Abstract
Proper synaptic function requires the spatial and temporal compartmentalization of RNA metabolism via transacting RNA-binding proteins (RBPs). Loss of RBP activity leads to abnormal posttranscriptional regulation and results in diverse neurological disorders with underlying deficits in synaptic morphology and transmission. Functional loss of the 68-kDa RBP Src associated in mitosis (Sam68) is associated with the pathogenesis of the neurological disorder fragile X tremor/ataxia syndrome. Sam68 binds to the mRNA of β-actin (actb), an integral cytoskeletal component of dendritic spines. We show that Sam68 knockdown or disruption of the binding between Sam68 and its actb mRNA cargo in primary hippocampal cultures decreases the amount of actb mRNA in the synaptodendritic compartment and results in fewer dendritic spines. Consistent with these observations, we find that Sam68-KO mice have reduced levels of actb mRNA associated with synaptic polysomes and diminished levels of synaptic actb protein, suggesting that Sam68 promotes the translation of actb mRNA at synapses in vivo. Moreover, genetic knockout of Sam68 or acute knockdown in vivo results in fewer excitatory synapses in the hippocampal formation as assessed morphologically and functionally. Therefore, we propose that Sam68 regulates synapse number in a cell-autonomous manner through control of postsynaptic actb mRNA metabolism. Our research identifies a role for Sam68 in synaptodendritic posttranscriptional regulation of actb and may provide insight into the pathophysiology of fragile X tremor/ataxia syndrome.
In neurons, the control of RNA transport and translation by RNA-binding proteins (RBPs) is necessary for proper synaptic function (1, 2). RBPs typically bind to and repress the translation of dendritically expressed mRNAs until receipt of an activating signal (3). This mechanism allows locally restricted translation to occur in an input-specific manner. Loss of RBP-mediated posttranscriptional regulation results in diverse neurological disorders (4, 5) caused by underlying deficits in dendritic spine morphology and synaptic transmission. The dendritic compartment contains numerous RBPs, of which only a small fraction have been well characterized (6). The RBP Src-associated in mitosis of 68kD (Sam68) is a member of the signal transduction and activation of RNA family of proteins (7) that is highly expressed in the hippocampus, where it has been observed in dendritic processes (8). Because the cytoplasmic function of Sam68 in neurons remains largely unexplored, we examined the capacity of Sam68 to regulate mRNA metabolism in the synaptodendritic compartment of hippocampal neurons.
Sam68 has been implicated in cytoplasmic–nuclear RNA transport (9), alternative splicing (10–12), and translational control (13). In contrast to other neuronal RBPs that act as repressors of translation (3), evidence suggests that Sam68 promotes translation of its cargo mRNAs (13). Among several transcripts, Sam68 binds to β-actin (actb) mRNA in vivo and in vitro through an extended KH-RNA–binding domain that recognizes a stem loop poly(U) motif in its 3′ UTR (14, 15). Actb is the primary cytoskeletal component of dendritic spines, and polymerization/depolymerization of actin subunits is essential for the formation and maintenance of spine architecture (16, 17). The contribution of local actb synthesis per se to the integrity of dendritic spines in vivo is unclear, but abnormal dendritic transport of actb mRNA results in altered dendritic morphology in neuronal cultures (18, 19). Dendritic transport of actb mRNA requires, in part, Zip code-binding protein 1 (ZBP1) (20). ZBP1 is expressed primarily during development (21, 22), and only 50% of actb-containing ribonucleoprotein granules colocalize with ZBP1 (23), suggesting that other RBPs, such as Sam68, are necessary to localize actb mRNA to the dendrite postnatally.
Loss of Sam68 function has been linked to the pathogenesis of the neurodegenerative disorder fragile X tremor/ataxia syndrome (FXTAS) (24) that is characterized by adult-onset ataxia and cognitive decline (25). Interestingly, Sam68-KO mice exhibit motor-coordination deficits similar to the ataxia phenotype observed in FXTAS patients (12, 26). The histological hallmark of FXTAS is the presence of intranuclear Sam68-containing inclusions, principally in hippocampal neurons (24), indicating that Sam68 might be crucial in the maintenance of proper hippocampal physiology.
Here we report that in neuronal cultures knocking down Sam68 or interfering with its binding to actb mRNA results in less synaptodendritic actb mRNA and decreased spine density. Moreover we find that Sam68-KO mice have less actb mRNA associated with polysomes in the synaptic compartment, lower levels of synaptic actb protein, and fewer synapses in the hippocampus as assessed morphologically and functionally. Acute knockdown of Sam68 in vivo results in a similar decrease in the number of synapses. These observations suggest that Sam68 regulates hippocampal synapse number by promoting the association of actb mRNA with synaptic polyribosomes, the sites of local translation at synapses.
Results
Sam68 is Present at Synapses.
Proteomic analyses demonstrate that the postsynaptic density (PSD) contains numerous RBPs, including Sam68 (6, 27). To determine if Sam68 is expressed in the synaptic compartment, we prepared subcellular fractions from acutely dissected rat brains and found, using Western blots, that Sam68 was present in synaptosomal and PSD fractions (Fig. 1A). Similar to the distribution observed for ZBP1, which has a well-characterized role in the dendritic metabolism of actb mRNA, only a fraction of Sam68 was cytoplasmic (Fig. 1A). Immunocytochemical analysis of Sam68 in rat primary hippocampal cultures at 21 d in vitro (DIV) confirmed that Sam68 was present in the nucleus, dendrites, and synapses (Fig. 1 B and C). We identified synapses by the colocalization of pre- and postsynaptic markers Bassoon and PSD95 and found that ∼38% of synapses colocalized with Sam68 (Fig. 1C). Knockdown of Sam68 using lentiviral shRNAs reduced its signal observed in immunocytochemistry (ICC) as well as Western blots (Fig. S1 and Fig. 2B), demonstrating the specificity of the antibody used. The presence of Sam68 at a substantial number of synapses suggests that it has a role in synaptic mRNA metabolism.
