Abstract
OBJECTIVE
Clinically, vaginal progesterone (VP) and 17 alpha-hydroxyprogestreone caproate (17P) have been shown to prevent preterm birth (PTB) in high risk populations. We hypothesize treatment with these agents may prevent PTB by altering molecular pathways involved in uterine contractility or cervical remodeling.
STUDY DESIGN
Using a mouse model, on days E14-E17 CD-1 pregnant mice were treated with either 1) 0.1cc of 25 mg/ml of 17P subcutaneously, 2) 0.1cc of castor oil subcutaneously, 3) 0.1 cc of 10 mg/ml of progesterone in Replens vaginally, or 4) 0.1cc of Replens vaginally, with four dams per treatment group. Mice were sacrificed six hours after treatment on E17.5. Cervices and uteri were collected for molecular analysis.
RESULTS
Exposure to VP significantly increased the expression of Defensin 1 compared to Replens (p<0.01) on E17.5. Neither VP nor 17P altered the expression of uterine contraction-associated proteins, progesterone mediated regulators of uterine quiescence, microRNAs involved in uterine contractility, or pathways involved in cervical remodeling. In addition, neither agent had an effect on immune cell trafficking or collagen content in the cervix.
CONCLUSION
Neither VP nor 17P had any effect on the studied pathways known to be involved in uterine contractility or quiescence. In the cervix, neither VP nor 17P altered pathways demonstrated to be involved in cervical remodeling. Administration of VP was noted to increase the expression of the antimicrobial protein Defensin 1. Whether this molecular change from VP results in a functional effect and is a key mechanism by which VP prevents PTB requires further study.
Keywords: Preterm Birth, Cervical Remodeling, Progesterone
Background
Preterm birth remains the leading cause of neonatal morbidity and mortality in the United States.1 In high risk populations, 17 alpha-hydroxyprogesterone caproate (17P)2 and vaginal progesterone (VP)3,4 have been shown to prevent preterm birth (PTB). However, the molecular mechanisms by which progesterone supplementation prevents preterm birth remain poorly understood.
While the precise pathways that govern preterm parturition remain to be discovered, an increase in uterine activity and premature cervical remodeling are believed to be critically involved in the pathogenesis of spontaneous preterm birth.5 Recent data suggests the use of progesterone may be important in maintaining uterine quiescence in the latter half of pregnancy by limiting the production of stimulatory prostaglandins and inhibiting the expression of contraction-associated protein genes (ion channels, oxytocin and prostaglandin receptors, and gap junctions) within the myometrium.6,7 While contraction-associated proteins have long been associated with myometrial activity, recent work has demonstrated the role of microRNAs in uterine activity. A conserved family of micro-RNAs (miRNAs), the miR-200 family, has been found to be significantly induced at term in both mice and humans. Additionally, two down-regulated targets ZEB1 and ZEB2 in this pathway act as transcriptional repressors and are downregulated at term.8 ZEB1, which has been shown to be up-regulated by progesterone in vivo, and ZEB2 inhibit the expression of the contraction associated genes oxytocin receptor and connexin-43.8 While human and mouse data support the role of these targets in uterine contractility, the effect of progesterone supplementation on these pathways has not been explored as a possible mechanism by which these agents prevent preterm birth.
In addition to uterine activity, premature cervical remodeling is believed to be an obligatory step in the pathogenesis of PTB.5 Clinical studies have shown a short cervix is a risk factor for preterm birth.9 Cervical remodeling and eventual cervical ripening is a biomechanical process that involves the gradual change of the connective tissue in the cervix throughout pregnancy.10,11 Maintenance of cervical integrity and appropriate timing of cervical remodeling is critical to maintain a pregnancy.
While similar pathways must be involved in the final phases of cervical ripening (to allow passage of the fetus), the inciting events that trigger cervical remodeling appear to differ in the preterm compared to term period.12-14 Preterm cervical remodeling pathways involve changes in immune cell trafficking, complement activation, and an altered collagen structure.12,15 In addition to these pathways, changes in the epithelial barrier have also been implicated in preterm cervical remodeling. Claudins, which in part regulate cell-cell adhesion and the permeability of the epithelial barrier, undergo changes in expression during cervical remodeling.16,17 Microarray data has also shown epithelial cell differentiation pathways are up-regulated with cervical remodeling as well.13
In other biological systems, such as the gastrointestinal system, there is a greater understanding of the pathways by which the immune system and the epithelial cell barrier interact leading to pathophysiological states.18,19 Once the epithelial barrier becomes a target for bacterial pathogens and/or their by-products, the mucosal immune response becomes essential in preventing disruption of the underlying stroma.15,16 In the epithelial barrier, mucosal immunity consists of antimicrobial proteins,20,21 along with immune cells and their related cytokines.22 Disruption of the cervical epithelial barrier from inflammation may lead to premature cervical remodeling and ultimately preterm birth.
