Abstract
Double-stranded DNA (dsDNA) derived from pathogen- or host-damaged cells triggers innate immune responses when exposed to cytoplasm. However, the machinery underlying the primary recognition of intracellular dsDNA is obscure. Here we show that the DNA damage sensor, meiotic recombination 11 homolog A (MRE11), serves as a cytosolic sensor for dsDNA. Cells with a mutation of MRE11 gene derived from a patient with ataxia-telangiectasia–like disorder, and cells in which Mre11 was knocked down, had defects in dsDNA-induced type I IFN production. MRE11 physically interacted with dsDNA in the cytoplasm and was required for activation of stimulator of IFN genes (STING) and IRF3. RAD50, a binding protein to MRE11, was also required for dsDNA responses, whereas NBS1, another binding protein to MRE11, was dispensable. Collectively, our results suggest that the MRE11–RAD50 complex plays important roles in recognition of dsDNA and initiation of STING-dependent signaling, in addition to its role in DNA-damage responses.
Keywords: signal transduction, innate immunity, pattern recognition receptor
Nucleic acids derived from pathogens, or released from host cells during tissue damage, have the potential to activate the immune system. Tremendous progress has been made in our understanding of how host cells recognize these nucleic acid molecules and trigger immune responses (1, 2). RNA-sensing mechanisms in particular, which are mediated by members of the Toll-like receptor (TLR) family and by retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs), have been assessed. In contrast, despite the important possibility that DNA derived from either pathogens or damaged host cells could trigger innate immune responses, the mechanisms underlying DNA sensing are relatively obscure.
TLR9 is a membrane-bound DNA sensor; it senses CpG DNA at the endosome to trigger immune responses, particularly in plasmacytoid dendritic cells (pDCs). It also contributes to antiviral responses by producing type I IFN (IFN), and its aberrant recognition of host DNA is linked to autoimmune diseases (3–5). However, it appears that there are TLR9-independent pathways that recognize double-stranded DNA (dsDNA) in the cytoplasm (6–8). DNA-dependent activator of IFN-regulatory factors (DAI; also known as ZBP1 or DLM-1) has been reported as an intracellular dsDNA sensor (9), but Dai-deficient mice are able to produce type I IFN after intracellular DNA stimulation (10), suggesting that the roles of DAI are either redundant or restricted to specific cell types.
An endoplasmic reticulum (ER) resident transmembrane protein, STING (stimulator of IFN genes, also known as MITA, ERIS, MPYS or TMEM173), was found to play essential roles for cytosolic dsDNA-mediated production of type I IFN and inflammatory cytokines through activating the TANK-binding kinase 1 (TBK1)-IFN regulatory factor 3 (IRF3) axis (11). Sting-deficient mice have lost their ability to produce type I IFN in response to dsDNA. Although the STING–TBK1–IRF3 axis is crucial for cytosolic DNA-induced IFN production, STING fails to bind and colocalize with DNA, suggesting existence of a DNA sensor(s) upstream of STING. Previous studies have also demonstrated that intracellular delivery of DNA, but not RNA, elicits dynamic translocation of STING from the ER to the Golgi apparatus, followed by formation of a vesicle-like structure in which TBK1 is recruited (11, 12). These studies showed that STING translocation is essential for activation of downstream events. However, the regulatory mechanisms for this trafficking of STING are largely unknown.
Absent in melanoma 2 (AIM2) functions as a cytosolic DNA sensor forming inflammasomes to release IL-1β, but it is not essential for type I IFN production (1). Recently, two DNA-binding proteins IFN, gamma-inducible protein 16 (IFI16) and DEAD box polypeptide 41 (DDX41) were identified to be involved in the STING pathway (13, 14). These proteins interact with DNA and STING and are critical for dsDNA-induced type I IFN production. However, the contributions of these molecules to responses against dsDNA under physiological conditions, and their roles in different cell types, are still unknown.
Early studies about the link between DNA damage and innate immune responses indicated that DNA damage events can trigger type I IFN induction (15, 16). However, it was shown that Ku70, DNA-PKcs, p53, or ataxia-telangiectasia mutated kinase (ATM) is dispensable for intracellular dsDNA-induced type I IFN production (8). Whereas other DNA repair-related genes were demonstrated to be involved in viral infection (17), their roles in innate immune responses to dsDNA have not been well characterized.
In the present study, we showed that a DNA damage sensor, meiotic recombination 11 homolog A (MRE11), serves as a key sensor for exogenous DNA and activates the STING-dependent pathway.
Results
Intracellular dsDNA Promotes Phosphorylation of ATM.
