Abstract
BACKGROUND AND OBJECTIVE:
Impoverished urban children suffer disproportionately from asthma and underuse preventive asthma medications. The objective of this study was to examine cost-effectiveness (CE) of the School-Based Asthma Therapy (SBAT) program compared with usual care (UC).
METHODS:
The analysis was based on the SBAT trial, including 525 children aged 3 to 10 years attending urban preschool or elementary school who were randomized to either UC or administration of 1 dose of preventive asthma medication at school by the school nurse each school day. The primary outcome was the mean number of symptom-free days (SFDs). The impact of the intervention on medical costs was estimated by using parent-reported child health services utilization data and average national reimbursement rates. We estimated the cost of running the program using wages for program staff. Productivity costs were estimated by using value of parent lost time due to child illness. CE of the SBAT program compared with UC was evaluated based on the incremental CE ratio.
RESULTS:
The health benefit of the intervention was equal to ∼158 SFD gained per each 30-day period (P < .05) per 100 children. The programmatic expenses summed to an extra $4822 per 100 children per month. The net saving due to the intervention (reduction in medical costs and parental productivity, and improvement in school attendance) was $3240, resulting in the incremental cost-savings difference of $1583 and CE of $10 per 1 extra SFD gained.
CONCLUSIONS:
The SBAT was effective and cost-effective in reducing symptoms in urban children with asthma compared with other existing programs.
KEY WORDS: randomized trial, cost-effectiveness, asthma, minority children, urban, preventive medicine
What’s Known on This Subject:
Urban children suffer disproportionately from asthma, and suboptimal treatment with preventive medications is common. Although several programs have been developed to reduce morbidity for urban children with asthma, their economic feasibility and sustainability remain unknown.
What This Study Adds:
Our study demonstrates that the school-based asthma therapy program could be an economically effective program for children aged 3 to 10 years attending preschool or elementary school in a city school district, at the cost of $10/symptom-free day.
Asthma is one of the most common chronic illnesses of childhood1–3 that often results in preventable hospitalizations.4–8 Asthma causes morbidity from daytime and nighttime symptoms, impairment of quality of life, and functional impairment, including limitation of activity, absenteeism from school, and missed days of work for caretakers. In the United States, poor and minority children suffer disproportionately from asthma,9–12 and suboptimal treatment with preventive medications is common.
Several intervention programs have been developed to reduce morbidity for urban children with asthma. Most of these have involved relatively intensive case-management and educational interventions, with varied effectiveness.13–26 We recently completed the School-Based Asthma Therapy (SBAT) trial and demonstrated effectiveness in improving outcomes.27,28 The purpose of this study was to examine the cost-effectiveness (CE) of the SBAT program compared with a usual care (UC) control group that did not receive the intervention.
Methods
Study Population
The University of Rochester Institutional Review Board approved the study protocol. During the beginning of 3 consecutive school years starting in 2006, we recruited children aged 3 to 10 years attending preschool or elementary school in the Rochester City School District in Rochester, NY, who had physician-diagnosed asthma with persistent symptoms based on National Heart, Lung, and Blood Institute Expert Panel guidelines.27–30 Persistent symptoms at screening were based on responses to questions that asked the caregiver to think about symptoms during a “typical week” in the past year, as well as the number of asthma attacks in the past year. Children were excluded if they had other medical conditions that could interfere with the assessment of asthma-related outcomes (cystic fibrosis, congenital heart disease, other lung disease), if the primary caregiver was unable to speak and understand English, if they were planning to leave the school district within 6 months, or if they had no access to a telephone for follow-up surveys (at home or an easily accessible alternate location).
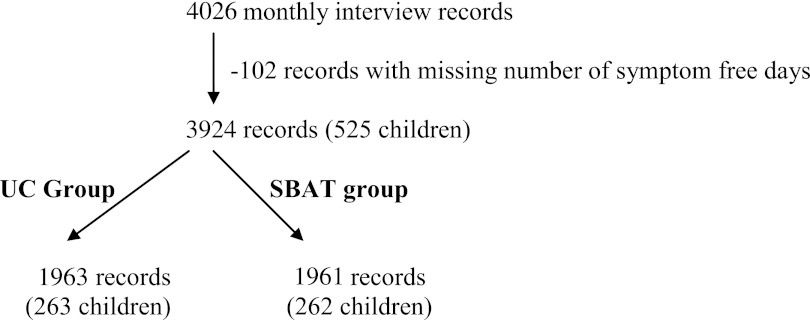
After the baseline assessment, children were stratified by exposure to tobacco smoke in the home and were assigned to the SBAT or UC group by blocked randomization in a 1:1 ratio. The intervention continued for ∼10 months, September through June of each year. The main analysis included 3924 person-month records from 525 children (Figure 2). We also repeated the analysis for those children who at the baseline assessment reported current persistent symptoms (>4 days of daytime symptoms and/or >1 night with nighttime symptoms over 2 weeks before baseline assessment; n = 338).
FIGURE 2.
Study flowchart. The chart explains the differences between the clinical trial sample and patients and observations included in the CE analysis.
Trial Design
The primary intervention for children in the SBAT group was directly observed administration of preventive asthma medication at school. Children were followed for 1 school year (7–9 months). Each child received 1 dose of medication (fluticasone propionate or fluticasone with salmeterol xinafoate) from the school nurse once each school day.27,28,31 Parents were responsible for medication administration on weekend days and other days the child did not attend school.
