Abstract
Multiple abnormalities in pain processing have been reported in patients with chronic musculoskeletal pain syndromes. These changes include mechanical and thermal hyperalgesia, decreased thresholds to mechanical and thermal stimuli (allodynia), and central sensitization, all of which are fundamental to the generation of clinical pain. Therefore, we hypothesized that quantitative sensory tests may provide useful predictors of clinical pain intensity of such patients. Our previous studies of fibromyalgia (FM) patients have shown statistically significant correlations of quantitative sensory test results with clinical pain intensity, including mechanical spatial summation, number of pain areas, wind-up, and wind-up aftersensations. Although these tests predicted up to 59% of the variance in FM clinical pain intensity, their expense and technical complexities limited widespread use in clinical practice and trials. Thus, we developed practical tests of primary (mechanical) and secondary (heat) hyperalgesia that also strongly predict clinical pain intensity in patients with chronic musculoskeletal pain disorders. Thirty-six individuals with FM, 24 with local musculoskeletal pain, and 23 normal controls underwent testing of mechanical and heat hyperalgesia at the shoulders and hands. All subjects rated experimental pains using an electronic visual analog scale. Using either heat or pressure pain ratings as well as tender point counts and negative affect as predictors, up to 49.4% of the patients' variance of clinical pain intensity could be estimated. Results of this study emphasize the important contributions of peripheral and central factors to both local and widespread chronic pain. Overall, measures of mechanical and heat hyperalgesia in combination with tender point and negative affect provided powerful predictors of clinical pain intensity in chronic musculoskeletal pain patients that can be readily used in clinical practice and trials.
Perspective
Simple tests of mechanical and heat hyperalgesia can predict large proportions of the variance in clinical pain intensity of chronic musculoskeletal pain patients and thus are feasible to be included in clinical practice and clinical trials.
Keywords: Mechanical, heat, hyperalgesia, tonic, fibromyalgia, back pain, chronic pain
Characteristic symptoms of chronic musculoskeletal pain include widespread pain, fatigue, and distress.28,69 The pain is most consistently described as dull and aching and appears to be related to deep tissue structures. Accordingly, many patients present with tenderness to mechanical stimulation of deep tissues3,8,24,42,57 that can be evaluated by pressure algometry.34,37,43,44 In addition, many chronic musculoskeletal pain patients have been found to be hyperalgesic to heat, cold, and electrical stimuli,15,24,31 and they frequently complain of fatigue, insomnia, and emotional distress.29,30,58,72,73 Tender point (TP) counts have been used to characterize chronic widespread pain sufferers,70,75 specifically fibromyalgia (FM) patients. TPs, however, seem to reflect not only tenderness but more importantly psychological distress in these patients.73
Peripheral and central abnormalities of nociception have been described in musculoskeletal pain patients.8,24,25,45 Important nociceptive systems in the skin and deep tissues of these patients seem to undergo profound changes, resulting in sensitization of the hyperpolarization-activated cyclic nucleotide-gated ion channels,18 transient receptor potential channels, acid-sensing ion channel receptors, and purinoreceptors.2 Tissue mediators of inflammation and nerve growth factors can excite these receptors and cause extensive changes in pain sensitivity.16 However, the exact contributions of these specific factors to overall peripheral mechanisms of musculoskeletal pain remain uncertain.
Positive associations between pain sensitivity and clinical pain intensity have been reported within groups of musculoskeletal pain patients.38 For example, low pressure pain thresholds were found to be associated with back pain intensity and deterioration of physical functioning among patients with chronic low back pain.10 Using trigger point manipulation at the shoulder of FM patients, investigators reported good correlations between referred pain areas and clinical pain (r2 = .36).20 However, many studies found that pain threshold measurements do not reliably predict clinical pain intensity in chronic musculoskeletal pain patients5,14; that is, cervical pressure pain thresholds (PPTs) and neck pain scores were only weakly correlated with clinical pain intensity (r = −.20 to −.33)35 in patients with whiplash-associated disorder, and there was no significant correlation between tibialis anterior PPT and pain score (r = −.01 to −.21).
We have previously shown that psychophysical measurements, including number of painful body areas,63 mechanical spatial summation aftersensations,62 and wind-up (WU) aftersensations,64 individually predicted up to 64% of the variance of FM patients' clinical pain. Most of these tests require special equipment and/or considerable expertise, which may have contributed to their lack of wide acceptance in clinical practice and clinical trials. Thus, we developed quantitative sensory tests of mechanical and heat hyperalgesia that can be readily made available to researchers and clinicians alike as reliable predictors of clinical pain intensity. We hypothesized that measures of mechanical and heat hyperalgesia would reflect relevant factors of peripheral and central pain processing and thus clinical pain. Accordingly, mechanical hyperalgesia and heat hyperalgesia were tested at body locations proximal (shoulders) and distal (hands) to spontaneously painful sites in musculoskeletal pain patients. In addition, we assessed negative affect, which has previously shown moderate correlations with clinical pain.56 We tested our hypotheses in patients with chronic widespread pain (FM) and local (neck and shoulder) musculoskeletal pain (LMP) disorders as well as normal pain-free controls (NCs).
Methods
The University of Florida Institutional Review Board approved all procedures described in this report. Informed consent was obtained from all subjects, and the study protocol conformed to the ethical guidelines of the Declaration of Helsinki.
Study Subjects
NC and LMP participants came from the local community. They had received information about the study from print or TV advertisements. FM subjects were recruited at the Health Science Center Outpatient Clinics and from FM support groups. Use of analgesics, including nonsteroidal anti-inflammatory drugs, tramadol, and acetaminophen, was not allowed during the study. No subject was taking narcotic analgesics during the trial. Medications needed for treatment of chronic medical conditions such as hypertension or hypothyroidism were permitted during the study.
Inclusion and Exclusion Criteria
Inclusion criteria for participants were 1) adults over the age of 18; 2) the ability to give informed consent; 3) NC subjects had to be healthy and pain free; 4) widespread pain patients had to fulfill the 1990 American College of Rheumatology (ACR) Criteria for FM75; and 5) LMP subjects had to have >3 months of localized chronic neck/shoulder pain. Exclusion criteria were 1) a relevant medical condition besides musculoskeletal pain; 2) current participation in another research protocol that could interfere or influence the outcome measures of the present study; 3) the inability to give informed consent; 4) current use of analgesic drugs, anxiolytic drugs, antidepressants except amitriptyline, flexeril, or trazodone (all ≤10 mg per day), or cough suppressants. All subjects taking analgesic drugs or antidepressants before enrollment went through a wash-out phase of 5 drug half-lives prior to study entry.
Ratings of Clinical Pain
A mechanical visual analog scale (VAS) (0–100) was used to rate somatic pain at the beginning and end of the experiments.48 Although the NC subjects were required to be pain free at enrollment, they were asked to rate any somatic pains before and after testing sessions to capture possible new-onset symptoms such as back pain and headaches.
Electronic VAS Used for Ratings of Experimental Pain
An electronic VAS (e-VAS) was used to rate experimental pain ranging from 0 to 100. Details and characteristics of this scale have been previously published.52 Briefly, the e-VAS is an electronic pain scale that is displayed on 2 separate 19-inch monitors, with only 1 display visible to the subject. This monitor depicts a horizontal black bar anchored on the left by “no pain at all” and on the right by “the most intense pain imaginable.”48 Turning a dial moves a second (red) bar across the black bar from left to right indicating the intensity of the sensation. On the second monitor, visible only to the investigator, this rating is displayed numerically between 0 and 100. All subjects were instructed to use the e-VAS to rate painful sensations continuously as well as for 1 minute after termination of the experimental pain stimulus (to rate aftersensations). During the experiments each subject's ratings was sampled at a frequency of 20 Hz.
