Abstract
Fibroblasts can be reprogrammed to induced pluripotent stem cells (iPSCs) by application of transcription factors octamer-binding protein 4 (Oct4), SRY-box containing gene 2 (Sox2), Kruppel-like factor 4 (Klf4), and c-Myelocytomatosis oncogene (c-Myc) (OSKM), but the underlying mechanisms remain unclear. Here, we report that exogenous Oct4 and Sox2 can bind at the promoter regions of mir-141/200c and mir-200a/b/429 cluster, respectively, and induce the transcription activation of miR-200 family during the OSKM-induced reprogramming. Functional suppression of miR-200s with specific inhibitors significantly represses the OSKM-caused mesenchymal-to-epithelial transition (MET, an early event in reprogramming of fibroblasts to iPSCs) and iPSC generation, whereas overexpression of miR-200s promotes the MET and iPSC generation. Mechanistic studies showed that miR-200s significantly repress the expression of zinc finger E-box binding homeobox 2 (ZEB2) through directly targeting its 3′ UTR and direct inhibition of ZEB2 can mimic the effects of miR-200s on iPSC generation and MET process. Moreover, the effects of miR-200s during iPSC generation can be blocked by ZEB2 overexpression. Collectively, our findings not only reveal that members of the miR-200 family are unique mediators of the reprogramming factors Oct4/Sox2, but also demonstrate that the miR-200/ZEB2 pathway as one critical mechanism of Oct4/Sox2 to induce somatic cell reprogramming at the early stage.
Keywords: pluripotency, microRNAs, SMAD interacting protein 1
Ectopic expression of four transcription factors, octamer-binding protein 4 (Oct4), SRY-box containing gene 2 (Sox2), Kruppel-like factor 4 (Klf4), and c-Myelocytomatosis oncogene (c-Myc) (OSKM), can directly convert mouse and human somatic cells to an embryonic stem cell (ES)-like pluripotent state (1–6). Fully reprogrammed induced pluripotent stem cells (iPSCs) are similar to ES cells in morphology, pluripotent gene expression pattern, teratoma formation, germ-line transmission competent chimeras, and tetraploid complementation (6–9). The iPSCs thus provide a valuable tool to study human disease and raise the possibility of somatic cell-based personalized therapy (10, 11). Mesenchymal-to-epithelial transition (MET) has been shown as an important early event in somatic cell reprogramming (12, 13). The factors activating MET, such as Bone Morphogenetic Proteins (BMPs) and TGF-β inhibitors, can promote iPSC generation (12). Among the OSKM factors, Oct4 and Sox2 have been proven to be crucial in somatic cell reprogramming (14, 15), but the mechanism of Oct4 and Sox2 in iPSC generation and MET process remains unclear.
The miR-200 family is comprised of five members (miR-200a, miR-200b, miR-200c, miR-141, and miR-429), which located within two clusters on two separate chromosomes. These miRNAs can be subdivided into two groups according to their seed sequences (group I: miR-200a and miR-141; group II: miR-200b, miR-200c, and miR-429). Previous studies found that miRNAs of the miR-200 family were enforcers of epithelial phenotype and key regulators of MET (16–18). Enforced constitutive expression of the miR-200s in human mesenchymal cells promotes MET, whereas inhibition of miR-200s induces the mesenchymal-like spindle cell morphology, accompanied by an enhancement in cell migration. Expression of the miR-200 family is normal in ES cells but down-regulated during epithelial-to-mesenchymal transition (EMT), and stalls differentiating ES cells at the epiblast-like stem cell stage (19). A recent report found that p53 serves as a transcriptional activator of miR-200c, but not of the miR-200a/b/429 in human mammary epithelial cells (20), whereas an integrative genomic approach identified p73 and p63 as activators of miR-200a/b/429 and miR-200c/141 in ovarian carcinoma (21). However, whether the miR-200 family can be regulated and is involved in OSKM-induced MET and iPSC generation remains unknown.
Using miRNA target prediction algorithms (22, 23), the most prominent targets of the miR-200 family are zinc finger E-box binding homebox 1 (ZEB1) (also known as Tcf8 and δEF1) and ZEB2 [also known as zinc finger homebox1B (ZFXH1B) and SMAD interacting protein 1 (SIP1)] with multiple target sites (24, 25). Both ZEB1 and ZEB2 are the key transcriptional repressors of E-cadherin (E-cad) and a number of master regulators of epithelial polarity (26). Control of ZEB1 and ZEB2 by the miR-200 family is critical for MET and conducive to maintain stable epithelial states in human cancers (17). Interestingly, ZEB1 was shown to repress miR-200c (27). These data indicate that miR-200 family and ZEB1/ZEB2 represent a well-organized signaling pathway to accurately regulate MET process. However, whether the miR-200/ZEB pathway also plays important roles in OSKM-induced MET and cell reprogramming remains unknown.
In the present study, we indentified that members of the miR-200 family, directly activated by Oct4 and Sox2, are able to help fibroblasts to overcome the MET barrier and facilitate iPSC generation. Inhibition of ZEB2 and overexpression of ZEB2 can mimic or block the effects of miR-200s on iPSC generation and MET process, respectively, indicating that the miR-200/ZEB2 pathway is critically involved in Oct4/Sox2-induced MET and iPSC generation.
