Abstract
Background
Recognition of adipose-related signaling in surgery is increasing, though direct interrogation of human adipose has been sparse. Few scenarios rival uremia for health impact. We hypothesized that adipose from uremic patients holds a relatively higher adipose derived hormone and pro-inflammatory adipokine signature; we simultaneously evaluated the impact of clinical parameters on adipose phenotype.
Materials and Methods
Adipose was harvested from surgical patients. Histology and protein analyses were completed for select mediators.
Results
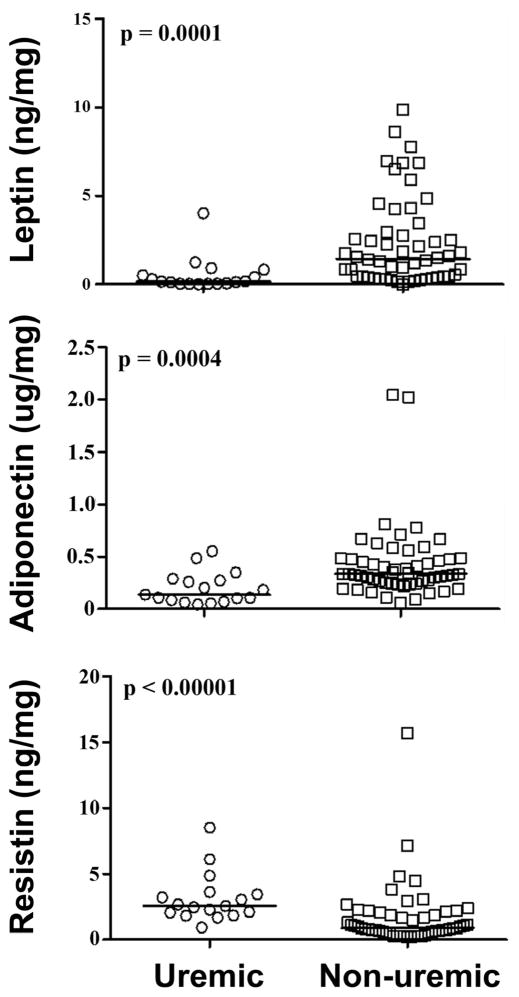
In the 71 patient cohort, mean age=63.4y; 63.3% had diabetes, 49.2% had hyperlipidemia and 53.5% had coronary disease. Compared to non-uremic patients, uremic patients had 1/10th the levels of leptin (p<0.001), 1/3rd the levels of adiponectin (p<0.001), and 3-fold higher resistin (p<0.001). Females had 6-fold higher leptin, 1.5-fold higher adiponectin and 2-fold higher TNF-α but equivalent resistin. There were differences in mediators when stratified by age. In both the obese/non-obese strata, we observed a concordant pattern of association (magnitude/significance) of uremia and leptin/adiponectin/resistin. No differentials in other mediators emerged upon BMI stratification. Multiple regression analysis for leptin/adiponectin/resistin (with age/gender/uremia as independent variables) showed uremia as the highest independent predictor of all three mediators.
Conclusions
Advanced chronic kidney disease is associated with perturbations in adipose derived hormones (leptin/adiponectin/resistin). Adipose adiponectin and leptin (in contrast to reported plasma levels) was lower in uremic patients; there is an inverse correlation between adipose resistin and renal function. Compared with other clinical parameters including BMI, uremia dominates overall in determining adipose phenotype, highlighting the complex biologic interplay between uremia and adipose biology.
Keywords: Uremia, chronic kidney disease, adipose tissue, leptin, adiponectin, resistin
INTRODUCTION
Adipose tissue is now recognized as a biologically active tissue that participates in signaling through endocrine, paracrine, and autocrine mechanisms (1, 2). Adipocyte-derived secreted proteins such as leptin, adiponectin, resistin and IL-6 have important roles in homeostasis, inflammation and glucose metabolism (3). While the overall mass of adipose tissue dominates other organs in humans, simple fat volume does not necessarily correlate with clinical phenotypes(4) and surgical outcomes (5). Substantial knowledge gaps exist regarding the determinants of adipose tissue phenotype and the true role of adipose tissue-related signaling networks in disease. The literature to date largely builds on animal models and human studies considering circulating adipose tissue derived mediator levels (6–8). By contrast, direct examination of clinically relevant human adipose tissue phenotype has been sparse (9–13).
Few clinical scenarios rival uremia for overall health impact and implications for surgical care. Patients with chronic kidney disease are seen as having a chronic inflammatory state and suffer from markedly increased risks of overall morbidity and mortality, especially cardiovascular complications (14–16). Single capture evaluations of circulating adiponectin(17), leptin, and resistin(18–21) have suggested that these adipose tissue-derived mediators are affected by uremia, but direct tissue interrogation has largely been lacking (9, 10). In one recent series, abdominal subcutaneous fat in patients with chronic kidney disease was found to have significantly up regulated gene expression of pro-inflammatory mediators such as interleukin 6 and down regulated gene expression of leptin and oxidative stress genes (9) compared to non-uremic controls, suggesting uremia-derived perturbation in local production and action of adipokines.