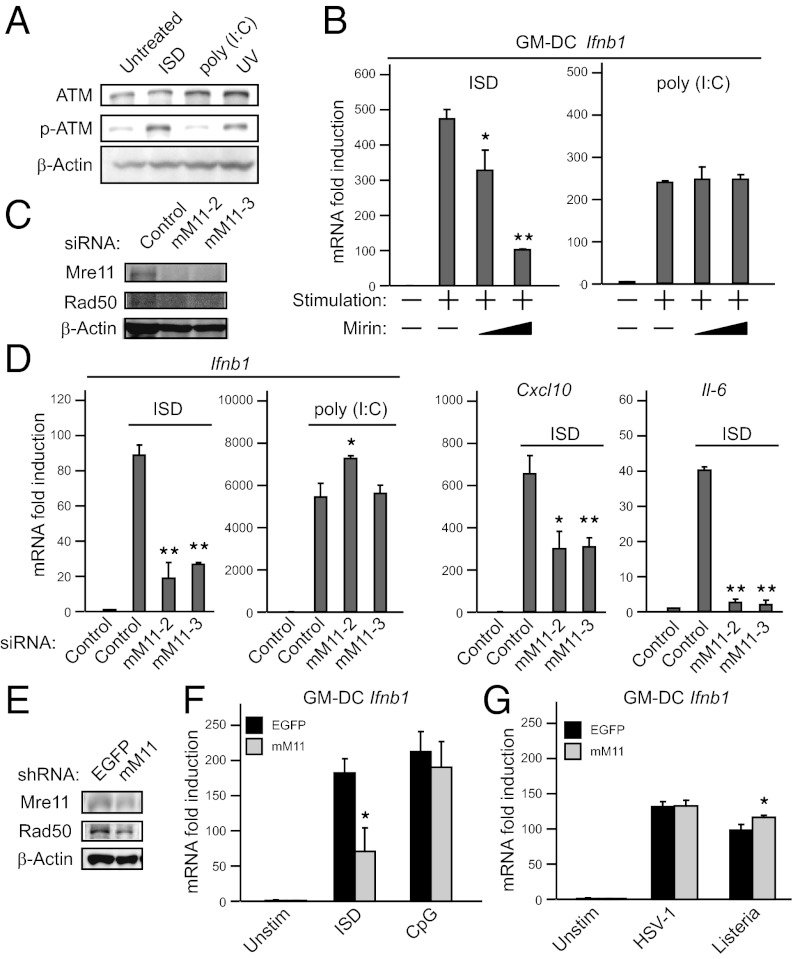
To identify molecules involved in innate immune responses to cytosolic dsDNA, we stimulated mouse embryonic fibroblast cells (MEFs) with dsDNA or dsRNA, and mRNA extracted from the cells was subjected to microarray analysis. Because the B-form dsDNA ligand, polydeoxyadenine-polydeoxythymidine [poly (dA:dT)], can trigger multiple pathways including RLRs (18, 19), we used a non–AT-rich sequence dsDNA ligand, IFN stimulatory DNA (ISD) (8). No genes were strongly and specifically induced by liposome-delivered ISD compared with a dsRNA synthetic analog polyinosinic-polycytidylic acid [poly (I:C)] [which is dependent on RIG-I/melanoma differentiation-associated gene 5 (MDA5) signaling] (Fig. S1A). However, we found that Atm mRNA was preferentially, albeit only weakly, up-regulated by ISD. We confirmed the increased Atm mRNA expression after ISD stimulation using quantitative reverse transcription-PCR (qPCR) assays (Fig. S1B). These findings imply that exogenous dsDNA mimics signaling associated with DNA double-strand breaks (DSBs). To verify this hypothesis, we stimulated MEFs with ISD or poly (I:C), and evaluated ATM activation. ISD, but not poly (I:C), promoted ATM phosphorylation, similar to UV treatment (Fig. 1A). Nevertheless, it has previously been reported that ATM is not required for type I IFN production in response to ISD (8). We also found that there was no obvious change in IFNB1 mRNA induction between ATM-deficient AT5BIVA human cells and ATM-complemented AT5BIVA cells (Fig. S1C). Collectively, these findings suggest the possibility that other DNA repair protein(s) may contribute to immune responses against intracellular dsDNA.
Fig. 1.
MRE11 is involved in dsDNA-mediated type I IFN production. (A) Immunoblot analysis of phospho-ATM proteins. HEK293 cells were transfected with 1 μg⋅mL−1 of ISD or poly (I:C) (with Lipofectamine 2000) for 2 h or were exposed to UV light (10 Jm−2). Data are from one experiment representative of two. (B) qPCR analysis in GM-DCs. GM-DCs were transfected with 1 μg⋅mL−1 of ISD or poly (I:C) for 6 h with DMSO or Mirin (10 μM or 100 μM). (C and D) Immunoblot analysis of MRE11 and RAD50 (C) and qPCR analysis of mouse Ifnb1, Cxcl10, and Il6 (D) in MEFs treated with a control siRNA or siRNAs targeting Mre11 (mM11-2 or mM11-3) and stimulated with 1 μg⋅mL−1 of ISD or poly (I:C) for 6 h. (E–G) Immunoblot analysis of MRE11 and RAD50 (E) and qPCR analysis of mouse Ifnb1 in GM-DCs infected with shRNA-coding retrovirus targeting Mre11 or EGFP (used as control) and stimulated with 1 μg⋅mL−1 of ISD or 250 nM CpG for 6 h (F) or infected with HSV-1 at a multiplicity of infection (MOI) of 10 or L. monocytogenes for 12 h (G). Results for mRNA are represented relative to those of control samples. Data are from three independent experiments in triplicate (mean and SD in B, D, F, and G). *P < 0.05 and **P < 0.005 compared with controls.