The medication dose varied depending on the child’s baseline asthma therapy, and medication adjustments were made at the primary care provider’s (PCP) discretion. Assessment for possible step-up in therapy occurred during the first 3 months of the intervention28 and recommendations for adjustments were relayed to the parents and PCPs. The process of symptom assessment, communication, and delivery of medications to schools and families was facilitated by the study team along with a nurse educator (a registered nurse with specific training in asthma care).
In the UC group, caregivers were encouraged to contact their PCP to discuss the child’s persistent asthma symptoms. Families were responsible for filling prescriptions and administering medications daily to the child.
Health Outcomes
All families were given diaries based on the school calendar to track their child’s symptoms. Outcomes were assessed by monthly telephone interviews by an independent research group blinded to group allocation. The completion rate was 90% and higher each month.
The primary SBAT trial outcome was the number of symptom-free days (SFDs) during the previous 2 weeks.27 Parents were asked to refer to their diaries and report the number of days their child experienced no symptoms of asthma (defined as a 24-hour period with no coughing, wheezing, chest tightness, or shortness of breath and no need for rescue medications) during the past 2 weeks. For the CE assessment, effectiveness was measured as the difference between the SBAT and UC groups in the average number of SFDs during the 30-day period before each assessment.
Evaluating Costs
Four main categories of costs were considered, including programmatic costs (total staff salaries divided by the number of children), health care (HC) costs, school attendance fees losses, and parents’ productivity losses, estimated at individual child (family) level. Costs associated with the study that would not exist as part of the intervention in a real-life setting were not included in the cost analysis. We did not include medication costs as part of the program costs, because according to the national guidelines all children should have been using a preventive asthma medication regardless of the study procedures.
To estimate HC costs, parents were asked monthly about their children’s hospitalizations, emergency department (ED) or physician visits, medical procedures, and tests for the past month. We defined an acute exacerbation as any visit for asthma where prednisone was prescribed. Medical records were reviewed for 10% of the sample to confirm office and ED visits and hospitalizations; visits were confirmed in 83% of cases.
The schools’ saved revenue due to reduced absenteeism was calculated based on the number of expected missed school days using the weighted average daily attendance (WADA) rate of $40 per absent day.32–36
The productivity/opportunity costs were determined based on the amount of time parents took off from work to care for sick children or to take them to see a doctor. We used a standard parent daily wage estimate of $80 to estimate value of parent time lost from work.37
CE Analysis
To determine the average numbers of SFDs, physician office and ED visits, as well as child’s missed school days due to asthma in each group, we used the generalized estimating equation Poisson log-linear regression models with group (SBAT versus UC) assignment as the only explanatory variable. We used robust SEs to control for dependence between multiple observations over time provided by the same child. To calculate the average number of hospital admissions due to asthma, we divided the number of hospitalizations by the number of months a child was enrolled in the study.
The goal of a CE assessment is to estimate or predict economic feasibility of an intervention once it is implemented in a real practice setting. Hence, instead of actual parameters (costs or health effects) from a particular randomized controlled trial or database, researchers often use predicted generalized estimates for a relevant/eligible population. In this case, the relevant population is all US urban children, so we used 2005–2009 Medical Expenditures Panel Survey data to obtain estimates of mean costs of asthma-related doctor office visits, ED visits, and hospital admissions for children with asthma for ages 3 to 10 (Table 2).38 The expected cost of HC use was calculated by multiplying estimated expected utilization by estimated unit costs adjusted to 2009 US dollars.37
TABLE 2.
Utilization of HC Related to Asthma
| Cost per Unit Mean (SE) [ 95% CI] | UC Group | SBAT Group | P Value | Overall | ||||
|---|---|---|---|---|---|---|---|---|
| Total No. of Visits | Mean No. of Visits (SE) | Total No. of Visits | Mean No. of Visits (SE) | Total No. of Visits | Mean No. of Visits (SE) | |||
| Office visits with prednisone | $97 ($7) [$83–$110] | 37 | 1.74 (0.35) | 29 | 1.45 (0.34) | .561 | 66 | 1.60 (0.25) |
| ED visits with prednisone | $306 ($51) [$206–$406] | 16 | 0.81 (0.20) | 13 | 0.66 (0.18) | .567 | 29 | 0.74 (0.13) |
| Hospitalization with prednisone | $2997 ($965) [$1005–$4990] | 2 | 0.10 | 0 | 0.00 | 2 | 0.05 | |
| Total office, ED, and hospital stays with prednisone | 55 | 2.58 (0.42) | 42 | 2.08 (0.42) | .412 | 97 | 2.33 (0.30) | |
| SFDs, mean (SE) | 2398 (35) | 2556 (28) | .001 | 2476 (23) | ||||
| Missed school days, mean (SE) | 84 (7) | 60 (7) | .014 | 72 (5) | ||||
The numbers of visits represent raw data reported by respondents (for varying length of the reference period), whereas the means are estimated for 30-d period per 100 children. The 3 cohorts that completed the study in 3 consecutive years were stacked together and analyzed as 1 study cohort.