Fig. 1.
Sam68 is present at the PSD. (A) Equal amounts of nonnuclear (Non-Nuc, supernatant after nuclear purification), nuclear (Nuc), whole-brain lysate (Total), synaptosomes (Syn), or PSD fractions were analyzed by Western blots probed for PSD (PSD95 and GluR1), presynaptic (SV2), and nuclear (c-FOS) markers and for the RBPs Sam68, and ZBP1. (B) Cultured hippocampal neurons (21 DIV) immunostained for Sam68 (green) and the dendritic marker MAP2 (blue) show that Sam68 exhibits a nuclear and punctate distribution along the dendrite. (C) ICC of dendrites shows that Sam68 (blue) is present at 37.7 ± 0.06% of synapses (n = 6 dendrites) identified by the colocalization of both pre- (Bassoon, red) and postsynaptic (PSD95, green) makers.
Fig. 2.
The dendritic localization of actb mRNA requires Sam68. (A) FISH analysis of dendrites from 21-DIV hippocampal cultures infected with control (scr virus), overexpression (Sam68 over) or knockdown (shS68#1) lentivirus show the effects of Sam68 abundance on the punctate distribution of actb mRNAs (red) in dendrites. Dendritic morphology was observed using expressed GFP (false-colored gray). (B) Western blots show that lentiviral-expressed shRNAs (shS68#1 or shS68#2) can knock down endogenous Sam68. Rescue was achieved using a virus that expresses an shRNA-insensitive eGFP-tagged Sam68 along with an shRNA (shS68#1). A virus expressing a Sam68 construct lacking the RNA-binding domain (ΔKH-S68) served as a rescue control. (C) Quantification of dendritic actb mRNA 10–60 µm from the soma by FISH after Sam68 knockdown or overexpression. (scr, 0. 75 ± 0.05, n = 5; shS68#1, 0.42 ± 0.05, n = 9; shS68#2, 0.26 ± 0.03, n = 7; Sam68 over, 1.20 ± 0.12, n = 6; rescue, 0.6 ± 0.04, n = 5). One way ANOVA F(4,27) = 27.3, P < 0.05. (D) RNA immunoprecipitations confirm that Sam68 binds to actb and CaMKIIα mRNA. The addition of an actb-blocking oligo (S68/actb oligo), but not an scr oligo, specifically disrupts the interaction of Sam68 with actb but not with CaMKIIα mRNA. (E) Quantification of dendritic actb mRNA in hippocampal cultures transfected with chimeric 2′-O-methyl DNA oligonucleotides antisense to the Sam68- or ZBP1-binding regions in the actb 3′ UTR (scr, 0.82 ± 0.1, n = 15; S68/actb, 0.42 ± 0.05, n = 16; S68/actb + ZBP1, 0.18 ± 0.03, n = 16). One way ANOVA F(2,45) = 23.5, P < 0.05.
Sam68 Is Required for the Dendritic Localization of Actb mRNA.
The transport of actb mRNA into neuronal processes requires its association with RBPs (18, 28). Because actb is a cargo of Sam68 in vivo (15), we asked if Sam68 was necessary for proper dendritic distribution of actb mRNA. Using FISH, we detected and quantified dendritic actb mRNA in a region 10–60 µm from the soma (Fig. 2A). Lentivirus-mediated knockdown of Sam68, which resulted in a 70% decrease in its abundance (Fig. 2B, shS68#1 and shs68#2), significantly reduced the level of actb mRNA in dendrites compared with a scrambled (scr) shRNA control (Fig. 2C, shS68#1). This decrease was evident throughout dendrites up to 100 µm from the soma (Fig. S2 A and C). We obtained similar results using a second lentiviral shRNA targeting a different region of Sam68, indicating that our results likely were not caused by off-target effects (Fig. 2 B and C, shS68#2). To confirm further the specificity of our knockdown experiments, we generated a rescue lentiviral construct that contained a Sam68-specific shRNA and an shRNA-insensitive eGFP-tagged Sam68 construct (Fig. 2B, rescue). Rescue of Sam68 (Fig. 2C, rescue) resulted in dendritic actb mRNA levels similar to those in controls. Overexpression of Sam68 in the absence of knockdown of endogenous protein increased the amount of actb mRNA observed in dendrites (Fig. 2C, Sam68 over). We found no changes in the total levels of actb mRNA across all experimental conditions (Fig. S2E). Moreover, Sam68 knockdown did not alter the dendritic localization of poly(A) mRNA as visualized by FISH analysis using an oligo(dT) probe (Fig. S2 B and D). These results suggest that Sam68 is necessary and sufficient for the localization of actb mRNA in dendrites.
The effects of Sam68 knockdown on actb mRNA localization could arise from a dysregulation of any number of Sam68 mRNA cargos (8, 15, 29). To determine if a direct interaction between Sam68 and actb mRNA mediates this effect, we designed 2′-O-methyl oligonucleotides targeting the Sam68-binding region in the 3′ UTR of actb mRNA. These oligos were designed to hinder sterically the interaction between Sam68 and the actb mRNA 3′ UTR while leaving the binding of Sam68 to its other cargos intact (20, 30). We tested their specificity using RNA-immunoprecipitation (RIP) assays and found that they could block the interaction of Sam68 with actb mRNA but not with another Sam68 target, CaMKIIα mRNA (Fig. 2D, S68/actb oligo). An scr oligo had no effect on either interaction (Fig. 2D, scr oligo). Transfection of these blocking oligos into primary hippocampal neurons reduced the amount of actb mRNA in dendrites, similar to knockdown experiments (Fig. 2E). These results suggest that the dendritic localization of actb mRNA depends on the binding of Sam68 to the 3′ UTR of actb.
