Abstract
To realize the potential of large molecular weight substances to treat neurological disorders, novel approaches are required to surmount the blood–brain barrier (BBB). We investigated whether fusion of a receptor-binding peptide from apolipoprotein E (apoE) with a potentially therapeutic protein can bind to LDL receptors on the BBB and be transcytosed into the CNS. A lysosomal enzyme, α-L-iduronidase (IDUA), was used for biological and therapeutic evaluation in a mouse model of mucopolysaccharidosis (MPS) type I, one of the most common lysosomal storage disorders with CNS deficits. We identified two fusion candidates, IDUAe1 and IDUAe2, by in vitro screening, that exhibited desirable receptor-mediated binding, endocytosis, and transendothelial transport as well as appropriate lysosomal enzyme trafficking and biological function. Robust peripheral IDUAe1 or IDUAe2 generated by transient hepatic expression led to elevated enzyme levels in capillary-depleted, enzyme-deficient brain tissues and protein delivery into nonendothelium perivascular cells, neurons, and astrocytes within 2 d of treatment. Moreover, 5 mo after long-term delivery of moderate levels of IDUAe1 derived from maturing red blood cells, 2% to 3% of normal brain IDUA activities were obtained in MPS I mice, and IDUAe1 protein was detected in neurons and astrocytes throughout the brain. The therapeutic potential was demonstrated by normalization of brain glycosaminoglycan and β-hexosaminidase in MPS I mice 5 mo after moderate yet sustained delivery of IDUAe1. These findings provide a noninvasive and BBB-targeted procedure for the delivery of large-molecule therapeutic agents to treat neurological lysosomal storage disorders and potentially other diseases that involve the brain.
Keywords: in vivo evaluation, LDL receptor-related protein 1, lysosomal storage diseases, neurological disorders, CNS protein delivery
Mucopolysaccharidosis type I (MPS I), one of the most common lysosomal storage diseases with CNS involvement, is caused by the deficiency of α-L-iduronidase (IDUA) and subsequent accumulation of glycosaminoglycans (GAGs) in multiple organs (1). In the severe form of MPS I (i.e., Hurler syndrome), CNS complications are characterized by hydrocephalus, learning delays, and mental retardation in patients who would die by 10 y of age if untreated. Metabolic correction, resulting from intercellular lysosomal enzyme transfer via mannose-6-phosphate receptor (M6PR)-mediated endocytosis (2), has provided the basis of treating such multiorgan disease with allogeneic hematopoietic stem cell (HSC) transplantation or enzyme therapy. HSC transplantation has demonstrated variable degrees of clinical response with significant CNS benefits in some patients with Hurler syndrome when treated before 2 y of age (3). However, it is also limited by a procedure-related mortality rate of ∼25%, risk of engraftment failure, and complications related to graft-vs.-host disease (4). Enzyme therapy by i.v. administration of recombinant enzyme has shown clinical improvement of visceral manifestations in patients with MPS I (5). However, it is unsuccessful in treating neurological complications as a result of poor penetration of the enzyme to the CNS (6). We have recently shown in a gene therapy study with a mouse model of Hurler syndrome that supraphysiological IDUA levels could be achieved continuously in the blood by reprogramming erythroid cells with a tissue-specific lentiviral vector (LV), leading to phenotypic correction in all systemic organs evaluated (7). Some CNS improvement were observed, but not a cure, most likely because of the lack of M6P receptor on brain blood microvasculature in adult mice (8).
The surface area of human brain microvasculature available for transport of therapeutic agents is ∼20 m2, embracing all neurons or glial cells within a 20-µm proximity (9). However, the blood–brain barrier (BBB) formed by brain capillary endothelial cells restricts the delivery of almost all neurotherapeutic agents from the blood to the brain (10). Only small lipophilic molecules (<0.5 kDa) or those that bind to one of the receptors naturally existing on the BBB can be transported effectively across the BBB (11). The LDL receptor (LDLR) superfamily (LDLRf) proteins, including LDLR-related protein 1 (LRP1) and very low-density lipoprotein receptor (VLDLR), but not LDLR, are abundantly expressed on BBB (12) and can bind apolipoproteins to facilitate their transcytosis across the BBB. The receptor-associated protein (RAP), an antagonist as well as a ligand for both LRP1 and VLDLR, has been shown to have higher permeability than transferrin in crossing the BBB (13), suggesting that these lipoprotein receptors may be an efficient target for delivery of therapeutic agents across the BBB. Moreover, gene expression mapping has shown high and widespread expression of LRP1 and VLDLR throughout the brain parenchyma (www.brain-map.org), providing secondary targeting for further distribution when the delivered agent has been transported into the brain. Thus, use of LDLRf-mediated transcytosis system for CNS delivery may provide effective targeting to the brain.
Two previous studies have tested the utility of the LDLRf system for protein delivery to the brain. One study demonstrated that the receptor-binding domain of apolipoprotein B (apoB) could facilitate fusion protein transport across the BBB (14), and the second study showed the conjugation of apolipoprotein E (apoE) to nanoparticles could trigger LDLRf-mediated transcytosis across the BBB in mice (15). However, the drawbacks of these studies are that apoB binds only to LDLR and has negligible affinity to LRP1 or other LDLRf proteins (16), and that the use of the entire apoE molecule may potentially interfere with its natural biological functions, including apoE isoform-specific effects on Alzheimer’s disease (17, 18). In the present study, we identified two receptor-binding peptides (Rb) from apoE by in vitro screening for desirable receptor-mediated binding, endocytosis, and transendothelial transport, as well as appropriate lysosomal enzyme trafficking and biological function. We demonstrated in a short-term in vivo study that high levels of IDUA-Rb in the blood could lead to elevated brain enzyme activities and protein delivery to nonendothelial perivascular cells, neurons, and astrocytes. Its therapeutic potential concerning the brain was substantiated by correction of brain GAG accumulation and normalization of elevated β-hexosaminidase 5 mo after systemic delivery of moderate levels of IDUA-Rb in MPS I mice.