Experimental Design
For the experiments we designed tonic mechanical and heat stimuli that slowly increased from baseline to a plateau phase resulting in preferential C-fiber activation47,49,50 (Fig 1). Mechanical measures were chosen because their pain ratings frequently reflect a combination of primary and secondary hyperalgesia.65 In contrast, pain ratings of heat stimuli are more exclusive measures of secondary hyperalgesia.60 We selected quantitative sensory testing (QST) sites at the shoulders and hands for several reasons: 1) the shoulders were chosen because most patients with FM or neck pain complain of shoulder pain (primary hyperalgesia); 2) in contrast, few FM and neck pain patients report painful hands, indicating their hand hyperalgesia as secondary. Prior to the experimental stimuli all subjects underwent a brief training period to familiarize themselves with the e-VAS. Subsequently they received 6 mechanical and 6 heat stimuli to the shoulders and hands in counterbalanced fashion while comfortably seated. Shoulder stimuli (mechanical and heat) were always applied to the center of the shoulders (midpoint between the cervical spine and acromion on the trapezius muscle), whereas painful pressure to the hands was placed on the dorsal webspace between the first and second fingers. All subjects received heat stimuli to the hands at the center of the thenar eminence. The interval between each experimental stimulus was at least 1 minute or until all painful aftersensations had disappeared. During testing, the subjects were asked to continuously use the e-VAS to rate pain intensity.
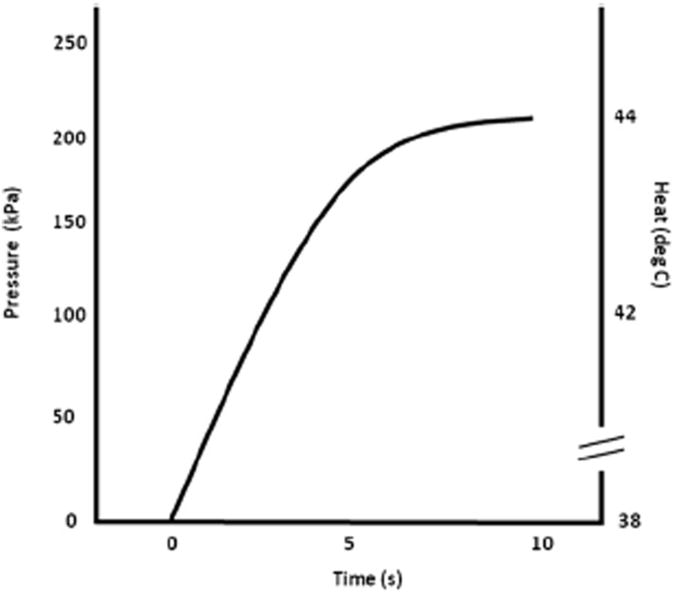
Figure 1.
Time course of pressure and heat pulses used at the shoulders and hands. The solid line represents the time course of either 200 kPa pressure or 44°C heat pulses. Duration of all experimental pain stimuli was 10 seconds. Pressure increased from baseline (0 kPa) to peak levels (200 kPa) in 6 seconds. Subsequently, they remained at peak levels for 4 seconds. Similarly heat pulses increased from baseline (38°C) to peak levels (44°C) in 6 seconds and remained at peak levels for 4 seconds.
Thermal Probe
A Peltier thermode with a contact surface of 3 × 3 cm (9 cm2) (TSA-2001; Medoc Advanced Medical Systems, Ramat Yishai, Israel) was used for the heat stimuli. For heat pain testing the probe was brought into firm contact with the skin of the trapezius or thenar eminence for 10 seconds.
Mechanical Probe
A calibrated electronic algometer (Somedic AB, Horby, Sweden) was utilized forthe pressure stimuli. The rubber tip of the algometer was 1 cm in diameter. The algometer has an electronic display showing the pressure (kPa) as well as the pressure changes applied to the subjects.
Mechanical Pain Stimuli
Six 10-second pressure stimuli were applied in counterbalanced order to the center of the shoulders (trapezius muscle) and the web space between first and second fingers (adductor pollicis muscle) of both hands. After the algometer was placed on the target area (shoulder or hand), pressure was gradually increased to 200 and 400 kPa in all subjects, respectively. The pressure increase varied (33 kPa/second for 200 kPa and 67 kPa/second for 400 kPa stimuli) to reach peak pressure within 6 seconds. Subsequently, peak pressure was maintained for 4 seconds (Fig 1). The subjects were instructed to continuously rate the intensity of pressure pain during this procedure using e-VAS.
Heat Pain Stimuli
Experimental heat pain was elicited by 10-second pulses to the skin overlying the trapezius muscle or thenar eminence of the hands. In order to preferentially activate C-fiber afferents the Peltier probe was programmed to gradually increase from ambient (38°C) to target temperature in 6 seconds. Subsequently, it remained at peak temperature for 4 seconds for a total stimulus duration of 10 seconds (Fig 1). Three 10-second heat pulses each were applied to each trapezius area and each thenar eminence in counterbalanced order. Stimulus intensities included 44°C and 46°C. The subjects were instructed to continuously rate the intensity of the heat pain during this procedure using e-VAS.
Tender Point Testing
Nine paired TPs as defined by the ACR Criteria75 were assessed by a trained investigator (E.E.W.) using a Wagner dolorimeter (Force Measurement, Greenwich, CT). The rubber tip of the dolorimeter was 1 cm in diameter. The dolorimeter was placed on the examination site and pressure was gradually increased by 1 kg/s. The subjects were instructed to report when the sensation at the examination site changed from pressure to pain. At that moment, pressure testing was stopped and the result recorded as positive if maximal pressure was ≤4 kg. If no pain was elicited at ≥4 kg the test result was listed as negative.
Questionnaires
The Medical College of Virginia Pain Questionnaire21,22 was applied to all study subjects. It has 2 domains, consisting of ratings of pain (VAS) and negative emotions related to chronic pain (VAS). All subjects were instructed to also complete the Beck's Depression Inventory (BDI-II)7 and the Spielberger State/Trait Anxiety Questionnaires.59 The BDI-II is a self-administered 21-item self-report rating inventory measuring characteristic attitudes and symptoms of depression. Scores can range from 0 to 63. A score of 19 and higher is indicative of clinical depression. Spielberger's State/Trait Anxiety Inventory consists of 20 items that ask how a person feels now and reflects situational factors that may influence anxiety levels. Scores range from 20 to 80; the higher the score, the greater the level of anxiety. All questionnaires except the BDI-II were only used to characterize the study subjects. The results of the BDI-II were entered into regression analyses of clinical pain intensity.
Data Analysis
Statistical analyses were calculated using SPSS 20.0 software (SPSS Inc, Chicago, IL). For group comparisons of mechanical and heat-related pain ratings, 1-way analysis of variance (ANOVAs) were used. Significant differences between groups were decomposed using Tukey's Honestly Significant Difference tests. Hierarchical regression analysis was used to determine the independent contributions of mechanical and heat peak pain ratings, TPs, and negative affect to overall clinical pain intensity in FM and LMP subjects. Only results of 200 kPa pressure and 44°C heat stimuli at the shoulders and hands are reported here, because results of 400 kPa pressure and 46°C heat stimuli did not provide fundamentally different predictors of clinical pain intensity.