MRE11 Is Involved in Cytosolic DNA Responses.
We next investigated whether or not upstream molecules involved in ATM phosphorylation trigger type I IFN responses. The MRN complex, containing MRE11, RAD50 homolog (RAD50), and nijmegen breakage syndrome 1 (NBS1), plays crucial roles in the early phase of DSBs and is required for ATM phosphorylation (20, 21). To address whether or not the MRN complex is involved in exogenous dsDNA responses, we stimulated granulocyte macrophage colony-stimulating factor (GM-CSF)-induced bone marrow-derived dendritic cells (GM-DCs) prepared from C57BL/6J mice with ISD in the presence or absence of Mirin, a chemical inhibitor of MRE11 (22). Mirin diminished the expression of Ifnb1 mRNA induced by ISD in a dose-dependent manner (Fig. 1B, Left), whereas Ifnb1 mRNA induction in response to poly (I:C) was not affected (Fig. 1B, Right). To confirm the contribution of MRE11 to dsDNA responses, we used a knockdown strategy using two distinct siRNAs targeting the Mre11 gene. MEFs treated with these siRNAs displayed a considerable reduction of Mre11 expression (Fig. 1C). In accordance with a previous study (23), reduction of Mre11 expression resulted in destabilization of Rad50. Knockdown of Mre11 led to reduced Ifnb1 induction in response to ISD, but not change of Ifnb1 induction in response to poly (I:C) (Fig. 1D, Left). In addition, the expression of chemokine (C-X-C motif) ligand 10 (Cxcl10) and Interleukin 6 (Il6) was suppressed by Mre11 knockdown (Fig. 1D, Right). Predictably, Rad50 knockdown displayed similar results (Fig. S1 D and E). Likewise, Mre11 knockdown in GM-DCs by shRNA abrogated (Fig. 1E), albeit not completely, the induction of Ifnb1 in response to ISD (Fig. 1F). In contrast, Mre11 knockdown did not impair the responses to CpG DNA (Fig. 1F). Moreover, production of IFNβ by ISD was also abolished by Mre11 knockdown, as measured by ELISA (Fig. S1F).
We next examined whether MRE11 is involved in pathogen-induced type I IFN production. We treated GM-DCs with herpes simplex virus (HSV)-1 and Listeria monocytogenes, which are understood to induce type I IFN production by exposing their genomic DNA. However, Mre11 knockdown did not impair type I IFN induction by these pathogens (Fig. 1G). These results further support the assertion that MRE11 is absolutely required for gene induction by cytoplasmic dsDNA in various cell types.
Cytosolic DNA Responses Are Abrogated in Ataxia-Telangiectasia–Like Disorder Cells.
We examined dsDNA-mediated type I IFN responses in cells derived from a patient with ataxia-telangiectasia–like disorder (ATLD), in which the MRE11 protein is truncated due to a genetic mutation, and both MRE11 and RAD50 proteins are destabilized (Fig. 2A) (20, 23). In ATLD cells, IFNB1 mRNA expression was not increased after stimulation with various dsDNA ligands such as ISD, Escherichia coli DNA, and plasmid DNA (Fig. 2B). In contrast, MRE11-complemented cells were able to induce IFNB1 against dsDNA stimulation (Fig. 2B). Both cell types have comparable ability to induce IFNB1 after poly (I:C) stimulation. The response to poly (dA:dT) was partially impaired by loss of MRE11 expression, consistent with a previous report that STING is partially involved in poly (dA:dT) responses (11). This unresponsiveness in ATLD cells was observed in a variety of DNA dosage conditions and IFNB1 mRNA expression was not induced by 24 h after stimulation (Fig. S2A). We also analyzed type I IFN production by ELISA and confirmed that production of IFNβ after ISD stimulation was dependent on MRE11 (Fig. 2C). Furthermore, we examined pathogen-induced IFNB1 induction in ATLD cells. HSV-1–induced IFNB1 expression was comparable between ATLD and MRE11-complemented cells (Fig. S2B). These results strongly suggest that MRE11 is crucial for type I IFN induction following cytosolic DNA stimulation but not HSV-1 infection.
Fig. 2.