We assessed health and economic benefits of the SBAT compared with UC by using the standard CE methodology.39,40 The 3 cohorts that completed the study in 3 consecutive years were stacked together and analyzed as 1 study cohort. Because most children in the study were eligible for Medicaid or the State Children’s Health Insurance Program (74%), the main analysis was conducted from the Medicaid perspective. The main outcome for the CE analysis from the society perspective was an incremental CE ratio (ICER), which is the ratio of net total intervention costs to the number of SFDs gained,
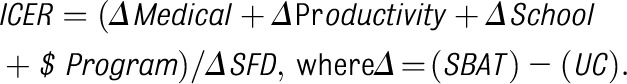 |
To estimate how much the results of our CE analysis depend on our assumptions and sources of information about SBAT-associated costs, we used the bootstrapping method41 and varied the unit costs to lower and upper bounds of a 95% confidence interval (CI). We also plotted CE acceptability curves, linking various values of 1 SFD obtained from the literature for other school-based asthma-management programs13–26 to probability of SBAT being cost-effective.42–45
Results
Population Descriptive Statistics
The children’s mean age was 7.1 years, and more than half were boys (58%, Table 1). Approximately two-thirds of the cohort were black (63%), and three-quarters were covered by Medicaid (74%). Asthma severity was similar at baseline with children in both groups having an average of 8 SFDs and 4 days using rescue medication per 2 weeks; 64% of all children had persistent symptoms at the time of the baseline assessment. As presented for the main study,27 there were no differences in demographic characteristics between children in the 2 study groups (Table 1).
TABLE 1.
Participant Characteristics at Baseline
| All Enrolled Children | Children With Persistent Symptoms at Baseline | |||||||
|---|---|---|---|---|---|---|---|---|
| UC | SBAT | UC | SBAT | |||||
| Overall | Group | Group | P | Overall | Group | Group | P | |
| (n = 525a) | (n = 263) | (n = 262) | (n = 338) | (n = 167) | (n = 171) | |||
| Child’s age, mean (SD), y | 7.13 (1.9) | 7.21 (1.89) | 7.06 (2.0) | .382 | 6.94 (2.0) | 7.04 (2.0) | 6.85 (2.0) | .4 |
| Male child, % | 58.29 | 55.89 | 60.69 | .265 | 57.4 | 52.69 | 62 | .08 |
| Child’s race, % | ||||||||
| White | 8.95 | 7.98 | 9.92 | .703 | 8.28 | 7.19 | 9.36 | .47 |
| Black | 63.43 | 63.5 | 63.36 | 64.5 | 67.66 | 61.4 | ||
| Other | 27.62 | 28.52 | 26.72 | 29.24 | 25.15 | 29.2 | ||
| Hispanic ethnicity, % | 28.38 | 26.24 | 30.53 | .275 | 28.11 | 23.95 | 32.2 | .09 |
| Medicaid insurance, % | 73.52 | 74.9 | 72.14 | .472 | 75.74 | 79.04 | 72.5 | .16 |
| Smoker at home, % | 53.52 | 54.37 | 52.67 | .696 | 56.21 | 55.69 | 56.7 | .85 |
| Cotinine level, mean (SD) | 1.41 (2.31) | 1.57(2.83) | 1.25 (1.61) | NS | ||||
| Caregiver education less than high school, % | 38.86 | 37.64 | 40.08 | .567 | 39.35 | 37.72 | 40.9 | .55 |
| Maternal depression, mean (SD) score | 19.7 (9.1) | 19.51 (8.9) | 19.87 (9.3) | .648 | 20.67 (9.3) | 20.4 (8.9) | 20.9 (9.8) | .59 |
| Baseline asthma severity in past 2 wk | ||||||||
| SFDs, mean (SD) | 8.04 (4.8) | 8.05 (4.8) | 8.03 (4.9) | .964 | 5.48 (4.1) | 5.46 (4.0) | 5.49 (4.2) | .95 |
| Days with rescue medication, mean (SD) | 3.94 (4.6) | 3.84 (4.5) | 4.03 (4.7) | .638 | 5.4 (4.9) | 5.2 (4.8) | 5.6 (5.0) | .52 |
| Days absent due to asthma, mean (SD) | 0.40 (1.1) | 0.37 (1.03) | 0.42 (1.08) | .523 | 0.58 (1.3) | 0.54 (1.2) | 0.62 (1.27) | .57 |
| Severe persistent asthma at baseline, % | 64.38 | 63.5 | 65.27 | .672 | ||||
The number of records per child varied from 1 to 10 with the average of 7.5 interviews per child (similar across the groups).
Health Care Utilization and Costs
Across all utilization categories, children in the SBAT group had 1.45 acute office visits with prednisone compared with 1.74 in the UC group (per 100 children per month, P = .56, Table 2). The mean number of all doctor visits (all types) across the 2 groups was 2.33 per 100 children over 1 month, with 2.08 in the SBAT group and 2.58 in UC group. The average number of missed school days was lower in the SBAT group (60 SBAT versus 84 UC per 100 children per month, P = .01).
The programmatic costs were estimated to be $4822 per 100 children per month assuming $66 000 a year for salary and benefits for a nurse educator and $10 per hour, for 40 hours a week, for 12 weeks for a research assistant to perform symptom screening and scheduling (Table 3). For a program working only with children with continued persistent symptoms, one would have to screen 156 children with asthma to identify 100 children with persistent symptoms.
TABLE 3.
