Abstract
Purpose
To develop a clinically relevant model system to study head and neck squamous cell carcinoma (HNSCC), we have established and characterized a direct-from-patient, tumorgraft model of Human Papillomavirus (HPV)-positive and HPV-negative cancers.
Experimental Design
Patients with newly diagnosed or recurrent HNSCC were consented for donation of tumor specimens. Surgically obtained tissue was implanted subcutaneously into immunodeficient mice. During subsequent passages, both formalin-fixed/paraffin embedded as well as flash frozen tissues were harvested. Tumors were analyzed for a variety of relevant tumor markers. Tumor growth rates and response to radiation, cisplatin, or cetuximab were assessed and early passage cell strains were developed for rapid testing of drug sensitivity.
Results
Tumorgrafts have been established in 22 of 26 patients to date. Significant diversity in tumorgraft tumor differentiation was observed with good agreement in degree of differentiation between patient tumor and tumorgraft (Kappa 0.72). Six tumorgrafts were HPV-positive on the basis of p16 staining. A strong inverse correlation between tumorgraft p16 and p53 or Rb was identified (Spearman correlations p=0.085 and p=0.002, respectively). Significant growth inhibition of representative tumorgrafts was demonstrated with cisplatin, cetuximab or radiation treatment delivered over a two-week period. Early passage cell strains showed high consistency in response to cancer therapy between tumorgraft and cell strain.
Conclusions
We have established a robust human tumorgraft model system for investigating HPV-positive and HPV-negative HNSCC. These tumorgrafts show strong correlation with the original tumor specimens and provide a powerful resource for investigating mechanisms of therapeutic response as well as preclinical testing.
Keywords: Head and neck cancer, animal models of cancer, DNA tumor viruses
Background
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common worldwide cancer with approximately 620,000 new diagnoses annually (1). The development of these cancers has been traditionally associated with tobacco and alcohol use. Evidence in recent years has identified an etiological role of human papillomavirus (HPV) in a substantial subset of HNSCC patients, many of whom do not have a strong history of tobacco and alcohol use (2).
Radiation alone or with concurrent chemotherapy is an important component of therapy for patients with HNSCC. Treatment can be quite toxic with significant acute and long-term side effects. Retrospective and prospective analyses confirm a striking difference in clinical outcome between HPV-positive and HPV-negative HNSCC (3). However, at present, there is no compelling data that therapy recommendations can be guided on the basis of HPV status. To facilitate the development of novel therapy approaches and to better understand molecular mechanisms that underlie therapeutic responses, robust preclinical model systems are needed.
We sought to establish and validate a direct-from-patient human tumorgraft model system of HNSCC that could be utilized to identify molecular targets, validate novel therapeutics, guide treatment recommendations, and facilitate studies regarding biological mechanisms of treatment sensitivity and/or resistance. Such tumorgraft model systems differ from traditional xenograft model systems in that the human tumors are grafted directly from human subjects into immunodeficient mice, rather than first being established as cell lines in tissue culture and then being engrafted onto mice. The biological relevance of the traditional cell line-based xenograft model system has come under increased scrutiny at various levels including recent system wide analyses suggesting that immortalized cell lines from a variety of tumor types retain greater gene expression similarity to each other than to their tissue of origin (4, 5). The tumorgraft model system is increasingly recognized as a preclinical model system that may provide greater biological relevance to human cancers at many levels including tumor pathology, growth, metastasis, disease outcome, and drug responsiveness (6–8).
Methods
Patient Selection
Patients with newly diagnosed or recurrent head and neck squamous cell carcinoma were approached for possible tissue donation. Patients consenting to participate in this IRB-approved protocol completed a brief questionnaire collecting information regarding tobacco use, alcohol use, prior malignancy, sex, age, and prior treatment received. At the time of surgery or staging biopsy, a section of tumor was collected for research use making sure not to compromise surgical margin or pathologic assessment.
Mice
Immunodeficient mice used for tumorgraft development included male and female NOD scid gamma (NSG, Jackson Laboratories, Bar Harbor, ME) and Hsd:athymic Nude-Foxn1nu (Harlan Laboratories, Madison, WI). All mice were kept in the American Association for Accreditation of Laboratory Animal Care approved Wisconsin Institute for Medical Research Animal Care Facility and studies with them were carried out in accordance with an approved animal protocol.
Establishment of tumorgrafts
Tumor was transported directly from the operating room to the laboratory in ice-cold culture media (DMEM with 10% fetal bovine serum, 1% penicillin/streptomycin, and 25 µg/ml amphotericin) and minced in a culture dish to <1mm3 pieces under sterile conditions. Minced tumor pieces were mixed 1:1 with reduced growth factor Matrigel (cat #354230, BD Biosciences, Inc, Bedford, MA) and injected subcutaneously into NSG mice with an 18 gauge needle as passage zero (P0). Every effort was made to accomplish this transfer within 1 hour of tumor harvest from the surgical procedure. Subsequent passages were made in a similar fashion into either NSG or athymic nude mice.
Cryopreservation of tumorgrafts was accomplished by mixing minced tumor pieces with transport media supplemented with 10% dimethylsulfoxide (DMSO). Tumors were frozen in controlled rate freezers (1C/min) to −80C overnight and transferred to liquid nitrogen for long-term storage. To thaw tumors, aliquots were warmed to 37C in a heated water bath, tumor tissue was washed twice in transport media (without DMSO) and immediately implanted into mice.
Histology of primary and tumorgrafts
At each passage, a section of the tumor was reserved for fixation in 10% neutral buffered formalin and subsequent embedding in paraffin blocks. 5 µm sections were cut and H&E stains performed on every 10th section. Each patient’s primary tumor was also stained by hematoxylin and eosin (H&E) and slides imaged on an Olympus BX51 microscope (Olympus America, Inc., Melville, NY). Comparisons between primary tumor, first passage tumorgraft, and subsequent tumorgraft passages were made by a surgical pathologist on the basis of differentiation, keratinization, and overall tumor architecture.