Hypomorphic mutation of MRE11 in ATLD cells drastically abolishes type I IFN production against various types of DNA ligands. (A) Immunoblot analysis of MRE11 in ATLD2SV and MRE11 complemented cells. (B) qPCR analysis of human IFNB1 in ATLD2SV cells transfected with 1 μg⋅mL−1 of the indicated nucleic acid ligands for 8 h. Results for mRNA are represented relative to those of control samples. Data are from three independent experiments in triplicate (mean and SD). (C) ELISA of human IFNβ in ATLD2SV cells transfected with 1 μg⋅mL−1 of ISD or poly (I:C) for 24 h. Data are from three independent experiments in triplicate (mean and SEM). (D) Immunoblot analysis of phospho-IRF3 proteins in ATLD2SV cells stimulated with 5 μg⋅mL−1 of ISD or poly (I:C) for 3 h. Data are from one experiment representative of two. (E) Immunofluorescence staining of stably expressed STING-HA in ATLD2SV cells stimulated with 5 μg⋅mL−1 of ISD for 1 h. The localization of STING and GM130 (Golgi apparatus marker) was observed by confocal microscopy. Data are from one experiment representative of three. **P < 0.005 compared with controls.
MRE11 Functions Upstream of the STING–TBK1–IRF3 Axis.
We next addressed whether or not MRE11 functions as a cytosolic sensor for exogenous dsDNA. Type I IFN responses induced by dsDNA are dependent on TBK1 (7), which promotes phosphorylation of IRF3 in cytoplasm, followed by translocation of IRF3 to the nucleus. As shown in Fig. 2D and Fig. S2C, ATLD cells had defective IRF3 phosphorylation and nuclear translocation in response to ISD. We then generated ATLD cells stably expressing HA-tagged STING (STING-HA) by retrovirus infection to visualize STING localization. STING-HA was localized at the ER and failed to translocate to the Golgi in ATLD cells during ISD stimulation. In contrast, the translocation was rescued by restoration of MRE11 expression (Fig. 2E). Similar loss of translocation of IRF3 and STING was observed in Mre11-knockdown MEFs (Fig. S2 D and E). Taken together, these results show that MRE11 is involved in DNA responses upstream of STING. Notably, although HSV-1– or L. monocytogenes-induced type I IFN production was dependent on STING, we were unable to observe STING translocation after treatment with these pathogens in MEFs, ATLD, and MRE11-complemented ATLD cells. This was consistent with our data suggesting that MRE11 and RAD50 are dispensable for type I IFN production by these pathogens.
MRE11 Senses dsDNA in Cytoplasm.
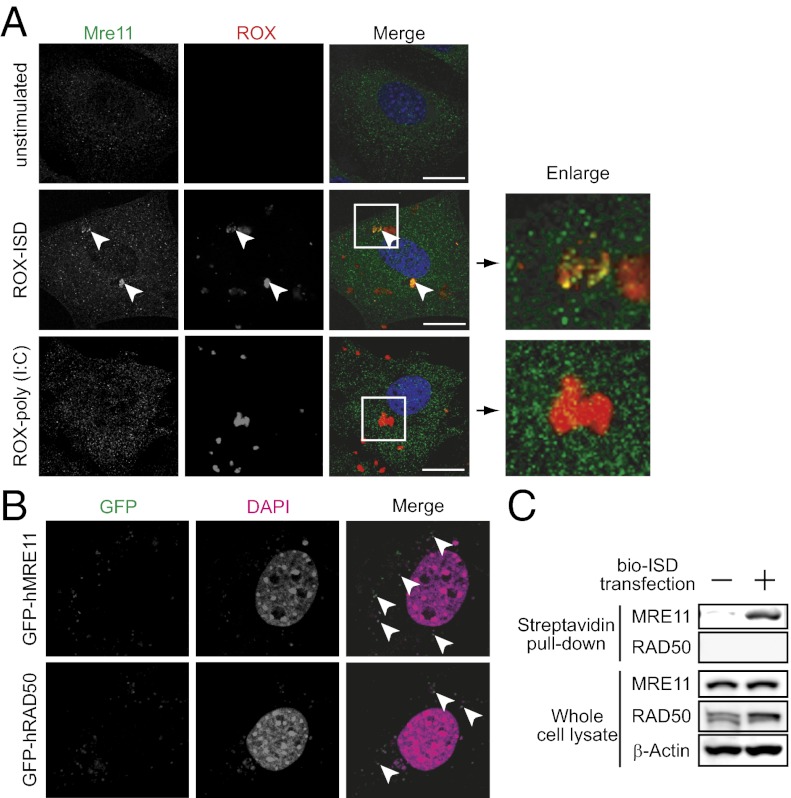
To address whether MRE11 recognizes exogenous dsDNA in the cytoplasm, we determined the cellular localization of ISD and MRE11. MRE11 was localized in both nuclei and cytoplasm with dot-like structures, and rhodamine (ROX)-labeled ISD was partially colocalized with MRE11 in the cytoplasm in MEF and HeLa cells (Fig. 3A and Fig. S3A). In contrast, there was no colocalization between MRE11 and ROX-poly (I:C). Furthermore, Rad50 was also colocalized with MRE11 and exogenous dsDNA (Fig. S3B). To confirm these results, we transiently transfected MEFs with expression plasmids for GFP-fused MRE11 or RAD50. Fig. 3B shows that ectopically expressed GFP-MRE11 and GFP-RAD50 were both colocalized with plasmid DNA in the cytoplasm. We further examined this interaction using a biochemical binding assay. Cell lysates prepared from HEK293 cells transfected with biotin-labeled ISD were subjected to a pull-down assay using streptavidin-conjugated beads. As shown in Fig. 3C, MRE11, but not RAD50, was detected by immunoblot, indicating an interaction between MRE11 and DNA. This was consistent with previous reports showing that MRE11 is the major component of the MRN complex for DNA binding (reviewed in ref. 21). In addition, MRE11 senses sugar-phosphate contacts and has no base interactions, which is consistent with previous results that intracellular DNA detection requires a native sugar-phosphate backbone and lacks sequence preference (8) and with our own results showing that MRE11 is required for various dsDNA ligand-induced immune responses (Fig. 2B). Thus, these findings suggest that MRE11, rather than RAD50, functions as a major component to recognize ISD.