The RBP ZBP1 also has a role in the dendritic transport of actb mRNA (30). To determine the relative contributions of Sam68 and ZBP1 to the transport of actb mRNA, we simultaneously transfected our Sam68/actb mRNA-blocking oligos with an oligo previously shown to block the binding of ZBP1 to the 3′ UTR of actb mRNA (30). Transfection of both oligonucleotides resulted in a further decrease in dendritic actb mRNA compared with the Sam68 oligo alone (Fig. 2E, S68/actb + ZBP1 oligo). All oligos were readily transfected into primary neurons as assayed using Cy3 (S68 oligo)- and Cy5 (ZBP1 oligo)-labeled oligos. We measured a transfection efficiency of 84 ± 0.04%, and 97 ± 0.05% of transfected cells were transfected with both oligos (Fig. S2F). Transfection of these oligos did not alter total levels of actb mRNA (Fig. S2E). This observation suggests that the combinatorial action of Sam68 and ZBP1, working in parallel, regulates the dendritic localization of actb mRNA in neuronal cultures.
Sam68 Is Required for the Postsynaptic Localization of Actb mRNA.
Because both Sam68 and actb mRNA are present in dendritic spines (23), we sought to determine the role of Sam68 in the postsynaptic targeting of actb mRNA. FISH analysis for actb mRNA in conjunction with ICC for PSD95 in hippocampal neurons at 21 DIV revealed that 37 ± 7% of PSD95 signal colocalized with actb mRNA (Fig. 3 A and B). Sam68 knockdown with either of two different Sam68-specific shRNAs significantly decreased the extent of this colocalization compared with control (scr virus) shRNAs (Fig. 3B, shS68#1 and shS68#2). As expected, rescue of Sam68 expression restored the postsynaptic localization of actb mRNA (Fig. 3B, rescue). However, re-expression of a Sam68 mutant lacking the KH-RNA–binding domain (∆KH-S68) failed to do so (Fig. 3B), demonstrating that the RNA-binding activity of Sam68 is essential for this process. The decrease in postsynaptic actb mRNA could result from a selective deficit in postsynaptic targeting of actb mRNA or from a general loss of actb mRNA in dendrites. To distinguish between these two possibilities, we measured the fraction of dendritic actb mRNA that colocalized with PSD95 to normalize our results for the amount of dendritic actb mRNA. We found that 13 ± 2% of dendritic actb mRNA colocalized with PSD95. Knockdown of Sam68 resulted in an ∼2.6-fold decrease (shS68#1, 5 ± 2%) in this colocalization, suggesting that Sam68 selectively localizes actb mRNA to synaptic junctions (Fig. 3C).
Fig. 3.
Knockdown of Sam68 results in selective loss of actb mRNA from the PSD. (A) FISH for actb mRNA (red) in conjunction with ICC for PSD95 (green) illustrates that actb mRNA is present in the dendrites and spines of 21-DIV cultured hippocampal neurons. (B) The fraction of PSD95 signal that colocalizes with actb mRNA was quantified using Mander’s coefficients [Java constraint solver (JACoP), ImageJ]. Sam68 knockdown significantly reduced this fraction compared with controls (scr virus, 37 ± 7%; shS68#1, 13.7 ± 1%; shS68#2, 14.3 ± 2%; n = 8 dendrites). Full-length Sam68 (rescue), but not ∆KH-S68, restored control levels of synaptic actb mRNA (rescue, 32.9 ± 4%; ∆KH-S68, 4.8 ± 1%; n = 8 dendrites). One-way ANOVA F(4,35) = 10.9, P < 0.05. (C) Sam68 knockdown significantly reduced the percentage of actb mRNA in dendrites that colocalized with PSD95 compared with control (scr virus, 13 ± 2%; shS68#1, 5 ± 2%, n = 10 dendrites for each condition, P < 0.05).
We also analyzed actb mRNA FISH in conjunction with Sam68 ICC and found that 40 ± 1% of the actb mRNA signal colocalized with Sam68, whereas 28 ± 2% of the Sam68 signal colocalized with actb mRNA (Fig. S3). Triple colocalization analysis of Sam68, actb mRNA, and PSD95 revealed that a majority (75 ± 4%) of the postsynaptic actb mRNA signal (actb mRNA colocalized with PSD95) contained Sam68 (Fig. S3C). These results support our hypothesis that Sam68 plays an important role in the synaptic localization of actb mRNA.
Sam68 Promotes the Association of Actb mRNA with Synaptic Polysomes in Vivo.
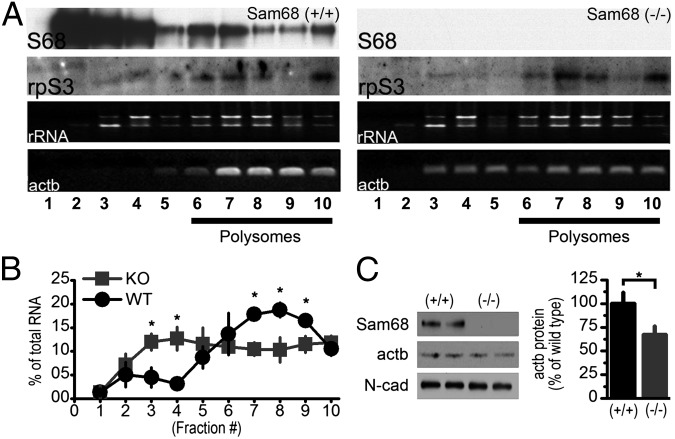
Sam68 associates with polysomes during mitotic divisions of germ cells (31) and after KCl-mediated depolarization of neuronal cultures (8). To investigate this interaction in a more physiological preparation, we obtained Sam68-KO mice from Stéphane Richard (McGill University, Montreal, Canada) (32), and isolated polysomes from synaptosomal fractions (synaptopolysomes) (33) of acutely dissected hippocampi. We found that Sam68 was present in the heavier polysome fractions, confirming an association between Sam68 and synaptic polysomes (Fig. 4A, Left). There was no Sam68 immunoreactivity in synaptopolysomes isolated from KO mice, further validating the specificity of our antibody (Fig. 4A, Right). The overall distribution of ribosomal subunits as revealed by staining for a ribosomal marker, the 40S protein rpS3, was similar in the two genotypes (Fig. 4A). Because polysomes are sites of active translation (34, 35), these results suggest that Sam68 plays a role in the translation of synaptic actb mRNA.