Results
Study Participants
We enrolled 23 NC subjects (20 females), 36 FM subjects (35 females), and 24 LMP subjects (18 females) into the study (Table 1). Whereas all NC and LMP subjects were recruited through advertising, 97% of all FM subjects came from University of Florida outpatient clinics. Only 3% of FM subjects came from FM support groups. The mean age (standard deviation [SD]) of study participants was 42.3 (13.6), 47.6 (12.8), and 39.5 (18.2) for NC, FM, and LMP subjects, respectively. A 1-way ANOVA demonstrated no significant effect of diagnostic group (P > .05). Average number of TPs (SD) was 3.4(3.5) for NC, 15.8 (3.5) for FM, and 9.3 (5.3) for LMP subjects. A 1-way ANOVA showed a significant effect for diagnostic group (P < .001). Post hoc comparisons demonstrated significantly higher TP counts in FM subjects compared to all other groups as well as LMP subjects compared to NC subjects (all P < .002).
Table 1. Demographics of Study Participants.
| NC | LMP | FM | |
|---|---|---|---|
| Screened subjects | 24 F; 3 M | 25 F; 9 M | 44 F; 6 M |
| Enrolled subjects | 20 F; 3 M | 18 F; 6 M | 35 F; 1 M |
| Age (SD) (years) | 42.3 (13.6) | 39.5 (18.2) | 47.6 (12.8) |
| Pain (SD) (0–100) | 2.0 (.3) | 29 (23) | 47 (25) |
| TP count (SD) | 3.2 (3.3) | 9.5 (5.4) | 14.6 (4.5) |
| BDI-II score (SD) | 3.4 (3.9) | 12.7 (8.0) | 14.8 (9.9) |
Abbreviations: F, female; M, male.
Clinical Pain Ratings
Overall clinical pain of NC subjects was minimal (2.0 VAS units on a 100-point scale), whereas FM and LMP subjects rated their average (SD) pain as 47 (25) and 29 (23) VAS units, respectively. A 1-way ANOVA showed a significant effect for diagnostic group (F[2,80] = 32.6; P < .001). Subsequent post hoc tests demonstrated significant differences in clinical pain between all groups (P < .01) (Fig 2).
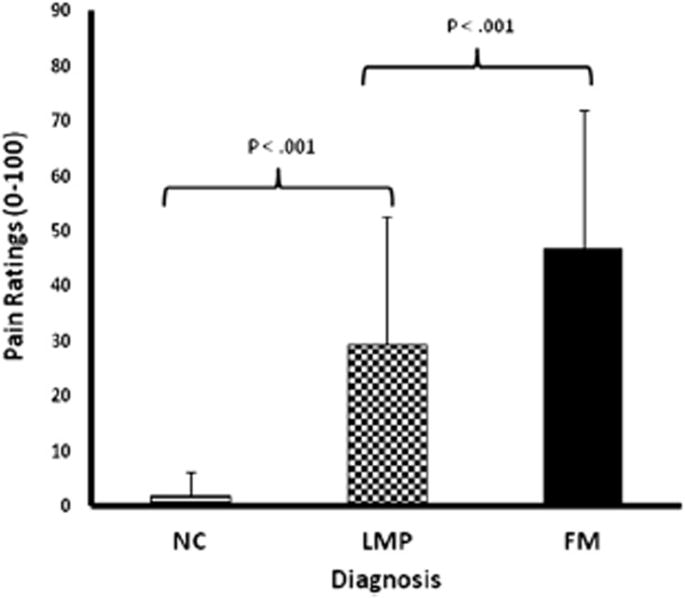
Figure 2.
Clinical pain ratings of all study subjects using a mechanical VAS (0–100). Mean (SD) pain ratings of LMP and FM subjects were 29.3 (23.4) and 46.8 (25.0), respectively. NC subjects reported only minimal incidental pains. Clinical pain ratings significantly differed among all groups (P < .001).
Ratings of Mechanical Pain Stimuli
Shoulder Stimuli
All subjects received three 200- and 400-kPa pressure stimuli to each shoulder. Mean (SD) peak e-VAS ratings of 200- and 400-kPa pressure stimuli by NC, LMP, and FM subjects are listed in Table 2. A 1-way ANOVA of 200-kPa ratings demonstrated a significant effect for diagnostic group (F[2,80] = 22.0; P < .001) (Fig 3). Post hoc tests showed pressure pain ratings as significantly higher for FM compared to all other diagnostic groups (P < .001).
Table 2. Average (SD) Ratings of Pressure and Heat Pain at Shoulders and Hands.
| NC | LMP | FM | |
|---|---|---|---|
| Shoulders - pressure | |||
| 200 kPa (peak) | 7.1 (11.8) | 13.9 (17.4) | 43.1 (29.4) |
| 400 kPa (peak) | 21 (22.3) | 27.0 (24.6) | 55.6 (29.0) |
| Shoulders - heat | |||
| 44°C (peak) | 12.8 (17.3) | 17.1 (23.6) | 29.0 (23.5) |
| 46°C (peak) | 22.6 (24.4) | 33.6 (26.4) | 48.7 (30.5) |
| Hands - pressure | |||
| 200 kPa (peak) | 10.9 (13.6) | 12.3 (11.3) | 34.3 (24.4) |
| 400 kPa (peak) | 23.7 (24.5) | 26.2 (18.8) | 51.3 (29.4) |
| Hands - heat | |||
| 44°C (peak) | 4.9 (4.8) | 14.3 (13.9) | 31.0 (26.1) |
| 46°C (peak) | 12.4 (9.1) | 26.6 (18.0) | 49.4 (27.5) |
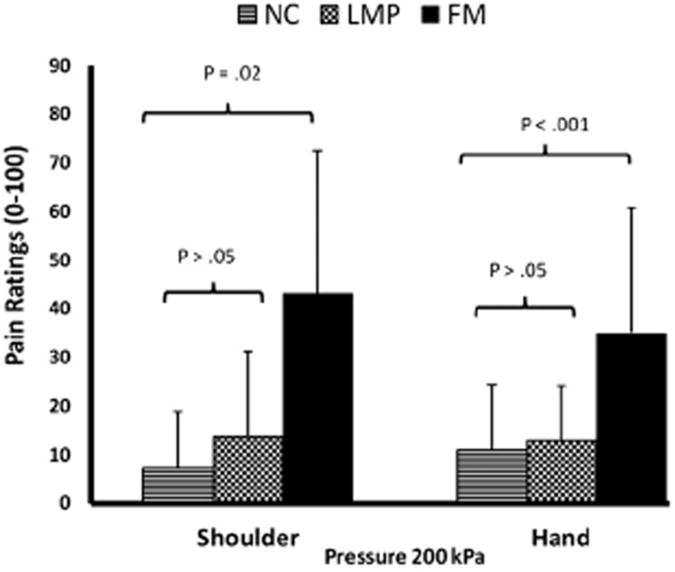
Figure 3.
Shoulder and hand pain ratings of all subjects during pressure stimuli. Experimental pressure stimuli were applied to the middle of the shoulders (trapezius muscle) or to the space between the first 2 fingers using an electronic algometer. After 10 seconds of 200-kPa pressure pain, ratings of FM subjects were significantly higher than either NC or LMP subjects. No significant differences in pressure pain ratings were noted between NC and LMP subjects (P > .05). Similar results were found at the hands.