Fig. 3.
MRE11 and RAD50 are recruited to exogenous dsDNA delivered into the cytosol. (A) Immunofluorescence staining of MRE11 in MEFs transfected with 0.5 μg⋅mL−1 of ROX-ISD or poly (I:C) for 2 h. Data are from one experiment representative of three. (B) Localization of ectopically expressed MRE11 and RAD50. MEFs were transiently transfected with expression plasmids for GFP-fused MRE11 or RAD50. After 48 h, GFP signals colocalized with DAPI stained plasmids were observed. Data are from one experiment representative of two. (C) Binding assay of exogenous DNA and MRE11. HEK293 cells were transfected with 5 μg⋅mL−1 of biotin-labeled ISD for 2 h. Cell lysates were incubated with streptavidin beads for 1 h and then beads were washed three times with lysis buffer. Purified proteins were analyzed by immunoblot with indicated antibodies. Data are from one experiment representative of two. (Scale bars, 2 μm.)
NBS1 Is Dispensable for Cytosolic DNA Responses.
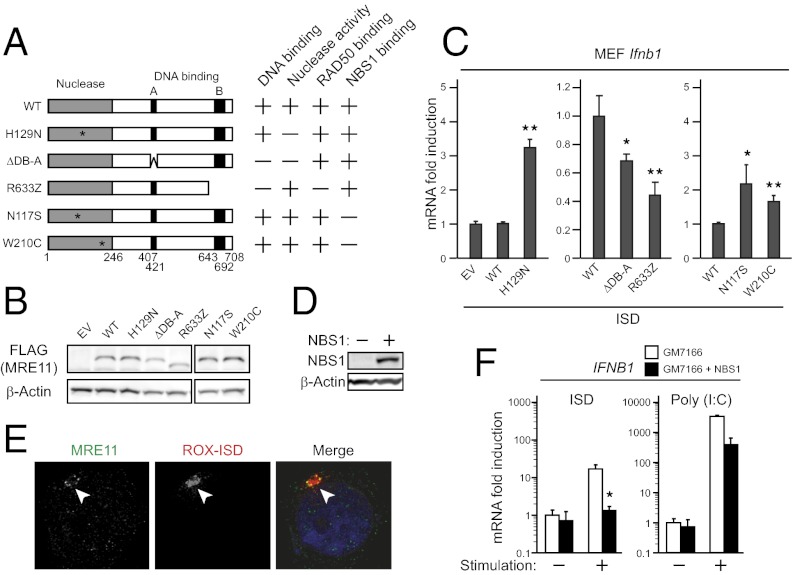
To further understand how MRE11 induces type I IFN responses to dsDNA, we constructed a series of MRE11 mutants (Fig. 4A). MEFs were infected with retroviruses encoding MRE11 mutants (Fig. 4B) and subjected to qPCR analysis after ISD stimulation. MRE11 has phosphoesterase motifs in the N-terminal region, which serve to process the end of DNA. Thus, we first examined whether nuclease activity is required for type I IFN induction. As shown in Fig. 4C, Left, retroviral introduction of FLAG-tagged wild-type hMRE11 (WT) in MEFs displayed similar type I IFN induction to that of empty vector-introduced control cells. In contrast, the H129N mutation, which lost the nuclease activity (24–26), was found to facilitate type I IFN induction, suggesting that the nuclease activity has inhibitory effects on downstream signal transduction. MRE11 contains two predicted DNA-binding regions in the central (DB-A) and the C-terminal (DB-B) regions (Fig. 4A). To further investigate the contributions of these DNA-binding regions, we generated two mutants: ΔDB-A and R633Z. The R633Z mutant, which is identical to a mutation found in ATLD cells, has lost the C-terminal DNA binding region. As shown in Fig. 4C, Center, stable expression of these mutants led to lower induction of type I IFN than control cells. Interestingly, DB-A showed a small contribution to type I IFN induction compared with DB-B. This result may reflect the function served by DB-A to exert nuclease activity by capping captured DNA, a function which is dispensable for forming the DSB complex. On the other hand, the requirement of DB-B is consistent with the defect of IFN induction in ATLD cells.