Costs and CE: Entire Cohort of Subjects
| Cost SBAT | Cost UC | Cost Difference | Effect SBAT (SFD) | Effect UC (SFD) | Effect Difference (SFD) | ICER $/SFD | |
|---|---|---|---|---|---|---|---|
| Program costs | $4822 | $0 | $4822 | ||||
| HC costs | $342 ($70) [224 to 503] | $719 ($234) [357 to 1309] | –$376 ($245) [–944 to 24] | ||||
| Productivitya | $4893 ($516) [4089 to 6193] | $6813 ($555) [5867 to 8081] | –$1920 ($751) [–3354 to –347] | ||||
| School costs | $2405 ($256) [2011 to 3049] | $3348 ($274) [2886 to 3966] | –$943 (371) [–1644 to –176] | ||||
| Total costs | $12 463 ($790) [11 251 to 14 461] | $10 880 (909) [9235 to 12 772] | $1583(1198) [–663 to 4188] | 2556 (28) [2484 to 2597] | 2398 (34) [2325 to 2453] | 158 (45) [60 to 238] | 10 (13) [–4 to 46] |
| Total direct costs | $4454 | — | — | 158 | 28 (15) [18 to 75] |
Costs and effects are shown as estimated mean per 30 days per 100 children (bootstrap SE), bootstrap [95% CI]. The cost of medication was not included in the analysis because all children with this level of asthma severity require medications (based on national guideline criteria) regardless of the study, and the vast majority of eligible children have drug coverage from Medicaid or the State Children’s Health Insurance Program.
We assumed that a parent missed a day from work for every day a child missed a day of school and that for every doctor appointment a parent had to take 2 hours from work; further, we assumed 1 day missed from work for each ED visit and 2 days for each hospitalization (1 for admission and 1 for discharge).
Among all participating children, the average costs associated with parents’ missed days from work were valued at $4893 (SE $516) for the SBAT group versus $6813 (SE $555) for the UC group, resulting in the incremental difference of $1920 (Table 3). Because of improved attendance attributable to the asthma intervention, the schools on average saved $943 (SE $371) in state WADA funding.
The difference in total HC costs (office visits, ED visits, and hospitalizations with prednisone) between groups is not significant, with the negative difference being interpreted as savings (–$376, 95% CI –$944 to $24 per 100 children per month). The total saving attributable to the intervention, including reduction in HC costs, reduction in productivity, and attendance losses, was $3240. These savings offset part of the programmatic costs, $4822 per 100 children per month ($48 per child per month), resulting in the overall difference of $1583 (Table 3).
CE
The health benefit of the intervention measured as additional asthma SFDs was significant and equal to 158 gained SFDs over a month per 100 children (Table 3). The cost per SFD was $10, 95% CI –$4 to $46; in other words, gaining 1 additional SFD costs $10 on average. If the indirect costs were not taken into account, then the ICER was equal to $28 per SFD, 95% CI $18 to $75 (Table 3).
For the children with more severe asthma, the SBAT resulted in additional 1.79 SFDs per month per child (Table 4). The savings were greater for all categories, including HC costs, productivity, and school attendance fees. Because of reduced absenteeism, the SBAT schools could save on average $1146 in lost revenue compared with the UC group. For the more severe children, the ICER was $5.62 per SFD, 95% CI –12.80 to 46.61, with all costs included.
TABLE 4.
Costs and CE: Children With Persistent Symptoms at Baseline
| Cost SBAT | Cost UC | Cost Difference | Effect SBAT (SFD) | Effect UC (SFD) | Effect Difference (SFD) | ICER $/SFD | |
|---|---|---|---|---|---|---|---|
| Program costs | $5056 | $0 | $5056 | ||||
| HC costs | $431 ($101) [243 to 640] | $997 ($361) [430 to 1828] | –$566 ($375) [–1512 to 3] | ||||
| Productivity | $6096 ($777) [4662 to 7730] | $8435 ($779) [7098 to 10 153] | –$2339 (1702) [–4602 to –504] | ||||
| School costs | $2996 ($387) [2276 to 3820] | $4143 ($384) [3490 to 4992] | –$1146 ($531) [–2278 to –232] | ||||
| Total | $14 579 (1182) [12 411 to 17 099] | $13 574 (1291) [11 404 to 16 606] | $1005 (1707) [–2597 to 4092] | 2471 (38) [2364 to 2525] | 2292 (47) [2151 to 2358] | 179 (60) [62 to 299] | $6 (19) [–13 to 47] |
Costs and effects are shown as estimated mean per 30 days per 100 children (bootstrap SE), bootstrap [95% CI].
We drew 1000 re-samples of children from the original sample. Because the randomization to the intervention and control groups was done separately within 2 strata defined by the exposure to cigarette smoke in the child’s home, the re-sampling process was done separately in each of 4 strata (120–143 kids in strata) defined by smoke exposure and treatment group. For each re-sample, the ICER was reestimated providing 1000 bootstrap values of the ICER. This distribution was used to calculate the bootstrapped SEs and 95% bootstrapped CI.
Table 5 presents costs and ICER when we modeled a reduced program cost scenario by excluding the research assistant for symptom screening and associated costs ($422 and $657 cost reduction for all children and children with more severe symptoms, respectively). Under this assumption, with the responsibility for symptom screening transferred to school administrative personnel (with 100% salary coverage from non–program-related sources), the CE for all children with asthma and for children with severe persistent asthma at baseline would improve (from $10/SFD to $7/SFD for all children and from $6/SFD to $2/SFD for more severe children).
TABLE 5.