Hand Stimuli
The experimental pressure pain protocol was repeated at the hands (dorsal webspace between first and second fingers) of all study subjects. The average (SD) peak e-VAS ratings of NC, LMP, and FM subjects for 200- and 400-kPa stimuli at the hand are listed in Table 2. A 1-way ANOVA demonstrated a significant effect of diagnostic group (F[2,73] = 13.1, P < .001). Post hoc tests showed that pressure pain ratings were significantly different between all diagnostic groups (P < .001). Because 400-kPa stimuli at the shoulders and hands did not provide fundamentally different predictors of clinical pain intensity than 200-kPa stimuli, only the analyses of 200-kPa stimuli are reported here.
These findings demonstrate that mechanical hyperalgesia is widespread in both LMP and FM subjects and can be used to detect significant group differences between all study subjects, including NC subjects.
Ratings of Heat Pain Stimuli
Shoulder Stimuli
All subjects received three 10-second heat stimuli at 44°C and 46°C to each shoulder in counterbalanced fashion. Mean (SD) peak e-VAS ratings of 44°C and 46°C stimuli are listed in Table 2. Because 46°C stimuli at the shoulders and hands did not provide fundamentally different predictors of clinical pain intensity than 44°C stimuli, only the analyses of 44°C stimuli are reported here. A 1-way ANOVA of 44°C stimulus ratings demonstrated a significant effect for diagnostic group (F[2,70] =4.0; P < .03; part η2 = .10; Fig 4). Post hoc testing showed heat pain ratings as significantly different only between NC and FM groups (P < .02). All other group comparisons were not statistically different (P > .05).
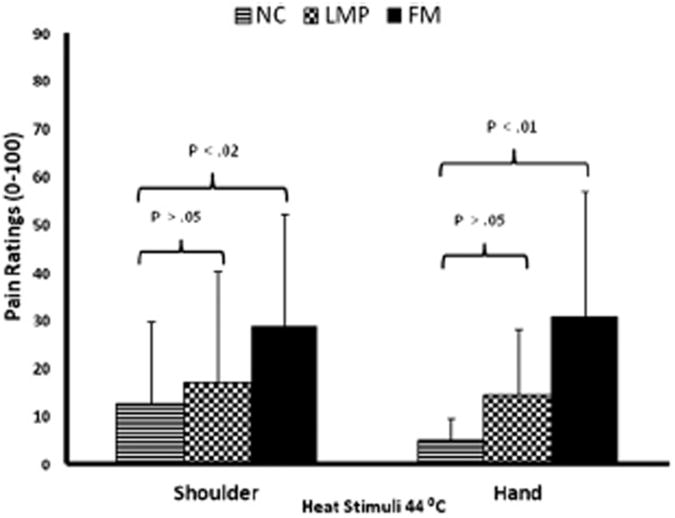
Figure 4.
Shoulder and hand pain ratings of all subjects during heat stimuli. 10 seconds heat stimuli were applied to the skin overlying the middle of the trapezius muscles or to the thenar eminence of the hands. Experimental pain ratings of FM subjects at the shoulders were significantly higher at 44°C than NC or LMP subjects (P < .01). However, there was no significant difference between heat pain ratings of LMP and NC subjects (P > .05). Similar findings were obtained at the hands.
Hand Stimuli
The experimental heat pain protocol was repeated at the hands (thenar eminence) of all study subjects. The average (SD) peak e-VAS ratings of NC, LMP, and FM subjects for 44°C and 46°C stimuli at the hand are listed in Table 2. A 1-way ANOVA of 44°C stimulus ratings demonstrated a significant effect for diagnostic group (F[2,61] = 9.6, P < .001). Subsequent post hoc testing showed heat pain ratings as significantly higher in FM than the other groups (P =.01). In addition, heat pain ratings of LMP subjects were significantly higher than those of NC subjects (P < .02).
These results show that heat hyperalgesia is widespread in LMP and FM subjects and that significant group differences are detectable at both the shoulders and hands of all study subjects.
Predicting Clinical Pain Intensity Using Pressure Stimuli
Several hierarchical regression analyses were used to determine whether pressure pain ratings can predict clinical pain intensity in FM and LMP subjects. Because our previous work with FM subjects has demonstrated significant contributions of TPs and negative affect to clinical pain,64 we decided a priori to include TP count and BDI-II scores in our model. To decrease the number of comparisons and thus spurious findings, multiple other factors were omitted from this analysis because we had no strong a priori hypotheses for their inclusion.
Using Pressure Pain Ratings at the Shoulders
A hierarchical regression analysis was performed using clinical pain intensity VAS ratings as the predicted variable. Shoulder pressure pain sensitivity at 200 kPa was entered as predictor variable in the first block, followed by TP count and BDI-II scores entered in separate blocks. The results of the regression indicated that 45.3% and 38% of the variance in clinical pain scores was predicted by pressure sensitivity of FM and LMP subjects, respectively (Table 3A). Besides pressure pain ratings, neither TP count nor BDI-II scores contributed significantly to the pain variance in FM and LMP subjects. Overall, 50% and 39.4% of the pain variance of FM and LMP subjects, respectively, was predicted by unique contributions of pressure pain ratings at the shoulders, TP count, and BDI-II scores (Table 3A).
Table 3A. Pressure Pain Ratings of FM and LMP Subjects as Predictors of Pain Intensity at Shoulders.
| ΔR2 | F Change | P | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Block | Variable | FM | LMP | FM | LMP | FM | LMP |
| 1 | 200 kPa rating | .453 | .380 | 24.83 | 12.89 | <.001 | <.01 |
| 2 | TP count | .022 | .001 | 1.39 | .02 | >.05 | >.05 |
| 3 | BDI-II | .025 | .013 | 1.21 | .43 | >.05 | >.05 |
|
| |||||||
| Beta (Standardized) | T | ||||||
|
| |||||||
| 1 | 200 kPa rating | .528 | .598 | 3.28 | 3.01 | <.003 | <.01 |
| TP count | .177 | .029 | 1.18 | .15 | >.05 | >.05 | |
| BDI-II | .152 | .152 | 1.02 | .12 | >.05 | >.05 | |
NOTE. Final Models: FM: R2 = .500, F = 9.32; P < .001; LMP: R2 = .394, F = 4.12; P = .021.
Using Pressure Pain Ratings at the Hands
As before, hierarchical regression analyses were performed using clinical pain intensity VAS ratings as the predicted variable. Hand pressure pain sensitivity at 200 kPa, TP count, and BDI-II scores were entered as predictor variables. The results of these regressions indicated that 32.9% and 47.3% of the variance in clinical pain scores was predicted by pressure sensitivity in FM and LMP subjects, respectively (Table 3B). Besides pressure pain ratings, neither TP count nor BDI-II scores contributed significantly to the pain variance in FM and LMP subjects.
Table 3B. Pressure Pain Ratings of FM and LMP Subjects as Predictors of Pain Intensity at Hands.
| ΔR2 | F Change | P | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Block | Variable | FM | LMP | FM | LMP | FM | LMP |
| 1 | 200 kPa rating | .329 | .473 | 14.72 | 13.49 | <.005 | <.005 |
| 2 | TP count | .074 | .001 | 3.60 | .02 | >.05 | >.05 |
| 3 | BDI-II | .062 | .018 | 3.26 | .45 | >.05 | >.05 |
|
| |||||||
| Beta (Standardized) | T | ||||||
|
| |||||||
| 1 | 200 kPa rating | .429 | .661 | 2.87 | 3.23 | <.01 | <.01 |
| TP count | .239 | .039 | 1.59 | .20 | >.05 | >.05 | |
| BDI-II | .262 | .138 | 1.81 | .67 | >.05 | >.05 | |
NOTE. Final Models: FM: R2 = .466, F = 8.13; P < .001; LMP:R2 = .492, F = 4.19; P < .03.