To advance understanding of the spectrum and determinants of human adipose tissue biology, we compared protein levels of key mediators isolated from subcutaneous adipose tissue from patients with and without uremia. Use of fresh, human clinical specimens from select anatomic locations offers insights into the variability and clinical determinants of human adipose tissue phenotypes. We hypothesized that there would be more variation between uremic and non-uremic patients than within these groups, and that patients with uremia would display a relatively higher pro-inflammatory adipokine signature. Finally, beyond comparisons between uremic and non-uremic patients, we also evaluated the impact of standard clinical parameters on adipose tissue phenotype.
MATERIALS AND METHODS
This prospective study consisted of a series of patients undergoing lower extremity major amputation (below knee or above knee), elective open orthopedic procedures, arteriovenous fistula creation for permanent hemodialysis access, or open plastic surgery procedures at Brigham and Women’s Hospital (Boston, MA, USA). Protocol approvals were obtained from the Partners Healthcare Institutional Review Board, and participating subjects all provided informed consent.
All samples were harvested from live patients intra-operatively by trained surgeons. Two grams of subcutaneous adipose tissue were collected from the site (where applicable, proximal end of amputation specimens to avoid confounding ischemia/infection). A portion of the sample was placed in formalin for standard fixation/paraffin embedding for histology, with the remainder being immediately flash frozen in liquid nitrogen, then stored at −80 degrees Celsius until the time of analysis. Proteins were isolated in ice-cold Dulbecco’s phosphate buffered saline with Protease Inhibitor Cocktail (Roche Applied Science, Indianapolis, IN), homogenized, and centrifuged (2,000g x 5 minutes) to remove gross debris. The homogenates were next centrifuged once more (10,000g x 10 minutes). The supernatant was then collected for quantitative protein analysis via multiple antigen flow microparticle bead assay Luminex (Luminex Corporation, Austin, TX) for levels of ten biologic mediators: IL-1β, IL-6, IL-8, leptin, TNF-α, MCP-1, adiponectin, resistin, PAI-1, and IL-10. For normalization purposes, protein was quantified via the Bradford assay.
Demographic and clinical data were collected from interview, examination and abstraction of medical records using a standardized instrument, per approved protocol. Study covariates included age, gender, race, diagnoses of hypertension, diabetes mellitus, hyperlipidemia, malignancy, renal disease, cerebrovascular disease, congestive heart failure, and coronary artery disease, as well as available measurements of hemoglobin A1c, body mass index (BMI), total cholesterol, high density lipoprotein, low density lipoprotein, creatinine, smoking history, and current medications. Coronary artery disease (CAD) was defined as a history of myocardial infarction, percutaneous coronary intervention or coronary artery bypass grafting or documentation of CAD by a cardiologist.
Estimated GFR (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation using the most recent pre-operative serum creatinine measurement. Uremia (term used here to denote advanced chronic kidney disease) was ascribed to subjects with eGFR < 20 mL/min/1.73m2 body surface area and to those on maintenance hemodialysis. Obesity was defined as body mass index (BMI) > 30 according to World Health Organization criteria.
All statistical analyses were performed with Microsoft Excel, GraphPad InStat or STATA 10.0MP. Continuous variables were expressed as means, standard deviations, medians, and interquartile ranges, as dictated by empiric distribution (i.e., normally or non-normally distributed); they were compared between groups using with the Mann-Whitney or the unpaired Student’s t-test. Categorical variables were expressed as counts and proportions and compared across groups using Fisher’s exact or Chi-square tests. Correlations were estimated using Spearman non-parametric correlation testing. Multiple regression analysis was performed for log transformed hormone levels; point estimates and confidence intervals were back transformed for presentation on the natural scale.
RESULTS
Cohort Demographics and Clinical Parameters
Characteristics of the study cohort are shown in Table 1. There were 17 uremic patients (10 and 7 who underwent vascular access and non-vascular access surgery, respectively) and 54 non-uremic patients (22, 21, 8 and 3 who underwent major amputation, orthopedic surgery, plastic surgery and vascular access surgery, respectively). In the overall cohort, mean age was 63.4 years; 33 (46.4%) had diabetes mellitus, 35 (49.2%) had hyperlipidemia and 38 (53.5%) had coronary artery disease. Compared to non-uremics, uremic patients are more likely to be non-caucasian, to have diabetes, CAD and to be on a beta blocker. Uremic patients had slightly lower mean hemoglobin A1c levels overall, but higher levels when consideration was limited to only diabetics within each group.
TABLE 1.