Results
Accessibility of IDUA for Ligand Insertion as Fusion Protein.
To determine the accessibility of IDUA for genetic modification, a human myc tag (Table S1) was fused in-frame to the 5′- or 3′ end of the human IDUA coding sequence (19). The addition of myc tag at C terminus (IDUA3′Myc), but not at the N terminus (IDUA5′Myc), as expected, had no effect on IDUA catalytic function or its trafficking to the extracellular space (Fig. S1). The released form of IDUA3′Myc retained the tag sequence (Fig. S2A) and was catalytically active while captured by epitope binding to anti-myc immune beads (Fig. S2B). As the M6PR system plays a key role in intercellular and intracellular trafficking of lysosomal enzymes, the intactness of M6PR-mediated lysosomal targeting was confirmed in fusion IDUA3′Myc by competitive uptake assay (Fig. S3A), and by immunostaining showing colocalization of internalized IDUA3′Myc in lysosomes (Fig. S3B). Thus, the IDUA3′Myc protein not only retained IDUA catalytic activity, lysosomal enzyme trafficking, and endogenous M6PR-mediated uptake, but also acquired an additional epitope-binding ability, suggesting that C terminus of IDUA is suitable for ligand insertion in the construction of a fusion protein.
In Vitro Screening for Optimal Rb in LRP1-Overexpressing Cell Lines.
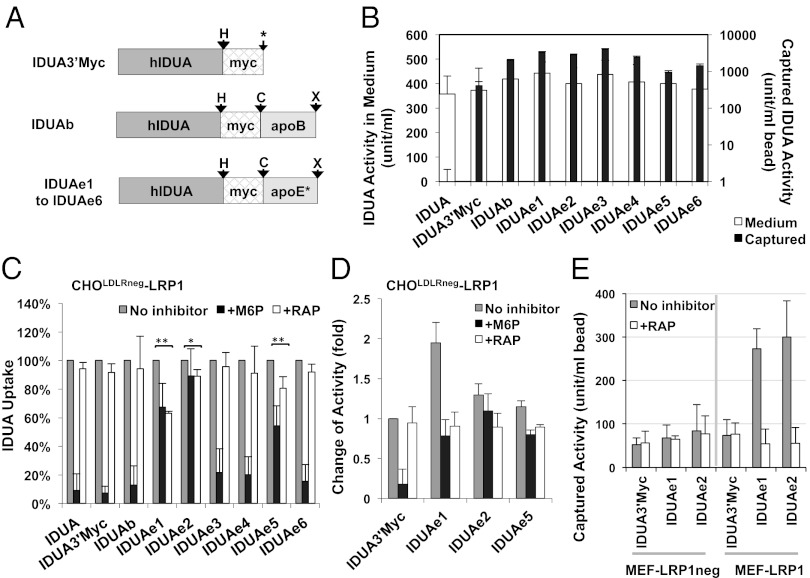
Studies on the apoE receptor-binding region have been reported to narrow its location to the vicinity of amino acid residues 130 to 150 of mature apoE for the binding to LDLRf (20, 21). Several synthetic oligopeptides derived from this region mimic certain aspects of receptor binding or apoE function in vitro and in vivo (22, 23). However, none of the LDLRf binding domain of apoE has been reported in a fusion protein setting for BBB delivery. As a first step toward screening for optimal Rb, we designed six candidate peptides within the vicinity of residues 148 to 173 of precursor apoE, the extended putative receptor-binding domain of apoE (as monomer or tandem dimers), and one peptide from the receptor-binding domain of apoB previously reported by others (14) (Table S1). Seven IDUA-Rb fusion candidates were constructed at the C terminus of IDUA3′Myc (Fig. 1A). All IDUA fusion candidates could be overexpressed and released into the extracellular space from 3T3 cells (Fig. S4A). The released forms of IDUA fusion proteins contained functioning myc-tag without proteolytic removal, as demonstrated by Western blot analysis with anti-myc antibody showing bands of appropriate size for all fusion candidates except IDUAe6 (Fig. S4B). Importantly, the released forms of all IDUA fusion candidates remained catalytically active while being captured by immunoprecipitation (IP) with anti-myc antibody (Fig. 1B), thereby confirming the presence of Rb tag and the preservation of IDUA function.
Fig. 1.
Engineered IDUA proteins introduce LRP1-mediated endocytosis in genetically modified cell lines. (A) Diagram of modified human IDUA proteins (hIDUA) fused in-frame with the myc-tag and the receptor-binding domain of apoB or various peptides from apoE (apoE*). Arrows indicate space linkers that were encoded by restriction sites for HpaI (marked “H”), ClaI (“C”), XhoI (“X”), or a polylinker (asterisk). (B) Epitope-binding and catalytic activity in released form of modified IDUA. Medium containing different IDUA-Rb with IDUA activities (open bars) were precipitated with mouse anti-myc monoclonal antibody. IDUA activities were determined (solid bars) by using immune-precipitated beads. Data were derived from two experiments, each in triplicate IP reactions with duplicate IDUA assays. (C) Uptake inhibition assays. CHOLDLRneg-LRP1 cells were cultured with medium containing similar activities of IDUA-Rb (500 U/mL) with or without M6P or RAP inhibitors. Data were derived from two or three independent experiments in duplicate wells. (D) Binding/internalization assay for selected IDUA-Rb candidates. CHOLDLRneg-LRP1 cells were exposed at 4 °C to various IDUA-Rb (500 U/mL) in the presence or absence of different inhibitors, followed by culture in fresh medium at 37 °C. Two independent experiments were performed in duplicate wells. (E) Comparison of selected IDUA-Rb for receptor binding in cells lacking (MEF-LRP1neg) or overexpressing LRP1 receptor (MEF-LRP1). Cells were exposed at 4 °C to IDUA fusion proteins (500 U/mL) in the presence or absence of RAP inhibitor. IP was performed with cell lysates by using rabbit anti-LRP1 antibody, followed by IDUA enzyme assay with washed immune beads.