Overall, 46.6% and 49.2% of the pain variance of FM and LMP subjects, respectively, was predicted by unique contributions of pressure pain ratings at the hands, TP count, and BDI-II scores (Table 3B).
Predicting Clinical Pain Intensity Using Heat Stimuli
Whether heat hyperalgesia ratings can predict clinical pain intensity in FM and LMP subjects was tested in several hierarchical regression analyses. Again, we decided a priori to include TP count and BDI-II scores in our final model.
Using Heat Pain Ratings at the Shoulders
Parallel to the regression analyses reported above, we tested heat hyperalgesia, TP count, and BDI-II scores as predictors for clinical pain intensity. Again, heat pain ratings at 44°C, TP count, and BDI-II scores were entered in separate blocks. 16.9% and 26.8% of the variance in clinical pain scores was predicted by heat pain ratings of FM and LMP subjects, respectively (Table 4A). While TP counts predicted 26.9% of FM subjects' pain variance, BDI-II scores did not significantly contribute to variability in clinical pain for either group. Overall, 48.3% of the pain variance of FM subjects was predicted by unique contributions of heat pain ratings at the shoulders, TP count, and BDI-II scores (Table 4A). In contrast, the final prediction model for clinical pain was not significant in LMP subjects.
Table 4A. Heat Pain Ratings of FM and LMP Subjects as Predictors of Pain Intensity at Shoulders.
| ΔR2 | F Change | P | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Block | Variable | FM | LMP | FM | LMP | FM | LMP |
| 1 | 44°C rating | .169 | .268 | 4.68 | 4.76 | <.05 | <.05 |
| 2 | TP count | .269 | .010 | 10.51 | .17 | <.005 | >.05 |
| 3 | BDI-II | .045 | .076 | 1.84 | 1.30 | >.05 | >.05 |
|
| |||||||
| Beta (Standardized) | T | ||||||
|
| |||||||
| 1 | 44°C rating | .181 | .383 | .97 | 1.24 | >.05 | >.05 |
| TP count | .498 | .168 | 3.09 | .55 | <.01 | >.05 | |
| BDI-II | .255 | .280 | 1.36 | 1.14 | >.05 | >.05 | |
NOTE. Final Models: FM: R2 = .483, F = 6.54; P = .003; LMP: R2 = .355, F = 2.02; P > .05.
Using Heat Pain Ratings at the Hands
As before, hierarchical regression analyses were performed using clinical pain intensity VAS ratings as the predicted variable. Hand heat pain sensitivity at 44°C, TP count, and BDI-II scores were entered as predictor variables. The results of these regressions indicated that 18.6% and 28.7% of the variance in clinical pain scores was predicted by heat pain sensitivity of FM and LMP subjects, respectively (Table 4B). Again, TP counts and BDI-II scores did not significantly contribute to variability in clinical pain. Overall, 49.4% of the pain variance of FM subjects was predicted by unique contributions of heat pain ratings at the hands, TP count, and BDI-II scores (Table 4B). The final model for predicting clinical pain was not significant in LMP subjects.
Table 4B. Heat Pain Ratings of FM and LMP Subjects as Predictors of Pain Intensity at Hands.
| ΔR2 | F Change | P | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Block | Variable | FM | LMP | FM | LMP | FM | LMP |
| 1 | 44°C rating | .186 | .287 | 6.18 | 6.84 | .02 | .02 |
| 2 | TP count | .128 | .011 | 4.84 | .24 | .04 | >.05 |
| 3 | BDI-II | .180 | .028 | 8.90 | .64 | <.01 | >.05 |
|
| |||||||
| Beta (Standardized) | T | ||||||
|
| |||||||
| 1 | 44°C rating | .368 | .513 | 2.57 | 2.38 | .02 | .03 |
| TP count | .440 | .108 | 2.98 | .49 | <.01 | >.05 | |
| BDI-II | .249 | .185 | 1.68 | .86 | >.05 | >.05 | |
NOTE. Final Models: FM: R2 = .494, F = 8.13; P < .001; LMP: R2 = .325, F = 2.41; P > .05.
Combining Pressure and Heat Pain Ratings
When experimental pressure and heat pain ratings were entered into a hierarchical regression analysis together with TP counts and BDI-II scores to predict the variability in clinical pain intensity, only experimental pressure pain made a unique contribution to the prediction of pain (r2 = 49.7, beta = .5; P < .05).
Discussion
Results from our study indicate that simple tests of heat and mechanical hyperalgesia at the hands or shoulders can distinguish local or widespread chronic musculoskeletal pain patients from each other and from NCs. More importantly, however, the same tests of heat and mechanical hyperalgesia predicted large proportions of the variance in clinical pain ratings of LMP and FM patients. Specifically, single measures that reflect a combination of primary and secondary hyperalgesia, such as ratings of mechanical hyperalgesia of shoulder muscles, predicted large proportions of the variance in clinical pain intensity (45.3%) and easily distinguished LMP and FM groups. Adding measures of TP and negative emotion to these mechanical tests added only minor additional predictability (total: 50%). In contrast, more exclusive measures of secondary hyperalgesia, such as pain ratings of heat stimuli applied to the hand/shoulder,60 accounted for 28.7% of the variance in clinical pain intensity, about 63% of that of provided by mechanical tests. These differences suggest a simple means of separately evaluating combinations of primary and secondary hyperalgesia (eg, mechanical testing) and exclusive contributions of secondary hyperalgesia (eg, heat tests). However, adding TP and negative affect measures to ratings of heat pain resulted in a model that demonstrated unique contributions from factors that reflect psychological distress and predict nearly 50% of the variance in clinical pain intensity. Although previous tests of mechanical spatial summation accounted for similar amounts of the variance in clinical pain intensity (56%),62 the required methodologies of those studies were technically elaborate and time-consuming, and they cannot easily be implemented in clinical practice and clinical trials. In contrast, QST used in the current study was brief, used only moderately intense stimuli, and did not require expensive instrumentation. Furthermore, little psychophysical expertise was required to perform these tests. The predictive value of these simple tests and statistical models of the present study are likely associated with many of the same factors that have shown predictive value in the past, including TPs, temporal summation of pain, and pain-related emotions.
Tenderness as Predictor of Clinical Pain
TP examination was one of the first quantitative sensory tests introduced into clinical practice. TPs seemed to reflect the deep tissue tenderness of FM patients via decreased pain thresholds (allodynia) at multiple body sites. In 1990, TPs were adopted by the ACR as an essential criterion for theFMsyndrome.75 Chronic pain patients fulfill this criterion if they report pain at ≥11 TP sites when stimulated with pressures of ≤4kg. Early on, however, the limitations of TP counts became apparent because they not only failed as predictors of widespread clinical pain conditions12,13,33 but also did not correlate highly with clinical pain intensity.26,32,41,54,71 However, TPs were strongly associated with psychological distress41,71 and appeared to be more useful as predictors of health care use and disability than pain intensity in FM patients.12 Although the results of our current study confirm these findings, they seem to indicate that TPs can capture a significant portion of the variance in clinical pain (13%) of FM but not LMP patients.