Costs and CE: Reduced Program Cost
| Cost SBAT | Cost UC | Cost Difference | Effect SBAT (SFD) | Effect UC (SFD) | Effect Difference (SFD) | ICER $/SFD | |
|---|---|---|---|---|---|---|---|
| Entire cohort of children | $12 040 ($790) [10 829 to 14 039] | $10 880 ($909) [9235 to 12 772] | $1160 (1198) [–1086 to 3766] | 2556 (28) [2484 to 2597] | 2398 (34) [2325 to 2453] | 158 (45) [60 to 238] | $7 ($12) [–6 to 42] |
| Children with persistent symptoms at baseline | $13 923(1182) [11 755 to 16 443] | $13 574 (1291) [11 404 to 16 606] | $349(1707) [–3253 to 3436] | 2471 (38) [2364 to 2525] | 2292 (47) [2151 to 2358] | 179 (60) [62 to 299] | $2 (17) [–17 to 37] |
Reduced program cost: we modeled a reduced program cost scenario by excluding the screener position and associated costs ($422 and $656 cost reduction). For instance, the responsibility of a screener could be transferred to school administrative personnel (with 100% salary coverage from non–program-related sources). Costs and effects are shown as estimated mean per 30 days per 100 children (bootstrap SE), bootstrap [95% CI].
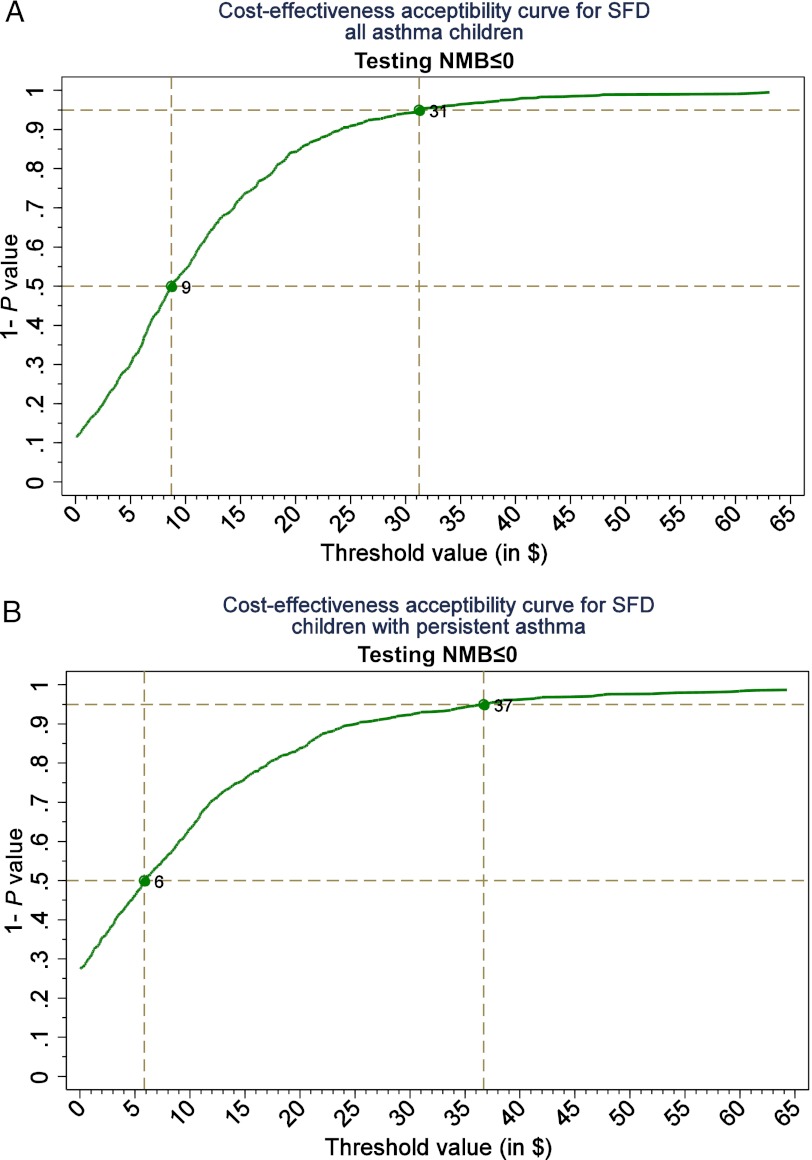
When the value of 1 SFD is valued at $10, the probability of the SBAT being cost-effective for all eligible children was ∼50% (Fig 1). At the value of $31 per SFD, the probability of the SBAT being cost-effective was at the 95% level (Fig 2). For children with persistent symptoms at baseline, the 50% and 95% probability of the SBAT being cost-effective corresponded to the SFD values of $6 and $37, respectively.
FIGURE 1.
A, CE acceptability curves for all asthma patients. B, Patients with persistent asthma only. CE acceptability curves present probability of SBAT being cost-effective at various values of 1 SFD. NMB, incremental net monetary benefit, the difference between the benefit of the SBAT expressed in monetary terms and the cost of the intervention.
Discussion
Our study demonstrates that the SBAT program, at the cost of $10 per 1 extra SFD gained, could be economically effective for children aged 3 to 10 years in a city school district. The cost of running the program summed to $4822 per 100 children and was determined mainly by qualifications and wages of the program personnel. The program resulted in $3240 in savings to the schools (preventing WADA funding loss owing to reduced absenteeism), parents (decreased productivity losses), and HC system (decreased HC costs) per 100 children per year. Limiting the program to children with continued persistent symptoms at the baseline assessment further improved its CE ($5.60/SFD).