Results
Oct4/Sox2 Directly Activates the miR-200 Clusters by Binding at Promoter Regions.
In exploring differentially expressed miRNAs among mouse embryonic fibroblasts (MEFs), iPSCs generated with OSKM, and embryonic stem cells (E14), we found that all members of the miR-200 family (miR-200a, miR-200b, miR-200c, miR-141, miR-429) had a significantly elevated level in pluripotent cells (iPS, E14) compared with MEF cells (Fig. S1A). During iPSC reprogramming, these miRNAs are significantly up-regulated after infection by using OSKM on day 7 (Fig. 1A), suggesting that the miR-200 family may correlate with pluripotency and their activation may promote the emergence of iPSCs.
Fig. 1.
Oct4/Sox2 directly regulates the miR-200 clusters. (A) The expression level of miR-200a, miR-200b, miR-200c, miR-141, and miR-429 in MEFs, and MEFs after infection with OSKM viruses on day 5 and day 7. U6 was used as an internal control. (B) The expression level of miR-200a, miR-200b, miR-200c, miR-141, and miR-429 in MEFs, MEFs after infection with pMx-Oct4 and pMx-Sox2 retroviruses. (C) The exact binding information of Oct4 and Sox2 on the promoter regions of the miR-200 clusters (mmu-mir-141/200c, mmu-mir-200a/b/429). (D) Luciferase reporter assay for Oct4/Sox2 bindings at the promoter regions of mmu-mir-141/200c and mmu-mir-200a/b/429 was carried out with the corresponding luciferase reporter vector (Rluc-mir-141/200c, Rluc-mir-200a/b/429) by transfection with 100 ng, 200 ng, and 400 ng of pMx-Oct4 and pMx-Sox2, respectively. The pMx-GFP vector was used as a negative control (Ctrl). (E) ChIP-qPCR analyses for the fold enrichment of Oct4 and Sox2 at the promoter regions of mmu-mir-141/200c (Left) and mmu-mir-200a/b/429 (Right) were performed with MEFs (Ctrl), pMx-Oct4–, and pMx-Sox2–infected MEF cells. Fold enrichment for Oct4 or Sox2 binding was normalized to IgG. Primers for regions about more than 1,000 nt from the binding site were used as the negative control (NC) regions. Error bars represent the SD of three independent experiments (*P < 0.05; **P < 0.01; ***P < 0.001; Student’s t test) (n = 3).
Positive regulation of Oct4/Sox2 on the expression of miR-200 family were observed in MEF cells on day 4 after infection with retroviruses containing Oct4 and Sox2 (Fig. 1B), but not for c-Myc or Klf4 (Fig. S1B). Characterization of these miRNAs showed that members of the miR-200 family are clustered on chromosome 4 (mir-200a/b/429) and chromosome 6 (mir-141/200c). Analyses for the promoter regions of both clusters, and the custom binding sites of transcription factors Oct4/Sox2, gave a direct binding region of Oct4/Sox2 at the promoters of these miRNAs (Fig. 1C). To investigate the direct activation of Oct4/Sox2 on these miRNAs, we carried out dual-luciferase reporter assay with vectors containing the promoter region of each miRNA cluster with the predicted binding sites. Results showed that Oct4 specifically activates the mir-141/200c cluster and Sox2 can activate mir-200a/b/429 cluster in a dose-dependent manner (Fig. 1D). However, when the corresponding binding sites were mutated, there was no effect of Oct4 or Sox2 on the mutant promoter vectors of the miR-200 clusters (Fig. S1C). Further, we also observed almost 8.0-fold enrichment of Oct4 at the promoter region of mir-141/200c in MEF cells after infection with retroviruses generated with the vector pMx-Oct4, and 2.3-fold enrichment of Sox2 at that of mir-200a/b/429 after infection with pMx-Sox2, whereas there was no detectable enrichment in MEF cells and the negative control regions (Fig. 1E). Supporting this speculation, our results indicated that members of the miR-200 family are specific and direct targets of key pluripotency-associated transcription factors Oct4/Sox2 in iPSC generation.
Members of the miR-200 Family Promote iPSC Reprogramming.
To explore the exact function of each member of the miR-200 family in iPSC generation, we constructed an individual retroviral vector containing the specific miRNA primary sequence and confirmed the ectopic expression level of these miRNAs by quantitative RT-PCR (qRT-PCR) (Fig. S1D). Then, we introduced the OSKM factors with miR-200a, miR-200b, miR-200c, miR-141, or miR-429.