Demographic and Clinical Characteristics
| Total cohort (n=71) | Uremia (n=17) | No uremia (n=54) | P value | |
|---|---|---|---|---|
| Age (years) | 63.4 ± 15.9 | 63.8 ± 12.3 | 63.3 ± 17.0 | 0.89 |
| Male gender n (%) | 32 (54.9) | 5 (29.6) | 27 (50) | 0.22 |
| Caucasian n (%) | 42 (59.2) | 5 (29.4) | 37 (68.5) | 0.01 |
| Diabetes n (%) | 33 (46.4) | 12 (70.5) | 21 (38.9) | 0.04 |
| Hypertension n (%) | 52 (73.2) | 12 (70.5) | 40 (74.1) | 0.76 |
| Coronary artery disease n (%) | 38 (53.5) | 12 (70.5) | 16 (29.6) | 0.01 |
| Hyperlipidemia n (%) | 35 (49.2) | 10 (58.8) | 25 (46.3) | 0.53 |
| Current or former smoker n (%) | 34 (47.9) | 9 (52.9) | 25 (46.2) | 0.84 |
| Total cholesterol (mg/dL) | 158.0 ± 44.5 | 134.5 (128.8–164.8) | 163.4 ± 43.8 | 0.14 |
| HDL (mg/dL) | 47.3 ± 14.0 | 45.7 ± 12.1 | 48.1 ± 15.0 | 0.47 |
| LDL (mg/dL) | 85.4 ± 40.0 | 75.9 ± 46.3 | 90.3 ± 36.4 | 0.11 |
| Triglycerides (mg/dL) | 125.2 ± 57.7 | 126.0 ± 48.1 | 124.8 ± 62.7 | 0.60 |
| HgbA1c (%) | 6.4 (5.7–7.7) | 6.4 ± 1.6 | 6.9 (6.2–8.4) | 0.04 |
| HgbA1C (%) in diabetics only | 6.9 (5.9–9.1) | 8.0±1.9 | 5.9 (5.3–7.2) | 0.03 |
| BMI (kg/m2) | 29.6 ± 7.6 | 26.9 ± 5.9 | 29.1 ± 8.2 | 0.25 |
| BMI ≥ 30 n (%) | 27 (38.0) | 6 (35.2) | 21 (38.9) | 0.79 |
| ASA n (%) | 43 (60.6) | 11 (64.7) | 32 (59.3) | 0.91 |
| Beta blocker n (%) | 38 (53.5) | 15 (88.2) | 23 (42.6) | 0.01 |
| Statin n (%) | 40 (56.3) | 11 (64.7) | 29 (53.7) | 0.93 |
| Metformin n (%) | 7 (9.9) | 0 | 7 (13.0) | 0.18 |
| Glitazone n (%) | 0 | 0 | 0 | 0 |
| ACE inhibitor and/or ARB n (%) | 30 (42.2) | 6 (35.3) | 24 (44.4) | 0.58 |
Histology
Histologic evaluation of subcutaneous adipose tissue from uremic patients, in comparison to non-uremic patients, showed increased interlobar fibrosis, increased microvascular density, and increased vascular wall hypertrophy. There was also noted to be scattered fat necrosis in most specimens from uremic subjects, and adipocyte volume was subjectively lower in uremic subjects than in non-uremic subjects (Figure 1).
FIG. 1.
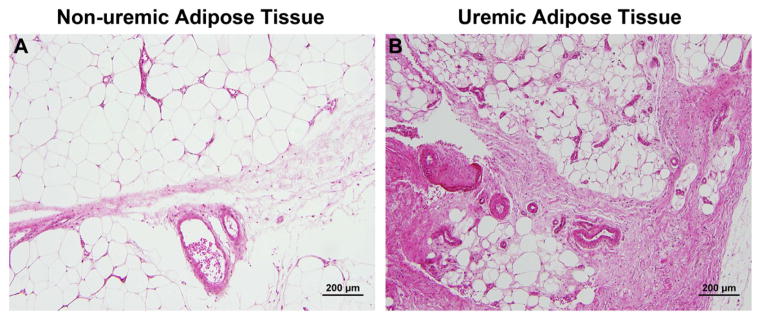
Representative hematoxylin and eosin stained microscopic images from non-uremic (panel A) and uremic (panel B) subjects are shown. Uremic subjects showed increased interlobar fibrosis, increased microvascular density, increased vascular wall hypertrophy, scattered fat necrosis, and lower adipocyte volumes as compared to non-uremic subjects. Scale bar = 200 μm.
Adipose tissue-derived inflammatory mediator profile
The distribution of adipose tissue-derived inflammatory mediators from subcutaneous fat in the total cohort is shown in Table 2. IL-1β was detectable in only 2 patients (one in the non-uremic group [42.2 pg/mg] undergoing amputation and one in the uremic group [0.8 pg/mg] undergoing vascular access creation). Due to assay difficulties, IL-10 was assessed in 54 (76%) patients; it was detectable in 24 (44%): median value was 0 pg/mg in both the uremic and non-uremic groups. Neither mediator was considered in subsequent analyses.
TABLE 2.
Adipose Tissue-Derived Mediators by Uremia
| Mediator (pg/mg) | Total Cohort (n=71) | Uremia (n=17) | No Uremia (n=54) | P value |
|---|---|---|---|---|
| IL-6 | 2.5 (0–7.0) | 2.1 (0–11.1) | 2.5 (0–6.2) | >0.90 |
| IL-8 | 2.0 (1.2–2.6) | 2.0 (0.6–2.4) | 2.1 (1.2–2.6) | 0.52 |
| Leptin | 922 (294–2473) | 155 (47–515) | 1,450 (445–2,905) | <0.001 |
| TNF-alpha | 0.5 (0.3–1.0) | 0.4 (0.3–0.8) | 0.6 (0.3–0.9) | 0.18 |
| MCP-1 | 52.7 (26.5–106.3) | 26.4 (12.3–100.2) | 53.5 (32.2–106.6) | 0.10 |
| Adiponectin | 31,060 (192,150–455,204) | 137,001 (85,019–272,364) | 335,068(242,732–475,587) | <0.001 |
| Resistin | 1,315 (685–2,426) | 2,563 (2,055–3,448) | 870 (621–2,068) | <0.001 |
| PAI-1 | 100.5 (59.1–158.2) | 100.5 (61.4–149.4) | 99.3 (58.1–162.9) | 0.96 |
Compared to non-uremics, uremic patients had significantly different adipose tissue mediator profiles: approximately one-tenth the adipose tissue levels of leptin, one-third the adipose tissue levels of adiponectin, and 3-fold higher adipose tissue levels of resistin (Table 2; Figure 2). Other demographic characteristics were associated with differential expression of these adipose tissue derived hormones (sex with leptin and adiponectin; CAD with adiponectin and resistin; amputation status with resistin; obesity with leptin; age and diabetes were not differentially associated with any) however, the pattern of association between uremia and these hormones was preserved qualitatively and in terms of statistical significance upon stratified analyses (Supplementary Tables 1–6).
