What if Gastroenterologists Were Accountable for Preventing Colorectal Cancer?
Gastroenterologists will soon have a choice regarding whether they want to guide colorectal cancer (CRC) screening programs within populations, as leaders, or to continue to function primarily as proceduralists who focus on providing colonoscopy services. With passage of the Patient Protection and Affordable Care Act (ACA), millions of currently uninsured patients will gain access to much needed gastroenterology services, particularly CRC screening. In addition to increasing access to care, the ACA will gradually shift reimbursement for Medicare services from a fee-for-service structure to a health systems model that rewards improved patient care in a population, higher efficiency, value, and innovation. As a result, Accountable Care Organizations (ACOs) and Integrated Healthcare Delivery Systems (IHDS) will assume increasing responsibility for population-based clinical outcomes such as CRC prevention. How can gastroenterologists – who traditionally serve patients on an individual referral basis – successfully help prevent CRC in a population?
CRC is one of the leading causes of cancer-related deaths in the United States (US) and a common cause of morbidity and mortality worldwide.1 The United States Preventive Services Task Force (USPSTF) and National Comprehensive Cancer Network (NCCN) both recommend all individuals aged 50 to 75 years, who are at average risk for CRC, to undergo CRC screening using one of the following: an annual high sensitivity fecal occult blood test (FOBT) or fecal immunochemical test (FIT), flexible sigmoidoscopy every 5 years, or colonoscopy every 10 years.2,3 Despite these recommendations and the existence of national consensus guidelines, CRC screening rates remain low; as of 2010, only 65% of eligible adults were up-to-date with CRC screening, far fewer than screening for breast and cervical cancer.4
Customizing screening to the individual
One of the key impediments to increasing CRC screening rates and minimizing CRC mortality may be our focus on “the single best screening test” rather than the overall best strategy.5,6 A one-size-fits-all approach to screening tests has significant limitations. Despite the demonstrated effectiveness of colonoscopy in detecting prevalent cancers and removing adenomas, there is concern that interval cancers can occur after a negative colonoscopy due to variability in the quality of the exam, aggressive tumor biology, and the long interval between each negative colonoscopy (≥10 years). The second most common screening option in the US, fecal-based testing (FIT and FOBT), is the most widely used screening test in all of Europe due to its non-invasiveness, high adherence rate, and low costs. However, while FIT has a high sensitivity and specificity for CRC, its ability to detect early or even advanced neoplasia is lower than that of colonoscopy when compared on a one-time basis. Therefore, FIT requires annual or biennial testing in order to effectively reduce CRC incidence and mortality.
Tailoring screening to patient preferences and to patient risk may provide the greatest reduction in CRC mortality; this approach may include combining different individual screening tests into novel screening strategies, customized to patient risk and prior screening history.5 If risk factors for advanced neoplasia can effectively stratify risk, then screening and surveillance can be tailored or targeted to persons with high risk, average risk, or low risk for advanced neoplasia with different intensities/frequencies. Furthermore, understanding the absolute risk of CRC and advanced neoplasia after a negative colonoscopy or several rounds of negative FITs in different populations, at different ages, combined with increased adoption of electronic medical records, can provide risk stratification tools to help assign the right test, to the right person at the right time and frequency. Higher risk patients may be best served entirely by frequent colonoscopies, for example, whereas lower risk patients may be best served by infrequent invasive tests, noninvasive tests such as FIT or fecal DNA, or by a combination of the two to both “clear out” polyps and to detect interval cancers.
The importance of patient preference also has been shown to impact CRC screening rates. In a recent study by Inadomi et al, average-risk participants from a racially diverse group of 997 people were randomized to receive recommendation for screening with colonoscopy, fecal testing, or their choice of colonoscopy or fecal testing.6 The primary outcome was completion of CRC screening within 12 months. When given a choice of screening methods, 69% of people were screened within the year. In contrast, when the physician recommended colonoscopy, only 38% of people followed through with screening. When fecal testing was recommended, 67% of people completed the screening test. The researchers also found that cultural influences may play a role in adherence to CRC screening. In particular, Caucasian participants preferred colonoscopy while African-American, Asian, and Latino participants preferred fecal testing as their screening test of choice. Based on this study, universally recommending a single test may actually reduce compliance to CRC screening, especially among our minority and underserved populations who already have the lowest CRC screening rates.
Improving global screening rates and decreasing cancer deaths will be important not only for the patients, but also for the financial viability of the Accountable Care Organizations and Integrated Healthcare Delivery Systems, after the introduction of value-based incentive payments for meeting CRC prevention quality measures.