Based on recent clinical studies demonstrating progestational agents can prevent preterm birth in high risk populations, we hypothesize these agents are modifying pathways that are critically involved in uterine activity and/or premature cervical remodeling. These studies sought to assess if supplementation with VP or 17P upregulated crucial aspects of these pathways as potential mechanisms by which they prevent preterm birth in clinical trials.
Materials and Methods
Mouse Model
All procedures were performed with Institutional Animal Care and Use Committee approval from the Perelman School of Medicine at the University of Pennsylvania. In this study, CD-1 out-bred, timed pregnant mice were used (Charles River Laboratories, Wilmington, Massachusetts). 17P (Boothwyn Pharmacy, Boothwyn PA) injections were prepared by diluting 0.1cc of 250 mg/ml in 0.9cc castor oil (Sigma Chemical Co, St. Louis, MO). Vaginal progesterone was prepared by diluting 40 mg of progesterone (Sigma Chemical Co, St Louis, MO) in 4ml of Replens. On days E14-E17, pregnant CD-1 mice were treated daily with either 1) 0.1cc of 25 mg/ml of 17P subcutaneously, 2) 0.1cc of castor oil subcutaneously, 3) 0.1 cc of 10 mg/ml of progesterone in Replens vaginally, or 4) 0.1cc of Replens vaginally. After administration of either VP or Replens, the mice were returned to their cages and none of the progesterone solution was noted to be leaking from the vagina. Prior work from our laboratory has demonstrated that administration of castor oil to mice does not induce preterm birth. Hence, an additional control group of mice exposed to castor oil was deemed unnecessary for these studies.23 The dose of VP and 17P represent a 25 and 17 fold increase compared to human dosing basing on 70 kilograms of body weight, respectively. Four dams per treatment group were used. A murine gestation lasts 19-21 days, and treatment on days E14-E17 would mimic late second and third trimester supplementation in humans. The same experiment was repeated twice with four dams per group for each set of experiments. The first set of experiments provided tissue for molecular analysis of uterine and cervical tissues. The second set provided cervical tissues for histological assessments as described below. On day E17.5, 6 hours after the last treatment, dams were euthanized. By harvesting tissue on E17.5, the uterine and cervical tissue could be analyzed prior to the progesterone withdrawl that occurs with term parturition in the murine model. Whole blood was collected from the aorta using a 25-gauge syringe. The blood was allowed to clot at room temperature for 2 hours and then spun-down for 20 minutes at 2,000 × g. The serum was collected and flash frozen in liquid nitrogen. Uterine tissue was harvested. Gestational sacs were removed and the decidua was left in situ. Cervical tissues were carefully dissected from the surrounding organs (bladder and bowel) and adjacent adipose tissue was removed. Tissues were rinsed in sterile saline solution and placed immediately in liquid nitrogen. Samples were stored at -80°C until processed for experiments described below.
Quantitative Polymerase Chain Reaction
Total RNA was extracted from the cervix and uterus with Trizol (Invitrogen) according to product protocol. Complementary DNA (cDNA) was generated from 2 μg of RNA/sample using a high capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, California). To assess expression, quantitative polymerase chain reaction (qPCR) was performed using equivalent dilutions of each sample on the Applied Biosystems Model 9700 sequence detector PCR machine. Primer sets, conjugated to Taqman MGB probes, were used for qPCR (Applied Biosystems). Uterine targets analyzed were contraction-associated proteins: connexin-43, oxytocin receptor and cyclooxygenase-2; and progesterone mediated regulators of uterine quiescence: stat 5b, zeb 1, zeb 2, and 20-aphahydroxysteroid dehydrogenase. Cervical targets analyzed included IL17, IL22, IL22R, Defensin 1, Defensin 3, Defensin 4, and SLP1. QPCR reactions were carried out with equivalent dilutions of each cDNA sample on the Applied Biosystems Model 7900 sequence detector PCR machine, as previously reported from our laboratory.24,25 The relative abundance of the target of interest was divided by the relative abundance of 18S in each sample to generate a normalized abundance for the target of interest. All samples were analyzed in duplicate. Statistical analyses were performed comparing means or medians depending on whether the data were parametric (student's t test) or non-parametric (Mann-Whitney). Analysis was performed by SigmaStat 3.5(Aspire Software International, Asburn, Virginia).