Fig. 4.
NBS1 is dispensable for intracellular DNA-mediated type I IFN induction. (A) Schematic representation of the series of human MRE11 mutants and their proposed properties. (B and C) Immunoblot analysis of MRE11 (B) and qPCR analysis of mouse Ifnb1 (C) in MEFs stimulated with 1 μg⋅mL−1 of ISD for 6 h. (D) Immunoblot analysis of NBS1 in GM7166 and complemented cells. (E) Immunofluorescence staining of MRE11 in GM7166 cells treated as in Fig. 3A and endogenous MRE11 was observed by immunofluorescence staining. Data are from one experiment representative of two. (F) qPCR analysis of human IFNB1 in GM7166 cells transfected with 1 μg⋅mL−1 of ISD or poly (I:C) for 8 h. Results for mRNA are represented relative to those of control samples. Data are from three independent experiments in triplicate (mean and SD in C and F). *P < 0.05 and **P < 0.005 compared with controls.
Furthermore, we addressed the effect of other mutations identified in patients with ATLD. We introduced N117S or W210C mutations, both of which had a loss of binding with NBS1 (23, 27, 28). However, there was no significant change in type I IFN induction by expression of these mutants (Fig. 4C, Right), suggesting that NBS1-binding is not essential for dsDNA-induced type I IFN production.
To further investigate the involvement of NBS1, we performed binding assays and microscopy analysis using GM7166 cells derived from a patient with Nijmegen breakage syndrome in which NBS1 expression was particularly low (Fig. 4D) (29). In these cells, MRE11 colocalized and interacted with exogenous DNA (Fig. 4E and Fig. S4A). Furthermore, ISD stimulation significantly increased levels of type I IFN expression in GM7166 cells (Fig. 4F). Notably, NBS1 complementation resulted in a reduction of IFNB1 expression. Because it had been reported that loss of NBS1 increases cytosolic distribution of MRE11 (30) (Fig. S4B) and transfected DNA is mainly sensed in the cytosol (31), this result implies that NBS1 expression decreases cytosolic DNA sensing by MRE11.
Taken together, these results suggest that MRE11 is the major component required for DNA sensing, whereas its nuclease activities and one of the binding partners, NBS1, are not required for type I IFN production.
5,6-Dimethylxanthenone-4-Acetic Acid Induction of Type I IFNs Depends on STING but Not MRE11.
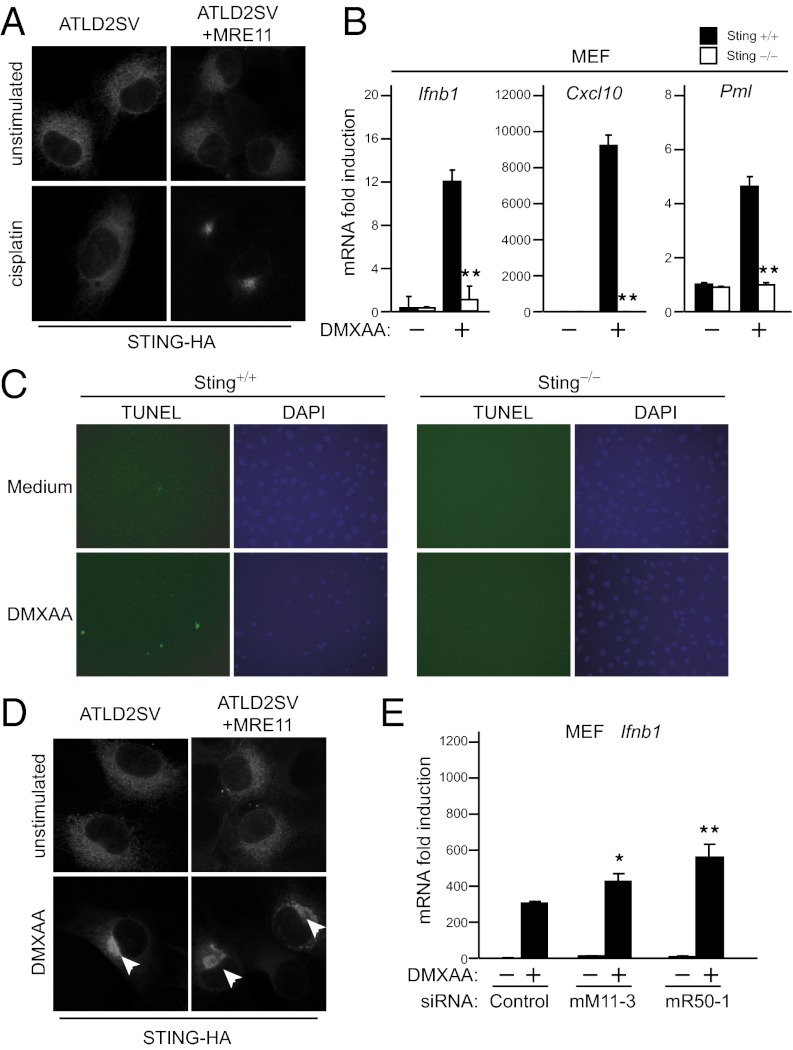
MRE11 plays crucial roles in DNA repair. In addition, previous reports indicated that DNA damage can induce type I IFN (15). Thus, we assessed whether or not DNA damage can trigger the MRE11–STING pathway. We found that cisplatin and etoposide treatment induced translocation of STING (Fig. S5A). In addition, this translocation was impaired in ATLD2SV cells, but rescued by replacement of MRE11 (Fig. 5A). However, this trafficking was observed in less than 0.1% cells. Indeed, these stimuli were not sufficient to induce gene expression in MEFs although they modestly induced IFNβ gene (Fig. S5B). Notably, Sting was not required for these weak responses. These results suggest that cisplatin and etoposide slightly activate the MRE11–STING pathway, but is insufficient for type I IFN induction.