Fig. 4.
Sam68 promotes the association of actb mRNA with synaptopolysomes in vivo. (A) (Upper) Western blots of Sam68 and the ribosomal marker rpS3 from synaptopolysomal fractions isolated from hippocampi of Sam68-KO mice and WT littermates. (Lower) Ribosomal RNA (rRNA) and RT-PCR of actb mRNA extracted from similar fractions. (B) The actb cDNA in each fraction was quantified by densitometry and graphed as a percentage of total actb cDNA from all fractions (n = 3 paired littermates). (C) Western blot of actb expression in synaptosomal fractions prepared from hippocampi of Sam68-KO mice and WT littermates and quantified by densitometry (WT, 100 ± 11.5%, n = 10 hippocampi; KO, 67 ± 9%, n = 10 hippocampi, P < 0.05). N-cadherin (N-cad) was used as a loading control.
To determine if Sam68 regulates the association of actb mRNA with polysomes in vivo, we isolated hippocampal synaptopolysomes from WT and KO mice and determined the amount of actb mRNA present by RT-PCR. We found less actb mRNA in the heavier synaptopolysome fractions and more actb mRNA in the lighter ribonucleoprotein (RNP) fractions in Sam68-KO mice than in control littermates (Fig. 4 A and B). The distribution of ribosomal RNA was similar in the two genotypes (Fig. 4A), suggesting that gross translation was not disturbed by loss of Sam68. Furthermore, there was no difference between the two genotypes in the association of actb mRNA with polysomes prepared from whole hippocampus (Fig. S4), indicating that translation of actb outside the synapse may not require Sam68. Consistent with the observation that there was less actb mRNA at translating synaptopolysomes, we observed an ∼30% decrease in the amount of actb protein in synaptosomes prepared from Sam68-KO mice compared with WT littermates (Fig. 4C). There was no decrease in the total levels of actb mRNA prepared from hippocampal lysates of KO animals (WT: 1.00 ± 0.05, n = 3 animals; KO: 0.99 ± 0.06, n = 3 paired littermates; levels are stated in arbitrary units normalized to WT) indicating that altered levels of synaptic actb protein were not caused by overall changes in actb mRNA degradation or transcription. These results are consistent with our hypothesis that Sam68 regulates the local translation of actb mRNA through polysomal occupancy and/or recruitment.
Loss of Sam68 in Vitro and in Vivo Reduces the Number of Dendritic Spines on Hippocampal Pyramidal Neurons.
Remodeling of the filamentous actin cytoskeleton enables dendritic spines to undergo changes in morphology. However, it is unclear if local translation of dendritic actb mRNA is necessary for this process. We therefore investigated if loss of Sam68, which results in decreased levels of synaptic actb protein, had an effect on dendritic spine density. Lentivirus-mediated knockdown of Sam68 in hippocampal primary cultures at 21 DIV resulted in fewer postsynaptic sites than in controls as measured by the number of PSD95-reactive puncta (Fig. 5A, shS68#1). This effect was rescued by exogenous expression of WT Sam68, but not ∆KH-S68 (Fig. 5A, rescue and ∆KH-S68), demonstrating that Sam68 regulates dendritic spine density in a manner that is dependent on its ability to bind RNA. To determine if this effect was caused by the loss of actb mRNA metabolism and not by the loss of other Sam68 mRNA cargos (8, 11, 15, 29), we used blocking oligos (Fig. 2) to interfere with the association between Sam68 and actb mRNA. Similar to our results with Sam68 knockdown, transfection of blocking oligonucleotides resulted in fewer postsynaptic sites in 21-DIV hippocampal neurons (Fig. 5A, S68/actb oligo). These results suggest that the observed decrease in postsynaptic sites in culture likely is caused by the loss of binding of Sam68 to the 3′ UTR of actb mRNA. However, we cannot fully exclude the possibility that other potential Sam68 mRNA cargos influence dendritic spine density.
Fig. 5.
Sam68 regulates dendritic spine density in hippocampal cultures and in vitro and in vivo. (A) The number of PSD95-reactive puncta along dendrites 10–60 µm from the soma were counted in hippocampal cultures transduced at 14 DIV and incubated for 7 d with indicated viruses. Sam68 knockdown reduces PSD95 puncta compared with controls (scr virus, 0.73 ± 0.04, n = 40 dendrites; shS68#1, 0.47 ± 0.05 n = 42 dendrites). This reduction was rescued by re-expression of full-length Sam68 (rescue) but not by ΔKH-S68 (rescue, 0.84 ± 0.04, n = 39 dendrites; ∆KH-S68, 0.45 ± 0.05, n = 34 dendrites). One-way ANOVA F(3,151) = 15.76, P < 0.05. Transfection of a Sam68/actb mRNA-blocking oligonucleotide resulted in a decrease in spine density similar to that seen with a control oligo (scr oligo, 0.83 ± 0.08, n = 5 dendrites; S68 oligo, 0.49 ± 0.05, n = 5 dendrites). (B) Golgi stain and 3D reconstruction of dendrites from CA1 pyramidal neurons from Sam68-KO mice and WT littermates. Sam68-KO animals have significantly reduced dendritic spine density (fewer spines per micrometer) than WT littermates (WT, 0.72 ± 0.13, n = 22 dendrites from three animals; KO, 0.43 ± 0.06, n = 33 dendrites from three animals; P < 0.05). (C) Whole-cell voltage-clamp recordings of mEPSCs from individual CA1 pyramidal neurons. (Left) Sample sweeps of mEPSCs from each genotype. (Inset) Composite average of hundreds of mEPSC events reveals similar kinetic profiles between genotypes. (Right) mEPSC frequency is reduced significantly in Sam68-KO animals compared with WT littermates (WT: 1.60 ± 0.2 Hz, n = 22 cells, seven animals; KO: 0.81 ± 0.06 Hz, n = 27 cells, seven animals; P < 0.05). No difference in mEPSC amplitude was observed (WT: 12.71 ± 0.58 pA, n = 22 cells, seven animals; KO: 11.56 ± 0.44 pA, n = 27 cells, seven animals, P > 0.05).