miRNA Quantitative Polymerase Chain Reaction
miRNA was isolated from mouse uterus using Trizol (Invitrogen) and the miRNeasy Isolation Kit (Qiagen). cDNA was generated from the isolated miRNA using the miScript Reverse Transcription II kit (Qiagen) and qPCR was performed on the 7900HT Real-Time PCR System (Applied Biosystems) using the miScript SYBR Green PCR kit (Qiagen) according to the manufacturers’ protocols. The ΔΔCT method was used for relative expression quantification using the RQ manager software v2.4 (Applied Biosystems). The endogenous reference gene RNU6B was used for miRNA quantification. All primer sets were purchased from Qiagen: miR-200a (MS00001813), 429 (MS00002366), and RNU6B (MS0001400). Statistical analyses were performed comparing means or medians depending on whether the data were parametric (student's t test) or non-parametric (Mann-Whitney). Analysis was performed by SigmaStat 3.5(Aspire Software International, Asburn, Virginia).
Staining of Cervical Tissue
For histological assessments of the cervix, after euthanizing the dam, the cervix was excised, removing all adjacent tissues and adipose and fixed in 4% paraformaldehyde for 24 hours. Samples were then immersed and stored in 20% sucrose at 4°C. Cervix were rehydrated in phosphate buffered saline, a graded series of alcohols, then embedded in paraffin and sectioned at 10 μm. As previously shown,13,26 longitudinal sections of cervix from each mouse were stained with picrosirius red to assess collagen content and cross-linked structure. Photomicrographs of birefringence of transmitted polarized light in sections were taken (Zeiss AxioImager A1, 20× objective). Multiple defined fields of view were analyzed for optical density using NIH Image J software (grey scale threshold determined with uncalibrated Rodbard standard curve). The optical density of birefringent polarized transmitted light is inversely related to the density of collagen content and structure. Thus, low optical density is indicative of areas bright red birefringence with high collagen content and complex dense crosslinking due to high transmittance of light. By contrast, areas with low birefringence and low collagen content and diffuse structure were dark and had proportionally high optical density values. Other sections were processed by immunohistochemistry to stain macrophages and counterstained with methyl green to identify cell nuclei. The BM-8 (F4/80) antibody was specific for macrophages because reports of cross-reactivity with other immune cells are not a consideration due to their absence or rare present in the murine cervix.27 Brightfield scans of sections were taken (Aperio ImageScope). In a snapshot of a calibrated fields of view for each section (approximately 1.251×106 μm3), cell nuclei and macrophages were counted. Data for collagen and immune cell counts were normalized to cell nuclei density for each individual to account for hypertrophy and hyperplasia of tissue. Data were evaluated by Levene's test for homogeneity of variance and determined to be normally distributed (P> 0.05). An unpaired Student's t-test was then used to assess statistical significance between control and treated mice.
Detection of Serum Progesterone
Progesterone levels were assessed with an enzyme-linked immunosorbent assay kit (ELISA) (USCN Life Science Inc., Houston TX). Four dams per treatment group were used. The detection range was 1.23-100 ng/ml, and the minimal detectable dose of progesterone was less than 0.48 ng/ml. Due to unknown levels of serum progesterone after VP and 17P administration, initial ELISAs were performed to determine optimal performance. Based on the detection range of the ELISAs and these initial experiments, maternal serum was diluted (1:10). We chose to test serum levels to determine if the administration of either VP or 17P had a measurable increase on circulating levels of progesterone indicating an increase in bioavailability at the cervix and uterus.
Results
Effect of Progestational Agents on Uterine Quiescence
Contraction Associated Proteins
Neither VP nor systemically administered 17P significantly altered the signal transduction pathways regulating the contraction associated proteins connexin-43, oxytocin receptor or cyclooxygenase-2 (Table 1).
Table 1.