Fig. 5.
DMXAA-mediated gene expression requires STING but not MRE11. (A) Immunofluorescence staining of stably expressed STING-HA in ATLD2SV cells stimulated with 10 μM cisplatin for 24 h. (B) qPCR analysis of mouse Ifnb1, Cxcl10, and Pml in wild-type and Sting−/− MEFs treated with 50 μg⋅mL−1 DMXAA for 4 h. (C) TUNEL assay in wild-type and Sting−/− MEFs treated with 50 μg⋅mL−1 DMXAA for 12 h. Data are from one experiment representative of two. (D) Immunofluorescence staining of stably expressed STING-HA in ATLD2SV cells stimulated with 100 μg⋅mL−1 DMXAA for 1 h. Data are from one experiment representative of two. (E) qPCR analysis of mouse Ifnb1 in MEFs treated with a control siRNA or siRNAs (mM11-3 or mR50-1) and stimulated with 100 μg⋅mL−1 DMXAA for 4 h. Results for mRNA are represented relative to those of control samples. Data are from three independent experiments in triplicate (mean and SD in B and E). *P < 0.05 and **P < 0.005 compared with controls.
Previous studies have shown that a vascular-disrupting agent (VDA) 5,6-dimethylxanthenone-4-acetic acid (DMXAA) has the ability to induce type I IFN via TBK1 and IRF3, but not TLR- and RLR-signaling pathways (32). Furthermore, DMXAA induces type I IFN without activation of NFκB or MAPKs. These similar responses to DNA prompted us to examine whether DMXAA can activate the MRE11–STING pathway. First, we investigated the dependency on STING. Ifnb1 and Cxcl10 induction after DMXAA treatment was impaired in Sting-deficient MEFs (Fig. 5B). Because DMXAA is implicated in induction of cell death through up-regulation of promyelocytic leukemia (Pml) (Fig. 5B, Right) and down-regulation of Bcl-2 in a type I IFN-dependent manner (32), we tested whether or not STING affects DMXAA-induced cell death. The TUNEL assay shown in Fig. 5C demonstrates that Sting-deficient cells are resistant to DMXAA-induced apoptosis, suggesting that STING-mediated gene induction contributes to DMXAA function. Next, we further assessed the effect of DMXAA on the STING pathway. DMXAA treatment triggered STING translocation, indicating that DMXAA activates the STING pathway. However, the translocation also occurred in ATLD cells (Fig. 5D) and Mre11 or Rad50 knockdown had no measurable effect on gene expression changes induced by DMXAA (Fig. 5E). These results indicate that DMXAA activates STING independently of MRE11.
Discussion
In the present study, we identified MRE11 as a sensor for exogenous dsDNA, which is required for STING trafficking and type I IFN induction. Our data show that MRE11 contributes to recognition of a broad spectrum of dsDNA and the contribution of MRE11 is not restricted to certain cell types. Analysis of a series of MRE11 mutants indicated that the nuclease activity of MRE11 is not required for type I IFN induction. Indeed, the H129N nuclease-inactive form of MRE11 induces higher amounts of IFNβ than wild type, indicating that nuclease activity may negatively regulate type I IFN induction. It is speculated that the interaction of MRE11 with dsDNA initially elicits the STING-dependent signaling pathway, and subsequent DNA processing via MRE11 nuclease activity terminates downstream signaling. Thus, MRE11 may also have a function to suppress excessive DNA responses, similar to Trex1 (33). Notably, we found that Mirin, which inhibits ATM activation by targeting MRE11, blocked type I IFN induction. Although it is considered that Mirin suppresses MRE11 nuclease activity, a recent study using H129N knock-in mice demonstrated that the nuclease activity of MRE11 is not required for ATM activation (26). Thus, the suppressive effect of Mirin on type I IFN induction is not restricted to inhibition of MRE11 nuclease activity. The precise mechanisms by which Mirin modifies MRE11 function to suppress dsDNA-induced type I IFN production require future investigation.