Although none of the other studies took into account productivity costs, our results are nevertheless similar to the studies that evaluated the CE of community-based asthma interventions for children.24,46–48 One of the largest community-based interventions, initiated as part of the National Collaborative Inner City Asthma Study, employed Master’s-level social workers to deliver a home-based educational intervention to poor urban families.18 The authors found an improvement in symptoms for an additional incremental cost of $9.20 per SFD gained.24 Another home-based environmental intervention carried out at 7 sites across the United States as a part of the Inner-City Asthma Consortium demonstrated benefit at $27.57/SFD.49 A cost-benefit analysis of childhood asthma management through school-based clinic programs supported our finding that medical savings alone ($1.69 billion for nationwide implementation) could not offset the expense of implementing an asthma-prevention program ($4.55 billion). However, when savings due to reduction of parent opportunity costs and child premature death ($23.13 billion) were considered, the benefit of the program far exceeded its cost.48 Furthermore, studies indicated that poorly controlled asthma has a negative long-term impact on academic achievement through its effect on cognition, school connectedness, and chronic absenteeism, which in turn could contribute to economic losses related to education attainment, crime, and future earnings.28,50
More information is needed to justify whether asthma-prevention programs are cost-saving or whether resulting health benefits are worth additional costs. One of the approaches to make this intervention more affordable is to predominantly use resources that are currently available to city schools; however, currently many schools are under substantial financial stress, resulting in fewer resources available.
Our study has several potential limitations. Although we made our best effort to differentiate the study-related expenses and services use from the services necessary to run the program in a real school setting, it is unknown whether the effectiveness of the intervention could be maintained in a real setting over time. Our cost estimates were sensitive to the type of insurance coverage the children had (Medicaid versus commercial plans) and the type of PCP they have seen (pediatrician versus family physician versus asthma specialist). Further, the original study included an additional home-based smoke reduction component that was not included in the cost analysis or subsequent SBAT studies.51 This decision was made based on our primary analysis that demonstrated that the improvement in asthma symptoms in the treatment group was independent of children’s cotinine level.27,51 We did, however, control for family smoking status in all analyses.
It is important to note that our study likely provides a conservative estimate of the effectiveness of the SBAT, as children in the UC arm improved more than expected during the course of the study. This may be because of regression to the mean (children with significant symptoms at baseline gradually improve over time independent of any intervention), as well as simply from participation in the study (because monthly calls inquiring about children’s health and symptoms and contact with families and providers may have served as a “weak” intervention to the children in the UC arm).
Although the Panel on Cost-Effectiveness39 recommends using quality-adjusted life years as a standard measure of effectiveness in CE studies, we chose to focus on outcome reporting using natural units, SFDs, which is consistent with the vast majority of other CE studies in asthma. This is thought to be reasonable, as the methods of utility assessment in children are poorly established and disease exacerbations affect not only the patient (a child) but family and caregivers as well.24,47,49
Several studies have demonstrated that case-management, self-management, and educational programs for asthma are effective22,52,53 but costly, both from the payer and from patient perspectives, and result in higher drug costs owing to better compliance22 and more physician visits for monitoring and education. Studies of prescription medication costs for childhood asthma among minority populations have reported an average expense of ∼$300 a year (2011 US$),49 much lower than the expected annual costs given the average monthly cost of common inhalers ($187 for fluticasone propionate and $286 for luticasone with salmeterol xinafoate54). Because the burden of childhood asthma disproportionally falls on low-income families, further research is needed to understand whether the cost of medication represents a substantial financial barrier to the families, even though a large proportion of affected children are eligible for Medicaid or the State Children’s Health Insurance Program.55 Furthermore, because medication costs were likely to be higher for children participating in the SBAT program compared with children receiving care as usual, the true incremental cost per SFD associated with the SBAT program may be higher than we reported here.
Conclusions
We demonstrated that the SBAT program could be economically effective for urban children aged 3 to 10 years attending school. Our study also demonstrates that this population experiences substantial economic burden associated with persistent asthma symptoms among children.
Glossary
- CE
cost-effectiveness
- CI
confidence interval
- ED
emergency department
- HC
health care
- ICER
incremental cost-effectiveness ratio
- PCP
primary care provider
- SBAT
School-Based Asthma Therapy
- SFD
symptom-free days
- UC
usual care
- WADA
weighted average daily attendance
Footnotes
Dr Noyes conceptualized and designed cost-effectiveness analysis, interpreted the results, drafted the initial manuscript, and approved the final draft as submitted; Ms Bajorska conducted the data analyses, helped interpret the results, assisted with drafting of the initial manuscript, and approved the final draft as submitted; Dr Fisher assisted in the planning of the trial and with preparation of the manuscript; Mr Sauer contributed to the design and implementation of the trial and assisted with data preparation for the manuscript; Ms Fagnano participated in the planning of the trial, directed the trial, and assisted with the conceptualization of the analysis and preparation of the manuscript; and Dr Halterman conceptualized and designed the trial, and assisted with the conceptualization of this analysis and preparation of the manuscript.
This trial has been registered at www.clinicaltrials.gov (identifier NCT01175369).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by a grant from the National Heart, Lung, and Blood Institute of the National Institutes of Health (R01HL079954). Dr Noyes was supported in part by grant 1 UL1 RR024160-01 from the National Center for Research Resources, a component of the National Institutes of Health and the NIH Roadmap for Medical Research. Funded by the National Institutes of Health (NIH).