Assessment of human papillomavirus
Early passage (P0–P2) tissue at the time of harvest from immunodeficient mice was snap frozen in liquid nitrogen. Total genomic DNA and total RNA from this tissue were isolated using DNeasy Blood and Tissue Kit, miRNeasy mini kit, and RNeasy minElute spin column (Cat#69504, 217004, and 74204, respectively, from Qiagen Inc. Valencia, CA). Multiple methods were used to assess for the presence of HPV.
Quantitative real-time PCR (qRT-PCR) was performed on a BioRad CFX96. Briefly, total RNA was harvested using the miRNeasy with MinElute Kit with snap frozen tissue samples from passage 0. cDNA was synthesized using the iScript Reverse Transcription Supermix kit (Bio-Rad Laboratories, Hercules, CA) and 1,000 ng of total RNA. qRT-PCR was performed by using IQ Multiplex Powermix with 10 ng cDNA per 10 µl reaction. GAPD, HPV-16 E5, E6, and E7 transcripts were detected using primers and probes (Supplemental Table 1) purchased from Integrated DNA Technologies, Inc. (Coralville, IA). The thermocycler was programmed for an initial 95°C for 7 minutes followed by 40 cycles of 94°C for 15 seconds and 60°C for 30 seconds.
Total genomic DNA was used to probe for HPV DNA using a nested PCR approach previously described (9). Briefly, for both rounds of PCR, a final concentration of 1× PCR buffer, 0.2 mmol/L dNTPs, 1.5 mmol/L MgCl2, and 0.2 U Taq was used. In the first round, 100 ng of purified DNA and MY09 and MY11 (Supplemental Table 1) primers (which detect multiple HPV subtypes) at 0.2 µmol/L were used. The thermocycler was programed for an initial 94°C for 4 minutes followed by 40 cycles of 94°C for 15 seconds, 55°C for 30 seconds, and 72°C for 1 minute with final extension at 72°C for 5 minutes. Final products from PCR reactions were run on a 1.5% agarose gel, stained with ethidium bromide, and imaged.
Southern blot was performed using 10 µg of BamHI digested total cellular DNA separated on a 1.25% agarose gel, transferred to Hybond N+ nylon membrane (Amersham, Pittsburgh, PA) and crosslinked. DNA probes were made by 5’ end labeling 10 pmoles of HPV16 specific oligonucleotides (Supplemental Table 1) in the presence of T4 polynucleotide kinase (New England Biolabs Inc, Ipswich, MA) with [γ-32P] ATP (6000 Ci/mmol) at 37°C for 1.5 hours. The membrane was pre-hybridized with Church hybridization buffer for 15 minutes at 52°C followed by probe hybridization for 18 hours at 52°C in a hybridization oven. Membrane was washed with Church wash buffer, exposed to a storage phosphor screen and scanned using a Typhoon 8610 imaging system (Amersham).
To assess for alternative HPV subtypes, an additional PCR for the HPV E1 gene was performed. Briefly, 100 ng of total genomic DNA was amplified using the degenerate E1 primers (Supplemental Table 1) with the thermocycler programmed for an initial 94°C for 5 minutes followed by 40 cycles of 95°C for 10 seconds, 50°C for 10 seconds, and 72°C for 30 seconds with final extension at 72°C for 5 minutes. Final products were run on a 1.5% agarose gel, stained with ethidium bromide, and imaged. Positive bands were individually gel purified and sent for Sanger sequencing using the E1 primers. Sequences were annealed, base discrepancies edited, and the resulting ideal sequence compared via BLAST search.
Radiation and chemotherapy growth delay
Tumor growth rates and therapeutic response were monitored by injecting athymic nude mice subcutaneously (n=12 per group) with tumors into bilateral flanks. Tumor volume was monitored twice weekly by measurement with Vernier calipers and calculated according to the equation V = (π)/6 × (large diameter) × (small diameter)2. When individual tumor volumes reached 200 mm3, mice were stratified into treatment groups based on tumor size such that each group had tumors with a similar range in size as determined by Wilcoxon rank-sum test. Treatment commenced the next day with cisplatin (2 mg/kg), cetuximab (0.2 mg), or vehicle control (0.95 normal saline) delivered by intraperitoneal (IP) injection twice weekly. Radiation (2 Gy/fraction twice weekly) was delivered via an X-RAD 320 biological irradiator (Precision X-Ray, North Branford, CT) using custom-designed mouse jigs to immobilize animals and limit radiation exposure to the tumors on the dorsal flanks. Time to tumor quadrupling was calculated from the first day of treatment. Curves were fit to an exponential growth equation and compared using the extra-sum-of-squares f-test using Graphpad Prism v 5.0d.
Immunohistochemistry
The expression of p16 (BD Pharmingen catalog #550834), p53, Rb, and EGFR was detected in histologic sections of tumorgrafts by standard immunohistochemistry (IHC). A one-step Gomori’s trichrome stain (ENG Scientific) was used to develop 5 micron paraffin sections of passage 0 tumorgrafts. Briefly, sections were deparaffinized, incubated in Bouins solution for 1 hour at 60C, washed in running tap water, and the nuclei stained with Weigert's iron hematoxylin for 15 minutes. Next, the slides were placed in Gomori's trichrome stain and incubated 20 minutes at room temperature. Finally, the tumor sections were rinsed with H20, dehydrated, cleared and coverslipped.
Images were acquired with a 20X objective using an Olympus BX51 fluorescent microscope and photographed with a SPOT RT CCD camera (Diagnostic Instruments, Inc., Sterling Heights, MI). A determination of positive vs. negative was made by a board certified pathologist based on cytoplasmic and/or nuclear positivity >2+ intensity in more than 70% of tumor cells (10). The intensity of p53 and Rb staining was scored as follows: negative when <5% of tumor cells displayed staining; 1+ when intensity was mild; 2+, moderate; 3+, when intensity was equal to the positive control; and 4+ when intensity was greater than the positive control (11).
Results
Establishment of tumorgrafts
A total of 37 patients with head and neck cancer have been consented for tumor collection and establishment as tumorgrafts. Twenty-six had tumor collected and implanted subcutaneously into NSG mice as described in the Methods. The remaining nine patients did not undergo tumor collection for the study due to limitations in tumor quantity, the altered timing of surgery, and/or the availability of laboratory personnel for immediate tissue transfer. Average age of donors was 61 (range 45–87). To date, 22 samples have given rise to viable tumors (take rate: 85%). Molecular characterization and assessment of human papillomavirus infection was performed for all tumors. Comprehensive tumor growth rate assessment and therapeutic evaluation have been performed for 3 tumorgraft models to date: UW-SCC6, UW-SCC14N, UW-SCC22.