We next asked if Sam68-KO animals also displayed fewer spines on the dendrites of CA1 pyramidal neurons, a region of the hippocampus with high endogenous expression of Sam68. We performed Golgi stains on whole brains extracted from perfused Sam68-KO mice and WT littermates. The 3D reconstructions of CA1 pyramidal cells from 150-µm-thick sagittal hippocampal slices revealed an ∼40% decrease in spine density in the stratum radiatum of Sam68-KO mice compared with WT littermate controls (Fig. 5B). Taken together, our observations in culture and in vivo suggest that Sam68 is an important determinant of hippocampal spine density.
We postulated that the loss of dendritic spines in the stratum radiatum might result in fewer functional synapses in the Sam68-KO mice. To verify this possibility, we performed whole-cell voltage-clamp recordings in CA1 of acute slices of AMPA receptor-mediated miniature excitatory postsynaptic currents (mEPSCs). We observed an ∼50% reduction in the frequency of events in Sam68-KO mice compared with WT littermate controls. This result was consistent with the ∼40% reduction in spine number we determined by Golgi staining. In contrast, there was no difference in the amplitude of mEPSCs in the two genotypes (Fig. 5C). Further analysis of mEPSC kinetics revealed no significant differences in the 10–90% rise time (WT: 2.14 ± 0.07 ms, n = 22 cells, seven animals; KO: 2.14 ± 0.14 ms, n = 27 cells, seven animals) or decay time constant tau (WT: 3.53 ± 0.21 ms, n = 22 cells, seven animals; KO: 3.85 ± 0.34 ms, n = 27 cells, seven animals). The reduced frequency of mEPSCs could be explained by fewer excitatory synapses or by a decreased probability of neurotransmitter release. To differentiate between these two possibilities, we measured the paired-pulse ratio (PPR), which is inversely related to release probability. Stimulation of Schaffer collaterals and recording extracellular field excitatory postsynaptic potentials in striatum radiatum revealed no difference between Sam68-KO mice and control littermates in PPRs (Fig. S5A), indicating a similar release probability in both genotypes. We also investigated synaptic function in the dentate gyrus (DG), another area of the hippocampal formation with high endogenous expression of Sam68. Similar to our experiments in CA1, we observed an ∼30% decrease in mEPSC frequency in dentate granule cells (DGCs) from Sam68-KO animals compared with WT littermates (Fig. S5B). Again, no differences in the amplitude (Fig. S5B) or kinetics of the mEPSCs or PPR were observed (Fig. S5C). Therefore, our results suggest that loss of Sam68 results in fewer synapses in at least two regions (i.e., CA1 and DG) of the hippocampal formation.
Acute Loss of Sam68 in Vivo Results in Fewer Hippocampal Synapses.
To rule out potential long-term or developmental deficits in the Sam68-KO mice that could contribute to the observed synaptic dysfunction, we injected lentiviruses expressing a Sam68-specific shRNA (shS68#1) into the DG of WT rats. We recorded from infected DGCs (visualized by GFP expression; Fig. 6A, Lower) and found an ∼50% decrease in mEPSC frequency but no change in amplitude, compared with adjacent uninfected neurons (Fig. 6B), mirroring our observations in the KO mice. We found no difference in mEPSC frequency or amplitude in neurons infected with an scr nontargeting virus, indicating that the decrease in mEPSC frequency after acute Sam68 knockdown is not caused by viral toxicity. These results show that developmental deficits likely do not play a role in the synaptic dysfunction observed and indicate that Sam68 plays an ongoing and cell-autonomous role in determining the number of functional synapses in the hippocampus.
Fig. 6.
Acute knockdown of Sam68 in vivo results in fewer synapses. (A) Whole-cell recordings of neurons from WT rats (postnatal day 21–22) injected into the DG with lentivirus containing either shS68#1 or an scr shRNA. (Upper) Low-magnification image showing injected DG region (dashed box). (Lower) Sample high-magnification image of a recording pipette accessing a GFP-expressing neuron. (B) Sample sweeps of mEPSCs from uninfected neurons (uninf) and from neurons infected with a control shRNA (scr) or knockdown shRNA (shS68#1). (Inset) Composite average of hundreds of mEPSC events reveals similar kinetic profiles in all conditions. (C) mEPSC frequency is reduced significantly in neurons infected with shS68 #1 compared with scr shRNA or uninfected neurons (uninf) (uninfected: 0.42 ± 0.04 Hz, n = 28 cells, eight animals; scr: 0.42 ± 0.06 Hz; shS68: 0.23 ± 0.05 Hz, n = 12 cells, four animals; P < 0.05). Sam68 does not alter mEPSC amplitude significantly (uninfected: 6.67 ± 0.15 pA, n = 28 cells, eight animals; scr: −6.24 ± 0.3 pA; shS68: −7.13 ± 0.45, n = 12 cells, four animals, P > 0.05).
Discussion
We show that Sam68 function is necessary for an appropriate number of synapses in the hippocampal formation, likely through regulation of local actb mRNA metabolism. Knocking down Sam68 in primary hippocampal cultures or interfering with the binding of Sam68 to the 3′ UTR of actb using blocking oligonucleotides results in a loss of synaptodendritic actb mRNA and fewer dendritic spines. These effects can be rescued by exogenous expression of WT Sam68 but not by expression of a mutant that is unable to bind to RNA. Consistent with these findings, Sam68-KO mice display less actb mRNA associated with synaptic polysomes, reduced levels of actb protein in the synaptodendritic compartment, and fewer synapses in the hippocampal formation. Synaptic dysfunction also is evident upon acute knockdown of Sam68 in vivo, suggesting that Sam68 regulates synapses in a cell-autonomous manner, independent of developmental effects.