In order to improve some of the shortcomings of pain threshold testing, the multiple random staircase (MRS) method has been used to assess tenderness of chronic musculoskeletal pain patients, including FM.21 However, MRS testing is time-consuming and requires special equipment, which may have prevented widespread use of this method in research and clinical practice. Pressure pain sensitivity as determined by MRS was found to predict up to 27% of the variance of clinical pain intensity of FM patients.21 Similar to our study, the proportion of clinical pain intensity predicted by heat pain sensitivity testing (5.7%) was significantly lower than that predicted by pressure pain.
Measures of Central Sensitization as Predictors of Pain Intensity
Besides mechanical allodynia, other psychophysical abnormalities have been well characterized in chronic musculoskeletal pain patients, including temporal summation of pain or WU.27,53,61,66,68
WU reflects mechanisms of early central sensitization (CS) manifested by increased excitability of neurons within the spinal cord.76 CS is demonstrated by increases in receptive field size, increased responsiveness to nociceptive and non-nociceptive stimuli, and increased ongoing impulse activity.11 Behaviorally, CS is related to heightened pain sensitivity with a spread to uninjured sites (secondary hyperalgesia) and the generation of pain by low threshold receptors (allodynia).67 These changes are fundamental to the mechanisms of some forms of clinical pain.
WU aftersensations, which are strongly affected by central sensitization, have been used as predictors of clinical pain in FM patients. When WU aftersensations were entered into regression models of FM pain intensity that also included TP count and pain-related negative affect, 50% of the pain variance could be predicted.64
Because WU aftersensations are dependent on temporal summation and central sensitization, these findings indirectly suggest that central sensitization is an important part of the mechanisms relevant for FM pain. Abnormalities of WU and its aftersensations, however, are not unique to FM. They are also found in back pain, complex regional pain syndrome, and trigeminal neuralgia.17,23,40,46,51
Negative Affect as a Predictor of Pain Intensity
Generally, there seems to be an association between clinical pain and negative mood,6,19,22,36,55 and this relationship has been detected in many chronic pain studies.1,4,9,39,71,74 Several investigations of chronic pain patients have shown that negative affect can predict a moderate amount of the clinical pain variance,19,22,36 and similar relationships have been described in FM patients.39,64 However, in the current investigation, negative affect did not make a significant contribution to clinical pain most likely due to low levels of depression in our study population.
Limitations
Some of the limitations of this study include its small sample size and its cross-sectional design, which will not allow determining cause-effect relationships of the results. The heterogeneity of the pain complaints by the LMP subjects may have limited the interpretation of the study findings, which nevertheless allowed meaningful comparisons across groups.
Conclusions
Our previous studies of chronic musculoskeletal pain patients have shown strong correlations of mechanical spatial summation, WU, WU aftersensations, and negative affect with clinical pain intensity. We now have added to these findings by showing that simple tests of mechanical and heat hyperalgesia can predict large proportions of the variance in clinical pain intensity of chronic musculoskeletal pain patients. Such strong correlations between experimental and clinical pain intensity suggest that peripheral nociceptive input is required for clinical pain in patients with chronic musculoskeletal pain disorders.65 Most critically, however, these simple tests will be far more feasible to be included in clinical practice and clinical trials.
Furthermore, results from psychophysical tests used in our study provide a means of separately assessing contributions of primary and secondary hyperalgesia to clinical pain, as well as contributions from factors related to psychological distress (BDI-II). Thus, measures of heat and mechanical hyperalgesia could easily be applied in clinical practice and trials for chronic pain disorders, including widespread (eg, FM) and regional (eg, low back, neck, arthritis) pain. Tests used in the present study may embody unique contributions of peripheral and central mechanisms to both local and widespread chronic pain. Yet, similar to previous studies,63 our results also suggest that pain-related negative emotions may make significant contributions to clinical pain intensity. Thus, mechanical and heat hyperalgesia as well as negative affect may represent relevant and separate mechanisms that contribute to chronic musculoskeletal pain syndromes and provide specific targets for treatments.
Acknowledgments
The expert technical assistance of Amber M. Schwier, Elena M. Moran, and Meriem Mokhtech is greatly appreciated.
References
- 1.Almay BG. Clinical characteristics of patients with idiopathic pain syndromes. Depressive symptomatology and patient pain drawings. Pain. 1987;29:335–346. doi: 10.1016/0304-3959(87)90048-0. [DOI] [PubMed] [Google Scholar]
- 2.Arendt-Nielsen L, Henriksson KG. Pathophysiological mechanisms in chronic musculoskeletal pain (fibromyalgia): The role of central and peripheral sensitization and pain disinhibition. Best Pract Res Clin Rheumatol. 2007;21:465–480. doi: 10.1016/j.berh.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Arroyo JF, Cohen ML. Abnormal responses to electrocutaneous stimulation in fibromyalgia. J Rheumatol. 1993;20:1925–1931. [PubMed] [Google Scholar]
- 4.Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian AV. Chronic pain and the emotional brain: Specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26:12165–12173. doi: 10.1523/JNEUROSCI.3576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banic B, Petersen-Felix S, Andersen OK, Radanov BP, Villiger PM, Arendt-Nielsen L, Curatolo M. Evidence for spinal cord hypersensitivity in chronic pain after whiplash injury and in fibromyalgia. Pain. 2004;107:7–15. doi: 10.1016/j.pain.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Basbaum AI. Distinct neurochemical features of acute and persistent pain. PNAS. 1999;96:7739–7743. doi: 10.1073/pnas.96.14.7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck AT, Beamesderfer A. Assessment of depression: The depression inventory. In: Pichot P, editor. Psychological Measurements in Psychopharmacology Modern Problems in Pharmacopsychiatry. Basel, CH: Karger; 1974. pp. 151–169. [DOI] [PubMed] [Google Scholar]
- 8.Berglund B, Harju EL, Kosek E, Lindblom U. Quantitative and qualitative perceptual analysis of cold dysesthesia and hyperalgesia in fibromyalgia. Pain. 2002;96:177–187. doi: 10.1016/s0304-3959(01)00443-2. [DOI] [PubMed] [Google Scholar]
- 9.Burckhardt CS, Clark SR, Bennett RM. A comparison of pain perceptions in women with fibromyalgia and rheumatoid arthritis: Relationship to depression and pain extent. Arthritis Care Res. 1992;5:216–222. doi: 10.1002/art.1790050406. [DOI] [PubMed] [Google Scholar]
- 10.Clauw DJ, Williams D, Lauerman W, Dahlman M, Aslami A, Nachemson AL, Kobrine AI, Wiesel SW. Pain sensitivity as a correlate of clinical status in individuals with chronic low back pain. Spine. 1999;24:2035–2041. doi: 10.1097/00007632-199910010-00013. [DOI] [PubMed] [Google Scholar]