Clinical characteristics of the 26 patients who had tumor collected and the relationship to tumor take rate are presented in Table 1. Briefly, by univariate analysis patient sex, tobacco use, alcohol use, and T-stage did not correlate with tumorgraft take rate. Successful tumorgraft take was also not correlated with primary tumor differentiation or HPV status. However, tumorgraft establishment was significantly higher (100% vs 64%, p=0.02) from subjects with lymph node metastases than from those without lymph node metastases, regardless of whether the tumor biopsy was taken from the primary tumor site or from a metastatic lymph node.
Table 1.
Clinical characteristics of patients and corresponding frequency of tumorgraft establishment by variable.
| Patient and tumor characteristics | Tumor take rate (%) |
|||
|---|---|---|---|---|
| Parameter | Variable | Patients (n) | Significance* | |
| Age | <60 | 13 | 85 | NS |
| ≥60 | 13 | 85 | ||
| Sex | Male | 18 | 83 | NS |
| Female | 8 | 88 | ||
| Tobacco use | Non-smoker | 7 | 100 | NS |
| Minimal use (<20 pack years) | 1 | 100 | ||
| > Minimal use | 18 | 78 | ||
| Alcohol use | None/light | 9 | 100 | NS |
| Moderate | 6 | 83 | ||
| Heavy | 11 | 73 | ||
| T stage | T1 or T2 | 12 | 83 | NS |
| T3 or T4 | 14 | 86 | ||
| Nodal status | Node negative | 11 | 64 | p=0.02 |
| Node positive | 15 | 100 | ||
| Differentiation | Well | 11 | 82 | NS |
| Moderate | 11 | 82 | ||
| Poor | 4 | 100 | ||
| HPV status | positive | 8 | 100 | NS |
| negative | 13 | 100 | ||
| Testing not done | 5 | 20 | ||
The frequency of tumor take compared via Fisher's Exact Test (age, sex, T stage, nodal status, and HPV status) or Chi squared test (tobacco use, alcohol use, differentiation).
Tumorgraft comparison with primary tumors
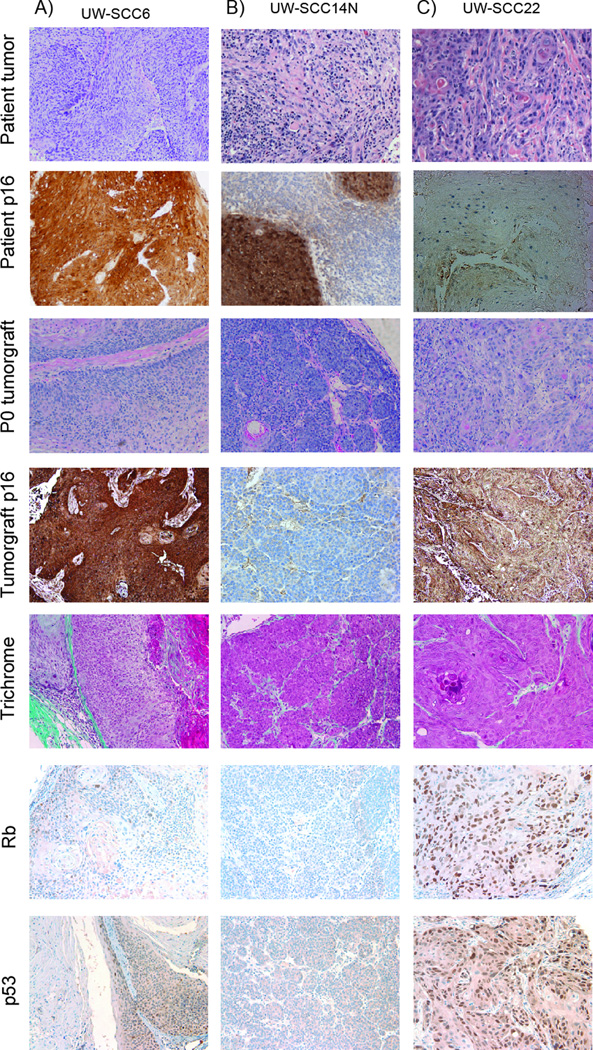
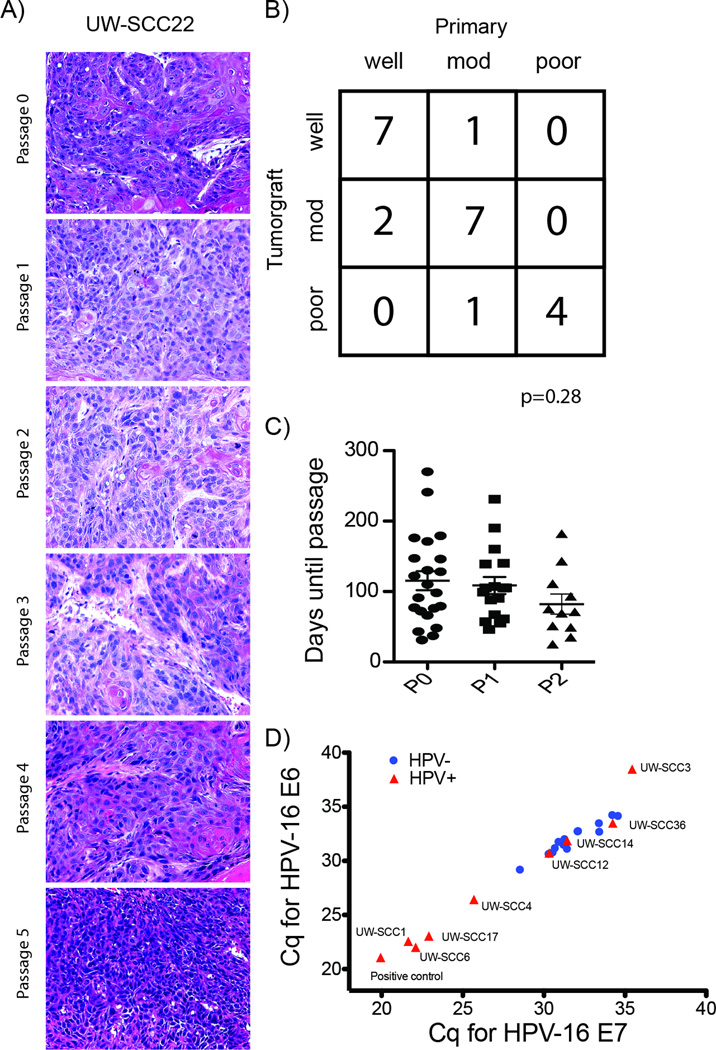
As shown in Table 2, tumorgrafts were established from multiple subsites of the head and neck including oropharynx (base of tongue and tonsil), oral cavity, and hypopharynx. The 22 successful tumorgrafts analyzed were composed of poorly differentiated tumor histology in 5 cases, moderately differentiated in 9 cases, and well differentiated in 8 cases. Figure 1 shows representative images from three tumorgrafts including H&E stain and p16 IHC of both the primary tumor and early passage tumorgraft, trichrome stain, and IHC of biomarkers p53, Rb, and EGFR. Strong retention of overall tumor histology including cell morphology, stromal component architecture, and the presence of cystic structures were observed between original patient tumor and tumorgraft (Figure 1) and in sections from multiple serial passages of the same tumorgraft (Figure 2A). In addition, good agreement with regard to the degree of tumor differentiation (i.e. poor, moderate, or well) was observed between patient specimen and tumorgraft (Figure 2B, unweighted Kappa 0.72). To date, seventeen tumors that were established in mice (P0 generation) have been successfully passaged with growth evident in the subsequent (i.e., P1) generation. Of those tumors that have been passaged at least once (n= 22), the mean time from initial tumorgraft implantation to first passage was 115 days and to second passage (n=17) was 109 days (Figure 2C, p=0.55). Eight tumors have been passaged at least 4 times. Comparisons of differentiation over multiple passages in a single tumorgraft have shown remarkable stability (Supplemental Table 2) with a probability of change of differentiation estimated at 3% (one sided 95% confidence interval: 15.8%).