Results of qRT-PCR on the effect of vaginal progesterone and 17 alpha-hydroxyprogesterone caproate on pathways associated with myometrial contractility.
| Gene Name | Replens | Vaginal Progesterone | P value | Castor Oil | 17 Alpha-Hydroxyprogesterone Caproate | P value |
|---|---|---|---|---|---|---|
| Contraction Associated Proteins: | ||||||
| Connexin-43 | 1.58±0.57 | 1.54±0.32 | 0.95 | 1.18±0.20 | 1.28±0.05 | 0.62 |
| Oxytocin Receptor | 0.06±0.02 | 0.05±0.01 | 0.77 | 0.06±0.03 | 0.03±0.01 | 0.39 |
| Cyclooxygenase-2 | 1.27±0.49 | 1.32±0.40 | 0.94 | 0.71±0.12 | 1.30±0.29 | 0.11 |
| Progesterone Mediated Regulators of Uterine Queiscence: | ||||||
| Stat 5b | 1.33±0.26 | 1.15±0.18 | 0.58 | 1.05±0.05 | 0.94±0.08 | 0.28 |
| Zeb 1 | 1.53±0.34 | 1.05±0.09 | 0.22 | 1.07±0.10 | 0.77±0.05 | 0.06 |
| Zeb 2 | 1.58±0.37 | 1.13±0.13 | 0.30 | 1.15±0.28 | 1.00±0.11 | 0.63 |
| 20-Alphahydroxysteroid Dehydrogenase | 1.45±0.35 | 0.97±0.24 | 0.30 | 1.07±0.07 | 1.00±0.29 | 0.81 |
| Mirco-RNAs: | ||||||
| miR-200a | 0.96±0.21 | 0.99±0.25 | 0.97 | 1.16±0.20 | 0.94±0.06 | 0.33 |
| miR-429 | 1.22±0.30 | 1.29±0.23 | 0.87 | 1.25±0.13 | 1.12±0.23 | 0.63 |
Progesterone Mediated Regulators of Uterine Quiescence
Neither treatment effected expression of recently discovered mediators of uterine quiescence: stat 5b, zeb 1, zeb 2, and 20-aphahydroxysteroid dehydrogenase (Table 1).
miRNA
Neither VP nor 17P effected the production of the micro-RNA miR-200a or mir-429 (Table 1).
Effect of Progestational Agents on Cervical Remodeling
Mucosal Immunity
Exposure to VP significantly increased the expression of Defensin 1 compared to Replens (p<0.01) (Figure 1). Treatment with either 17P or VP did not have a statistically significant effect on the production of IL22R or SLP1 (data not shown). Baseline mRNA values of IL17, IL22, Defensin 3, and Defensin 4 were very low and expression was not altered by either progesterone treatment.
Figure 1.
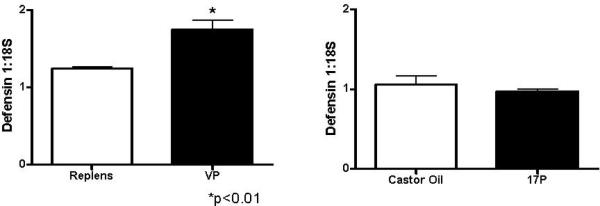
Bar graphs depicting the mean and standard error of the mean (SEM) (n=4 per treatment group) demonstrating the effect of vaginal progesterone compared to Replens and 17P compared to CO on the expression of Defensin 1 in the cervix. Exposure to VP significantly increased the expression of Defensin 1 compared to Replens (*p<0.01), unpaired Student's t-test.
Collagen Content
Characteristics associated with remodeling of the cervix in mice as pregnancy nears term did not vary with respect to progestational treatment.28 The number of methyl green stained cell nuclei per area of cervix section was not different in control mice given Replens or castor oil, and unaffected by VP or 17P treatment (p>0.05, Student's t-test). Picrosirius red- stained collagen fibers were loosely packed and disoriented in the stroma of cervix sections of controls and treated mice. No significant differences were found in the optical density of birefringent polarize light in sections of cervix between control groups and progesterone-treated mice (Figure 2).
Figure 2.