Focusing on quality over quantity
More colonoscopies do not necessarily equate to better outcomes. Recent studies have suggested that colonoscopy is less effective in the right side as compared to the left side of the colon and that interval cancers can occur after a negative or clearing colonoscopy.7,8 Among the possible reasons that contribute to interval cancers include aggressive tumor biology, inadequate bowel preparation, suboptimal examination technique, low adenoma detection rates (ADRs), and lack of cecal intubation. In addition, production pressure and fatigue can adversely affect the quality and safety of the colonoscopic examination. Compounding the problem of interval cancers, ironically, is the overuse of colonoscopies to monitor low-risk patients who are unlikely to benefit from aggressive surveillance. In a study by Goodwin et. al., overuse of screening colonoscopies was commonly seen in a large proportion of Medicare patients.9 In fact, patients aged 75-79 years or 80 years or older at the time of the initial negative screening colonoscopy, 46% and 33%, respectively, received a repeat examination within 7 years.9 Moreover in a national survey, over 50% of US gastroenterologists and surgeons recommended surveillance colonoscopies every 3 years for patients with a small adenoma (<1cm), even though guidelines suggest that 5-10 years is an appropriate surveillance interval.10
Within the past few years, there has been increased awareness that the success of colonoscopy in preventing CRC and minimizing complications is dependent on the skill and competence of the endoscopist. Recent studies have shown that failure of cecal intubation rates and low ADRs are associated with increased risk for interval cancers. In addition, bowel preparation, withdrawal time, and withdrawal technique have been linked to ADR, which is currently our best surrogate marker for quality.
As we move towards accountability and a pay-for-performance structure, gastroenterologists will be rated and judged by the quality of their colonoscopy. In fact, by 2013, insurance companies and the Centers for Medicaid and Medicare Services will start rolling out programs similar to Physician Quality Reporting System (PQRS) to allow patients to select their gastroenterologists based on participation in quality reporting initiatives. However, does measuring and recording quality improve patient outcomes? The verdict is still out, but Rex et al. recently demonstrated that measuring and reporting quality can improve patient care.11 In his study, endoscopists were secretly videotaped performing colonoscopies. Afterwards, endoscopists were later informed that they were going to be videotaped to assess the attainment of five quality measures. Compared with the stealth baseline videos, measurable improvements were demonstrated for all endoscopists on all parameters. This suggests that the focus should be on providing high-quality exams instead of performing more frequent, but low-quality, colonoscopies.
Developing organized versus opportunistic screening
Organized screening offers the promise of uniformly screening all eligible members of a population with a risk- and preference-based approach. An organized approach identifies who needs to be screened and contacts them to arrange screening; it creates a screening process rather than using a “convenience” approach whereby screening is mainly offered during health care visits conducted for other purposes. In addition, this approach provides much greater attention to the quality of the screening process, including timely referrals and appropriate follow-up of participants, and it provides greater protection against the harms of screening, including overuse and misuse of screening tests.
In contrast to the approach described below, CRC screening, as currently practiced in most US practice settings, uses an opportunistic approach, meaning that patients who come to the physician’s office for a general checkup or other unrelated issues are offered screening with a colonoscopy or a fecal-based test. As a result, those who visit a doctor regularly are more likely to be screened than those who do not. Not surprisingly, a recent report shows that only 65% of the population is up-to-date with CRC screening4 and some populations are more disproportionately affected than others; for example; blacks and Hispanics have lower screening rates than non-Hispanic whites, and urban areas have more CRC screening capacity than rural areas.4 Further adding to the issue of CRC screening disparity with an opportunistic approach is the overuse, underuse, and misuse of screening tests, which to date remains a significant problem in the US.
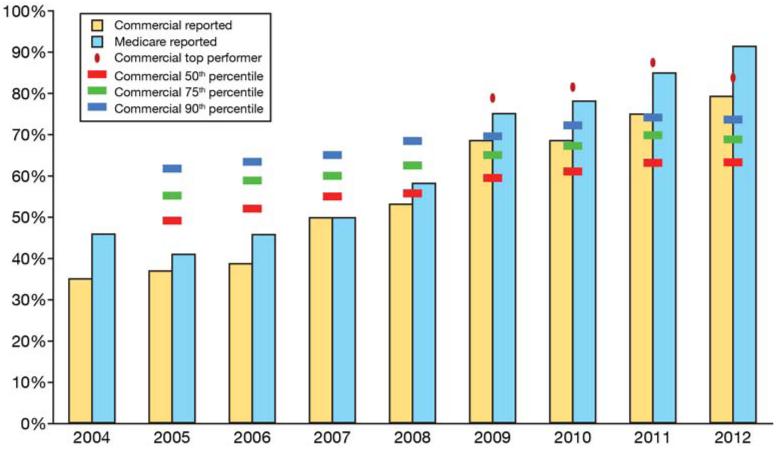
Although many examples of organized screening exist, one illustration of an integrated approach is the program in Kaiser Permanente, Northern California (KPNC), and a health care delivery system with over 3.2 million members. In addition to opportunistic referral for screening colonoscopy, since 2007 KPNC has used its electronic medical records to identify persons due for screening and targeted them with a supplemental organized population-based mailed outreach of FIT screening kits. Opportunistic in-reach supplements the mailed outreach, using electronic health record prompts to identify patients who are due for screening at the time of an office visit.12 Directed by a gastroenterologist, and orchestrated by the gastroenterology chief of each hospital, the program makes the entire organization accountable for CRC screening. From 2005, when the Healthcare Effectiveness Data and Information Set (HEDIS) CRC screening rates were first publicly reported, to 2011, the proportion of the commercially insured population screened in accordance with HEDIS measures at KPNC, has increased from 37% to 79%; the proportion of the Medicare population screened has increased form 41% to 91% (Figure 1). More importantly, unpublished data indicate this increase in screening has been associated with a change in cancer stage and even the incidence of CRC in the KPNC population (manuscript in preparation).