Table 2.
Immunohistochemical characteristics and HPV status of tumorgraft at early passage.
| Tumorgraft | Tumor site | Differentiation of primary |
Differentiation of tumorgraft |
p53* | Rb% | Primary tumor p16 status (% of cells >=2+ for p16) |
Tumorgraft p16 status (% of cells >=2+ for p16) |
HPV-16 E6/E7 qRT-PCR$ |
MY9/11& | Southern blot& | degenerate E1 PCR& |
HPV subtype from E1 sequencing& |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UW-SCC1P | Base of Tongue | Moderate | Moderate | ++ | ++ | positive (70%) | positive (80%) | + | + | + | + | 16 |
| UW-SCC3P | Tonsil | Poor | Poor | + | +++ | positive (95%) | negative (30%) | − | − | − | − | |
| UW-SCC4P | Floor of Mouth | Well | Well | + | + | positive (70%) | positive (70%) | + | + | + | + | 16 |
| UW-SCC6P | Tonsil | Moderate | Moderate | + | + | positive (90%) | positive (90%) | + | + | + | + | 16 |
| UW-SCC10P | Oral Tongue | Moderate | Poor | ++ | +++ | negative (0%) | negative (30%) | − | − | − | − | |
| UW-SCC12P | Base of Tongue | Well | Well | +++ | ++ | positive (90%) | negative (30%) | − | − | − | − | |
| UW-SCC13P | Oral Tongue | Well | Well | +++ | +++ | negative (0%) | negative (10%) | − | − | − | − | |
| UW-SCC14N | Base of Tongue | Moderate | Moderate | + | − | positive (90%) | negative (0%) | − | − | − | − | |
| UW-SCC15P | Floor of Mouth | Poor | Poor | ++ | ++ | negative (5%) | negative (0%) | − | + | − | + | 16 |
| UW-SCC17P | Tonsil | Poor | Poor | + | + | positive (95%) | positive (95%) | + | + | + | + | 16 |
| UW-SCC20P | Floor of Mouth | Well | Moderate | NE | NE | ND | NE | NE | NE | NE | NE | |
| UW-SCC22P | Floor of Mouth | Well | Well | +++ | +++ | negative (20%) | negative (0%) | − | − | − | − | |
| UW-SCC23P | Floor of Mouth | Well | Well | +++ | +++ | negative (10%) | negative (0%) | − | − | − | − | |
| UW-SCC24P | Floor of Mouth | Well | Well | +++ | ++ | negative (0%) | negative (20%) | − | − | − | − | |
| UW-SCC25P | Floor of Mouth | Moderate | Moderate | +++ | − | negative (10%) | positive (95%) | − | + | − | +/− | negative |
| UW-SCC30P | Floor of Mouth | Moderate | Well | +++ | ++ | negative (0%) | negative (0%) | − | − | − | − | |
| UW-SCC31P | Hypopharynx | Poor | Poor | + | +++ | negative (0%) | negative (0%) | − | − | − | − | |
| UW-SCC32P | Buccal mucosa | Well | Well | +++ | ++ | negative (10%) | negative (10%) | − | − | − | − | |
| UW-SCC33P | Supraglottis | Moderate | Moderate | +++ | +++ | negative (40%) | negative (50%) | − | − | − | − | |
| UW-SCC34P | Buccal mucosa | Moderate | Moderate | + | + | negative (0%) | negative (10%) | − | − | − | − | |
| UW-SCC35P | Aveolar ridge | Well | Moderate | ++ | ++ | negative (10%) | negative (20%) | − | − | − | − | |
| UW-SCC36P | Tonsil | Moderate | Moderate | + | − | positive (90%) | positive (80%) | − | + | − | + | 16 |
Correlation between p16 and Rb and between primary tumor HPV and Rb (Spearman correlation p=0.002 and 0.034, respectively)
Correlation between p16 and p53 and between primary tumor HPV and p53 (Spearman correlation p=0.085 and 0.005, respectively)
ND not done
NE: not evaluable
performed on total genomic DNA
performed on cDNA generated from mRNA
Figure 1.