To evaluate LRP1 receptor-specific uptake pathway, we generated LRP1-overexpressing cell lines based on CHOLDLRneg (24) or mouse embryonic fibroblasts derived from LRP1 knockout mice (MEF-LRP1neg) (Fig. S5), which still maintained endogenous M6PR-mediated uptake pathway as shown in dose-dependent competitive uptake assay (Fig. S6). To examine Rb candidates for LRP1-mediated internalization, an uptake/inhibition study was conducted by using CHOLDLRneg -LRP1 cells in the absence or presence of RAP (Fig. 1C). In comparison with IDUA3′Myc, the uptake of IDUAe1, IDUAe2, or IDUAe5 was significantly inhibited by RAP. A highly efficient LRP1-mediated internalization of IDUAe1, comparable to M6PR-mediated uptake, was evidenced by a twofold higher intracellular enzyme level that could be partially blocked (∼50%) upon RAP inhibition as shown by binding/internalization assay (Fig. 1D). A similar pattern was also observed for the internalization of IDUAe2 and IDUAe5, but to a lesser extent. The specific binding of IDUAe1 and IDUAe2 to LRP1 was further supported by IP assay using anti-LRP1 antibody (Fig. 1E). IDUAe1 and IDUAe2 introduced significantly higher LRP1 binding that was blocked by RAP in LRP1-overexpressing, but not in LRP1-negative, MEF cells.
Correction of Lysosomal Defect by IDUA-Rb in Cells Derived from a Patient with MPS I.
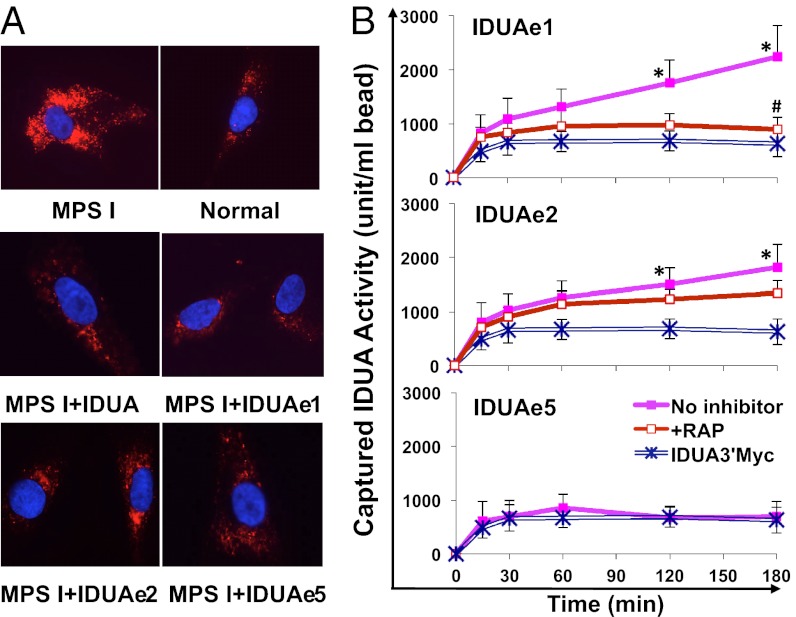
The functional integrity of the selected IDUA-Rb was determined by using primary fibroblasts from a patient with MPS I by in situ immunostaining using a fluorescent dye that can be endocytosed into lysosomes (Fig. 2A). The untreated MPS I fibroblasts exhibited excessive abundance of lysosomes and enlarged lysosomal compartment. The majority of the patient’s cells exposed to unmodified IDUA or fusion IDUA-Rb exhibited a normalized lysosomal pattern that was comparable to that in control cells derived from a healthy individual. Thus, we verified that the released forms of IDUAe1, IDUAe2, and IDUAe5 are fully functional and suitable for uptake by cells via M6PR- and LRP1-mediated endocytosis. The findings indicate that IDUA-Rb may be used for correction of phenotypic defects in cells from patients with MPS I.
Fig. 2.
Fusion IDUA proteins normalize lysosomal accumulation in patient fibroblasts and facilitate LRP1-dependent transendothelial protein transport. (A) Fibroblasts derived from a patient with MPS I (GM00497; Coriell Cell Repositories) were cocultured with HEK293 cells that overexpressed unmodified IDUA or selected fusion IDUA for 24 h by using a Transwell system. Representative photomicrographs are shown following immunofluorescence staining with LysoTracker for lysosomes (red) and DAPI for nuclei (blue). (B) Bovine brain capillary endothelial cells were cultured in the upper chambers of collagen-coated Transwell inserts, followed by exposure to modified IDUA at 4 °C and subsequent culture at 37 °C in fresh medium. IDUA in the lower chambers was captured at different time points by IP with anti-myc antibody and quantified by enzyme assay. Data were derived from two or three independent experiments with triplicate wells and expressed as mean ± SEM. (*P < 0.05 vs. IDUA3′Myc controls; #P = 0.08 vs. uptake without RAP).
Evaluation of Transcytosis Using an in Vitro BBB Model.
IDUAe1, IDUAe2, and IDUAe5 were selected for further evaluation of their ability to mediate transendothelial transport by using an in vitro BBB model with bovine brain capillary endothelial cells cultured on collagen-coated Transwell inserts (Fig. 2B). Nontranscytotic leakage of the cell layer was monitored by parallel experiments using IDUA3′Myc control at the same enzymatic levels. Transcytosis of IDUAe1 and IDUAe2, but not IDUAe5, was indicated by a steady increase of captured enzymatic levels found in lower chambers (as much as 3.6-fold vs. plateau levels of IDUA3′Myc) after the removal of fusion proteins from the upper chambers. The elevated transport process of IDUAe1 was mediated by LRP1 as suggested by RAP inhibition.
Short-Term in Vivo Evaluation of IDUA-Rb for Brain Delivery in MPS I Mice.
