Chediak-Higashi syndrome (CHS) (OMIM #214500) is a rare autosomal recessive disorder caused by mutations in the lysosomal trafficking regulator gene, LYST, or CHS1. Typically, CHS presents, with variable degrees of oculocutaneous albinism, immunodeficiency, bleeding diathesis and hemophagocytic lymphohistiocytosis (HLH or the “accelerated phase”).1 Neurological involvement in CHS can include intellectual impairment, sensory-motor neuropathy, cerebellar disease, and dementia.2, 3 Parkinsonism and its response to dopaminergic therapy has rarely been reported.2,4,5 A subset of CHS patients have a muted pigmentary or hematological presentation while their neurological symptoms dominate their disease.6, 7 We a provide video illustration of his therapeutic response to levodopa along with skin pigment dilution features, brain imaging and leukocyte morphology in a young adult male with CHS whose clinical presentation is dominated by motor and non-motor parkinsonian symptoms with a brisk and sustained therapeutic response to levodopa therapy.
Case Report
We report a 21-year-old African American male who had been gainfully employed and physically active, participating in martial arts and weight lifting until the age of 20. Over the ensuing 18 months, he developed progressive low back pain, most severe with prolonged standing; it was unresponsive to traditional pain management, including narcotics. The patient reported progressive fatigue and increasing difficulties initiating and maintaining sleep. He developed a stooped posture and began experiencing difficulty with balance (particularly with turning and picking objects up from the ground), frequent falls, intermittent bilateral hand tremor, decreased dexterity and poor endurance.
The patient carried a diagnosis of CHS, based initially on an incidental finding of giant granules on a peripheral blood smear collected in infancy. Outside of two episodes of osteomyelitis in childhood, gingival bleeding and recurrent epistaxis, his CHS was relatively mild and did not require bone marrow transplantation. Mutation analysis identified one nonsense mutation (c.1507C>T, p.R503X in exon 5) and one missense mutation (c.9925G>A, p.G3309S in exon 43) in the LYST gene.
The initial physical examination at the NIH Clinical Center revealed a young man with truncal obesity and a speckled skin appearance reflecting areas of hyper and hypopigmentation scattered diffusely over the body [Figure 1A]. There was mild psychomotor slowness. Facial expression demonstrated hypomimia. Speech was soft and muffled. There was a general paucity of spontaneous movements. Cranial nerves were normal except mild saccadic intrusion during smooth pursuit. Motor examination revealed moderate bilateral bradykinesia and cogwheel-type rigidity in the upper and lower extremities. There was mild (4+/5) weakness of toe extensors and foot dorsiflexor muscles bilaterally. Posture was stooped. He ambulated with hesitant short steps, diminished arm swing and mild dystonic posturing of the right hand. He turned “en bloc” and had difficulties recovering from an unexpected retropulsive tug. His reflexes were hypoactive throughout. His plantar responses were extensor on the right and neutral on the left. The UPDRS Part III (Motor Examination) score was 31 points. (See video segment 1.)
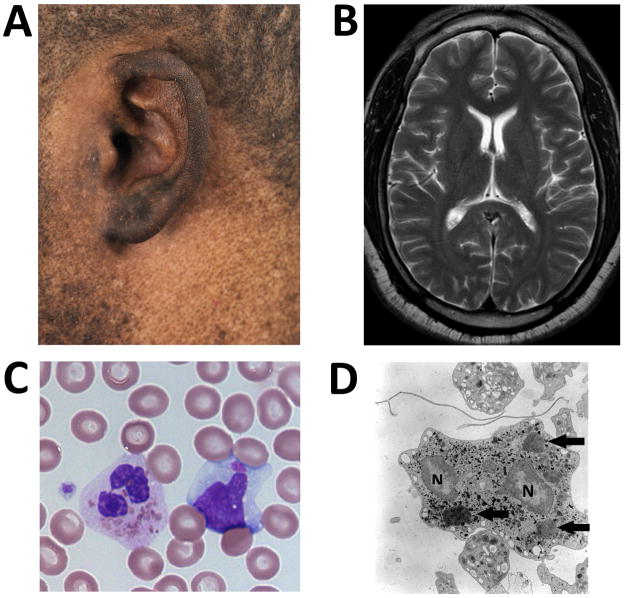
Figure 1.