Histopathologic features of three patient tumors and corresponding tumorgrafts. UW-SCC6 (A), UW-SCC14N (B), and UW-SCC22 (C). Shown are photomicrografts of primary tumor H&E and p16 IHC top two rows in addition to tumorgraft passage 1 H&E, p16 IHC, Trichrome, Rb, and p53. Overall a strong correlation between Rb and p53 staining was observed (Spearman correlation p=0.04). Images at 200×.
Figure 2.
A) Well differentiated primary tumor along with consistency between passages. B) Overall correlation between patient and tumorgraft differentiation showing good agreement (unweighted Kappa = 0.72, Std error = 0.13). C) Scatterplot depicting time from implantation to passage for the initial implantation (P0) and the second (P1), and third (P2) passages. One-way ANOVA, p=0.28. D) qRT-PCR with primers specific for HPV-16 E6 and HPV-16 E7 RNA showed high correlation in Cq values between E6 and E7 (HPV-negative, circles; HPV+, triangles), Pearson r=0.96, p<0.0001.
Cryopreservation of tumorgrafts
One challenge of utilizing direct from patient tumorgrafts is that tumor characteristics may change over time. While we have not seen this to date, we have attempted to cryopreserve tumorgrafts to allow us to reanimate early passage tumors at later time points. To date, 16 tumorgrafts have been cryopreserved and 10 have been thawed and re-implanted into NSG mice. At this time, six of ten (60%) have successfully grown additional tumor.
Assessment of HPV
In light of the causal association and prognostic significance of human papillomavirus infection in head and neck cancer, we assessed the HPV status of all tumorgrafts. HPV status was assessed by p16 staining of the primary tumor (i.e., surgical specimen) in all but one patient. The primary pathologic specimens of eight patients were HPV positive by p16 staining; six on the basis of at least 70% of cells having ≥2+ p16 staining, and two on p16 staining scored as positive vs. negative at an outside institution for which slides were not available for quantification of p16 positivity (Table 2).
All tumorgrafts were assessed for HPV status using four distinct tests: 1) expression of p16, a surrogate marker for HPV infection, was assessed by IHC showing 6 of 22 tumorgrafts positive for p16 expression (Figure 1); 2) qRT-PCR for HPV-16 E6 and E7 RNA, showing 4 of 22 tumorgrafts positive by qRT-PCR (Table 2, Figure 2D); 3) PCR-based detection was used to identify the presence of HPV DNA from total genomic DNA using two different set of degenerate primers known to detect multiple mucosotropic HPV genotypes (9), showing 7 of 22 tumorgrafts positive by HPV-specific PCR (Table 2, Supplemental Figure 1A); and, 4) Southern blot of total genomic DNA for HPV-16 and HPV-18, the two most frequently associated papillomaviruses, was performed using a procedure that can detect down to 0.1 copy per cell, showing 4 of 22 cases positive (Table 2, Supplemental Figure 1B).
In addition, samples from tumorgrafts that were positive on E1 degenerate PCR were assessed by Sanger sequencing to determine the specific HPV subtype. All showed near 100% identity with HPV-16. One sample, UW-SCC25P was faintly positive upon PCR with the MY9/11 primers and showed a positive doublet product by PCR using primers specific for E1. However, upon sequencing, these products, they showed no identity to any HPV subtype.
Differences between p16 staining of the primary surgical specimen and resultant tumorgraft were seen in 4 cases. In 3 of these cases, a different cutoff value for HPV positivity would have led to agreement between patient and tumorgraft. For example, UW-SCC3 and UW-SCC12 showed 90% of cells p16 positive in the primary and 30% of cells p16 positive in the tumorgraft. Alternatively, UW-SCC25 was scored as p16-negative in the primary (10% of cells p16 positive) and p16-positive in the tumorgraft (95% of cells p16 positive). One case, UW-SCC14N had strong p16 staining in the primary tumor, isolated cells positive for p16 in the lymph node and no p16 positive cells in the tumorgraft derived from the lymph node. Those tumors with four of more passages were assessed for p16 staining in each passage and showed no alteration in p16 staining over time (Supplemental Table 2).
Molecular markers – p53, Rb
Due to their known importance in HPV-associated malignancy and their central role as tumor suppressor proteins, we assessed the tumorgrafts for p53 and Rb expression by immunohistochemistry. A wide range of staining for both markers was identified (Figure 1 and Table 2) with a strong inverse correlation between p16 and p53 and between p16 and Rb detection (Spearman correlations p=0.03 and p<0.001, respectively) as would be expected based on the mechanism of HPV oncogenesis.