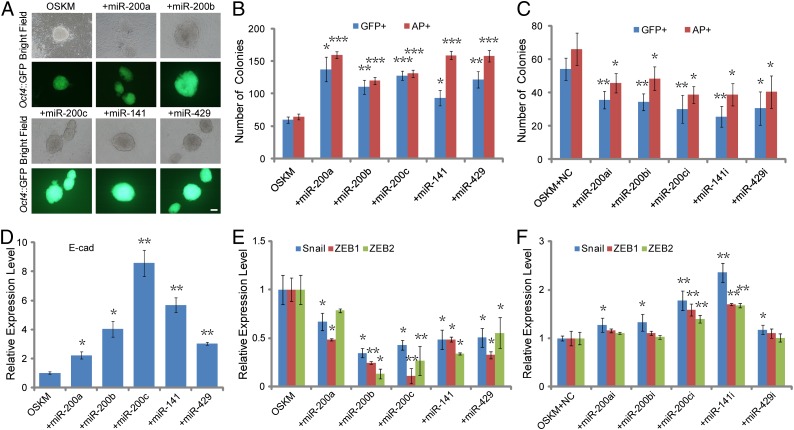
When members of the miR-200 family were included in iPSC induction with OSKM, numbers of both GFP- and Alkaline Phosphatase (AP)-positive colonies were significantly increased approximately twofold in Oct4::GFP MEFs (OG-MEFs) after infection with OSKM and miR-200a, miR-200b, miR-200c, miR-141, or miR-429 compared with that of OSKM group (Fig. 2 A and B and Fig. S1E). In contrast, the numbers of both GFP- and AP-positive colonies were significantly decreased in OG-MEFs after infection with OSKM and inhibitors for members of the miR-200 family (Fig. 2C), indicating that all members of the miR-200 family play important roles in somatic cell reprogramming.
Fig. 2.
The miR-200 family promotes iPSC generation at the early stage. (A) Morphology of typical Oct4::GFP-positive (GFP+) colonies for OSKM (Left), and OSKM in combination with member of the miR-200 family (Center and Right). (B) Quantification of GFP+ colonies on day 12 and AP-positive (AP+) colonies on day 8 after infection of OSKM, and OSKM+miR-200 member. (C) Quantification of GFP+ and AP+ colonies after infection of OSKM in combination with transfection of inhibitors for individual miRNA of the miR-200 family and the inhibitor negative control (NC). (D) qRT-PCR analysis for the expression level of epithelial gene (E-cad) in MEF cells after infection with OSKM, and OSKM in combination with member of the miR-200 family. (E) Quantification for the expression level of mesenchymal genes (Snail, ZEB1, ZEB2) as described in D. (F) qRT-PCR analysis for the expression level of mesenchymal genes (Snail, ZEB1, ZEB2) in MEF cells after infection with OSKM, and OSKM in combination with transfection of inhibitors for miR-200a, miR-200b, miR-200c, miR-141, miR-429, and the inhibitor negative control (NC). GAPDH was used as an internal control. Error bars represent the SD of three independent experiments (*P < 0.05; **P < 0.01; ***P < 0.001; Student’s t test) (n = 3). (Scale bars: 100 μm.)
Members of the miR-200 Family Promote the MET Process of iPSC Reprogramming.
The MET process has been shown with critical roles at the initial stage of somatic cell reprogramming. Consistently, we found that the expression level of mesenchymal genes (Snail, ZEB1, ZEB2, N-cadherin) were decreased and that of epithelial marker (E-cad, Ocln) were increased dramatically in MEF cells after infection with OSKM on day 5 and day 7 (Fig. S1F), indicating an effective MET process in OSKM-induced iPSC generation. However, the mechanism of Oct4/Sox2 facilitating MET remains to be investigated. Because Oct4/Sox2 could directly activate the miR-200 family, we speculated that Oct4/Sox2 may promote the MET process of iPSC generation by activating the miR-200 family.
Interestingly, we observed the number of ES-like colonies in OSKM+miRNA group was significantly higher than that in OSKM group (Fig. S2A). Quantification assay for the expression level of the epithelial marker (E-cad) (Fig. 2D), the E-cad–positive colony number (Fig. S2B), and the proportion of E-cad–positive cells (Fig. S2C) further confirmed that the increased level of E-cad and MET promotion induced by these miRNAs. The expression level of mesenchymal genes (Snail, ZEB1, ZEB2) were dramatically decreased in combination of OSKM and miR-200a, miR-200b, miR-200c, miR-141, or miR-429 on day 7 after infection compared with OSKM alone (Fig. 2E), whereas functional inhibition of these miRNAs significantly prevented the decrease of the mesenchymal genes compared with the negative control group (Fig. 2F). Moreover, overexpression of miR-200s can increase the expression level of some early reprogramming predictors [estrogen-related receptor beta (Esrrb), Utf1, Dppa2], whereas functional inhibition of these miRNAs significantly represses the expression of those early predictors (Fig. S2D). Overall, introduction of miR-200a, miR-200b, miR-200c, miR-141, or miR-429 facilitates MET and promotes reprogramming of MEFs to iPSCs, which may suggest that the miR-200s enhance iPSC generation at the early stage.
Pluripotency Characterization of iPSCs Generated with the miR-200 Family.