- 11.Cook AJ, Woolf CJ, Wall PD, McMahon SB. Dynamic receptive field plasticity in rat spinal cord dorsal horn following C-primary afferent input. Nature. 1987;325:151–153. doi: 10.1038/325151a0. [DOI] [PubMed] [Google Scholar]
- 12.Croft P, Schollum J, Silman A. Population study of tender point counts and pain as evidence of fibromyalgia. BMJ. 1994;309:696–699. doi: 10.1136/bmj.309.6956.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Croft PR, Burt J, Schollum J, Thomas E, Macfarlane G, Silman A. More pain, more tender points: Is fibromyalgia just one end of a continuous spectrum? Ann Rheum Dis. 1996;55:482–485. doi: 10.1136/ard.55.7.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curatolo M, Arendt-Nielsen L, Petersen-Felix S. Evidence, mechanisms, and clinical implications of central hypersensitivity in chronic pain after whiplash injury. Clin J Pain. 2004;20:469–476. doi: 10.1097/00002508-200411000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Desmeules JA, Cedraschi C, Rapiti E, Baumgartner E, Finckh A, Cohen P, Dayer P, Vischer TL. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum. 2003;48:1420–1429. doi: 10.1002/art.10893. [DOI] [PubMed] [Google Scholar]
- 16.Dubner R. Neuronal plasticity and pain following peripheral tissue inflammation or nerve injury. In: Bond M, Charlton E, Woolf CJ, editors. Proceedings of Vth World Congress on Pain Pain Research and Clinical Management. Elsevier; Amsterdam: 1991. pp. 263–276. [Google Scholar]
- 17.Dubner R, Sharav Y, Gracely RH, Price DD. Idiopathic trigeminal neuralgia: Sensory features and pain mechanisms. Pain. 1987;31:23–33. doi: 10.1016/0304-3959(87)90003-0. [DOI] [PubMed] [Google Scholar]
- 18.Emery EC, Young GT, Berrocoso EM, Chen L, McNaughton PA. HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science. 2011;333:1462–1466. doi: 10.1126/science.1206243. [DOI] [PubMed] [Google Scholar]
- 19.Gaskin ME, Greene AF, Robinson ME, Geisser ME. Negative affect and the experience of chronic pain. J Psychosom Res. 1992;36:707–713. doi: 10.1016/0022-3999(92)90128-o. [DOI] [PubMed] [Google Scholar]
- 20.Ge HY, Wang Y, Fernandez-de-las-Penas C, Graven-Nielsen T, Danneskiold-Samsoe B, Arendt-Nielsen L. Reproduction of overall spontaneous pain pattern by manual stimulation of active myofascial trigger points in fibromyalgia patients. Arthritis Res Ther. 2011;13:R48. doi: 10.1186/ar3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geisser ME, Gracely RH, Giesecke T, Petzke FW, Williams DA, Clauw DJ. The association between experimental and clinical pain measures among persons with fibromyalgia and chronic fatigue syndrome. Eur J Pain. 2007;11:202–207. doi: 10.1016/j.ejpain.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Geisser ME, Robinson ME, Keefe FJ, Weiner ML. Catastrophizing, depression and the sensory, affective and evaluative aspects of chronic pain. Pain. 1994;59:79–83. doi: 10.1016/0304-3959(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 23.George SZ, Wittmer VT, Fillingim RB, Robinson ME. Sex and pain-related psychological variables are associated with thermal pain sensitivity for patients with chronic low back pain. J Pain. 2007;8:2–10. doi: 10.1016/j.jpain.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Giesecke T, Gracely RH, Grant MAB, Nachemson A, Petzke F, Williams DA, Clauw DJ. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613–623. doi: 10.1002/art.20063. [DOI] [PubMed] [Google Scholar]
- 25.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–1343. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 26.Granges G, Littlejohn G. Pressure pain threshold in pain-free subjects, in patients with chronic regional pain syndromes, and in patients with fibromyalgia syndrome. Arthritis Rheum. 1993;36:642–646. doi: 10.1002/art.1780360510. [DOI] [PubMed] [Google Scholar]
- 27.Graven-Nielsen T, Aspegren-Kendall S, Henriksson KG, Bengtsson M, Sorensen J, Johnson A, Gerdle B, Arendt-Nielsen L. Ketamine reduces muscle pain, temporal summation, and referred pain in fibromyalgia patients. Pain. 2000;85:483–491. doi: 10.1016/S0304-3959(99)00308-5. [DOI] [PubMed] [Google Scholar]
- 28.Hall AM, Kamper SJ, Maher CG, Latimer J, Ferreira ML, Nicholas MK. Symptoms of depression and stress mediate the effect of pain on disability. Pain. 2011;152:1044–1051. doi: 10.1016/j.pain.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Henriksson CM. Longterm effects of fibromyalgia on everyday life. A study of 56 patients. Scand J Rheumatol. 1994;23:36–41. doi: 10.3109/03009749409102133. [DOI] [PubMed] [Google Scholar]
- 30.Hudson JI, Goldenberg DL, Pope HG, Jr, Keck PE, Jr, Schlesinger L. Comorbidity of fibromyalgia with medical and psychiatric disorders. Am J Med. 1992;92:363–367. doi: 10.1016/0002-9343(92)90265-d. [DOI] [PubMed] [Google Scholar]
- 31.Hurtig IM, Raak RI, Kendall SA, Gerdle B, Wahren LK. Quantitative sensory testing in fibromyalgia patients and in healthy subjects: Identification of subgroups. Clin J Pain. 2001;17:316–322. doi: 10.1097/00002508-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Jacobs JW, Rasker JJ, van-der-Heide A, Boersma JW, de-Blecourt AC, Griep EN, van-Rijswijk MH, Bijlsma JW. Lack of correlation between the mean tender point score and self-reported pain in fibromyalgia. Arthritis Care Res. 1996;9:105–111. doi: 10.1002/1529-0131(199604)9:2<105::aid-anr1790090206>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 33.Jensen B, Wittrup IH, Rogind H, Danneskiold-Samsoe B, Bliddal H. Correlation between tender points and the fibromyalgia impact questionnaire. J Musculoskelet Pain. 2000;8:19–29. [Google Scholar]
- 34.Jensen K, Andersen HO, Olesen J, Lindblom U. Pressure-pain threshold in human temporal region. Evaluation of a new pressure algometer. Pain. 1986;25:313–323. doi: 10.1016/0304-3959(86)90235-6. [DOI] [PubMed] [Google Scholar]
- 35.Kamper SJ, Maher CG, Hush JM, Pedler A, Sterling M. Relationship between pressure pain thresholds and pain ratings in patients with whiplash-associated disorders. Clin J Pain. 2011;27:495–501. doi: 10.1097/AJP.0b013e31820e1185. [DOI] [PubMed] [Google Scholar]
- 36.Keefe FJ, Wilkins RH, Cook WAJ, Crisson JE, Muhlbaier LH. Depression, pain, and pain behavior. J Consult Clin Psychol. 1986;54:665–669. [PubMed] [Google Scholar]
- 37.Kosek E, Ekholm J, Nordemar R. A comparison of pressure pain thresholds in different tissues and body regions. Long-term reliability of pressure algometry in healthy volunteers. Scand J Rehabil Med. 1993;25:117–124. [PubMed] [Google Scholar]
- 38.Lautenbacher S, Fillingim RB. Pathophysiology of Pain Perception. Springer; 2004. [Google Scholar]
- 39.Malt EA, Olafsson S, Lund A, Ursin H. Factors explaining variance in perceived pain in women with fibromyalgia. BMC Musculoskelet Disord. 2002;3:12–20. doi: 10.1186/1471-2474-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mannion RJ, Woolf CJ. Pain mechanisms and management: A central perspective. Clin J Pain. 2000;16:S144–S156. doi: 10.1097/00002508-200009001-00006. [DOI] [PubMed] [Google Scholar]
