosis of Alzheimer Disease by Measuring the Cerebrospinal Fluid Ratio of Phosphorylated tau Protein to β-Amyloid Peptide 42. Arch Neurol 60, 1202–1206 (2003). [DOI] [PubMed] [Google Scholar]
- Seppälä T. T. et al. CSF biomarkers for Alzheimer disease correlate with cortical brain biopsy findings. Neurology 78, 1568–75 (2012). [DOI] [PubMed] [Google Scholar]
- Blennow K. & Hampel H. Review CSF markers for incipient Alzheimer's disease CSF markers for incipient AD. Lancet Neurol 2, 605–613 (2003). [DOI] [PubMed] [Google Scholar]
- Visser P. J. et al. Prevalence and prognostic value of CSF markers of Alzheimer's disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol 8, 619–27 (2009). [DOI] [PubMed] [Google Scholar]
- Herukka S.-K., Hallikainen M., Soininen H. & Pirttilä T. CSF Abeta42 and tau or phosphorylated tau and prediction of progressive mild cognitive impairment. Neurology 64, 1294–7 (2005). [DOI] [PubMed] [Google Scholar]
- Wang K. et al. Altered functional connectivity in early Alzheimer's disease: a resting-state fMRI study. Hum Brain Mapp 28, 967–78 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai F. et al. Default-mode network activity distinguishes amnestic type mild cognitive impairment from healthy aging: a combined structural and resting-state functional MRI study. Neurosci Lett 438, 111–5 (2008). [DOI] [PubMed] [Google Scholar]
- Jones D. T. et al. Age-related changes in the default mode network are more advanced in Alzheimer disease. Neurology 77, 1524–31 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. Y. et al. Resting Brain Connectivity: Changes during the Progress of Alzheimer Disease. Radiology 256, 598–606 (2010). [DOI] [PubMed] [Google Scholar]
- Luo L. et al. Constrained source-based morphometry identifies structural networks associated with default mode network. Brain Connect 2, 33–43 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak H. K. F. et al. Quantitative assessment of cerebral hemodynamic parameters by QUASAR arterial spin labeling in Alzheimer's disease and cognitively normal Elderly adults at 3-Tesla. J Alzheimers Dis 31, 33–44 (2012). [DOI] [PubMed] [Google Scholar]
- Codispoti K.-E. T. et al. Longitudinal brain activity changes in asymptomatic Alzheimer disease. Brain Behav 2, 221–30 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosun D., Schuff N., Mathis C. a, Jagust, W. & Weiner, M. W. Spatial patterns of brain amyloid-beta burden and atrophy rate associations in mild cognitive impairment. Brain 134, 1077–88 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad B. et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 256, 240–6 (2004). [DOI] [PubMed] [Google Scholar]
- Andreasen N. et al. Cerebrospinal fluid β-amyloid (1-42) in Alzheimer disease: differences between early-and late-onset Alzheimer disease and stability during the course of disease. Arch Neurol 56, 673–680 (1999). [DOI] [PubMed] [Google Scholar]
- Koch W. et al. Effects of aging on default mode network activity in resting state fMRI: does the method of analysis matter? Neuroimage 51, 280–7 (2010). [DOI] [PubMed] [Google Scholar]