A: Pigment dilution in this patient of African-American descent over the skin surrounding his left ear.
B: T2 weighted MRI image at the level of the head of the caudate nuclei.
C: Several pathognomonic giant intracellular inclusions within a neutrophil and a single giant inclusion in a lymphocyte on light microscopy of a peripheral blood smear.
D: Giant inclusions within the neutrophil on electron microscopy. Arrows identify giant inclusions. “N” identify portions of the segmented nucleus.
Cranial [Figure 1B] and lumbar spine MRIs were normal. EMG/NCV showed features of mild axonal sensory neuropathy. Giant leukocyte granules were noted in his peripheral blood smear and by electron microscopy examination [Figure 1C and D]. Platelet dense bodies were absent on electron microscopy examination. Symptoms of overt parkinsonism associated with significant impairment of quality of life prompted a trial of levodopa therapy. Carbidopa/levodopa tablets were rapidly escalated to a dose of 37.5/150 mg, three-times daily; the medication was well tolerated. Within 3 weeks of initiating levodopa therapy, the patient reported complete resolution of low back pain and better quality of sleep. His general sense of discomfort and fatigue improved. Motor dexterity and ambulatory capacity improved as well. UPDRS Part III score six months after initiation of therapy was 17 points. Four consecutive attempts at the 20-foot timed walk averaged 13.7 ± 1.23 seconds pre-treatment. The same 20-foot timed walk improved to 5.22 ± 1.21seconds 6 months after therapy. (See video segment 2). The patient continues to do well on carbidopa/levodopa therapy.
Discussion
Our patient presented with a phenotype of non-motor and motor parkinsonian complaints. Both elements had an unequivocal response to levodopa therapy implicating a dopaminergic deficit as a main mechanism of symptoms. Variable degrees of motor symptoms improvement to antiparkinsonian agents such as levodopa, dopamine agonists and amantadine have been previously reported in patients with CHS.2,4,5 Like our patient, age at the onset of motor complaints is usually in the second or third decade of life. Unlike our case, most prior reports describe patients with combination of other neurological deficits including cerebellar ataxia, foot drop, dystonia, cortical atrophy and cognitive decline as part of their CHS neurological phenotype. Unique features of our case were the prominences of non-motor symptoms, in particular, back pain and sleep difficulties, and the relatively pure clinical parkinsonian phenotype.
Since a yet unknown fraction of patients with CHS might present primarily with a neurological phenotype due to relatively muted hematological and pigmentary manifestations, we suggest that patients such as ours may present to movement disorder specialists for evaluation of parkinsonism. Contrary to patients manifesting primarily neurological symptoms, classic CHS is a serious and life-threatening disease. Mortality is high in the first decade of life secondary to infection due to quantitative and qualitative leukocyte defects, bleeding tendency caused by markedly diminished or absent platelet dense bodies, or development of HLH1, all of which are amenable to treatment by bone marrow transplantation.8 In contrast, neurological deficits in CHS generally appear in adolescence or early adulthood and are not ameliorated by BMT.3
The gene mutated in CHS patients, LYST, encodes a protein that regulates lysosome-related organelle size and movement.9 The presence of a neurological phenotype in CHS, suggests that the LYST protein plays a yet uncharacterized role in neuronal function and that this function may be relevant to understanding at least some aspects of the dopaminergic deficits that accompany other forms of parkinsonism. We recommend consideration of a CHS diagnostic evaluation for young patients being evaluated for parkinsonism, particularly those with skin, hair or eye pigment dilution, excess bleeding or an infectious diathesis.
Supplementary Material
Video Legend Segment 1. Excerpts of the neurological exam pre-treatment.
Video Legend Segment 2. 20 foot timed walk before and 6 months after carbidopa/levodopa treatment initiation.