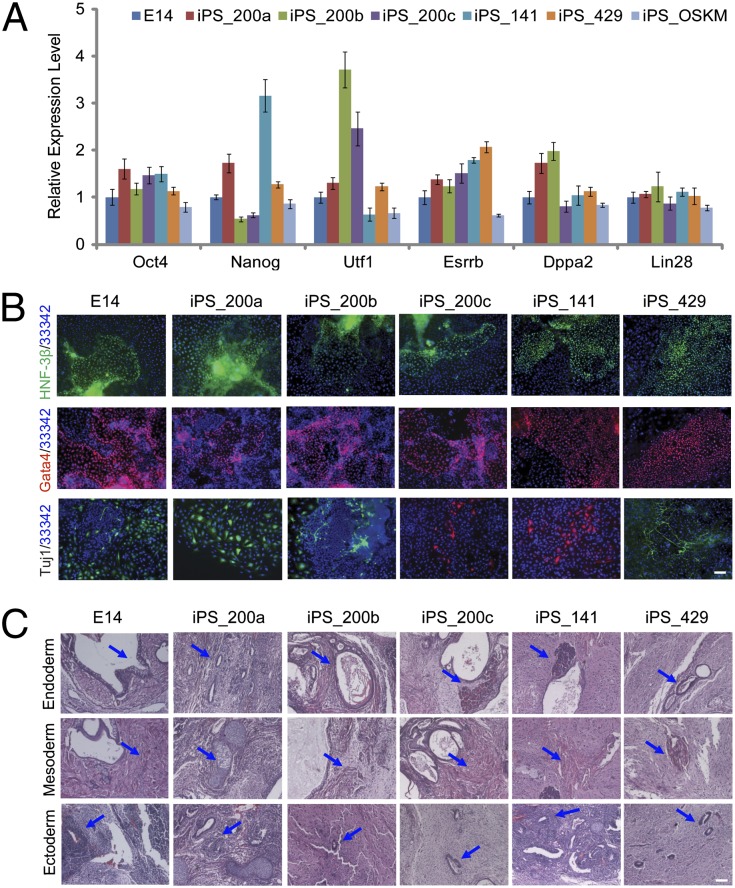
To examine whether introduction of the miR-200 family affects pluripotency during cell reprogramming, we derived iPSC lines from OSKM (iPS_OSKM), OSKM in combination with miR-200a (iPS_200a), miR-200b (iPS_200b), miR-200c (iPS_200c), miR-141 (iPS_141), and miR-429 (iPS_429). Genetic integration of Oct4, Sox2, Klf4, c-Myc, mir-200a, mir-200b, mir-200c, mir-141, and mir-429 retrovirus insertion in the corresponding iPSC lines showed that the colonies were derived from MEF cells infected by OSKM and corresponding miRNA simultaneously (Fig. S3A and Table S1). Endogenous Oct4, Sox2, Klf4, and c-Myc were activated, whereas exogenous transgenic OSKM were silenced in all iPSC lines (Fig. S3B and Table S2). Expression of pluripotency markers [Oct4, Nanog, Utf1, (Esrrb)] and predictors for successful reprogramming (Dppa2, Lin28) were also confirmed by qRT-PCR (Fig. 3A) and immunostaining assay for Oct4, Nanog, and SSEA-1 (Fig. S3C). Moreover, inconsistent with the activation of endogenous Oct4 and Nanog, the methylation level of Oct4 and Nanog promoter regions was lower in OSKM+miRNA-derived iPSCs than that in OG-MEF cells, and similar to that in E14 and OSKM-derived iPSCs (Fig. S3D and Table S3). Therefore, these iPSC lines derived by OSKM with members of the miR-200 family are pluripotent and have been successfully reprogrammed in terms of the activation of pluripotency-associated genes.
Fig. 3.
Pluripotency and differentiation potentials of OSKM+miRNA-derived iPSCs. (A) qRT-PCR analysis of Oct4, Nanog, Utf1, Esrrb, Dppa2, and Lin28 in mouse ES cells (E14), OSKM+miRNA-derived iPSCs, and OSKM-derived iPSC (iPS_OSKM). Error bars represent the SD of three independent experiments (n = 3). (B) Immunostaining shows OSKM+miRNA-derived iPSCs have in vitro differentiation potentials as expressing characteristic markers of the three germ layers, endoderm (HNF-3β), mesoderm (Gata4), and ectoderm (Tuj1), as similar to E14. Hochest 33342 (33342) was used for nucleus staining (blue). (C) H&E staining of teratomas for endoderm (epithelium and glandular), mesoderm (skeletal muscle), and ectoderm (neural tissue) were marked with blue arrows. (Scale bars: 100 μm.)
Differentiation Potentials of iPSCs Generated with the miR-200 Family.
To investigate whether these OSKM+miRNA-derived colonies have fully differentiation potentials like ES cells, embryoid body (EB) and teratoma formation assays were performed. qRT-PCR analyses indicated that these differentiated EBs expressed relevant three germ layer markers (Laminin B1, Sox17, BMP4, Mixl1, FGF5), whenever the expression of pluripotency markers Nanog and Esrrb was markedly decreased (Fig. S3E). Moreover, EBs generated from these iPSCs (iPS_200a, iPS_200b, iPS_200c, iPS_141, and iPS_429) exhibited positive immunostaining for lineage markers such as hepatocyte nuclear factor-3 beta (HNF-3β) (endoderm), GATA-binding protein 4 (Gata4) (mesoderm), and neuronal Class III β-tubulin (Tuj1) (ectoderm) (Fig. 3B). For differentiation potential detection in vivo, these OSKM+miRNA-derived iPSCs were injected to form teratomas with E14 cells as the positive control. Histological analysis revealed that these iPSCs can differentiate into all three germ layers like ES cells, including epithelium and glandular (endoderm), skeletal muscle (mesoderm), and neural tissue (ectoderm) (Fig. 3C). Taken together, members of the miR-200 family can promote iPSC reprogramming without sacrificing the differentiation potentials of iPSCs.