- 41.McBeth J, Macfarlane GJ, Benjamin S, Morris S, Silman AJ. The association between tender points, psychological distress, and adverse childhood experiences: A community-based study. Arthritis Rheum. 1999;42:1397–1404. doi: 10.1002/1529-0131(199907)42:7<1397::AID-ANR13>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 42.O'Neill S, Manniche C, Graven-Nielsen T, Arendt-Nielsen L. Generalized deep-tissue hyperalgesia in patients with chronic low-back pain. Eur J Pain. 2007;11:415–420. doi: 10.1016/j.ejpain.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Ohrbach R, Gale EN. Pressure pain thresholds in normal muscles: Reliability, measurement effects, and topographic differences. Pain. 1989;37:257–263. doi: 10.1016/0304-3959(89)90189-9. [DOI] [PubMed] [Google Scholar]
- 44.Ohrbach R, Gale EN. Pressure pain thresholds, clinical assessment, and differential diagnosis: Reliability and validity in patients with myogenic pain. Pain. 1989;39:157–169. doi: 10.1016/0304-3959(89)90003-1. [DOI] [PubMed] [Google Scholar]
- 45.Petzke F, Clauw DJ, Ambrose K, Khine A, Gracely RH. Increased pain sensitivity in fibromyalgia: Effects of stimulus type and mode of presentation. Pain. 2003;105:403–413. doi: 10.1016/S0304-3959(03)00204-5. [DOI] [PubMed] [Google Scholar]
- 46.Price DD, Bennett GJ, Rafii A. Psychophysical observations on patients with neuropathic pain relieved by a sympathetic block. Pain. 1989;36:273–288. doi: 10.1016/0304-3959(89)90086-9. [DOI] [PubMed] [Google Scholar]
- 47.Price DD, Browe AC. Responses of spinal cord neurons to graded noxious and non-noxious stimuli. Brain Res. 1973;64:425–429. doi: 10.1016/0006-8993(73)90199-6. [DOI] [PubMed] [Google Scholar]
- 48.Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain. 1994;56:217–226. doi: 10.1016/0304-3959(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 49.Price DD, Dubner R. Mechanisms of first and second pain in the peripheral and central nervous systems. J Invest Dermatol. 1977;69:167–171. doi: 10.1111/1523-1747.ep12497942. [DOI] [PubMed] [Google Scholar]
- 50.Price DD, Hu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3:57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- 51.Price DD, Long S, Huitt C. Sensory testing of pathophysiological mechanisms of pain in patients with reflex sympathetic dystrophy. Pain. 1992;49:163–173. doi: 10.1016/0304-3959(92)90139-3. [DOI] [PubMed] [Google Scholar]
- 52.Price DD, Patel R, Robinson ME, Staud R. Characteristics of electronic visual analogue and numeric scales for ratings of experimental pain in healthy subjects and fibromyalgia patients. Pain. 2008;140:158–166. doi: 10.1016/j.pain.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 53.Price DD, Staud R, Robinson ME, Mauderli AP, Cannon RL, Vierck CJ. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain. 2002;99:49–59. doi: 10.1016/s0304-3959(02)00053-2. [DOI] [PubMed] [Google Scholar]
- 54.Quimby LG, Block SR, Gratwick GM. Fibromyalgia: Generalized pain intolerance and manifold symptom reporting. J Rheumatol. 1988;15:1264–1270. [PubMed] [Google Scholar]
- 55.Robinson ME, Riley JL. The role of emotion in pain. In: Gatchel RJ, Turk DC, editors. Psychosocial Factors in Pain. New York, NY: Guilford Press; 1998. pp. 74–89. [Google Scholar]
- 56.Rudy TE, Kerns RD, Turk DC. Chronic pain and depression: Toward a cognitive-behavioral mediation model. Pain. 1988;35:129–140. doi: 10.1016/0304-3959(88)90220-5. [DOI] [PubMed] [Google Scholar]
- 57.Simms RW, Goldenberg DL, Felson DT, Mason JH. Tenderness in 75 anatomic sites. Distinguishing fibromyalgia patients from controls. Arthritis Rheum. 1988;31:182–187. doi: 10.1002/art.1780310205. [DOI] [PubMed] [Google Scholar]
- 58.Soderberg S, Lundman B, Norberg A. The meaning of fatigue and tiredness as narrated by women with fibromyalgia and healthy women. J Clin Nurs. 2002;11:247–255. doi: 10.1046/j.1365-2702.2002.00606.x. [DOI] [PubMed] [Google Scholar]
- 59.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (STAI) (Self Evaluation Questionnaire) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 60.Staud R, Bovee CE, Robinson ME, Price DD. Cutaneous C-fiber abnormalities of fibromyalgia patients are specifically related to temporal summation. Pain. 2008;139:315–325. doi: 10.1016/j.pain.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Staud R, Cannon RC, Mauderli AP, Robinson ME, Price DD, Vierck CJ. Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain. 2003;102:87–95. doi: 10.1016/s0304-3959(02)00344-5. [DOI] [PubMed] [Google Scholar]
- 62.Staud R, Koo E, Robinson ME, Price DD. Spatial summation of mechanically evoked muscle pain and painful aftersensations in normal subject and fibromyalgia patients. Pain. 2007;130:177–187. doi: 10.1016/j.pain.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Staud R, Price DD, Robinson ME, Vierck CJ. Body pain area and pain-related negative affect predict clinical pain intensity in patients with fibromyalgia. J Pain. 2004;5:338–343. doi: 10.1016/j.jpain.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 64.Staud R, Robinson ME, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Ratings of experimental pain and pain-related negative affect predict clinical pain in patients with fibromyalgia syndrome. Pain. 2003;105:215–222. doi: 10.1016/s0304-3959(03)00208-2. [DOI] [PubMed] [Google Scholar]
- 65.Staud R, Robinson ME, Weyl EE, Price DD. Pain Variability In Fibromyalgia Is Related To Activity And Rest: Role of peripheral tissue impulse input. J Pain. 2010;11:1376–1383. doi: 10.1016/j.jpain.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165–175. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 67.Torebjork HE, Lundberg LE, LaMotte RH. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia inhumans. J Physiol. 1992;448:765–780. doi: 10.1113/jphysiol.1992.sp019069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vierck CJ, Staud R, Price DD, Cannon RL, Mauderli AP, Martin AD. The effect of maximal exercise on temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. J Pain. 2001;2:334–344. doi: 10.1054/jpai.2001.25533. [DOI] [PubMed] [Google Scholar]
- 69.Wegener ST, Castillo RC, Haythornthwaite J, Mackenzie EJ, Bosse MJ. Psychological distress mediates the effect of pain on function. Pain. 2011;152:1349–1357. doi: 10.1016/j.pain.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 70.White KP, Harth M, Speechley M, Ostbye T. A general population study of fibromyalgia tender points in noninstitutionalized adults with chronic widespread pain. J Rheumatol. 2000;27:2677–2682. [PubMed] [Google Scholar]
- 71.Wolfe F. The relation between tender points and fibromyalgia symptom variables: Evidence that fibromyalgia is not a discrete disorder in the clinic. Ann Rheum Dis. 1997;56:268–271. doi: 10.1136/ard.56.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wolfe F, Hawley DJ, Wilson K. The prevalence and meaning of fatigue in rheumatic disease. J Rheumatol. 1996;23:1407–1417. [PubMed] [Google Scholar]
- 73.Wolfe F, Ross K, Anderson J, Russell IJ. Aspects of fibromyalgia in the general population: Sex, pain threshold, and fibromyalgia symptoms. J Rheumatol. 1995;22:151–156. [PubMed] [Google Scholar]
- 74.Wolfe F, Russell IJ, Vipraio G, Ross K, Anderson J. Serotonin levels, pain threshold, and fibromyalgia symptoms in the general population. J Rheumatol. 1997;24:555–559. [PubMed] [Google Scholar]
- 75.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Fam AG, Farber SJ, Fiechtner JJ, Franklin CM, Gatter RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, McCain GA, Reynolds WJ, Romano TJ, Russell IJ, Sheon RP. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 76.Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]