Our analyses in primary cultures indicate that Sam68 is present in a substantial fraction of synapses (37 ± 0.06%). A similar fraction of postsynaptic densities (37 ± 7%) also colocalizes with actb mRNA. These measurements suggest that Sam68 is intimately linked to the synaptic localization of actb mRNA. Indeed, we observe that Sam68 is present at the PSD in the majority (∼75 ± 4%) of cases where actb is present also. It is unclear what determines the presence of Sam68 and/or actb mRNA at synapses. The association of Sam68/actb mRNA with synapses is likely to be dynamic and responsive to synaptic activity. Alternatively, Sam68 may associate with dendritic spines of a particular morphology or with those in proximity to the rough endoplasmic reticulum, given its role in loading mRNA cargos onto polysomes.
Interestingly, we observe that loss of Sam68 results in an ∼30% decrease in the amount of synaptic actb protein, suggesting that a substantial fraction of the synaptic pool of actb protein is locally translated. Such a notion is consistent with the results of two studies that found significant contributions of local translation to the synaptic pools of CaMKIIα and BDNF protein (36, 37). Both studies found that restricting transcripts to the soma resulted in a substantial loss of the corresponding protein in the dendritic fraction, suggesting that a large fraction of dendritic CaMKIIα and BDNF is translated from dendritic transcripts. Our results similarly suggest that a significant amount of synaptic actb protein may arise from locally translated mRNAs and that this pool is an important determinant of hippocampal synapse number.
Recently, Sam68 has been implicated in neuronal activity-dependent alternative splicing of Neurexin 1 (Nrxn1) mRNA in the cerebellum (12). Because neurexins are involved in synaptogenesis, abnormal splicing in the absence of Sam68 could partially account for the loss of hippocampal synapses we observe. However, unlike the cerebellum, where aberrant splicing was evident, we did not observe a difference in the alternative splicing of Nrxn1 mRNA in the hippocampus of Sam68-KO animals (Fig. S6). Additionally, interfering with the binding of Sam68 to actb mRNA using targeted oligonucleotides decreases the amount of dendritic spines in hippocampal cultures. These two pieces of evidence strongly implicate inappropriate actb mRNA metabolism and not deficient splicing of Nrxn1 in the loss of hippocampal synapses observed in Sam68-KO animals.
The RBP ZBP1 has a well-established role in the synaptodendritic localization and translational repression of actb mRNA in embryonic neuronal cultures (18). Approximately 50% of actb-containing granules in cultures colocalize with ZBP1 (23), suggesting that Sam68 may regulate the metabolism of a distinct pool of actb mRNAs. Alternatively, because the binding sites for Sam68 and ZBP1 in the 3′ UTR of actb mRNA do not overlap, the two RBPs could bind cooperatively to regulate actb mRNA metabolism bidirectionally. Indeed although ZBP1 functions as a repressor of actb translation, a process relieved by Src phosphorylation (20), Sam68 appears to promote actb translation, which may require phosphorylation by MAPK (13). If Sam68 and ZBP1 concurrently bind to a single actb mRNA, such a configuration would allow different signaling pathways to control the translation of a single mRNA. Our results support the notion that Sam68 and ZBP1 coordinate parallel aspects of actb mRNA metabolism, such as transport and translation.
Our study supports a role for Sam68 in the localization and translation of synaptic mRNAs, and introduces another protein to the field of dendritic mRNA metabolism. In contrast to the heavily studied repressors of translation, fragile X mental retardation protein and ZBP1, our work presents evidence of an RBP that acts as a promoter of translation in the synaptodendritic compartment. As a translational promoter, Sam68 activation could allow the coordinated derepression of a subpopulation of RNP granules in response to synaptic activity. For example, members of the Src kinase family (7) and MAPK (10, 31) phosphorylate Sam68, which alters its affinity for RNA (38, 39) and promotes the translation of its mRNA cargos (13). The possibility that the translation of Sam68 cargos may be regulated by MAPK phosphorylation (13) is of particular interest, because the MAPK pathway is a major transducer for translation in neuronal dendrites (40). The presence of promoters in addition to repressors of translation in RNP granules would allow fine tuning of protein synthesis along the length of the dendrite.
Finally, our observations in Sam68-KO mice may provide insight into the pathophysiology of the neurodegenerative disorder FXTAS. Development of FXTAS is common (∼40%) in elderly males who carry a premutation CGG trinucleotide expansion (55–200 copies) in the 5′ UTR of the fragile X mental retardation 1 (FMR1) gene (41). The general prevalence of FXTAS in males over 50 y old has been estimated at 1 in 3,000, making it one of the most common single-gene causes of tremor and ataxia in the elderly (41). An RNA gain-of-function model has been proposed for the pathogenesis of FXTAS in which mutated FMR1 mRNA sequesters Sam68 into intranuclear inclusions, resulting in loss of function of Sam68 and the development of the clinical features of FXTAS (24). Intriguingly, Sam68-KO mice display ataxia reminiscent of the symptoms observed in FXTAS patients (12, 26). Moreover a recent study shows that neurons derived from fibroblasts taken from an FXTAS patient exhibit a decrease in the number of PSD95 puncta (42), similar to the effects of Sam68 knockdown in cultured neurons. Therefore the development of the Sam68-KO mice as a disease model for FXTAS may be useful for understanding the molecular mechanisms underlying FXTAS as well as for testing potential therapies.
Methods
Statistical Analysis.
One-way ANOVAs were performed on experiments with multiple groups, followed by post hoc Bonferroni correction. In these cases, an asterisk denotes a P value of < 0.05, and “n.s” indicates a P value > 0.05. All values are ± SEM. Quantitative analysis of ICC and FISH experiments were performed blind to the identity of the viruses used for infection.
Cell Cultures, Lentiviral Vectors, and Blocking Oligonucleotides.