References
- 1.Adams PF, Marano MA. Current estimates from the National Health Interview Survey, 1994. Vital Health Stat 10. 1995;(193 pt 1):1–260 [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Surveillance for asthma—US, 1960–1995. CDC Surveillance Summaries. 1995;47:1022–1025
- 3.National Heart Laboratory. Data Fact Sheet. Asthma Statistics. Bethesda, MD: National Institutes of Health; 1999 [Google Scholar]
- 4.Centers for Disease Control and Prevention Asthma mortality and hospitalization among children and adults—United States, 1980–1993. MMWR Morb Mortal Wkly Rep. 1996;45(17):350–353 [PubMed] [Google Scholar]
- 5.Gergen PJ, Weiss KB. Changing patterns of asthma hospitalization among children: 1979 to 1987. JAMA. 1990;264(13):1688–1692 [PubMed] [Google Scholar]
- 6.Akinbami LJ, Schoendorf KC. Trends in childhood asthma: prevalence, health care utilization, and mortality. Pediatrics. 2002;110(2 pt 1):315–322 [DOI] [PubMed] [Google Scholar]
- 7.The Canadian Burden of Illness Study Group . Burden of illness of multiple sclerosis: part I: cost of illness. Can J Neurol Sci. 1998;25(1):23–30 [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention Hospital Discharges by First-Listed Diagnosis Among Children. Atlanta, GA: Centers for Disease Control and Prevention; 2012 [Google Scholar]
- 9.Carr W, Zeitel L, Weiss KB. Variations in asthma hospitalizations and deaths in New York City. Am J Public Health. 1992;82(1):59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang DM, Polansky M. Patterns of asthma mortality in Philadelphia from 1969 to 1991. N Engl J Med. 1994;331(23):1542–1546 [DOI] [PubMed] [Google Scholar]
- 11.Targonski PV, Persky VW, Orris P, Addington W. Trends in asthma mortality among African Americans and whites in Chicago, 1968 through 1991. Am J Public Health. 1994;84(1830):1833 [DOI] [PMC free article] [PubMed]
- 12.Weiss KB, Wagener DK. Changing patterns of asthma mortality. Identifying target populations at high risk. JAMA. 1990;264(13):1683–1687 [PubMed] [Google Scholar]
- 13.Wissow LS, Warshow M, Box J, Baker D. Case management and quality assurance to improve care of inner-city children with asthma. Am J Dis Child. 1988;142(7):748–752 [DOI] [PubMed] [Google Scholar]
- 14.Clark NM, Feldman CH, Evans D, Levison MJ, Wasilewski Y, Mellins RB. The impact of health education on frequency and cost of health care use by low income children with asthma. J Allergy Clin Immunol. 1986;78(1 pt 1):108–115 [DOI] [PubMed] [Google Scholar]
- 15.Taggart VS, Zuckerman AE, Sly RM, et al. You can control asthma: evaluation of an asthma education program for hospitalized inner-city children. Patient Educ Couns. 1991;17(1):35–47 [DOI] [PubMed] [Google Scholar]
- 16.Evans D, Mellins R, Lobach K, et al. Improving care for minority children with asthma: professional education in public health clinics. Pediatrics. 1997;99(2):157–164 [DOI] [PubMed] [Google Scholar]
- 17.Shields MC, Griffin KW, McNabb WL. The effect of a patient education program on emergency room use for inner-city children with asthma. Am J Public Health. 1990;80(1):36–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans R, III, Gergen PJ, Mitchell H, et al. A randomized clinical trial to reduce asthma morbidity among inner-city children: results of the National Cooperative Inner-City Asthma Study. J Pediatr. 1999;135(3):332–338 [DOI] [PubMed] [Google Scholar]
- 19.Hughes DM, McLeod M, Garner B, Goldbloom RB. Controlled trial of a home and ambulatory program for asthmatic children. Pediatrics. 1991;87(1):54–61 [PubMed] [Google Scholar]
- 20.Bonner S, Zimmerman BJ, Evans D, Irigoyen M, Resnick D, Mellins RB. An individualized intervention to improve asthma management among urban Latino and African-American families. J Asthma. 2002;39(2):167–179 [DOI] [PubMed] [Google Scholar]
- 21.Kelly CS, Morrow AL, Shults J, Nakas N, Strope GL, Adelman RD. Outcomes evaluation of a comprehensive intervention program for asthmatic children enrolled in Medicaid. Pediatrics. 2000;105(5):1029–1035 [DOI] [PubMed] [Google Scholar]
- 22.Greineder DK, Loane KC, Parks P. Reduction in resource utilization by an asthma outreach program. Arch Pediatr Adolesc Med. 1995;149(4):415–420 [DOI] [PubMed] [Google Scholar]
- 23.Madge P, McColl J, Patton J. Impact of a nurse-led home management training program in children admitted to hospital with acute asthma. Thorax. 1997;52(3):223–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sullivan SD, Weiss KB, Lynn H, et al. National Cooperative Inner-City Asthma Study (NCICAS) Investigators . The cost-effectiveness of an inner-city asthma intervention for children. J Allergy Clin Immunol. 2002;110(4):576–581 [DOI] [PubMed] [Google Scholar]
- 25.Gerald LB, McClure LA, Mangan JM, et al. Increasing adherence to inhaled steroid therapy among schoolchildren: randomized, controlled trial of school-based supervised asthma therapy. Pediatrics. 2009;123(2):466–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Millard MW, Johnson PT, McEwen M, et al. A randomized controlled trial using the school for anti-inflammatory therapy in asthma. J Asthma. 2003;40(7):769–776 [DOI] [PubMed] [Google Scholar]