Figure 1.
Publicly reported HEDIS colorectal cancer screening rates for Kaiser Permanente, Northern California, for each year from 2004 to 2012. The Medicare population (light blue bars) are reported separately from the commercial population (green bars). The red, green, and blue hash marks represent the commercial 50th, 70th, and 90th percentiles, respectively. The red dots represent the commercial top performer each year. Note that each year’s reported results refer to screening performance as of the end of the prior year.
Conclusion
In the very near future, the CRC “screening” focus so prominent in the US in 2012 will change to a focus of “CRC prevention” as IHDN’s begin to assume both financial and performance risk for patient populations. As the effects of the ACA begin to take shape in the coming years, gastroenterologists will experience changes in models of reimbursement and in their role in the management of gastrointestinal and liver disorders in their communities. Anticipate these changes to continue irrespective of the outcome of the 2012 election. Gastroenterologists will have to consider whether their practices will transition to population-based management and prevention of CRC or remain primarily focused on providing referral-based colonoscopy services. With anticipated reductions in fee for service reimbursement, staying focused only on colonoscopy may not be a sustainable business model for our specialty.
Although we have made great strides in reducing CRC rates over the past three decades, many people remain unscreened and we are far from our goal of eliminating deaths from CRC. Gastroenterologists are uniquely equipped to develop and optimize screening strategies by tailoring our CRC screening to individual risk rather than focusing on a “single best test,” respecting patient preferences, providing high-quality colonoscopies, and developing an organized and resource-efficient screening approach. Healthcare reform will transform the way we provide CRC screening; gastroenterologists are at a pivotal point in deciding how to be involved in that change.
Acknowledgments
Funding: This work was supported by the grant T32DK007007 from the National Institutes of Health (JKL), Kaiser Permanente Community Benefits program, and from the National Cancer Institute (U54 CA163262)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Summary:
All authors have no conflict of interest in this manuscript.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics 2010. CA Cancer J Clin. 2010;60:277–96. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Screening for colorectal cancer: U. S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology — v.1.2009. NCCN; Jenkintown, PA: 2009. Colorectal cancer screening. [DOI] [PubMed] [Google Scholar]
- 4.Richardson LC, Tai E, Rim SH, et al. Vital signs: colorectal cancer screening, incidence, and mortality – United States, 2002-2010. MMWR Morb Mortal Wkly Rep. 2011;60:884–9. [PubMed] [Google Scholar]
- 5.Corley DA. The future of colon cancer screening: what do we recommend and will it be too much, too little, or just right? Gastroenterology. 2011;141:1956–1958. doi: 10.1053/j.gastro.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012;172:575–82. doi: 10.1001/archinternmed.2012.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baxter NN, Goldwasser MA, Paszat LF, et al. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 8.Brenner H, Chang-Claude J, Seiler CM, et al. Interval cancers after negative colonosocopy: population-based case-control study. Gut. 2012;61:1576–82. doi: 10.1136/gutjnl-2011-301531. [DOI] [PubMed] [Google Scholar]
- 9.Goodwin JS, Singh A, Reddy N, et al. Overuse of screening colonoscopy in the medicare population. Arch Intern Med. 2011;171:1335–1343. doi: 10.1001/archinternmed.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mysliwiec PA, Brown ML, Klabunde CN, et al. Are physicians doing too much colonoscopy? A national survey of colorectal surveillance after polypectomy. Ann Intern Med. 2004;141:264–271. doi: 10.7326/0003-4819-141-4-200408170-00006. [DOI] [PubMed] [Google Scholar]
- 11.Rex DK, Hewett DG, Raghavendra M, et al. The impact of videorecording on the quality of colonoscopy performance: a pilot study. Am J Gastroenterol. 2010;105:2312–7. doi: 10.1038/ajg.2010.245. [DOI] [PubMed] [Google Scholar]
- 12.Levin TR, Jamieson L, Burley DA, et al. Organized colorectal cancer screening in integrated health care systems. Epidemiol Rev. 2011;33:101–110. doi: 10.1093/epirev/mxr007. [DOI] [PubMed] [Google Scholar]