Members of the miR-200 Family Specifically Repress ZEB2 Expression.
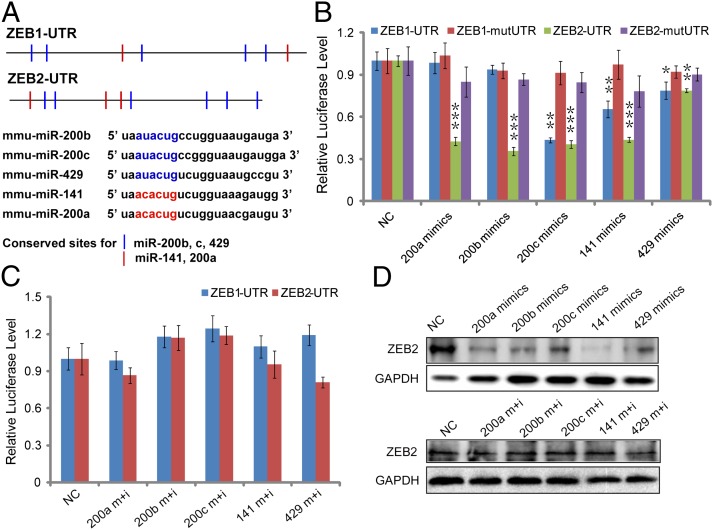
To further understand the mechanism underlying the miR-200 family effects on reprogramming, we explored the targets of miR-200a, miR-200b, miR-200c, miR-141, and miR-429. All of the five members can be classified into two groups based on the conserved binding sites (seed sequences). Combined with the previous studies and target prediction with three databases including TargetScan, Miranda, and PicTar, we obtained ZEB1 and ZEB2 as the candidate targets shared by these miRNAs in MET of iPSC reprogramming (Fig. 4A). Further, we performed the luciferase assay by using the ZEB1 and ZEB2 wild-type 3′ untranslated region (UTR) luciferase reporters. As shown in Fig. 4B, the wild-type 3′ UTR luciferase reporter activities of ZEB2 were significantly repressed by miRNA mimics compared with the control mimics. Cotransfection with inhibitors and mimics for these miRNAs could rescue the effect of corresponding mimics on the reporter gene activities (Fig. 4C). Regulation of the miR-200 family on ZEB1 and ZEB2 were further confirmed by the observations that there was little effect on the activities of ZEB1 and ZEB2 mutant 3′ UTR (Fig. 4B). Taken together, members of the miR-200 family have more significant effects on ZEB2 than ZEB1, suggesting that the miR-200 family mainly target ZEB2. Consistently, the protein level of ZEB2 was significantly inhibited by specific miRNA mimics of miR-200 family and blocked by the corresponding inhibitors (Fig. 4D). These results indicated that ZEB2 may be the preferred target gene of the miR-200 family in vivo.
Fig. 4.
The miR-200 family directly and specifically targets ZEB2. (A) Conserved target sites and seed sequences of miR-200b/c/429 (blue) and miR-141/200a (red) in the 3’ UTRs of mouse ZEB1 and ZEB2. (B) Luciferase reporter assay performed with vectors containing the wild-type ZEB1-UTR and ZEB2-UTR, and vectors containing DNA fragments with mutant target sites in the 3’ UTRs of ZEB1 (ZEB1-mutUTR) and ZEB2 (ZEB2-mutUTR). (C) Luciferase reporter assays performed with ZEB1-UTR and ZEB2-UTR after cotransfected with the miRNA mimics and inhibitors. (D) Western blotting analysis to confirm the effect of miR-200s on ZEB2 in MEF cells by transfection with mimics or mimics in combination with inhibitors. Mimics control was used as the negative control (NC). GAPDH was used as a loading control. Error bars denote the SD derived from three independent experiments (*P < 0.05; **P < 0.01; ***P < 0.001; Student’s t test) (n = 3).
miR-200 Family Promotes MET and iPSC Generation by Targeting ZEB2.