Sprague–Dawley rats were killed using CO2 in compliance with the guidelines of the Albert Einstein College of Medicine Institutional Animal Care and Use Committee. Primary hippocampal neuronal cultures from embryonic day19 embryos of either sex were prepared as described (43). We used pTRIP vectors to generate lentiviral shRNA vectors for knockdown or for overexpression following the methods described in ref. 44. (Sequences are given in SI Methods.) Chimeric 2′-O-methyl DNA oligos antisense to the actb 3′ UTR were designed and ordered from Fisher Scientific. (Sequences are given in SI Methods.)
Golgi Staining.
KO and WT Sam68 mice on a C57/BL6 background (gift of Stéphane Richard, McGill University, Montreal, Canada) were killed using CO2. Whole brains were removed quickly and processed for Golgi staining according to the manufacturer’s protocol (Golgi–Cox Variant Kit from Cornell Center for Technology). Dendritic spines were reconstructed and counted using Imaris Bitplane (Filament Tracer module). See SI Methods for details.
FISH.
FISH for actb mRNA was performed with and without ICC on primary hippocampal neurons at 21 DIV. See SI Methods for details.
Electrophysiology.
The experimenter was blind to genotype. Sam68 mice and WT rats were anesthetized and killed, and acute transverse hippocampal slices (400 μm thick, or 300 μm for visualized patching) were prepared. Whole-cell voltage-clamp recordings were made from CA1 pyramidal cells and DGCs. mEPSCs were analyzed with Mini Analysis (Synaptosoft) or AxoGraph X (AxoGraph Scientific). See SI Methods for details.
Supplementary Material
Acknowledgments
We thank Dr. Stéphane Richard for the generous gift of Sam68-KO mice, Adina Buxbaum for her invaluable technical help and Jaafar Tindi for his critical review of this manuscript. This work was supported by National Institutes of Health Grants F31-NS073200 (to M.E.K.), K01-MH073759 and R01-AG039521 (to B.A.J.), and R01-MH081935 and R01-DA17392 (to P.E.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1209811110/-/DCSupplemental.
References
- 1.Morris AR, Mukherjee N, Keene JD. Systematic analysis of posttranscriptional gene expression. Wiley Interdiscip Rev Syst Biol Med. 2010;2(2):162–180. doi: 10.1002/wsbm.54. [DOI] [PubMed] [Google Scholar]
- 2.Holt CE, Bullock SL. Subcellular mRNA localization in animal cells and why it matters. Science. 2009;326(5957):1212–1216. doi: 10.1126/science.1176488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besse F, Ephrussi A. Translational control of localized mRNAs: restricting protein synthesis in space and time. Nat Rev Mol Cell Biol. 2008;9(12):971–980. doi: 10.1038/nrm2548. [DOI] [PubMed] [Google Scholar]
- 4.Gallo JM, et al. The role of RNA and RNA processing in neurodegeneration. J Neurosci. 2005;25(45):10372–10375. doi: 10.1523/JNEUROSCI.3453-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranum LP, Cooper TA. RNA-mediated neuromuscular disorders. Annu Rev Neurosci. 2006;29:259–277. doi: 10.1146/annurev.neuro.29.051605.113014. [DOI] [PubMed] [Google Scholar]
- 6.Zhang G, Neubert TA, Jordan BA. RNA binding proteins accumulate at the postsynaptic density with synaptic activity. J Neurosci. 2012;32(2):599–609. doi: 10.1523/JNEUROSCI.2463-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lukong KE, Richard S. Sam68, the KH domain-containing superSTAR. Biochim Biophys Acta. 2003;1653(2):73–86. doi: 10.1016/j.bbcan.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Grange J, et al. Specific interaction between Sam68 and neuronal mRNAs: Implication for the activity-dependent biosynthesis of elongation factor eEF1A. J Neurosci Res. 2009;87(1):12–25. doi: 10.1002/jnr.21824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Liu Y, Kim BO, He JJ. Direct participation of Sam68, the 68-kilodalton Src-associated protein in mitosis, in the CRM1-mediated Rev nuclear export pathway. J Virol. 2002;76(16):8374–8382. doi: 10.1128/JVI.76.16.8374-8382.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matter N, Herrlich P, König H. Signal-dependent regulation of splicing via phosphorylation of Sam68. Nature. 2002;420(6916):691–695. doi: 10.1038/nature01153. [DOI] [PubMed] [Google Scholar]
- 11.Chawla G, et al. Sam68 regulates a set of alternatively spliced exons during neurogenesis. Mol Cell Biol. 2009;29(1):201–213. doi: 10.1128/MCB.01349-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iijima T, et al. SAM68 regulates neuronal activity-dependent alternative splicing of neurexin-1. Cell. 2011;147(7):1601–1614. doi: 10.1016/j.cell.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paronetto MP, et al. Sam68 regulates translation of target mRNAs in male germ cells, necessary for mouse spermatogenesis. J Cell Biol. 2009;185(2):235–249. doi: 10.1083/jcb.200811138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin Q, Taylor SJ, Shalloway D. Specificity and determinants of Sam68 RNA binding. Implications for the biological function of K homology domains. J Biol Chem. 1997;272(43):27274–27280. doi: 10.1074/jbc.272.43.27274. [DOI] [PubMed] [Google Scholar]
- 15.Itoh M, Haga I, Li QH, Fujisawa J. Identification of cellular mRNA targets for RNA-binding protein Sam68. Nucleic Acids Res. 2002;30(24):5452–5464. doi: 10.1093/nar/gkf673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matus A. Actin-based plasticity in dendritic spines. Science. 2000;290(5492):754–758. doi: 10.1126/science.290.5492.754. [DOI] [PubMed] [Google Scholar]
- 17.Cingolani LA, Goda Y. Actin in action: The interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci. 2008;9(5):344–356. doi: 10.1038/nrn2373. [DOI] [PubMed] [Google Scholar]