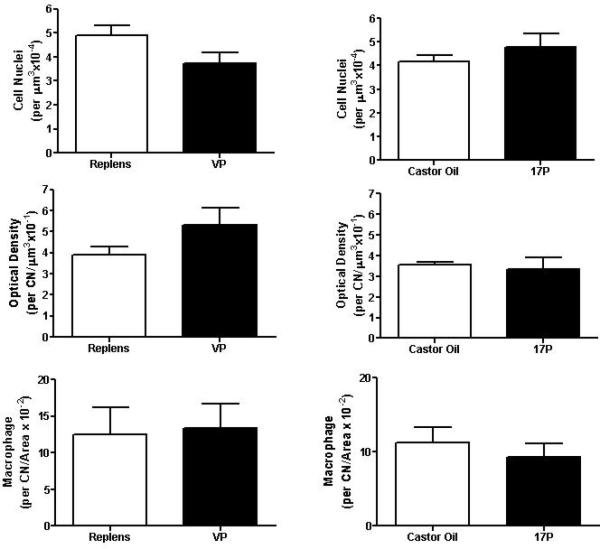
Bar graphs depicting the mean and the SEM (n=3 per treatment group) of the number of cell nuclei per area, optical density of birefringence of transmitted polarized light, and number of macrophages in the cervix from mice receiving the four treatment groups, unpaired Student's t-test.
Census of Immune Cells
Macrophages stained brown by immunohistochemistry were distributed in the subepithelial stroma, between smooth muscle cells in the perimetrium, and around blood vessels in the cervix. The presence of macrophages did not change with respect to type of progesterone treatment compared to respective controls (Figure 2).
Serum Progesterone
Exposure to VP significantly increased serum levels of progesterone when compared to Replens (p<0.05), and treatment with 17P significantly increased progesterone levels when compared to and CO (p<0.005) (Figure 3).
Figure 3.
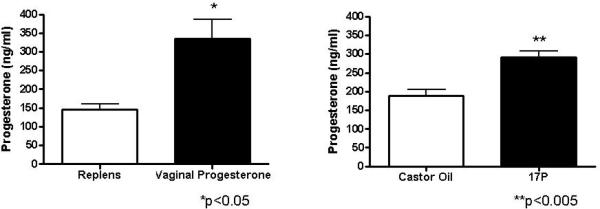
Bar graphs depicting the mean and SEM (n=4 per treatment group) demonstrating the effect of vaginal progesterone compared to Replens and 17P compared to CO on serum progesterone levels. Exposure to VP significantly increased serum levels of progesterone when compared to Replens (*p<0.05), and treatment with 17P significantly increased progesterone levels when compared to CO (**p<0.005), unpaired Student's t-test. There was no difference in circulating progesterone levels between Replens and castor oil with a p value of 0.09, unpaired Student's t-test.
Discussion
Although both VP and 17P are used to prevent preterm birth in high-risk patients, the mechanism of action for these agents has yet to be uncovered. We performed an exploration to assess if supplementation with progestational agents up-regulated crucial aspects on molecular pathways known to regulate uterine quiescence and cervical remodeling. Neither VP nor 17P had any effect on pathways known to be involved in uterine contractility or quiescence. In the cervix, neither VP nor 17P altered pathways demonstrated to be involved in cervical remodeling. Specifically, neither progestational agent altered collagen content, immune cell trafficking, or pathways associated with mucosal inflammation. Thus, contrary to the expectation that progesterone blocks pathways associated with structural remodeling of the prepartum cervix, administration of VP was only noted to increase the expression of the antimicrobial protein Defensin 1.
In many biological systems, epithelial cells serve as the first line of defense to bacteria. Infection of epithelial cells in the oral pharyngeal or gastrointestinal tract is a classic mechanism of disease in these systems.29 Similarly, infection of epithelial cells in the vagina and cervix has been demonstrated to be a mechanism by which Gonorrhea infection occurs.30 Human beta defensins are a component of the innate immune system and produced by epithelial cells. The observed increase in Defensin 1 with VP may serve to enhance the antimicrobial properties of the epithelial barrier, preventing a breakdown in the epithelial barrier from infectious processes. However, progesterone treatment or antagonism of the progesterone receptor does not appear to have an effect on Defensin 1 expression in vaginal epithelial cells.31,32 Of note, since we performed in vivo studies, VP could be affecting both vaginal and cervical epithelial cells. Thus, it is possible that VP could alter Defensin 1 expression in cervical cells, although no data regarding the effect of progesterone on these cells and Defensin 1 has been reported. We recognize that while statistically significant, the change in Defensin 1 mRNA expression may not have a biological effect. If progesterone does indeed modify Defensin 1 expression, further functional work is needed to determine if alterations in Defensin 1 levels in the cervicovaginal space is an essential mechanism by which VP prevents PTB.