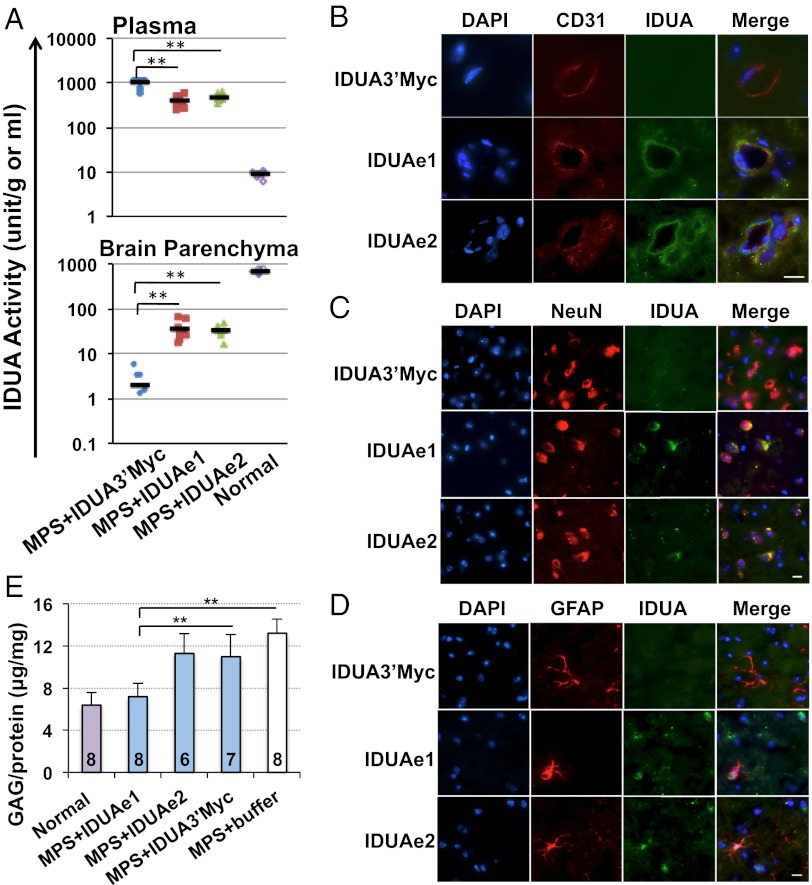
To evaluate the effectiveness of IDUAe1 and IDUAe2 for brain delivery in vivo, we performed hydrodynamic tail-vein injections of naked plasmid DNA that has been shown to induce high transgene expression in the liver of small rodents (25, 26). To eliminate any fusion protein generation in the CNS, we further restricted the expression of IDUA fusion candidates in liver by using a liver-specific hybrid promoter (27). Robust (peaking on day 1) and relatively continuous (to 3 d) protein production were achieved in the circulation of MPS I mice (Fig. S7), thus presenting a practical time window for in vivo evaluation of delivery to the brain. Two days after injection, 55 to 112 fold of normal heterozygous plasma IDUA activities were observed in all three injected groups, with IDUA3′Myc-treated mice exhibiting the highest levels (Fig. 3A). In contrast, capillary-depleted brain tissues (26) from IDUAe1- or IDUAe2-injected MPS mice exhibited 10- to 30-fold higher IDUA levels than IDUA3′Myc controls.
Fig. 3.
Liver-generated IDUA fusion proteins in the circulation can be transported into the CNS and normalize the accumulation of GAG in the brain of MPS I mice. Whole brains were collected from well perfused animals 2 d after hydrodynamic injection. (A) Enzyme activities in plasma and capillary-depleted brain parenchyma (n = 7–9 mice per group). Black bars represent mean activities. (B–D) Representative images from immunofluorescence staining of brain sections. Samples were stained with antibodies against IDUA protein (green) and endothelial marker (CD31; red) (B), terminally differentiated neuron marker (NeuN; red) (C), or astrocyte marker (GFAP; red) (D). All sections were counterstained with DAPI for nuclei (blue). (Scale bars, 10 μm.) (E) The accumulation of GAG in MPS brain was reduced by liver-generated IDUA-Rb. N for each group is indicated under each bar, and the average plasma IDUA activities were 1,771 U/mL for the IDUA3′Myc group, 808 U/mL for the IDUAe1 group, and 456 U/mL for the IDUAe2 group (*P < 0.05 and **P < 0.001 in A and E).
We then carried out immunofluorescence microscopy to identify the types of brain cells that accumulate IDUA-Rb (Fig. 3 B–D). The passage of IDUAe1 and IDUAe2 across the BBB was visualized by IDUA-positive staining in the abluminal side of the BBB-forming capillary endothelial cells labeled by CD31 marker (Fig. 3B). Based on their location adjacent to the endothelium, astrocyte end-feet and/or pericytes are presumed to be involved in the uptake of the trans-BBB–delivered IDUAe1 and IDUAe2. Delivery of liver-derived IDUAe1 and IDUAe2 to neurons was also demonstrated by colocalization of the IDUA protein with NeuN, a nuclear marker associated with the majority of neurons throughout the brain (Fig. 3C). In contrast, lack of colocalization was found in brains treated with IDUA3′Myc. We also observed cells that stained positively for IDUA and GFAP, a marker for astrocytes, in forebrain of MPS I mice injected with IDUAe1 or IDUAe2 plasmids, but not with IDUA3′Myc controls (Fig. 3D). These findings demonstrate that the BBB-targeted IDUA-Rb can be delivered across the BBB to nonendothelial perivascular cells, neurons, and astrocytes in the CNS.
To assess the biological functionality of CNS-delivered IDUA-Rb for its therapeutic potential, we measured brain GAG levels 2 d after hydrodynamic injection into 7- to 8-wk-old MPS I mice (Fig. 3E). GAG accumulation was normalized in MPS I mice treated with IDUAe1-expressing plasmid, suggesting metabolic correction in the brain of these animals. IDUAe2 and IDUA3′Myc caused reduction of GAG that was significantly less compared with IDUAe1. IDUAe1 was therefore selected for long-term evaluation in vivo.
CNS Delivery of IDUAe1 in MPS I Mice 5 mo After Gene Therapy with Erythroid-Specific LV.