FIG. 2.
Comparison of leptin, adiponectin and resistin levels between uremic and non-uremic patients.
Correlations among the mediators
A summary of the correlations among leptin, adiponectin and resistin is shown in Table 3. Adiponectin and leptin correlated positively. Both adiponectin and resistin correlated negatively but only the correlation between leptin and resistin achieved statistical significance.
TABLE 3.
Correlation Analysis between Mediators in Entire Cohort
| Spearman r | 95% Confidence Interval | P value | |
|---|---|---|---|
| Adiponectin vs Resistin | −0.19 | −0.41 to 0.06 | 0.12 |
| Adiponectin vs Leptin | 0.59 | 0.41 to 0.73 | <0.001 |
| Leptin vs Resistin | −0.28 | −0.49 to −0.04 | 0.02 |
Sensitivity analyses
Because the non-uremic group included 8 specimens taken from the breast, and because source site may influence adipose tissue metabolic profiles, we conducted sensitivity analysis excluding breast specimens from the control group. The pattern of association between eGFR and leptin, adiponectin and resistin was quantitatively and qualitatively similar to that seen in the initial analysis (Table 4). In subanalysis, we also compared breast fat to extremity fat among the non-uremic group and found that breast fat had significantly lower levels of MCP-1 and higher levels of resistin (Table 4).
TABLE 4.
Adipose Tissue-Derived Mediator Profile by Uremia and Adipose Depots
| Mediator (pg/mg) | Sensitivity Analysis (Extremity Adipose Tissue)
|
P value | Sensitivity analysis (Extremity vs Breast Adipose Tissue in Non-uremic Patients)
|
P value | ||
|---|---|---|---|---|---|---|
| Uremia (n=17) | Non-uremia (n=46) | Extremity Fat (n=46) | Breast Fat (n=8) | |||
| IL-6 | 2.1 (0–11.1) | 2.6 (0–7.4) | 0.99 | 2.6 (0–7.4) | 2.0 (1.5–2.6) | 0.76 |
| IL-8 | 2.0 (0.7–2.4) | 1.9 (1.2–2.7) | 0.68 | 1.9 (1.2–2.7) | 2.4 (2.1–2.7) | 0.26 |
| Leptin | 155 (47–515) | 1,372 (406–4,054) | <0.001 | 1,372 (406–4,054) | 1,635 (1,221–2,301) | 0.69 |
| TNF-alpha | 0.4 (0.3–0.8) | 0.6 (0.4–0.9) | 0.17 | 0.6 (0.4–0.9) | 0.8 (0.4–1.0) | 0.74 |
| MCP-1 | 26.4 (12.3–100.2) | 65.7 (36.8–158.0) | 0.06 | 65.7 (36.8–158.0) | 30.6 (17.8–37.0) | 0.01 |
| Adiponectin | 137,001(85,019–272,364) | 329,345 (240,588–471,108) | <0.001 | 329,345 (240,588–471,108) | 412,001 (296,999–610,579) | 0.29 |
| Resistin | 2,563 (2,055–3,448) | 809 (531–1,797) | <0.001 | 809 (531–1,797) | 1,639 (1,051–2,265) | 0.04 |
| PAI-1 | 100.5 (61.4–149.4) | 99.3 (50.2–149.1) | 0.87 | 99.3 (50.2–149.1) | 94.0 (75.8–243.1) | 0.72 |
To examine the robustness the associations between uremia and adiponectin, leptin and resistin, we considered a series of multiple regression models in which the association was adjusted separately for age, sex, diabetes, CAD, CLI and obesity. In addition, we fit a “best model” for each response parameter in which we included all covariates that were associated with the hormone of interest in bivariable testing. Reassuringly, we observed a concordant pattern of association (in terms of magnitude and significance) between uremia and each adipose tissue derived hormone (Table 5).
TABLE 5.