- 18.Eom T, Antar LN, Singer RH, Bassell GJ. Localization of a beta-actin messenger ribonucleoprotein complex with zipcode-binding protein modulates the density of dendritic filopodia and filopodial synapses. J Neurosci. 2003;23(32):10433–10444. doi: 10.1523/JNEUROSCI.23-32-10433.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perycz M, Urbanska AS, Krawczyk PS, Parobczak K, Jaworski J. Zipcode binding protein 1 regulates the development of dendritic arbors in hippocampal neurons. J Neurosci. 2011;31(14):5271–5285. doi: 10.1523/JNEUROSCI.2387-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hüttelmaier S, et al. Spatial regulation of beta-actin translation by Src-dependent phosphorylation of ZBP1. Nature. 2005;438(7067):512–515. doi: 10.1038/nature04115. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen J, et al. A family of insulin-like growth factor II mRNA-binding proteins represses translation in late development. Mol Cell Biol. 1999;19(2):1262–1270. doi: 10.1128/mcb.19.2.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donnelly CJ, et al. Limited availability of ZBP1 restricts axonal mRNA localization and nerve regeneration capacity. EMBO J. 2011;30(22):4665–4677. doi: 10.1038/emboj.2011.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tiruchinapalli DM, et al. Activity-dependent trafficking and dynamic localization of zipcode binding protein 1 and beta-actin mRNA in dendrites and spines of hippocampal neurons. J Neurosci. 2003;23(8):3251–3261. doi: 10.1523/JNEUROSCI.23-08-03251.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sellier C, et al. Sam68 sequestration and partial loss of function are associated with splicing alterations in FXTAS patients. EMBO J. 2010;29(7):1248–1261. doi: 10.1038/emboj.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brouwer JR, Willemsen R, Oostra BA. The FMR1 gene and fragile X-associated tremor/ataxia syndrome. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(6):782–798. doi: 10.1002/ajmg.b.30910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lukong KE, Richard S. Motor coordination defects in mice deficient for the Sam68 RNA-binding protein. Behav Brain Res. 2008;189(2):357–363. doi: 10.1016/j.bbr.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Jordan BA, et al. Identification and verification of novel rodent postsynaptic density proteins. Mol Cell Proteomics. 2004;3(9):857–871. doi: 10.1074/mcp.M400045-MCP200. [DOI] [PubMed] [Google Scholar]
- 28.Rossoll W, et al. Smn, the spinal muscular atrophy-determining gene product, modulates axon growth and localization of beta-actin mRNA in growth cones of motoneurons. J Cell Biol. 2003;163(4):801–812. doi: 10.1083/jcb.200304128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tremblay GA, Richard S. mRNAs associated with the Sam68 RNA binding protein. RNA Biol. 2006;3(2):90–93. doi: 10.4161/rna.3.2.3204. [DOI] [PubMed] [Google Scholar]
- 30.Kislauskis EH, Zhu X, Singer RH. Sequences responsible for intracellular localization of beta-actin messenger RNA also affect cell phenotype. J Cell Biol. 1994;127(2):441–451. doi: 10.1083/jcb.127.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paronetto MP, et al. The nuclear RNA-binding protein Sam68 translocates to the cytoplasm and associates with the polysomes in mouse spermatocytes. Mol Biol Cell. 2006;17(1):14–24. doi: 10.1091/mbc.E05-06-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richard S, et al. Ablation of the Sam68 RNA binding protein protects mice from age-related bone loss. PLoS Genet. 2005;1(6):e74. doi: 10.1371/journal.pgen.0010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muddashetty RS, Kelić S, Gross C, Xu M, Bassell GJ. Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X syndrome. J Neurosci. 2007;27(20):5338–5348. doi: 10.1523/JNEUROSCI.0937-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiler IJ, et al. Fragile X mental retardation protein is translated near synapses in response to neurotransmitter activation. Proc Natl Acad Sci USA. 1997;94(10):5395–5400. doi: 10.1073/pnas.94.10.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bagni C, Mannucci L, Dotti CG, Amaldi F. 2000. Chemical stimulation of synaptosomes modulates alpha -Ca2+/calmodulin-dependent protein kinase II mRNA association to polysomes. J Neurosc 20(10):RC76. [DOI] [PMC free article] [PubMed]
- 36.An JJ, et al. Distinct role of long 3′ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell. 2008;134(1):175–187. doi: 10.1016/j.cell.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller S, et al. Disruption of dendritic translation of CaMKIIalpha impairs stabilization of synaptic plasticity and memory consolidation. Neuron. 2002;36(3):507–519. doi: 10.1016/s0896-6273(02)00978-9. [DOI] [PubMed] [Google Scholar]
- 38.Wang LL, Richard S, Shaw AS. P62 association with RNA is regulated by tyrosine phosphorylation. J Biol Chem. 1995;270(5):2010–2013. doi: 10.1074/jbc.270.5.2010. [DOI] [PubMed] [Google Scholar]
- 39.Maroni P, Citterio L, Piccoletti R, Bendinelli P. Sam68 and ERKs regulate leptin-induced expression of OB-Rb mRNA in C2C12 myotubes. Mol Cell Endocrinol. 2009;309(1-2):26–31. doi: 10.1016/j.mce.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 40.Bramham CR, Wells DG. Dendritic mRNA: Transport, translation and function. Nat Rev Neurosci. 2007;8(10):776–789. doi: 10.1038/nrn2150. [DOI] [PubMed] [Google Scholar]
- 41.Jacquemont S, et al. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. JAMA. 2004;291(4):460–469. doi: 10.1001/jama.291.4.460. [DOI] [PubMed] [Google Scholar]
- 42.Liu J, et al. Signaling defects in iPSC-derived fragile X premutation neurons. Hum Mol Genet. 2012;21(17):3795–3805. doi: 10.1093/hmg/dds207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osten P, et al. Mutagenesis reveals a role for ABP/GRIP binding to GluR2 in synaptic surface accumulation of the AMPA receptor. Neuron. 2000;27(2):313–325. doi: 10.1016/s0896-6273(00)00039-8. [DOI] [PubMed] [Google Scholar]
- 44.Janas J, Skowronski J, Van Aelst L. Lentiviral delivery of RNAi in hippocampal neurons. Methods Enzymol. 2006;406:593–605. doi: 10.1016/S0076-6879(06)06046-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.