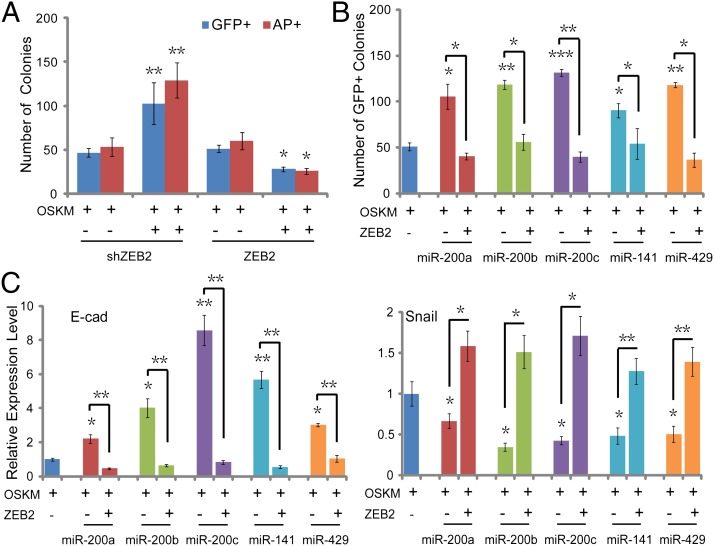
To address whether ZEB2 plays important roles in iPSC reprogramming, we carried out the ectopic expression and inhibition of ZEB2 (Fig. S4A) in OSKM-induced iPSC generation. It was shown that overexpression of ZEB2 led to a significant decrease in the number of GFP- and AP-positive colonies in OSKM+ZEB2 group compared with that of OSKM alone (Fig. 5A) and elevated the expression level of mesenchymal genes, and decreased that of epithelial genes (Fig. S4B). Inhibition of ZEB2 by shRNA can significantly mimic the effects of miR-200s on the expression level of MET genes (Fig. S4B) and GFP- and AP-positive colony numbers (Fig. 5A). Further, we compared the effects on MET and cell reprogramming efficiency after infection with OSKM, OSKM+miRNA, and OSKM+miRNA+ZEB2. Results showed that the addition of members of the miR-200 family improved the reprogramming efficiency and promoted MET, whereas combination of the individual miRNA with ZEB2 blocked the effects of these miRNAs on the number of AP- (Fig. S4C) and GFP-positive (Fig. 5B) colonies, and MET process (Fig. 5C and Fig. S4D) in iPSC generation. These findings suggested that members of the miR-200 family enhance MET of somatic cell reprogramming by directly targeting ZEB2 and first provided evidence for the critical role of the miR-200/ZEB2 pathway involved in Oct4/Sox2-mediated MET and iPSC generation.
Fig. 5.
The miR-200 family promote MET in iPSC generation by targeting ZEB2. (A) Quantification of the number of GFP+ and AP+ colonies on day 12 and day 8 after infection with OSKM and ZEB2-shRNA (OSKM+ZEB2-shRNA), OSKM and ZEB2 (OSKM+ZEB2) compared with OSKM and the corresponding control vector. (B) Quantification for the number of GFP+ colonies after infection with OSKM, OSKM+miRNA, and OSKM+miRNA+ZEB2 on day 12. (C) The expression level of epithelial gene (E-cad) (Left) and mesenchymal gene (Snail) (Right) after infection with OSKM, OSKM+miRNA, and OSKM+miRNA+ZEB2 on day 7. Error bars denote the SD derived from three independent experiments (*P < 0.05; **P < 0.01; ***P < 0.001; Student’s t test). (n = 3).
Discussion
Reprogramming from somatic cells to patient-specific iPSCs provides a valuable tool for studying human disease and personalized therapy. Among the defined transcriptional factors OSKM, Oct4 and Sox2 play critical roles in somatic cell reprogramming (14, 15). However, the exact roles and mechanisms of Oct4/Sox2 in iPSC generation are not clear. In the present study, we found that activation of the miR-200/ZEB2 pathway is an unrevealed and important function of Oct4/Sox2 at the early stage of iPSC generation.
It is becoming increasingly evident that miRNAs play crucial roles in somatic cell reprogramming, self-renewal, and differentiation. Previous studies showed that ES-specific miRNAs (miR-290 cluster; miR-302/367; miR-106a cluster) are under the control of the key transcription regulators such as Oct4, Sox2, and Nanog via occupying the promoters of miRNAs and play critical roles in maintaining ES cell pluripotency and self-renewal (28, 29). Among these miRNAs, miR-302 cluster is comprised of a cluster of eight related miRNAs and is directly regulated by Oct4 and Sox2 through binding at the promoter (29). miR-302 and ES-specific miR-290 cluster have been proven to promote the iPSC generation (30, 31), and miRNAs serve as sequence-specific posttranscriptional regulators, suggesting that direct regulation of miRNA expression by core transcription factors may represent one of the crucial mechanisms for transcription factors specifically regulating definite gene expression and cell reprogramming. Consistent with these reports, we found that expression of endogenous miR-200s obviously increased during the OSKM-induced cell reprogramming, or after infection of cells with Oct4 and Sox2 individually. Genomic assay showed that five members of the miR-200 family located within two clusters on two chromosomes in mouse (Chromosome 4: mir-200a/b/429; Chromosome 6: mir-141/200c). However, little is known about the role and intrinsic regulators of the miR-200 family in iPSC induction. Based on our data, Oct4 and Sox2 could bind to the promoter region of mir-141/200c and mir-200a/b/429, respectively, and activate the transcription of miR-200s. Combined with our findings that miR-200s are required for OSKM-induced iPSC generation, our study revealed that during iPSC induction, endogenous miR-200s serve as the unique mediators of Oct4 and Sox2 for the induction of cell reprogramming.