Beta Coefficient (95% CI) for Association between Uremia and Adipose Tissue Derived Hormones
| Unadjusted | Adjusted for
|
Best model | ||||||
|---|---|---|---|---|---|---|---|---|
| age | gender | diabetes | CAD | amputation | obesity | |||
| Ln (Lepin) | −2 (−2.7 to −1.3) | −2 (−2.0 to 1.3) | −1.8 (−2.4 to −1.1) | −2 (−2.8 to −1.3) | −1.9 (−2.7 to −1.1) | −2 (−2.7 to −1.3) | −1.9 (−2.6 to −1.3) | −1.7 (−2.4 to −1.1) |
| P<0.001 | P<0.001 | P<0.001 | P<0.001 | P<0.001 | P<0.001 | P<0.01 | P<0.01 Adjusted for gender, CAD, obesity |
|
| Ln (Adiponectin) | −0.8 (−1.2 to −0.5) | −0.8 (−1.2 to −0.5) | −0.07 0 |
−0.8 (−1.2 to −0.4) | −0.7 (−1.1 to −0.3) | −0.9 (−1.2 to −0.5) | −0.9 (−1.2 to −0.5) | −0.7 (−1.1 to −0.3) |
| P<0.001 | P<0.001 | P<0.001 | P<0.001 | P=0.001 | P<0.001 | P<0.001 | P<0.001 Adjusted for gender, CAD |
|
| Ln (Resistin) | 1 (0.5 to 1.5) | 1 (0.5 to 1.5) | 1 (0.5 to 1.5) | 1 (0.5 to 1.5) | 0.8 (0.3 to 1.3) | 1 (0.5 to 1.4) | 1 (0.5 to 1.4) | 0.8 (0.3 to 1.3) |
| P<0.001 | P<0.001 | P<0.001 | P<0.001 | P=0.002 | P<0.001 | P<0.001 | P=0.002 Adjusted for CAD, amputation, Oesity |
|
DISCUSSION
This is a prospective study of the subcutaneous adipose tissue protein levels of core biologic mediators in surgical patients with and without advanced chronic kidney disease. Based on direct open biopsy of living human adipose tissue, we find that there are statistically significant differential protein levels of adiponectin, leptin and resistin in uremic patients compared to controls. Relative to other clinical parameters including BMI, uremia dominated overall in determining adipose tissue phenotype.
Adipocyte derived hormones, such as leptin, adiponectin, and resistin are renally cleared. Impaired renal function leads to progressive accumulation of nitrogenous wastes, resulting in the uremic state. The uremic milieu is an environment of increased oxidative stress that activates phagocytes and leads to increased production of reactive oxygen species and pro-inflammatory cytokines(22). Accordingly, C-reactive protein (CRP) is elevated in dialysis patients and is predictive of worse cardiovascular outcomes(23). Uremia dramatically impacts overall health status (15, 16, 24). The increased burden of accelerated atherosclerosis(25) and chronic inflammation(26) with resultant cardiovascular morbidity is seen even with partial decline of renal function(24, 27–29).
The level of renal function decline is delineated as the amount of filtrate in mL/min/1.73m2 body surface area, calculated using the serum creatinine. While eGFR< 15mL/min/1.73m2 is the cut off for end stage renal disease (ESRD), for this study, we selected eGFR eGFR < 20 mL/min/1.73m2 or dialysis dependent as the surrogate for uremia, since patients with eGFR between 15 and 20 are near to requiring renal replacement and functionally have similar uremic milieu. Not unexpectedly, our uremic cohort had a higher incidence of diabetes and coronary artery disease, representative of ESRD patients, and had a higher percentage of African American compared to control, but was otherwise similar to the non-uremic group in demographics and medical history.
Published studies have shown that circulating adiponectin is approximately two-fold higher in hemodialysis patients and correlates inversely with C-reactive protein levels and body mass index(17, 30). Interestingly, our study found that uremic patients have lower subcutaneous adipose tissue adiponectin (which was independent of BMI). Since adiponectin is solely synthesized in adipose tissue, elevated circulating levels are likely due to decreased renal clearance. Serum concentrations of leptin and resistin have also been reported to be higher in patients with chronic kidney disease(18–20) and the levels of resistin found in uremic patients may directly inhibit neutrophil function(31). Here we report lower adipose tissue leptin with uremia, though elevated adipose tissue resistin. Again, the circulating leptin is likely elevated due to decreased clearance.
Finally, our dataset cannot discern the relative contributions of increased production versus decreased clearance with respect to elevated circulating resistin in uremia. Finally, in a recent report subcutaneous fat in patients with Stage 5 CKD (compared to non-uremic controls) displayed upregulation of pro-inflammatory pathway genes such as IL-6 and down-regulation of leptin and oxidative stress genes(9). Our results confirm via protein analyses this leptin down-regulation in adipose tissue(18). Like Teplan et al (10) (who studied obese Stage 3–4 CKD patients) we did not observe increased IL-6 expression with uremia. The subtle discrepancies among these prior reports and our data may be related to differences in the patient cohorts. It should also be emphasized that our work is based on actual protein levels rather than RNA dynamics (9, 10).
Adiponectin, leptin and resistin are all adipose tissue derived hormones. Adiponectin is synthesized in adipocytes, secreted into the blood, and is inversely associated with insulin resistance, risk of diabetes mellitus, and obesity. Sex and age-adjusted adiponectin plasma levels are inversely correlated with other inflammatory markers such as CRP and IL-6 independent of obesity(32). One can speculate that the relatively attenuated adiponectin expression in uremic human adipose tissue may drive a low grade “adiposopathy”(33, 34) and may underlie increased cardiovascular disease rates associated with uremia(14–16, 24, 25, 27–29). Leptin affects the hypothalamus to regulate body weight, fat deposition and blood pressure. Plasma levels are elevated in chronic kidney disease (18), probably due to reduced renal clearance of leptin(35). Weight loss in dialysis patients likely relates at least in part to a leptin-stimulated increase in metabolic rate and oxygen consumption(36, 37). Finally, resistin mediates insulin resistance in animal models, but in humans, plasma resistin levels correlate with decreasing eGFR, age and decreasing leptin levels but not glucose or plasma insulin(38).