In a mouse model of preterm birth, both cervical collagen content and structure are reduced as well as recruitment of macrophages are increased within six hours of treatment with LPS.13 These changes in the cervix are blocked when medroxyprogesterone acetate (MPA) is administered prior to LPS treatment.13 In this study, in normal murine pregnancy, neither VP nor 17P altered collagen content, with similar levels of collagen in an unripened cervix based on previous studies.33 While other work has demonstrated high levels of progesterone does not change collagen content,34 no prior studies have investigated if exogenous administration of progestational agents could alter collagen structure. By administering VP or 17P on days E14-E17, we were seeking to assess if progestational agents modified the cervix prior to term cervical remodeling in the absence of an inflammatory challenge. Therefore, these results demonstrate that in a non-inflammatory late-gestation mouse cervix, neither treatment served to fortify the cervix by increasing cervical collagen content or mitigating recruitment of macrophages. These findings do not exclude the possibility that progestational treatments affect activity of resident immune cells in the prepartum cervix .35 Whether these agents, similar to MPA, might limit inflammation-induced cervical remodeling has not yet been studied.
A strength of our study is that we explored the effect of these two agents on several pathways involved in uterine quiescence as well as those involved in cervical remodeling. Our results indicated treatments with these two agents did, in fact, raise circulating progesterone levels 3-4 fold compared to previously published murine progesterone E17 levels.36 This demonstrates the use of these agents resulted in a measurable increase in progesterone, indicating these increased levels would be available to the cervix and uterus for a biological effect. Although the ELISA used in this analysis measured total serum progesterone and did not differentiate between the different progesterone metabolites, the increase in levels with VP and 17P indicates these agents were well absorbed and resulted in an increase in bioavailability at the cervix and uterus. Despite the increase in serum progesterone levels, these agents appeared to have little effect on previously studied pathways.
A limitation of our study is the use of a mouse model. In mice, progesterone levels decrease prior to delivery.37 In contrast, a systemic progesterone withdrawal is not evident in humans. However, in humans, a functional progesterone withdrawal is believed to occur that contributes to the onset of parturition. Since we studied mice in mid-late gestation, prior to a systemic progesterone withdrawal, this difference in regards to progesterone withdrawal at term between mice and humans should have little impact on the results from these experiments. Thus, the studies remain of biological interest for our clinical use of progestational agents. In addition, such studies are not feasible in human pregnancy. These mouse studies provide insight into possible molecular mechanisms by which progestational agents prevent PTB. While we explored many pathways known to be involved in uterine contractility and/or cervical remodeling, these studies are limited as there are likely other pathways, not yet discovered, that are involved in these processes. Recognizing this limitation, our exploration was fairly comprehensive and investigated those pathways reported to be of significance based on available evidence regarding cervical remodeling and uterine contractility in term and preterm labor.
Since this work focused on the effect of progestational agents in normal pregnancy, we did not study the effect of VP or 17P in the setting of an inflammation-induced preterm birth. Clinically, the administration of exogenous progesterone may prevent PTB in women who have active inflammatory processes in the uterus and/or cervix. If this is the mechanism by which these agents prevent preterm birth, these studies herein are unable to address this possibility. Future studies using models of inflammation-induced PTB can provide insight into this possible mechanism.
In summary, these findings suggest neither VP nor 17P had significant effect on pathways involved in uterine activity or cervical remodeling in a normal murine pregnancy. These studies draw into question the mechanisms by which these agents prevent preterm birth in high risk women. By continuing to increase our understanding of the pathways leading to preterm parturition as well as the mechanisms by which progestational agents prevent PTB, we may be able to further our therapeutic strategies to decrease this significant adverse pregnancy outcome.
Condensation.
Neither vaginal progesterone nor 17 alpha-hydroxyprogestreone caproate had an effect on studied uterine contractility pathways, although vaginal progesterone was noted to increase the expression of the antimicrobial protein Defensin 1 in the cervix of CD-1 pregnant mice.
Acknowledgments
The animal experiments and molecular analysis was supported by the Maternal and Child Heath Research Fund at the University of Pennsylvania. Cervical cell staining and immune cell analysis was performed by Loma Linda University School of Medicine Advanced Imaging and Microscopy core facility and supported in part by NIH HD054931, Loma Linda University School of Medicine Dean, as well as for effort to photomicrograph, analyze, and process data by Donald Chase, MD, Department of Pathology and Human Anatomy, Abigail E. Dobyns, and Ryan Strilaeff.
This study was conducted at the University of Pennsylvania in Philadelphia, Pennsylvania and Loma Linda University in Loma Linda, California.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflict of interest.