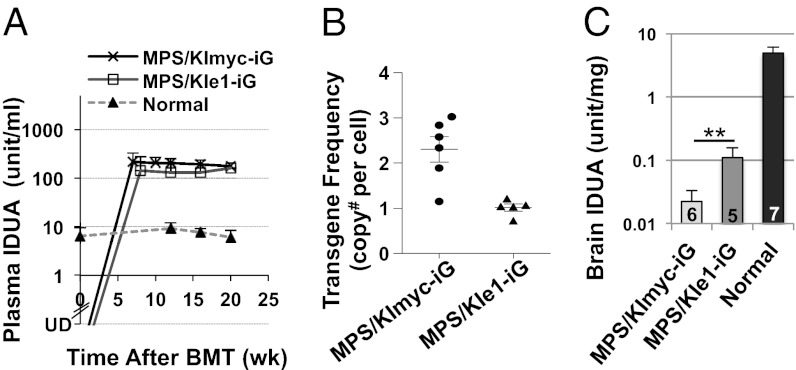
To further assess long-term brain delivery from moderate but achievable levels of peripheral IDUAe1, we conducted HSC-mediated gene transfer in MPS I mice by using a hybrid human ankyrin-1 promoter for restricted IDUA expression in maturing red blood cells, but not in myeloid or lymphoid cells (7). HSC-enriched bone marrow cells from MPS I mice were transduced with LVs coexpressing eGFP with IDUAe1 (KIe1-iG) or IDUA3′Myc (KImyc-iG), followed by transplantation into MPS I mice (Fig. 4). All treated mice survived until the end of the observation period, when age-matched MPS I controls started to die. Starting 7 to 8 wk after transplantation, plasma IDUA activity levels in both groups increased and persisted at supraphysiological levels (12–20-fold of normal heterozygous littermates) until the end of the observation period (Fig. 4A). The transgene frequencies in bone marrow were determined by real-time quantitative PCR (Fig. S8), resulting in 1.0 ± 0.2 copy per cell for the MPS/KIe1-iG group and 2.3 ± 0.7 copies per cell for the MPS/KImyc-iG group (Fig. 4B). Brain IDUA activities were fivefold higher in MPS/KIe1-iG mice than in control MPS/KImyc-iG mice (barely detectable; Fig. 4C). These brain IDUA levels (equivalent to 2%–3% of normal levels) are presumed to be significant, considering that minimum amounts of enzyme (e.g., 1%–5% of normal serum enzyme levels) have been associated with clinical benefits in patients with MPS I after successful HSC transplantation (28).
Fig. 4.
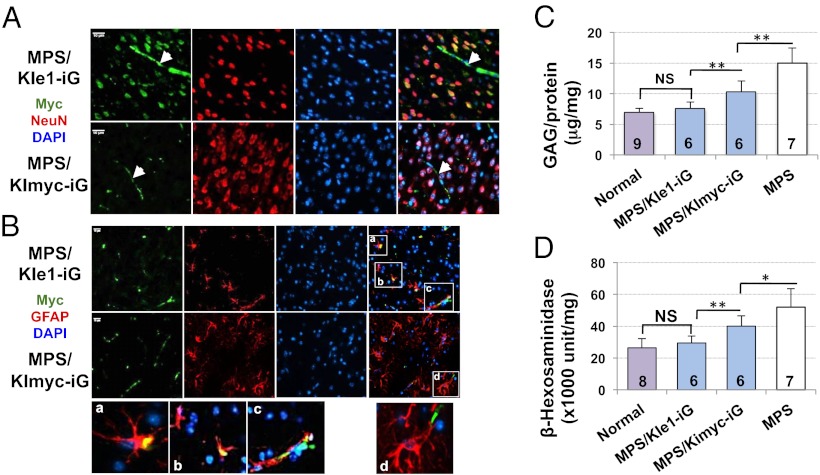
Long-term expression of IDUAe1 and brain enzyme elevation in MPS I chimeras after transduction with erythroid-specific LV. (A) Plasma IDUA levels. MPS I mice were transplanted at 7 to 8 wk of age with MPS Lin− cells that were transduced with LV-KImyc-iG (MPS/KImyc-iG) or LV-KIe1-iG (MPS/KIe1-iG). Normal, heterozygous littermates; UD, undetectable. Data were derived from five to seven mice per group. (B) Transgene frequencies determined by real-time quantitative PCR in bone marrow 5 mo after transplantation. Each sample was assayed in two or three experiments with duplicate reactions. (C) Brain IDUA activities. Whole brains were collected from well perfused animals 5 mo after BMT. N is indicated under each bar. Undetectable level of IDUA (<0.01 U/mg) was found in MPS I brain (**P = 0.025).
Immunofluorescence analysis was performed with the use of anti-myc antibody to evaluate the distribution of full-length IDUAe1 protein in brain after long-term delivery (Fig. 5 A and B). Myc-positive, capillary-like structures were observed sporadically in the cerebral cortex of IDUAe1 and IDUAmyc control groups (Fig. 5A). Colocalization of myc+ and NeuN+ staining was widespread in the forebrain of only MPS/KIe1-iG mice, suggesting IDUAe1 delivery into neuronal cells. IDUAe1-containing astrocytes were detected as cells that were positive for myc and GFAP staining in MPS/KIe1-iG mice (Fig. 5B). These results confirm CNS penetration of full-length IDUAe1 protein.
Fig. 5.
Delivery of peripheral IDUAe1, but not IDUAmyc, generated by maturing red blood cells to neurons and astrocytes leads to normalization of GAG and hexosaminidase in the brain of MPS I mice. Five months after transplantation of HSCs transduced with erythroid-specific LV, whole brains were collected from thoroughly perfused animals. (A and B) Representative images from immunofluorescence staining of brain sections from cerebral cortex. Samples were stained with antibodies against myc-tag (green) and NeuN (red in A) or GFAP (red in B). All sections were counterstained with DAPI for nuclei (blue). (Scale bars, 10 μm.) Arrowheads indicate capillary-like structure. Amplified areas within the white borders are also illustrated (Bottom panel), highlighting colocalization (yellow). (C) Brain GAG accumulation. (D) Brain β-hexosaminidase activity. N for each group is indicated under each bar. (*P < 0.05 and **P < 0.001 in C and D; NS, no significance).