Response to therapy
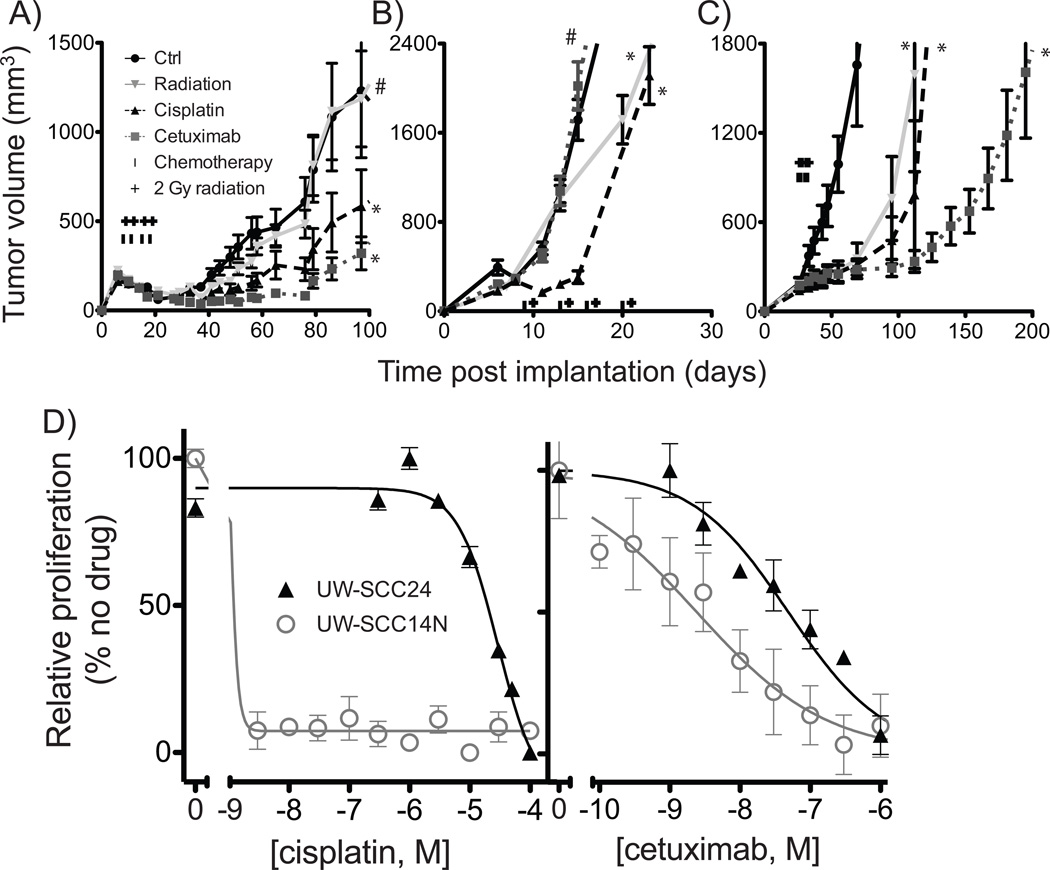
As an initial effort to assess the utility of tumorgrafts for therapeutic response, tumorgrafts from three different patients were tested in detail for response to radiation, cisplatin, and cetuximab (Figure 3A–C). Tumorgrafts from each patient exhibited notably distinct growth patterns and response profiles to these three treatments. UW-SCC14N, which derived from a metastatic cervical lymph node, displayed the fastest growth rate and limited response to radiation (Table 3). In contrast, the two tumorgrafts derived from primary lesions, UW-SCC6 and UW-SCC22, despite very different overall growth profiles (Figure 3), responded well to radiation, demonstrating a two-fold increase in time to tumor quadrupling. Both of these tumorgrafts responded more briskly to cetuximab than to cisplatin, which was not the case for UW-SCC14N.
Figure 3.
Effects of radiation, cetuximab, and cisplatin in three tumorgrafts: UW-SCC6 (A), UW-SCC14N (B), and UW-SCC22 (C). Treatments, started when subcutaneously growing tumors reached a volume of approximately 200 mm3, were administered twice weekly for two weeks as described in the methods. Growth curves plotting the mean (+/− SEM) tumor volume over time are shown. For each group, n=8–10 mice with dual tumors began treatment. *p<0.01, #p=not significant. D) Early passage cell strains developed from UW-SCC24 and UW-SCC14N were assessed for proliferative potential in the presence of indicated drugs. Cell number was estimated by CCK8 assay and standardized to the maximum relative value of vehicle control.
Table 3.
Time to tumorgraft quadrupling (d) under control conditions and after radiation (2 Gy twice weekly × 4), cisplatin (0.2 mg/kg twice weekly × 4), or cetuximab (2 mg/kg twice weekly × 4). TGD = tumor growth delay, the mean percentage increase in time to tumor quadrupling for each treatment relative to control.
| Ctrl | Radiation | Cisplatin | Cetuximab | |
|---|---|---|---|---|
| UW-SCC6 | 44 | 79 | 91.5 | 123 |
| UW-SCC14 | 13 | 13 | 25 | 17.0 |
| UW-SCC22 | 55 | 125 | 125 | 181 |
| % TGD (Mean +/− SD) | 169 +/− 53 | 209 +/− 14 | 247 +/− 84 | |
The patient donating tissue for UW-SCC6 presented with a T2N2BM0 oropharyngeal cancer and was treated with radiation and concurrent cisplatin. The patient developed lung metastases approximately 17 months after primary treatment, but remained controlled at the site of the primary and in the neck. UW-SCC14N presented with T1N2B oropharyngeal cancer and underwent an initial neck dissection followed by radiation and concurrent cetuximab chemotherapy. The patient’s disease is controlled 17 months after initial therapy. UW-SCC22 presented with a T4aN1M0 oral cavity tumor that was initial treated with surgical resection. Approximately 2 months after surgery the tumor recurred at both the site of the primary disease and in the neck. The patient was treated with radiation and concurrent cisplatin. One year later, the patient presented with locally recurrent disease at which time the biopsy for tumorgraft was taken. The patient died several months later of progressive disease.
Finally, early passage cell strains were generated directly from patient tumors if additional cells remained after the initial tumorgraft implantation, or from subsequent tumorgraft passages. To date, six cell strains have been generated and passaged. No difference in primary tumor characteristic appears to be predictive of cell strain development, although the power to detect such a difference is severely limited by the number of strains. The cell strains from UW-SCC14N and UW-SCC24 were tested in proliferation assays and demonstrated in vitro response to both cisplatin and cetuximab (Figure 3D). Interestingly, a very good response to cisplatin was seen in UW-SCC14N, a tumor that also demonstrated a good in vivo response to cisplatin alone (Figure 3B).
Discussion
Over several decades, many investigators have relied upon tumor cell line and xenograft model systems for testing novel therapeutics. However, these systems carry significant limitations based on adaptation to growth in tissue culture including upregulation of survival genes, alterations in multidrug resistance genes, and often greater similarities to other cultured cells than to the primary tumors they were originally intended to represent (4). In addition, only five HPV-positive head and neck squamous cancer cell lines have been described to date (12–16), significantly limiting our ability to investigate differences between HPV-positive and HPV-negative head and neck cancers. The tumorgrafts and cell strains described in the current study represent a promising system under development by which to investigate molecular alterations underlying the growth behavior of head and neck cancers, to serve as a preclinical model system for testing novel therapeutics either alone or in combination with radiotherapy. While we would hope that this model system could someday play a useful role in the selection of optimal therapy for personalized medicine, the mean time required for tumorgraft establishment (nearly 4 months) precludes the use for initial therapeutic selection. However, Hidalgo and colleagues have successfully used tumorgrafts to identify efficacious therapies following initial therapeutic failure (7).