References
- 1.Goldenberg R, Culhane J, Iams J, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meis P, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Eng J Med. 2003 Jun 12;348(24):2379–85. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 3.Fonseca EB, Celik E, Parra M, et al. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357:462–469. doi: 10.1056/NEJMoa067815. [DOI] [PubMed] [Google Scholar]
- 4.Hassan S, Romero R, Vidyadhari D, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011 Jul;38(1):18–31. doi: 10.1002/uog.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romero R, Lockwood C. Pathogenesis of spontaneous preterm labor. In: Creasy RK, Resnik R, Iams JD, Lockwood CJ, Romero R, editors. Creasy and Resnik's maternal fetal medicine: principles and practice. Saunders; Philadelphia, PA: 2009. pp. 521–544. [Google Scholar]
- 6.Norwitz E, Robinson J, Challis J. The Control of Labor. N Engl J Med. 1999;341:660–666. doi: 10.1056/NEJM199908263410906. [DOI] [PubMed] [Google Scholar]
- 7.Challis J, Matthews S, Gibb W, Lye S. Endocrine and Paracrine Regulation of Birth at Term and Preterm. Endocr Rev. 2000;21:514–550. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- 8.Renthal N, Chen C, Williams K, et al. miR-200 family and targets, ZEB1 and ZEB2, modulate uterine quiescence and contractility during pregnancy and labor. Proc Natl Acad Sci U S A. 2010 Nov 30;107(48):20828–33. doi: 10.1073/pnas.1008301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iams JD, Goldenberg RL, Meis PJ, et al. The Length of the Cervix and the Risk of Spontaneous Premature Delivery. N Engl J Med. 1996;334:567–573. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 10.Word RA, Li XH, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod med. 2007;25:69–79. doi: 10.1055/s-2006-956777. [DOI] [PubMed] [Google Scholar]
- 11.Maul H, Mackay L, Garfield RE. Cervical ripening: biochemical, molecular, and clinical considerations. Clin Obstet Gynecol. 2006;49:551–63. doi: 10.1097/00003081-200609000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Mahendroo M. Cervical remodeling in term and preterm birth: insights from an animal model. Reproduction. 2012 Apr;143(4):429–38. doi: 10.1530/REP-11-0466. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez J, Xu H, Chai J, et al. Preterm and term cervical ripening in CD1 Mice (Mus musculus): similar or divergent molecular mechanisms? Biol Reprod. 2009 Dec;81(6):1226. doi: 10.1095/biolreprod.108.075309. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez M, Dong Z, Romero R, Girardi G. Cervical remodeling/ripening at term and preterm delivery: the same mechanism initiated by different mediators and different effector cells. PLoS One. 2011;6(11):e26877. doi: 10.1371/journal.pone.0026877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yellon S, Ebner S, Elovitz M. Medroxyprogesterone acetate modulates remodeling, immune cell census, and nerve fibers in the cervix of a mouse model for inflammation-induced preterm birth. Reprod Sci. 2009 Mar;16(3):257–64. doi: 10.1177/1933719108325757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu H, Gonzalez JM, Ofori E, Elovitz MA. Preventing cervical ripening: the primary mechanism by which progestational agents prevent preterm birth? Am J Obstet Gynecol. 2008 Mar;198(3):314, e1–8. doi: 10.1016/j.ajog.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 17.Timmons B, Mitchell S, Gilpin C, Mahendroo M. Dynamic changes in the cervical epithelial tight junction complex and differentiation occur during cervical ripening and parturition. Endocrinology. 2007 Mar;148(3):1278–87. doi: 10.1210/en.2006-0851. [DOI] [PubMed] [Google Scholar]
- 18.Kimura H, Sawada N, Tobioka H, et al. Bacterial lipopolysaccharide reduced intestinal barrier function and altered localization of 7H6 antigen in IEC-6 rat intestinal crypt cells. J Cell Physiol. 1997;171:284–290. doi: 10.1002/(SICI)1097-4652(199706)171:3<284::AID-JCP6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 19.Xiao W-D, Chen W, Sun L-H, et al. The protective effect of enteric glial cells on intestinal epithelial barrier function is enhanced by inhibiting inducible nitric oxide synthase activity under lipopolysaccharide stimulation. Mol Cell Neurosci. 2011 Feb;46(2):527–34. doi: 10.1016/j.mcn.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Horne A, Stock S, King A. Innate immunity and disorders of the female reproductive tract. Reproduction. 2008 Jun;135(6):739–49. doi: 10.1530/REP-07-0564. [DOI] [PubMed] [Google Scholar]