Acknowledgments
The authors are indebted to Dr. William A. Gahl for his support, Dr. James G. White at the Department of Laboratory Medicine, Pathology and Pediatrics, University of Minnesota for gratefully providing the EM illustration and Catherine Groden for her dedicated clinical care. We specially thank the patient and his family for their cooperation.
Footnotes
Author Roles
1. Research project: A. Conception, B. Organization, C. Execution
2. Statistical Analysis: NA
3. Manuscript: A. Writing of the first draft, B. Review and Critique
Vikas Bhambhani: 1A, 1B, 1C, 3A, 3B
Wendy J. Introne: 1A, 1B, 1C, 3A, 3B
Catherine Groden: 1B, 1C
Codrin Lungu: 1C, 3B
Andrew Cullinane: 1C, 3A, 3B
James G. White: 1B, 1C
William A. Gahl: 1A, 1B, 1C, 3B
Camilo Toro: 1A, 1B, 1C, 3A, 3B
Full Financial Disclosures of all Authors for the Past Year
Vikas Bhambhani: None
Wendy J. Introne: None
Catherine Groden: None
Codrin Lungu: None
Andrew Cullinane: None
James G. White: None
William A. Gahl: None.
Camilo Toro: None
Financial Disclosure related to the work covered in this article:
This work was conducted through funding from the NHGRI Intramural Research Program.
There is no potential conflict of interest for any of the authors.
References
- 1.Introne W, Boissy RE, Gahl WA. Clinical, molecular, and cell biological aspects of Chediak-Higashi syndrome. Mol Genet Metab. 1999;68(2):283–303. doi: 10.1006/mgme.1999.2927. [DOI] [PubMed] [Google Scholar]
- 2.Uyama E, Hirano T, Ito K, et al. Adult Chediak-Higashi syndrome presenting as parkinsonism and dementia. Acta Neurol Scand. 1994;89(3):175–183. doi: 10.1111/j.1600-0404.1994.tb01657.x. [DOI] [PubMed] [Google Scholar]
- 3.Tardieu M, Lacroix C, Neven B, et al. Progressive neurologic dysfunctions 20 years after allogeneic bone marrow transplantation for Chediak-Higashi syndrome. Blood. 2005;106(1):40–42. doi: 10.1182/blood-2005-01-0319. [DOI] [PubMed] [Google Scholar]
- 4.Hauser RA, Friedlander J, Baker MJ, et al. Adult Chediak-Higashi parkinsonian syndrome with dystonia. Mov Disord. 2000;15(4):705–708. doi: 10.1002/1531-8257(200007)15:4<705::aid-mds1016>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 5.Silveira-Moriyama L, Moriyama TS, Gabbi TVB, et al. Chediak-Higashi syndrome with Parkinsonism. Mov Disord. 2004;19(4):472–475. doi: 10.1002/mds.10677. [DOI] [PubMed] [Google Scholar]
- 6.Karim MA, Suzuki K, Fukai K, et al. Apparent genotype-phenotype correlation in childhood, adolescent, and adult Chediak-Higashi syndrome. Am J Med Genet. 2002;108(1):16–22. [PubMed] [Google Scholar]
- 7.Westbroek W, Adams D, Huizing M, et al. Cellular defects in Chediak-Higashi syndrome correlate with the molecular genotype and clinical phenotype. J Invest Dermatol. 2007;127(11):2674–2677. doi: 10.1038/sj.jid.5700899. [DOI] [PubMed] [Google Scholar]
- 8.Introne WJ, Westbroek W, Golas GA, Adams D. GeneReviews at GeneTests Medical Genetics Information Resource (database online) Copyright, University of Washington; Seattle: 1997–2012. [Accessed [07/25/2012]]. Chediak-Higashi Syndrome. (Last Update: February 16, 2012) Available at http://www.genetests.org. [Google Scholar]
- 9.Kaplan J, De Domenico I, Ward DM. Chediak-Higashi syndrome. Curr Opin Hematol. 2008;15(1):22–29. doi: 10.1097/MOH.0b013e3282f2bcce. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video Legend Segment 1. Excerpts of the neurological exam pre-treatment.
Video Legend Segment 2. 20 foot timed walk before and 6 months after carbidopa/levodopa treatment initiation.