We commenced the current work anticipating that only a small fraction of patient tumors would grow successfully as tumorgrafts. However, the early success observed to date with 22 of 26 tumorgrafts has been highly gratifying, and may in part reflect the rapid transfer of tumor directly from patient to mouse within one hour, the use of matrigel to facilitate establishment of the tumorgraft and/or the use of NSG mice, which are highly immunodeficient, as recipients. Much akin to the diverse clinical presentations of cancer patients, we have observed considerable diversity in tumor differentiation, primary site location, lymph node status, tobacco history, and HPV status (Tables 1 and 2) in the tumorgrafts. In addition, these tumorgrafts have been established from patients undergoing well defined clinical treatments for which detailed outcome data is being carefully collected. The high tumor take rate in our study (85%) provides preliminary confidence that the process of tumorgrafting itself is not a significant selective pressure. The increased take rate of tumorgrafts from patients with lymph node metastases (regardless of the site of tissue) suggests that intrinsic factors reflecting the biology of the individual tumors may play a role in influencing tumorgraft take rates. Perhaps pooled analysis from multiple disease sites may identify critical molecular alterations associated with tumorgraft take rates.
Overall, we have observed strong histologic stability across serial passage of a single tumorgraft. While additional comparisons over multiple passages are needed to confirm phenotypic stability, our experience to date suggests high intrapatient fidelity in terms of tumor differentiation and p16 status. An exception to this pattern is the change observed in HPV-status in select patients. For example, UW-SCC14N is p16 positive in the patient’s primary tumor, but negative for all tests in the tumorgraft. On the other hand, UW-SCC25 is p16 negative in the patient, but p16 positive in the tumorgraft. There are several possible explanations for these differences. It may simply reflect a difference in the percentage of cells staining p16-positive suggesting a potential selection bias in our model system. Alternatively, we and others have previously described p16-positive, HPV-negative tumors (10, 17, 18). It is unclear in our model whether the loss of p16 expression represents loss of episomal HPV-genomes, false positive testing, alternative molecular pathway activation, or coincident tumor development. In most cases, p16 status and degenerate PCR were in agreement, those cases with discrepancies may represent false positive results as a third test for high-and low-risk HPV using degenerate primers failed to detect a product in these cases. This appears to predominantly reflect differences in the percentage of cells staining p16-positive, thus may not represent a true difference in biology. We and others have previously described both p16-negative but HPV positive cases, as well as p16-positive but HPV negative cancers (10, 17, 18). We did, however, identify the expected correlation between HPV-positivity and low p53 and low Rb (Table 2). Interestingly, not all groups confirm this expected correlation between HPV-positive HNC and p53 expression intensity (18–20) suggesting that there may be variation in expression of E6 and consequently incomplete p53 degradation; alternatively, mutations in p53 may be present in a subset of HPV+ HNC patients resulting in variable p53 expression.
Preclinical validation of therapeutic targets and response profiling remains an expensive and time-intensive process. There is considerable concern that human cancer cell lines, either in vitro or as tumor xenografts, often demonstrate limited ability to predict patient response to cancer therapy (21). This may reflect the tremendous selection pressure required to grow human tumor cells in artificial tissue culture systems with adherence to plastic ware and/or reliance on culture media. The primary tumorgraft system described in this report reflects a systematic effort to more faithfully preserve molecular, genetic, architectural, and treatment response characteristics of the original human tumor specimen. These HNSCC tumorgrafts may prove useful not only for the investigation of radiation and chemotherapy response profiles but also to uncover distinctions between HPV+ and HPV− tumors with regard to growth characteristics and response to conventional as well as new molecular therapies. Only systematic investigation over time will confirm if these tumorgraft model systems can prove consistently more faithful and predictive of true clinical response and outcome. A potential limitation of this model system is the use of immunodeficient mice necessary to enable tumorgraft growth. It has been suggested that the presence of an intact immune response in HPV+ HNC results in improved tumor control (22). However, the published data also suggests that cytotoxic therapies can result in tumor control even in the absence of an intact immune system.
An important component of the current studies is the development of cell strains. These non-immortalized early passage cells may more faithfully represent patient response patterns; to date they demonstrate a high correlation with tumorgraft response in our hands (Figure 3). Successful establishment of cell strains may provide a less expensive and more efficient system in which to evaluate or screen therapeutic regimens. While additional validation is necessary, our results thus far suggest that cell strains may provide a powerful adjunct to the human tumorgrafts.
In conclusion, we have described a large panel of human head and neck cancer squamous cell carcinoma tumorgrafts that reflect both HPV-positive and HPV-negative tumors. The tumorgrafts display a spectrum of differentiation typical of clinical histopathologic specimens, and a high degree of consistency between original patient tumor and tumorgraft. The combined use of early passage cell strains and tumorgrafts provides a powerful system for investigating novel therapeutics, combination therapies, and for testing hypotheses of mechanisms of therapeutic response and resistance in human head and neck cancer.
Supplementary Material
Supplemental Figure 1. Detection of multiple HPV subtypes by degenerate PCR of genomic DNA (A) or by HPV-16 specific Southern blot (B).
Statement of Translational Relevance.
We describe and characterize a direct-from-patient tumorgraft model system of human head and neck cancer including tumors derived from both human papillomavirus associated cancers and tobacco-associated cancers. Comparisons of primary tumor and tumorgrafts in addition to the significant diversity of tumor morphology suggest this resource represents an outstanding platform for investigating novel therapeutics and combinations of chemotherapy and/or radiation. These tumorgrafts may prove to be a valuable resource for optimizing therapy for specific subgroups of patients with head and neck cancer and may shed light into the molecular mechanisms underlying differential therapeutic responses between HPV-positive and HPV-negative head and neck cancer.
Acknowledgments
Financial support and disclosures: Supported by UWCCC pilot grant to PL and PH and by R01 CA 113448-01(PH), R01 DE017315 (PL), and U01 CA141583 (PL). RK is supported by the UW Kaye Fellowship in Head and Neck Cancer Research, K99 CA160639-01, Radiological Society of North American Research Fellow Grant and AACR/Bristol Myers Squibb Fellowship in Clinical Cancer Research.