The correlation between adipose tissue levels of adiponectin, leptin and resistin with uremia were independent of age, diabetes, or BMI, but both leptin and adiponectin were higher in females. Patients with a history of coronary artery disease had lower adipose tissue levels of leptin and adiponectin independent of uremia. The opposite pattern was seen in an observational cohort study looking at plasma leptin and coronary events (39). The correlation between plasma adiponectin and cardiovascular risk profile (i.e. is higher or lower more favorable) in non-uremics remains controversial (40–44). Regardless, even after controlling for the common cardiovascular comorbidities of diabetes, CAD and obesity, uremia remained the strongest predictor of adipose tissue levels of adiponectin, leptin and resistin in our study.
Our report is limited by the small sample size; as such we were not powered to detect differences that were modest in size. We caution against drawing conclusions about these associations until a larger study is undertaken. Additionally, we highlight relative protein levels, but cannot make mechanistic claims about biologic activity or causality. It is certainly possible that derangements in adipose biology could drive renal dysfunction. The cellular origin of the proteins is not delineated, nor is the balance of synthesis/degradation, but the results do paint a general picture of the overall adipose tissue phenotype for clinically relevant parameters. Our specific dataset does not have visceral adipose tissue or serum for relative comparisons. However, these have been widely described in the literature (17–20, 30).
In summary, utilizing human clinical tissues, we found that advanced chronic kidney disease is associated with perturbations in the adipose tissue derived hormones leptin, adiponectin, and resistin. Interestingly, we observed that adipose tissue levels of adiponectin and leptin, in contrast to reported plasma levels, were significantly lower in uremic patients. We report an inverse correlation between adipose tissue levels of resistin and renal function, which has also been observed with serum levels. Compared with other clinical parameters including BMI, uremia dominated overall in determining adipose tissue phenotype, which points to a potential biological effect of uremia on these three adipose derived hormones at the tissue level. Future studies will involve ascertainment of receptor levels and activity in adipose tissue, alteration in gene and protein expression of both adipokines and receptors in models of uremia, elucidation of the biological pathways between uremia and adipokine expression, and development of modulators in these signaling networks.
Supplementary Material
Supplemental Table 1. Comparison of leptin, adiponectin and resistin levels in patient cohort stratified by age.
Supplemental Table 2. Comparison of leptin, adiponectin and resistin levels in patient cohort stratified by gender.
Supplemental Table 3. Comparison of leptin, adiponectin and resistin levels in patient cohort stratified by diagnosis of diabetes mellitus.
Supplemental Table 4. Comparison of leptin, adiponectin and resistin levels in patient cohort stratified by diagnosis of coronary artery disease.
Supplemental Table 5. Comparison of leptin, adiponectin and resistin levels in patient cohort stratified by amputation status.
Supplemental Table 6. Comparison of leptin, adiponectin and resistin levels in patient cohort stratified by obesity.
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute T32HL007734, American Heart Association 12GRNT9510001, and the Carl and Ruth Shapiro Family Foundation.
ABBREVIATIONS
- ACE inhibitor
angiotensin converting enzyme inhibitor
- ARB
angiotensin receptor blocker
- ASA
aspirin
- BMI
body mass index
- HDL
high density lipoprotein
- HgbA1c
hemoglobin A1c
- IL-6
interleukin 6
- IL-8
interleukin 8
- LDL
low density lipoprotein
- MCP-1
monocyte chemotactic protein-1
- PAI-1
plasminogen activator inhibitor-1
- TNF-alpha
tumor necrosis factor alpha
Footnotes
Conflict of interest statement: None of the authors hold any commercial affiliations (including consultancies, stock or equity interests, and patent-licensing arrangements) that are a conflict of interest.
STATEMENT OF AUTHOR CONTRIBUTIONS
KJH—experimental design, data analyses,
HX—experimental design, data acquisition, data analyses
CM—experimental design, data acquisition
BN—experimental design, data acquisition
PY—experimental design, data acquisition
MT—experimental design, data acquisition, data analyses
MA—data acquisition, data analyses
SM—experimental design, data analyses
CKO—experimental design, data acquisition, securing funding
All authors participated in writing the manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chaldakov GN, Stankulov IS, Hristova M, Ghenev PI. Adipobiology of disease: adipokines and adipokine-targeted pharmacology. Current pharmaceutical design. 2003;9:1023–1031. doi: 10.2174/1381612033455152. [DOI] [PubMed] [Google Scholar]