To evaluate functional efficacy of IDUAe1 after long-term CNS delivery in the diseased brain, we assessed brain GAG accumulation and β-hexosaminidase activities, which are abnormally elevated in tissues of patients with MPS I and in mouse models (29) (Fig. 5 C and D). Five months after peripheral delivery of IDUAe1, MPS I mice exhibited normalized brain GAG (P = 0.38), a level that is dramatically reduced from IDUAmyc-treated or untreated, age-matched MPS I mice. Moreover, brain β-hexosaminidase activities in IDUAe1-treated mice were comparable to those found in normal heterozygous littermates (P = 0.29). The findings demonstrate that long-term peripheral delivery of IDUAe1 can lead to metabolic correction in the brain of MPS I mice.
Discussion
We discovered receptor-binding peptides by in vitro screening and in vivo evaluation that can translocate proteins across the BBB into the brain when engineered in a fusion-protein setting. By using LRP1-overexpressing cells and a cultured BBB model, we identified two of seven IDUA-Rb candidates with desirable receptor-mediated binding, endocytosis, and transendothelial transport, as well as appropriate lysosomal enzyme trafficking and biological function. Robust peripheral IDUAe1 or IDUAe2 levels (as much as 500-fold of heterozygous values) generated by hepatic expression led to elevated enzyme levels in capillary-depleted, enzyme-deficient brain tissues that were 10 to 30 fold greater than those in IDUAmyc controls. Immunofluorescence analysis revealed protein delivery into nonendothelial perivascular cells, neurons, and astrocytes of MPS I mice. Two to three percent of normal brain IDUA activities were detected in gene therapy-treated MPS I mice 5 mo after long-term delivery of moderate levels of IDUAe1 (as much as 14 fold) derived from maturing red blood cells, despite constant consumption/inactivation of IDUAe1 in enzyme-deficient cells of the delivery path. Widespread CNS delivery of full-length IDUAe1 was observed in neurons and astrocytes throughout the brain. Therapeutic potential was demonstrated by correction of accumulated GAG and elevated β-hexosaminidase in the brain of MPS I mice 5 mo after moderate yet sustained delivery of IDUAe1, but not IDUAmyc controls. The findings document the development of potent peptide(s) for BBB-targeted protein delivery.
The “impermeability” of the BBB has hindered effective treatment of CNS disorders. Direct brain injections have been used to bypass the BBB, but benefits are largely limited by practical considerations such as volume restriction and poor diffusion of a drug or vector from the injection sites. Transient BBB disruption by a variety of means has been explored for CNS drug delivery (30), but was often found to be associated with vascular pathologic conditions, astrogliosis, and chronic neuropathologic changes in the brain. Liposomes and nanoparticles, as well as cationization or protein transduction domains, have been developed to exploit the lipophilicity of the BBB for brain delivery (31). However, these approaches lack specific brain targeting and lead to rapid disappearance of the agents from the circulation and inadequate, if any, delivery to the brain. Selective BBB transport may be possible by taking advantage of endogenous receptor-mediated transcytosis for the entrance of proteins such as transferrin, insulin, or apolipoproteins. Current approaches have involved liposomes, nanoparticles (32), or i.v. injection of recombinant fusion proteins by “piggybacking” them with a natural substance or an antibody as ligands (10). Mature IDUA proteins fused with the heavy chains of chimeric monoclonal antibodies to insulin receptor or transferrin receptor have been explored for brain delivery (33, 34). However, the delivered therapeutic agent has to compete with endogenous natural substance for target binding. In the present study, we developed a potent 18-aa tag from apoE to effectively transport IDUA across the BBB into enzyme-deficient brain. Compared with the entire apoE molecule or antibody against LRP1, such small peptides can be readily attached to diagnostic or therapeutic agents with minimum risks of jeopardizing the biological functions of “cargo” agents. It can also theoretically reduce the risk of disrupting the important physiologic roles of apoE or stimulating immune response against the fusion protein, although further evaluation will be needed.
In the present short-term in vivo study, IDUAe1 and IDUAe2 produced comparably increased levels of brain IDUA activities, and targeted the delivery to similar types of cells in the brain of MPS I mice. However, unlike IDUAe1, which introduced normalization of brain GAG in MPS I mice, IDUAe2 reduced GAG levels to a much lesser degree. The difference in subcellular localization of IDUAe1 and IDUAe2 indicated by less M6PR-mediated internalization of IDUAe2 might likely affect GAG normalization in the CNS parenchyma. The difference between IDUAe1 and IDUAe2 was also noticeable in that IDUAe2 introduced high enzyme uptake in LDLR-negative, LRP1-overexpressing cells that could only be partially inhibited by the combination of RAP and M6P. The observations suggest significant involvement of an alternative receptor within the LDLR superfamily. The variation of receptor-binding affinities between IDUAe1 and IDUAe2 may lead to varying preferences in different applications depending upon the cargo agents.
In summary, we established a BBB-targeted, LRP1-binding Rb tag that can function as a “key” to selectively permit therapeutic agents to cross the BBB. This development has significant potential for the treatment of CNS diseases, especially neurological lysosomal storage diseases. This approach not only provides systemic brain drug/protein delivery by adapting a naturally existing receptor-mediated transport system on the BBB, but also offers an additional uptake pathway that can facilitate further/secondary distribution within the brain after the agents reach the CNS as a result of the widespread expression of LDLRf members within the brain parenchyma. Regardless of application strategies, i.e., enzyme replacement therapy or cell-based, gene-based therapy, quantity and distribution of therapeutic agents within the brain will have significant impact on the clinical outcome of disease treatment. This study and further exploration of such an approach by screening receptor-binding domain of other brain-targeted proteins may open the BBB to large-molecule neurotherapeutic agents.