- 21.Hubert P, Herman L, Maillard C, et al. Defensins induce the recruitment of dendritic cells in cervical human papillomavirus-associated (pre)neoplastic lesions formed in vitro and transplanted in vivo. FASEB J. 2007 Sep;21(11):2765–75. doi: 10.1096/fj.06-7646com. [DOI] [PubMed] [Google Scholar]
- 22.Monteleone I, Sarra M, Pallone F, Monteleone G. Th17-related cytokines in inflammatory bowel diseases: friends or foes? Curr Mol Med. 2012 Jun;12(5):592–7. doi: 10.2174/156652412800620066. [DOI] [PubMed] [Google Scholar]
- 23.Elovitz M, Mrinalini C. The use of progestational agents for preterm birth: lessons from a mouse model. Am J Obstet Gynecol. 2006 Oct;195(4):1004–10. doi: 10.1016/j.ajog.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Elovitz M, Wang Z. Medroxyprogesterone acetate, but not progesterone, protects against inflammation-induced parturition and intrauterine fetal demise. Am J Obstet Gynecol. 2004;190:693–701. doi: 10.1016/j.ajog.2003.10.693. [DOI] [PubMed] [Google Scholar]
- 25.Elovitz M, Mrinalini C, Sammel M. Elucidating the early signal transduction pathways leading to fetal brain injury in preterm birth. Pediatr Res. 2006;59:50–5. doi: 10.1203/01.pdr.0000191141.21932.b6. [DOI] [PubMed] [Google Scholar]
- 26.Yellon S, Oshiro B, Chhaya T, et al. Remodeling of the cervix and parturition in mice lacking the progesterone receptor B isoform. Biol Reprod. 2011 Sep;85(3):498–502. doi: 10.1095/biolreprod.111.091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Payne K, Clyde L, Weldon A, et al. Residency and activation of myeloid cells during remodeling of the prepartum murine cervix. Biol Reprod. 2012 Nov 1;87(5):106. doi: 10.1095/biolreprod.112.101840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirby L, Kirby M, Warren J, et al. Increased innervation and ripening of the prepartum murine cervix. J Soc Gynecol Investig. 2005 Dec;12(8):578–85. doi: 10.1016/j.jsgi.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Lim J, Yoon J, Hovde C. A brief overview of Escherichia coli O157:H7 and its plasmid O157. J Microbiol Biotechnol. 2010 Jan;20(1):5–14. [PMC free article] [PubMed] [Google Scholar]
- 30.Edwards J, Butler E. The Pathobiology of Neisseria gonorrhoeae Lower Female Genital Tract Infection. Front Microbiol. 2011;2:102. doi: 10.3389/fmicb.2011.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han J, Kim M, Lee M, et al. Modulation of human beta-defensin-2 expression by 17beta-estradiol and progesterone in vaginal epithelial cells. Cytokine. 2010 Feb;49(2):209–14. doi: 10.1016/j.cyto.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Narvekar N, Lakha F, Critchley H, et al. Changes in vaginal morphology, steroid receptor and natural antimicrobial content following treatment with low-dose mifepristone. Contraception. 2007 Apr;75(4):271–80. doi: 10.1016/j.contraception.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 33.Clyde L, Lechuga T, Ebner C, et al. Transection of the pelvic or vagus nerve forestalls ripening of the cervix and delays birth in rats. Biol Reprod. 2011 Mar;84(3):587–94. doi: 10.1095/biolreprod.110.086207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akins M, Luby-Phelps K, Bank R, Mahendroo M. Cervical softening during pregnancy: regulated changes in collagen cross-linking and composition of matricellular proteins in the mouse. Biol Reprod. 2011 May;84(5):1053–62. doi: 10.1095/biolreprod.110.089599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Payne K, Clyde L, Weldon A, et al. Residency and activation of myeloid cells during remodeling of the prepartum murine cervix. Biol Reprod. 2012 Nov 1;87(5):106. doi: 10.1095/biolreprod.112.101840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Virgo B, Bellward D. Serum progesterone levels in the pregnant and postpartum laboratory mouse. Endocrinology. 1974 Nov;95(5):1486–90. doi: 10.1210/endo-95-5-1486. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell B, Taggart M. Are animal models relevant to key aspects of human parturition? Am J Physiol Regul Integr Comp Physiol. 2009 Sep;297(3):R525–45. doi: 10.1152/ajpregu.00153.2009. [DOI] [PubMed] [Google Scholar]