Up to date, the role of the miR-200 family in pluripotency acquirement of iPS and ES cell differentiation is not clear. miR-200 family members have been found to inhibit ES cell differentiation through directly targeting Cadherin11 and Neuropilin1 (32), and with the reprogramming factors together, miR-200b and miR-200c can promote MET at the early stage of reprogramming (12). On the contrary, some studies showed that the miR-200 members promoted differentiation through repressing the expression of B lymphoma Mo-MLV insertion region 1 homolog (Bmi1), a polycomb repressor that acts to promote “stemness” in ES cells (33), and the overexpression of miR-200c in normal stem cells or cancer stem cells reduced their clonogenic or tumor-initiating capacities (34, 35). Results of the present study showed that the expression levels of miR-200s are significantly higher in pluripotent stem cells than that in MEF cells. Overexpression of the miR-200 family members can promote, whereas functional suppression of miR-200s with specific inhibitors significantly represses the OSKM-induced MET and iPSC generation. Recently, it has been demonstrated that the MET process is an important early event of iPSC generation (12) and the activation of EMT is associated with the maintenance of stem-cell properties (36). Our results thus indicated that the miR-200 family can promote the formation of iPSCs at the early stage. This conclusion was further supported by our findings that the miR-200s could up-regulate the expression level of Esrrb, Utf1, and Dppa2, the recently proven early predictors for successful reprogramming (37).
ZEB2 is a member of the ZFHX1 family of two-handed zinc finger/homeodomain proteins and was initially discovered with the yeast two-hybrid system as a binding partner of SMAD1 and SMAD2/3 (38). The best characterized role of ZEB2 is the induction of cell transformation and metastasis during the induction of EMT process, a phenomenon occurring normally during embryonic development, wound healing, and carcinogenesis (39). However, the function of ZEB2 in iPSC generation remains unclear. Based on the effects of overexpression and inhibition of ZEB2 on iPSC generation, the present study proved the unrevealed function of ZEB2 in iPSC reprogramming.
Previous studies found that members of the miR-200 family mainly function through directly targeting the mesenchymal markers ZEB1 and/or ZEB2 in the EMT process of cancer metastasis (18, 20), where the ZEB2 protein is responsible for repressing the key MET gene E-cadherin (40). It has been indicated that ZEB1 and ZEB2 could form a double-negative feedback loop with miRNAs to control EMT and MET programs in both development and tumorigenesis (41). Here, we found that ZEB2 is the in vivo functional target of miR-200s, inhibition of ZEB2 can mimic the effects of miR-200s, and overexpression of ZEB2 can significantly rescue the effects of miR-200s in MET and iPSC generation. These data prove that the miR-200/ZEB2 pathway plays critical roles in Oct4/Sox2 facilitating MET and iPSC reprogramming.
In conclusion, we found that Oct4 and Sox2 can directly activate the expression of a specific cluster of the miR-200 family by binding to their promoter regions, whereas the expression of miR-200s significantly repress ZEB2 expression through directly targeting its 3′ UTR. This miR-200/ZEB2 pathway helps fibroblasts to overcome the MET barrier and facilitates OSKM-induced iPSC generation. These results not only suggest that miR-200s are the unique mediator of Oct4 and Sox2 for the iPSC induction, but also demonstrate that the miR-200/ZEB2 pathway plays important roles in Oct4/Sox2-initiated MET process and somatic cell reprogramming.
Materials and Methods
Oct4::GFP MEFs (OG-MEFs) were used for iPSC induction with pMX-Oct4, Sox2, Klf4, c-Myc (3), and corresponding miRNA vectors constructed with primers in Table S4. iPSCs were maintained on feeder layers of mitomycin C (Sigma)-treated MEF cells. qRT-PCR assay with primer sequences listed in Table S5 for gene expression, and the Bulge-LoopTM miRNA qPCR Primer Sets (Ribobio) were used to detect the expression of miRNAs. ChIP-PCR assay was carried out with antibody for rabbit control IgG (Millipore), anti-Oct4 (Abcam), anti-Sox2 (Abcam), and primers around the binding site (<200 nt) and negative control region (>1,000 nt from the binding region of mmu-mir-141/200c, chromosome 6: 124677751–124677900; and mmu-mir-200a/b/429, chromosome 4: 154908051–154908150) (Table S5). The expression level was quantized by the relative standard curve method. Inhibitors for miRNAs chemically synthesized single-stranded RNA and antisense oligonucletides of mature miRNA by Ribobio were cotransfected with OSKM as described (42). Luciferase reporter assay, FACS, AP staining and immunostaining, embryoid body formation and in vitro differentiation, teratoma formation and H&E staining, and other protocols are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by Ministry of Science and Technology Grants 2011CB965100, 2011CBA01100, 2011DFA30480, 2010CB944900, 2010CB945000, and 2012CB966603; National Natural Science Foundation of China Grants 91219305, 31101061, 31210103905, 31071306, 31000378, 31171432, and 81170499; Science and Technology Commission of Shanghai Municipality Grants 11ZR1438500 and 11XD1405300; Ministry of Education Grants IRT1168 and 20110072110039; and the “Chen Guang” project, which is supported by Shanghai Municipal Education Commission and Shanghai Education Development Foundation Grant 12CG19, and the Fundamental Research Funds for the Central Universities.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1212769110/-/DCSupplemental.