Materials and Methods
SI Materials and Methods includes additional information on experimental methods.
Plasmid and Fusion Protein Construction.
The IDUA5′myc and IDUA3′myc fusion cassette were derived by TOPO-cloning to introduce human c-myc tag (410-419) and a flanking polylinker (Table S1) in-frame at 5′ (after the start codon) or at 3′ (immediately before the stop codon) of human IDUA cDNA (35). All seven Rb coding sequences (Table S1) were inserted between Cla I and Xho I sites within the polylinker of IDUA3′myc. Plasmids overexpressing different IDUA fusion candidates from the CMV promoter were constructed by insertion into pcDNA 3.1 (Invitrogen) between Hind III and XbaI sites. The pcDNA3.1/Zeo-LRP1 was generated by inserting the full-length cDNA of LRP-1 (National Center for Biotechnology Information Reference Sequence NM_002332.2) into the Xho I and Not I sites of pcDNA3.1/Zeo. To restrict gene expression in liver, the pBS-HCRHPI-A plasmid (27) was used by subcloning selected IDUA-Rb coding sequences into the Ndel and EcoRV sites.
Cell Lines and Experimental Animals.
The cell lines that overexpressed various IDUA fusion proteins or LRP1 were generated by transient cotransfection of pcDNA3.1-IDUA-Rb or pcDNA/Zeo-LRP1 with an EGFP-expressing plasmid (36) to monitor transfection efficiency (45–65%), followed by selection with G418 (0.45 mg/mL; Invitrogen) or Zeocin (250-400 µg/mL). MPS I mice (B6.129-iduatm1Clk) and WT C57/Bl6 mice were obtained from Jackson Laboratory. After in-house backcrossing with C57/Bl6 for more than nine generations, experimental groups were generated in a pathogen-free facility at Cincinnati Children’s Hospital Medical Center (CCHMC) and genotyped as described previously (37). Experimental procedures were approved by CCHMC Institutional Animal Care and Use Committee.
Statistical Analysis.
Quantitative assays were performed in duplicate or triplicate from at least two individual experiments. Data are presented as mean ± SD unless specified otherwise. Comparisons between two groups were performed by using two-tailed Student t tests. P values of less than 0.05 were considered to indicate statistical significance.
Supplementary Material
Acknowledgments
We thank Meghan Bromwell and the Comprehensive Mouse Core for technical assistance. We thank Dr. C. Miao (Seattle Children's Research Institute) for the pBS-HCRHPI-A plasmid and Dr. M. Krieger (Massachusetts Institute of Technology) for the CHOLDLRneg cell line. This work was supported by the National Institutes of Health Grants NS064330, DK074932, and U54 HL06-008.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1222742110/-/DCSupplemental.
References
- 1.Neufeld ES, Muenzer J. The mucopolysaccharidoses. In: Valle D, et al., editors. The Online Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill; 2007. Part 16, Chap 136. [Google Scholar]
- 2.Pan D. Cell- and gene-based therapeutic approaches for neurological deficits in mucopolysaccharidoses. Curr Pharm Biotechnol. 2011;12(6):884–896. doi: 10.2174/138920111795542679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staba SL, et al. Cord-blood transplants from unrelated donors in patients with Hurler’s syndrome. N Engl J Med. 2004;350(19):1960–1969. doi: 10.1056/NEJMoa032613. [DOI] [PubMed] [Google Scholar]
- 4.Schiffmann R, Brady RO. New prospects for the treatment of lysosomal storage diseases. Drugs. 2002;62(5):733–742. doi: 10.2165/00003495-200262050-00002. [DOI] [PubMed] [Google Scholar]
- 5.Pastores GM. Laronidase (Aldurazyme): Enzyme replacement therapy for mucopolysaccharidosis type I. Expert Opin Biol Ther. 2008;8(7):1003–1009. doi: 10.1517/14712598.8.7.1003. [DOI] [PubMed] [Google Scholar]
- 6.Sly WS. Enzyme replacement therapy: From concept to clinical practice. Acta Paediatr Suppl. 2002;91(439):71–78. doi: 10.1111/j.1651-2227.2002.tb03115.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang D, et al. Reprogramming erythroid cells for lysosomal enzyme production leads to visceral and CNS cross-correction in mice with Hurler syndrome. Proc Natl Acad Sci USA. 2009;106(47):19958–19963. doi: 10.1073/pnas.0908528106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urayama A, Grubb JH, Sly WS, Banks WA. Developmentally regulated mannose 6-phosphate receptor-mediated transport of a lysosomal enzyme across the blood-brain barrier. Proc Natl Acad Sci USA. 2004;101(34):12658–12663. doi: 10.1073/pnas.0405042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks: A step further in neuroglial and gliovascular interactions. Nat Rev Neurosci. 2010;11(2):87–99. doi: 10.1038/nrn2757. [DOI] [PubMed] [Google Scholar]
- 10.Pardridge WM. Blood-brain barrier delivery. Drug Discov Today. 2007;12(1-2):54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Pardridge WM. Drug and gene targeting to the brain with molecular Trojan horses. Nat Rev Drug Discov. 2002;1(2):131–139. doi: 10.1038/nrd725. [DOI] [PubMed] [Google Scholar]
- 12.Ueno M, et al. Transporters in the brain endothelial barrier. Curr Med Chem. 2010;17(12):1125–1138. doi: 10.2174/092986710790827816. [DOI] [PubMed] [Google Scholar]
- 13.Pan W, et al. Efficient transfer of receptor-associated protein (RAP) across the blood-brain barrier. J Cell Sci. 2004;117(pt 21):5071–5078. doi: 10.1242/jcs.01381. [DOI] [PubMed] [Google Scholar]