Footnotes
Conflicts of Interest: none
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer. 2010 doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 3.Lassen P. The role of Human papillomavirus in head and neck cancer and the impact on radiotherapy outcome. Radiother Oncol. 2010;95:371–380. doi: 10.1016/j.radonc.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Gillet JP, Calcagno AM, Varma S, Marino M, Green LJ, Vora MI, et al. Redefining the relevance of established cancer cell lines to the study of mechanisms of clinical anti-cancer drug resistance. Proc Natl Acad Sci U S A. 2011;108:18708–18713. doi: 10.1073/pnas.1111840108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uva P, Lahm A, Sbardellati A, Grigoriadis A, Tutt A, de Rinaldis E. Comparative Membranome expression analysis in primary tumors and derived cell lines. PLoS One. 2010;5 doi: 10.1371/journal.pone.0011742. e11742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeRose YS, Wang G, Lin YC, Bernard PS, Buys SS, Ebbert MT, et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med. 2011;17:1514–1520. doi: 10.1038/nm.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hidalgo M, Bruckheimer E, Rajeshkumar NV, Garrido-Laguna I, De Oliveira E, Rubio-Viqueira B, et al. A pilot clinical study of treatment guided by personalized tumorgrafts in patients with advanced cancer. Mol Cancer Ther. 2011;10:1311–1316. doi: 10.1158/1535-7163.MCT-11-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sivanand S, Pena-Llopis S, Zhao H, Kucejova B, Spence P, Pavia-Jimenez A, et al. A validated tumorgraft model reveals activity of dovitinib against renal cell carcinoma. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003643. 137ra75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snijders PJ, van den Brule AJ, Schrijnemakers HF, Snow G, Meijer CJ, Walboomers JM. The use of general primers in the polymerase chain reaction permits the detection of a broad spectrum of human papillomavirus genotypes. J Gen Virol. 1990;71(Pt 1):173–181. doi: 10.1099/0022-1317-71-1-173. [DOI] [PubMed] [Google Scholar]
- 10.Schache AG, Liloglou T, Risk JM, Filia A, Jones TM, Sheard J, et al. Evaluation of human papilloma virus diagnostic testing in oropharyngeal squamous cell carcinoma: sensitivity, specificity, and prognostic discrimination. Clin Cancer Res. 2011;17:6262–6271. doi: 10.1158/1078-0432.CCR-11-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant SW, Kyshtoobayeva AS, Kurosaki T, Jakowatz J, Fruehauf JP. Mutant p53 correlates with reduced expression of thrombospondin-1, increased angiogenesis, and metastatic progression in melanoma. Cancer Detect Prev. 1998;22:185–194. doi: 10.1046/j.1525-1500.1998.0oa18.x. [DOI] [PubMed] [Google Scholar]
- 12.Tang AL, Hauff SJ, Owen JH, Graham MP, Czerwinski MJ, Park JJ, et al. UM-SCC-104: A New human papillomavirus-16-positive cancer stem cell-containing head and neck squamous cell carcinoma cell line. Head Neck. 2011 doi: 10.1002/hed.21962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao M, Sano D, Pickering CR, Jasser SA, Henderson YC, Clayman GL, et al. Assembly and Initial Characterization of a Panel of 85 Genomically Validated Cell Lines from Diverse Head and Neck Tumor Sites. Clin Cancer Res. 2011;17:7248–7264. doi: 10.1158/1078-0432.CCR-11-0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferris RL, Martinez I, Sirianni N, Wang J, Lopez-Albaitero A, Gollin SM, et al. Human papillomavirus-16 associated squamous cell carcinoma of the head and neck (SCCHN): a natural disease model provides insights into viral carcinogenesis. Eur J Cancer. 2005;41:807–815. doi: 10.1016/j.ejca.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 15.Gwosdz C, Balz V, Scheckenbach K, Bier H. p53, p63 and p73 expression in squamous cell carcinomas of the head and neck and their response to cisplatin exposure. Adv Otorhinolaryngol. 2005;62:58–71. doi: 10.1159/000082473. [DOI] [PubMed] [Google Scholar]
- 16.Steenbergen RD, Hermsen MA, Walboomers JM, Joenje H, Arwert F, Meijer CJ, et al. Integrated human papillomavirus type 16 and loss of heterozygosity at 11q22 and 18q21 in an oral carcinoma and its derivative cell line. Cancer Res. 1995;55:5465–5471. [PubMed] [Google Scholar]
- 17.El-Naggar AK, Westra WH. p16 expression as a surrogate marker for HPV-related oropharyngeal carcinoma: a guide for interpretative relevance and consistency. Head Neck. 2012;34:459–461. doi: 10.1002/hed.21974. [DOI] [PubMed] [Google Scholar]
- 18.Harris SL, Thorne LB, Seaman WT, Hayes DN, Couch ME, Kimple RJ. Association of p16(INK4a) overexpression with improved outcomes in young patients with squamous cell cancers of the oral tongue. Head Neck. 2011;33:1622–1627. doi: 10.1002/hed.21650. [DOI] [PubMed] [Google Scholar]
- 19.Kumar B, Cordell KG, Lee JS, Worden FP, Prince ME, Tran HH, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26:3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fury MG, Drobnjak M, Sima CS, Asher M, Shah J, Lee N, et al. Tissue microarray evidence of association between p16 and phosphorylated eIF4E in tonsillar squamous cell carcinoma. Head Neck. 2011;33:1340–1345. doi: 10.1002/hed.21621. [DOI] [PubMed] [Google Scholar]
- 21.de Bono JS, Ashworth A. Translating cancer research into targeted therapeutics. Nature. 2010;467:543–549. doi: 10.1038/nature09339. [DOI] [PubMed] [Google Scholar]
- 22.Spanos WC, Nowicki P, Lee DW, Hoover A, Hostager B, Gupta A, et al. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009;135:1137–1146. doi: 10.1001/archoto.2009.159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Detection of multiple HPV subtypes by degenerate PCR of genomic DNA (A) or by HPV-16 specific Southern blot (B).