- 2.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. The Journal of clinical investigation. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Molecular and cellular endocrinology. 2010;316:129–139. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Bluher M. The distinction of metabolically ‘healthy’ from ‘unhealthy’ obese individuals. Current opinion in lipidology. 2010;21:38–43. doi: 10.1097/MOL.0b013e3283346ccc. [DOI] [PubMed] [Google Scholar]
- 5.Jackson RS, Black JH, 3rd, Lum YW, Schneider EB, Freischlag JA, et al. Class I obesity is paradoxically associated with decreased risk of postoperative stroke after carotid endarterectomy. J Vasc Surg. 2012;55:1306–1312. doi: 10.1016/j.jvs.2011.11.135. [DOI] [PubMed] [Google Scholar]
- 6.Vela D, Buja LM, Madjid M, Burke A, Naghavi M, et al. The role of periadventitial fat in atherosclerosis. Archives of pathology & laboratory medicine. 2007;131:481–487. doi: 10.5858/2007-131-481-TROPFI. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, et al. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circulation research. 2009;104:541–549. doi: 10.1161/CIRCRESAHA.108.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takaoka M, Suzuki H, Shioda S, Sekikawa K, Saito Y, et al. Endovascular injury induces rapid phenotypic changes in perivascular adipose tissue. Arterioscler Thromb Vasc Biol. 2010;30:1576–1582. doi: 10.1161/ATVBAHA.110.207175. [DOI] [PubMed] [Google Scholar]
- 9.Witasp A, Carrero JJ, Heimburger O, Lindholm B, Hammarqvist F, et al. Increased expression of pro-inflammatory genes in abdominal subcutaneous fat in advanced chronic kidney disease patients. Journal of internal medicine. 2011;269:410–419. doi: 10.1111/j.1365-2796.2010.02293.x. [DOI] [PubMed] [Google Scholar]
- 10.Teplan V, Jr, Vyhnanek F, Gurlich R, Haluzik M, Racek J, et al. Increased proinflammatory cytokine production in adipose tissue of obese patients with chronic kidney disease. Wiener klinische Wochenschrift. 2010;122:466–473. doi: 10.1007/s00508-010-1409-y. [DOI] [PubMed] [Google Scholar]
- 11.Pachler C, Ikeoka D, Plank J, Weinhandl H, Suppan M, et al. Subcutaneous adipose tissue exerts proinflammatory cytokines after minimal trauma in humans. Am J Physiol Endocrinol Metab. 2007;293:E690–696. doi: 10.1152/ajpendo.00034.2007. [DOI] [PubMed] [Google Scholar]
- 12.Ikeoka DT, Pachler C, Mader JK, Bock G, Neves AL, et al. Lipid-Heparin Infusion Suppresses the IL-10 Response to Trauma in Subcutaneous Adipose Tissue in Humans. Obesity (Silver Spring, Md. 2011;19:715–721. doi: 10.1038/oby.2010.227. [DOI] [PubMed] [Google Scholar]
- 13.Bremer AA, Devaraj S, Afify A, Jialal I. Adipose tissue dysregulation in patients with metabolic syndrome. The Journal of clinical endocrinology and metabolism. 2011;96:E1782–1788. doi: 10.1210/jc.2011-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 15.Foley RN. Clinical epidemiology of cardiac disease in dialysis patients: left ventricular hypertrophy, ischemic heart disease, and cardiac failure. Seminars in dialysis. 2003;16:111–117. doi: 10.1046/j.1525-139x.2003.160271.x. [DOI] [PubMed] [Google Scholar]
- 16.Baigent C, Burbury K, Wheeler D. Premature cardiovascular disease in chronic renal failure. Lancet. 2000;356:147–152. doi: 10.1016/S0140-6736(00)02456-9. [DOI] [PubMed] [Google Scholar]
- 17.Rao M, Li L, Tighiouart H, Jaber BL, Pereira BJ, et al. Plasma adiponectin levels and clinical outcomes among haemodialysis patients. Nephrol Dial Transplant. 2008;23:2619–2628. doi: 10.1093/ndt/gfn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menon V, Wang X, Greene T, Beck GJ, Kusek JW, et al. Factors associated with serum leptin in patients with chronic kidney disease. Clinical nephrology. 2004;61:163–169. doi: 10.5414/cnp61163. [DOI] [PubMed] [Google Scholar]
- 19.Malyszko J, Malyszko JS, Kozminski P, Pawlak K, Mysliwiec M. Elevated resistin is related to inflammation and residual renal function in haemodialysed patients. Nephrology (Carlton, Vic. 2007;12:246–253. doi: 10.1111/j.1440-1797.2007.00782.x. [DOI] [PubMed] [Google Scholar]
- 20.Nusken KD, Kratzsch J, Wienholz V, Stohr W, Rascher W, et al. Circulating resistin concentrations in children depend on renal function. Nephrol Dial Transplant. 2006;21:107–112. doi: 10.1093/ndt/gfi084. [DOI] [PubMed] [Google Scholar]
- 21.Pilz S, Weihrauch G, Seelhorst U, Wellnitz B, Winkelmann BR, et al. Implications of resistin plasma levels in subjects undergoing coronary angiography. Clinical endocrinology. 2007;66:380–386. doi: 10.1111/j.1365-2265.2007.02743.x. [DOI] [PubMed] [Google Scholar]
- 22.Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney international. 2002;62:1524–1538. doi: 10.1046/j.1523-1755.2002.00600.x. [DOI] [PubMed] [Google Scholar]
- 23.Arici M, Walls J. End-stage renal disease, atherosclerosis, and cardiovascular mortality: is C-reactive protein the missing link? Kidney international. 2001;59:407–414. doi: 10.1046/j.1523-1755.2001.059002407.x. [DOI] [PubMed] [Google Scholar]
- 24.Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9:S16–23. [PubMed] [Google Scholar]
- 25.McCullough PA, Agrawal V, Danielewicz E, Abela GS. Accelerated atherosclerotic calcification and Monckeberg’s sclerosis: a continuum of advanced vascular pathology in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1585–1598. doi: 10.2215/CJN.01930408. [DOI] [PubMed] [Google Scholar]