- 27.Halterman JS, Szilagyi PG, Fisher SG, et al. Randomized controlled trial to improve care for urban children with asthma: results of the School-Based Asthma Therapy trial. Arch Pediatr Adolesc Med. 2011;165(3):262–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halterman JS, Borrelli B, Fisher S, Szilagyi PG, Yoos L. Improving care for urban children with asthma: design and methods of the School-Based Asthma Therapy (SBAT) trial. J Asthma. 2008;45(4):279–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Asthma Education and Prevention Program. NAEPP Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma: Update on Selected Topics 2002. Bethesda, MD: National Heart Lung and Blood Institute, National Institutes of Health; 2002. Publication No. 02-5075 [Google Scholar]
- 30.National Asthma Education and Prevention Program. Expert Panel Report III: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute, National Institutes of Health; 2007. Publication No. 97-4051 [Google Scholar]
- 31.LaForce CF, Pearlman DS, Ruff ME. Efficacy and safety of dry powder fluticasone propionate in children with persistent asthma. Ann Allergy Asthma Immunol. 2000;85(5):407–415 [DOI] [PubMed]
- 32.University of Nevada. Using Average Daily Attendance in Missouri Public Schools. Las Vegas, NV: University of Nevada; 2012
- 33.Texas Association of School Business Officials. Average daily attendance. 2012. Available at: www.tasbo.org/files-public/training/academies/2011%20Business%20Managers%20Academy/1b_charts_and_examples.pdf. Accessed August 21, 2012
- 34.Texas Education Agency. Funding of Texas Public Schools. 2012 Austin, TX
- 35.The State Education Department. Analysis of school finances in New York State school district. 2002. Available at: www.oms.nysed.gov/faru/Analysis/00-01/htmlversion/analysisof_school_finances_00-01.htm. Accessed August 21, 2012
- 36.ChalkBoard Project. Using Average Daily Attendance as a Basis for Distributing State School Revenue. Salem, OR: Oregon State Department of Education; 2012
- 37.US Department of Labor Bureau of Labor Statistics. Consumer Price Index History. Washington, DC: Bureau of Labor Statistics; 2012
- 38.AHRQ. Medical Expenditures Panel Survey. Rockville, MD: Agency for Healthcare Research and Quality (AHRQ);2012
- 39.Gold MR, Siegel J, Russell L, Weinstein MC. Cost-effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996 [Google Scholar]
- 40.Drummond MF, Sculpher M, Torrance GW, O'Brien BJ, Stoddart G. Methods for the Economic Evaluation of Health Care Programmes. 3rd ed. New York, NY: Oxford University Press; 2005 [Google Scholar]
- 41.Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci. 1986;1(1):54–75 [Google Scholar]
- 42.Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics. 2000;17(5):479–500 [DOI] [PubMed] [Google Scholar]
- 43.Claxton K, Sculpher M, McCabe C, et al. Probabilistic sensitivity analysis for NICE technology assessment: not an optional extra. Health Econ. 2005;14(4):339–347 [DOI] [PubMed] [Google Scholar]
- 44.Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves—facts, fallacies and frequently asked questions. Health Econ. 2004;13(5):405–415 [DOI] [PubMed] [Google Scholar]
- 45.O’Brien BJ, Briggs AH. Analysis of uncertainty in health care cost-effectiveness studies: an introduction to statistical issues and methods. Stat Methods Med Res. 2002;11(6):455–468 [DOI] [PubMed] [Google Scholar]
- 46.Weiss KB, Sullivan SD, Lyttle CS. Trends in the cost of illness for asthma in the United States, 1985–1994. J Allergy Clin Immunol. 2000;106(3):493–499 [DOI] [PubMed] [Google Scholar]
- 47.Rutten-van Mölken MPMH, Van Doorslaer EKA, Jansen MCC, Kerstjens HAM, Rutten FFH. Costs and effects of inhaled corticosteroids and bronchodilators in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995;151(4):975–982 [DOI] [PubMed] [Google Scholar]
- 48.Tai T, Bame SI. Cost-benefit analysis of childhood asthma management through school-based clinic programs. J Community Health. 2011;36(2):253–260 [DOI] [PubMed] [Google Scholar]
- 49.Kattan M, Stearns SC, Crain EF, et al. Cost-effectiveness of a home-based environmental intervention for inner-city children with asthma. J Allergy Clin Immunol. 2005;116(5):1058–1063 [DOI] [PubMed] [Google Scholar]
- 50.Basch CE. Asthma and the achievement gap among urban minority youth. J Sch Health. 2011;81(10):606–613 [DOI] [PubMed] [Google Scholar]
- 51.Halterman JS, Sauer J, Fagnano M, et al. Working toward a sustainable system of asthma care: development of the School-Based Preventive Asthma Care Technology (SB-PACT) trial. J Asthma. 2012;49(4):395–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liljas B, Lahdensuo A. Is asthma self-management cost-effective? Patient Educ Couns. 1997;32(suppl 1):S97–S104 [DOI] [PubMed] [Google Scholar]
- 53.Greineder DK, Loane KC, Parks P. A randomized controlled trial of a pediatric asthma outreach program. J Allergy Clin Immunol. 1999;103(3 pt 1):436–440 [DOI] [PubMed] [Google Scholar]
- 54.New York State Department of Health. Pharmacy Pricelist. Albany, NY: New York State Department of Health; 2012 [Google Scholar]
- 55.Carlson A, Nesvold JH, Liu A. Population-based assessment of asthma symptom burden in children. J Urban Health. 2011;88(suppl 1):164–173 [DOI] [PMC free article] [PubMed] [Google Scholar]