- 14.Spencer BJ, Verma IM. Targeted delivery of proteins across the blood-brain barrier. Proc Natl Acad Sci USA. 2007;104(18):7594–7599. doi: 10.1073/pnas.0702170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreuter J, et al. Covalent attachment of apolipoprotein A-I and apolipoprotein B-100 to albumin nanoparticles enables drug transport into the brain. J Control Release. 2007;118(1):54–58. doi: 10.1016/j.jconrel.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Hui DY, Innerarity TL, Mahley RW. Lipoprotein binding to canine hepatic membranes. Metabolically distinct apo-E and apo-B,E receptors. J Biol Chem. 1981;256(11):5646–5655. [PubMed] [Google Scholar]
- 17.Bu G. Apolipoprotein E and its receptors in Alzheimer’s disease: Pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009;10(5):333–344. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12(12):723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan D, Aronovich E, McIvor RS, Whitley CB. Retroviral vector design studies toward hematopoietic stem cell gene therapy for mucopolysaccharidosis type I. Gene Ther. 2000;7(21):1875–1883. doi: 10.1038/sj.gt.3301298. [DOI] [PubMed] [Google Scholar]
- 20.Croy JE, Brandon T, Komives EA. Two apolipoprotein E mimetic peptides, ApoE(130-149) and ApoE(141-155)2, bind to LRP1. Biochemistry. 2004;43(23):7328–7335. doi: 10.1021/bi036208p. [DOI] [PubMed] [Google Scholar]
- 21.Sauer I, Dunay IR, Weisgraber K, Bienert M, Dathe M. An apolipoprotein E-derived peptide mediates uptake of sterically stabilized liposomes into brain capillary endothelial cells. Biochemistry. 2005;44(6):2021–2029. doi: 10.1021/bi048080x. [DOI] [PubMed] [Google Scholar]
- 22.Dobson CB, Sales SD, Hoggard P, Wozniak MA, Crutcher KA. The receptor-binding region of human apolipoprotein E has direct anti-infective activity. J Infect Dis. 2006;193(3):442–450. doi: 10.1086/499280. [DOI] [PubMed] [Google Scholar]
- 23.Swertfeger DK, Hui DY. Apolipoprotein E receptor binding versus heparan sulfate proteoglycan binding in its regulation of smooth muscle cell migration and proliferation. J Biol Chem. 2001;276(27):25043–25048. doi: 10.1074/jbc.M102357200. [DOI] [PubMed] [Google Scholar]
- 24.Sege RD, Kozarsky K, Nelson DL, Krieger M. Expression and regulation of human low-density lipoprotein receptors in Chinese hamster ovary cells. Nature. 1984;307(5953):742–745. doi: 10.1038/307742a0. [DOI] [PubMed] [Google Scholar]
- 25.Herweijer H, Wolff JA. Gene therapy progress and prospects: Hydrodynamic gene delivery. Gene Ther. 2007;14(2):99–107. doi: 10.1038/sj.gt.3302891. [DOI] [PubMed] [Google Scholar]
- 26.El-Amouri SS, Cao P, Miao C, Pan D. Secreted luciferase for in vivo evaluation of systemic protein delivery in mice. Mol Biotechnol. 2013;53(1):63–73. doi: 10.1007/s12033-012-9519-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miao CH, Ye X, Thompson AR. High-level factor VIII gene expression in vivo achieved by nonviral liver-specific gene therapy vectors. Hum Gene Ther. 2003;14(14):1297–1305. doi: 10.1089/104303403322319381. [DOI] [PubMed] [Google Scholar]
- 28.Peters C, et al. Outcome of unrelated donor bone marrow transplantation in 40 children with Hurler syndrome. Blood. 1996;87(11):4894–4902. [PubMed] [Google Scholar]
- 29.Zheng Y, et al. Treatment of the mouse model of mucopolysaccharidosis I with retrovirally transduced bone marrow. Mol Genet Metab. 2003;79(4):233–244. doi: 10.1016/s1096-7192(03)00116-1. [DOI] [PubMed] [Google Scholar]
- 30.Bellavance MA, Blanchette M, Fortin D. Recent advances in blood-brain barrier disruption as a CNS delivery strategy. AAPS J. 2008;10(1):166–177. doi: 10.1208/s12248-008-9018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Boer AG, Gaillard PJ. Strategies to improve drug delivery across the blood-brain barrier. Clin Pharmacokinet. 2007;46(7):553–576. doi: 10.2165/00003088-200746070-00002. [DOI] [PubMed] [Google Scholar]
- 32.Koziara JM, Lockman PR, Allen DD, Mumper RJ. The blood-brain barrier and brain drug delivery. J Nanosci Nanotechnol. 2006;6(9-10):2712–2735. doi: 10.1166/jnn.2006.441. [DOI] [PubMed] [Google Scholar]
- 33.Boado RJ, et al. Genetic engineering of a lysosomal enzyme fusion protein for targeted delivery across the human blood-brain barrier. Biotechnol Bioeng. 2008;99(2):475–484. doi: 10.1002/bit.21602. [DOI] [PubMed] [Google Scholar]
- 34.Boado RJ, Hui EK, Lu JZ, Zhou QH, Pardridge WM. Reversal of lysosomal storage in brain of adult MPS-I mice with intravenous Trojan horse-iduronidase fusion protein. Mol Pharm. 2011;8(4):1342–1350. doi: 10.1021/mp200136x. [DOI] [PubMed] [Google Scholar]
- 35.Wang D, Worsham DN, Pan D. Co-expression of MGMT(P140K) and alpha-L-iduronidase in primary hepatocytes from mucopolysaccharidosis type I mice enables efficient selection with metabolic correction. J Gene Med. 2008;10(3):249–259. doi: 10.1002/jgm.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Worsham DN, Schuesler T, von Kalle C, Pan D. In vivo gene transfer into adult stem cells in unconditioned mice by in situ delivery of a lentiviral vector. Mol Ther. 2006;14(4):514–524. doi: 10.1016/j.ymthe.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan D, Sciascia A, 2nd, Vorhees CV, Williams MT. Progression of multiple behavioral deficits with various ages of onset in a murine model of Hurler syndrome. Brain Res. 2008;1188:241–253. doi: 10.1016/j.brainres.2007.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.