- 26.Swaminathan S, Shah SV. Novel inflammatory mechanisms of accelerated atherosclerosis in kidney disease. Kidney international. 2011;80:453–463. doi: 10.1038/ki.2011.178. [DOI] [PubMed] [Google Scholar]
- 27.Lindner A, Charra B, Sherrard DJ, Scribner BH. Accelerated atherosclerosis in prolonged maintenance hemodialysis. The New England journal of medicine. 1974;290:697–701. doi: 10.1056/NEJM197403282901301. [DOI] [PubMed] [Google Scholar]
- 28.Snyder JJ, Foley RN, Gilbertson DT, Vonesh EF, Collins AJ. Body size and outcomes on peritoneal dialysis in the United States. Kidney international. 2003;64:1838–1844. doi: 10.1046/j.1523-1755.2003.00287.x. [DOI] [PubMed] [Google Scholar]
- 29.Drey N, Roderick P, Mullee M, Rogerson M. A population-based study of the incidence and outcomes of diagnosed chronic kidney disease. Am J Kidney Dis. 2003;42:677–684. doi: 10.1016/s0272-6386(03)00916-8. [DOI] [PubMed] [Google Scholar]
- 30.Chudek J, Adamczak M, Karkoszka H, Budzinski G, Ignacy W, et al. Plasma adiponectin concentration before and after successful kidney transplantation. Transplant Proc. 2003;35:2186–2189. doi: 10.1016/j.transproceed.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Cohen G, Horl WH. Resistin as a cardiovascular and atherosclerotic risk factor and uremic toxin. Seminars in dialysis. 2009;22:373–377. doi: 10.1111/j.1525-139X.2009.00583.x. [DOI] [PubMed] [Google Scholar]
- 32.Hung J, McQuillan BM, Thompson PL, Beilby JP. Circulating adiponectin levels associate with inflammatory markers, insulin resistance and metabolic syndrome independent of obesity. International journal of obesity (2005) 2008;32:772–779. doi: 10.1038/sj.ijo.0803793. [DOI] [PubMed] [Google Scholar]
- 33.Bays H, Abate N, Chandalia M. Adiposopathy: sick fat causes high blood sugar, high blood pressure and dyslipidemia. Future cardiology. 2005;1:39–59. doi: 10.1517/14796678.1.1.39. [DOI] [PubMed] [Google Scholar]
- 34.Bays HE. Adiposopathy is “sick fat” a cardiovascular disease? Journal of the American College of Cardiology. 2011;57:2461–2473. doi: 10.1016/j.jacc.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 35.Cumin F, Baum HP, de Gasparo M, Levens N. Removal of endogenous leptin from the circulation by the kidney. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 1997;21:495–504. doi: 10.1038/sj.ijo.0800428. [DOI] [PubMed] [Google Scholar]
- 36.Mak RH, Cheung W, Cone RD, Marks DL. Leptin and inflammation-associated cachexia in chronic kidney disease. Kidney international. 2006;69:794–797. doi: 10.1038/sj.ki.5000182. [DOI] [PubMed] [Google Scholar]
- 37.Cheung W, Yu PX, Little BM, Cone RD, Marks DL, et al. Role of leptin and melanocortin signaling in uremia-associated cachexia. The Journal of clinical investigation. 2005;115:1659–1665. doi: 10.1172/JCI22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kielstein JT, Becker B, Graf S, Brabant G, Haller H, et al. Increased resistin blood levels are not associated with insulin resistance in patients with renal disease. Am J Kidney Dis. 2003;42:62–66. doi: 10.1016/s0272-6386(03)00409-8. [DOI] [PubMed] [Google Scholar]
- 39.Wallace AM, McMahon AD, Packard CJ, Kelly A, Shepherd J, et al. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS) Circulation. 2001;104:3052–3056. doi: 10.1161/hc5001.101061. [DOI] [PubMed] [Google Scholar]
- 40.Matsushita K, Yatsuya H, Tamakoshi K, Wada K, Otsuka R, et al. Comparison of circulating adiponectin and proinflammatory markers regarding their association with metabolic syndrome in Japanese men. Arterioscler Thromb Vasc Biol. 2006;26:871–876. doi: 10.1161/01.ATV.0000208363.85388.8f. [DOI] [PubMed] [Google Scholar]
- 41.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, et al. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 42.Schulze MB, Shai I, Rimm EB, Li T, Rifai N, et al. Adiponectin and future coronary heart disease events among men with type 2 diabetes. Diabetes. 2005;54:534–539. doi: 10.2337/diabetes.54.2.534. [DOI] [PubMed] [Google Scholar]
- 43.Cavusoglu E, Ruwende C, Chopra V, Yanamadala S, Eng C, et al. Adiponectin is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction in patients presenting with chest pain. European heart journal. 2006;27:2300–2309. doi: 10.1093/eurheartj/ehl153. [DOI] [PubMed] [Google Scholar]
- 44.Pilz S, Mangge H, Wellnitz B, Seelhorst U, Winkelmann BR, et al. Adiponectin and mortality in patients undergoing coronary angiography. The Journal of clinical endocrinology and metabolism. 2006;91:4277–4286. doi: 10.1210/jc.2006-0836. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Comparison of leptin, adiponectin and resistin levels in patient cohort stratified by age.
Supplemental Table 2. Comparison of leptin, adiponectin and resistin levels in patient cohort stratified by gender.
Supplemental Table 3. Comparison of leptin, adiponectin and resistin levels in patient cohort stratified by diagnosis of diabetes mellitus.
Supplemental Table 4. Comparison of leptin, adiponectin and resistin levels in patient cohort stratified by diagnosis of coronary artery disease.
Supplemental Table 5. Comparison of leptin, adiponectin and resistin levels in patient cohort stratified by amputation status.
Supplemental Table 6. Comparison of leptin, adiponectin and resistin levels in patient cohort stratified by obesity.