References
- 1.Lowry WE, et al. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci USA. 2008;105(8):2883–2888. doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park IH, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134(5):877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 6.Wernig M, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448(7151):318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 7.Kang L, Wang J, Zhang Y, Kou Z, Gao S. iPS cells can support full-term development of tetraploid blastocyst-complemented embryos. Cell Stem Cell. 2009;5(2):135–138. doi: 10.1016/j.stem.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448(7151):313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 9.Zhao XY, et al. iPS cells produce viable mice through tetraploid complementation. Nature. 2009;461(7260):86–90. doi: 10.1038/nature08267. [DOI] [PubMed] [Google Scholar]
- 10.Nishikawa S, Goldstein RA, Nierras CR. The promise of human induced pluripotent stem cells for research and therapy. Nat Rev Mol Cell Biol. 2008;9(9):725–729. doi: 10.1038/nrm2466. [DOI] [PubMed] [Google Scholar]
- 11.Park IH, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451(7175):141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 12.Samavarchi-Tehrani P, et al. Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell. 2010;7(1):64–77. doi: 10.1016/j.stem.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Li R, et al. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell. 2010;7(1):51–63. doi: 10.1016/j.stem.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Shi Y, et al. A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;2(6):525–528. doi: 10.1016/j.stem.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Huangfu D, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26(11):1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 16.Gregory PA, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10(5):593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 17.Gregory PA, Bracken CP, Bert AG, Goodall GJ. MicroRNAs as regulators of epithelial-mesenchymal transition. Cell Cycle. 2008;7(20):3112–3118. doi: 10.4161/cc.7.20.6851. [DOI] [PubMed] [Google Scholar]
- 18.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22(7):894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill JG, et al. Snail and the microRNA-200 family act in opposition to regulate epithelial-to-mesenchymal transition and germ layer fate restriction in differentiating ESCs. Stem Cells. 2011;29(5):764–776. doi: 10.1002/stem.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang CJ, et al. p53 regulates epithelial-mesenchymal transition and stem cell properties through modulating miRNAs. Nat Cell Biol. 2011;13(3):317–323. doi: 10.1038/ncb2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knouf EC, et al. An integrative genomic approach identifies p73 and p63 as activators of miR-200 microRNA family transcription. Nucleic Acids Res. 2012;40(2):499–510. doi: 10.1093/nar/gkr731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 23.Griffiths-Jones S. miRBase: The microRNA sequence database. Methods Mol Biol. 2006;342:129–138. doi: 10.1385/1-59745-123-1:129. [DOI] [PubMed] [Google Scholar]
- 24.Burk U, et al. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9(6):582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korpal M, Lee ES, Hu G, Kang Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem. 2008;283(22):14910–14914. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peter ME. Let-7 and miR-200 microRNAs: Guardians against pluripotency and cancer progression. Cell Cycle. 2009;8(6):843–852. doi: 10.4161/cc.8.6.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bracken CP, et al. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res. 2008;68(19):7846–7854. doi: 10.1158/0008-5472.CAN-08-1942. [DOI] [PubMed] [Google Scholar]
- 28.Marson A, et al. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell. 2008;134(3):521–533. doi: 10.1016/j.cell.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Card DA, et al. Oct4/Sox2-regulated miR-302 targets cyclin D1 in human embryonic stem cells. Mol Cell Biol. 2008;28(20):6426–6438. doi: 10.1128/MCB.00359-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Judson RL, Babiarz JE, Venere M, Blelloch R. Embryonic stem cell-specific microRNAs promote induced pluripotency. Nat Biotechnol. 2009;27(5):459–461. doi: 10.1038/nbt.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin SL, et al. Regulation of somatic cell reprogramming through inducible mir-302 expression. Nucleic Acids Res. 2011;39(3):1054–1065. doi: 10.1093/nar/gkq850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin CH, Jackson AL, Guo J, Linsley PS, Eisenman RN. Myc-regulated microRNAs attenuate embryonic stem cell differentiation. EMBO J. 2009;28(20):3157–3170. doi: 10.1038/emboj.2009.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wellner U, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11(12):1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- 34.Mongroo PS, Rustgi AK. The role of the miR-200 family in epithelial-mesenchymal transition. Cancer Biol Ther. 2010;10(3):219–222. doi: 10.4161/cbt.10.6312548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimono Y, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138(3):592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buganim Y, et al. Single-cell expression analyses during cellular reprogramming reveal an early stochastic and a late hierarchic phase. Cell. 2012;150(6):1209–1222. doi: 10.1016/j.cell.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verschueren K, et al. SIP1, a novel zinc finger/homeodomain repressor, interacts with Smad proteins and binds to 5′-CACCT sequences in candidate target genes. J Biol Chem. 1999;274(29):20489–20498. doi: 10.1074/jbc.274.29.20489. [DOI] [PubMed] [Google Scholar]
- 39.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7(6):415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 40.Comijn J, et al. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001;7(6):1267–1278. doi: 10.1016/s1097-2765(01)00260-x. [DOI] [PubMed] [Google Scholar]
- 41.Hill L, Browne G, Tulchinsky E. ZEB/miR-200 feedback loop: At the crossroads of signal transduction in cancer. Int J Cancer. 2013;132(4):745–754. doi: 10.1002/ijc.27708. [DOI] [PubMed] [Google Scholar]
- 42.Li Z, Rana TM. Using microRNAs to enhance the generation of induced pluripotent stem cells. Curr Protoc Stem Cell Biol. 2012 doi: 10.1002/9780470151808.sc04a04s20. 20:4A.4.1–4A.4.14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.