Results from the microscopy experiments and the analysis of MRE11 mutants indicate that RAD50 is also involved in intracellular DNA responses. However, unlike MRE11, RAD50 is mainly present in the nucleus in resting cells and is recruited to the MRE11–DNA complex in the cytoplasm after DNA stimulation (Fig. S3B). RAD50 has a binding protein, RAD50 interacting protein 1 (RINT1), which is distributed in the ER during interphase and forms a complex to regulate ER–Golgi traffic (34, 35), suggesting the possibility that RINT1 may act as a bridge between MRE11/RAD50 and STING by mediating membrane trafficking. Further studies are required to clarify how RAD50 contributes to exogenous DNA responses. Given that Rad50 knockdown only partially abrogated poly (I:C)-mediated response (Fig. S1E), it may be possible that Rad50 is involved in RNA-mediated innate immune responses, which should be clarified in the future. Another component of the MRN complex, NBS1, is not essential for cytosolic DNA responses. NBS1 was shown to be important for recruitment of MRE11 to endogenous DSB foci, and for the interaction between MRE11 and ATM (30, 36), neither of which seem to be necessary for the exogenous DNA-induced STING pathway. Furthermore, loss of NBS1 increases cytosolic MRE11, suggesting that MRE11 recognition of exogenous dsDNA in the cytoplasm is enhanced by a loss of NBS1.
Our present results reveal that MRE11 is not necessary for type I IFN responses against pathogens such as HSV-1 or L. monocytogenes, which are considered to activate immune responses via intracellular DNA sensors. Although these pathogens induce type I IFN production through STING (11), we failed to observe STING translocation after treatment with these pathogens in various conditions. Indeed, our results indicate that MRE11 is specifically involved in the responses associated with STING trafficking (Fig. 2E). These findings propose that there are multiple pathways; dsDNA induces STING translocation and pathogens induce STING activation at the ER membrane. The latter pathway may also be important in the case of infection with RNA viruses such as vesicular stomatitis virus (VSV), because VSV induces type I IFN production through STING (11) in the absence of STING translocation. It remains unclear why HSV-1 and L. monocytogenes do not induce STING trafficking. One possibility is the location at which DNA responses are triggered. HSV-1 generally replicates in the nucleus and does not appear to expose genomic DNA to the cytoplasm of host cells. IFI16 has been shown to localize in the nucleus in many cells and sense Kaposi's sarcoma-associated herpesvirus in nucleus (37). Another study has also demonstrated that nuclear localization sequence of IFI16 locates IFI16 in the nucleus, where it recognizes HSV-1 (31). Furthermore, a cytosolic DNA sensor, AIM2, is unlikely required for inflammasome activation by HSV-1 (38). In addition, MRE11/RAD50 seems to affect the trafficking systems between the ER and the Golgi apparatus, suggesting that cytoplasmic DNA recognition by MRE11/RAD50 influences STING trafficking, which is linked to activation of downstream signaling. Alternatively, HSV-1 may possess the evasion machinery to avoid STING trafficking by collapsing the MRN complex, because HSV-1 circumvents host defense machinery and exploits the MRN complex for replication (17). On the other hand, L. monocytogenes resides in the cytoplasm and its genomic DNA is considered a ligand for the STING pathway (8). It is currently unknown why L. monocytogenes infection does not cause STING translocation. However, recent studies have shown that cyclic di-GMP of bacteria is a major component responsible for type I IFN induction and, notably, STING directly recognizes cyclic di-GMP and activates downstream signaling (39, 40), suggesting that L. monocytogenes directly activates STING without MRE11 and RAD50. Meanwhile, we also found that STING is critical for DMXAA-induced immune responses without MRE11. This result implies that DMXAA (the structure of which is more similar to cyclic di-GMP than to dsDNA) may interact with STING directly. However, we cannot exclude the possibility that other unknown receptors for DMXAA trigger the STING pathway.
Our observations that MRE11/RAD50 mediates recognition of dsDNA rather than pathogens suggest that the biological significance of MRE11-mediated intracellular DNA recognition is to respond to damaged host cells, rather than defense against foreign pathogens. In resting eukaryote cells, DNA is strictly stored in particular compartments such as the nucleus and mitochondria and the release of endogenous DNA to the cytoplasm or extracellular milieu provides signals that alert the host cell to danger. Whereas DNases mediate the clearance of self DNA in the apoptotic state (5), an excessive amount of DNA escaping from DNases is responsible for induction of type I IFN, probably through activation of DNA sensors. In addition to its function within the nucleus to guard the integrity of the genome, MRE11 may have acquired additional functions against various stresses from the extracellular environment over the course of evolution, in a similar manner to ATM, which has been shown to function as a redox sensor (41). A number of studies have demonstrated that inappropriate production of type I IFN is harmful and leads to autoimmune diseases (5). Further studies with regard to MRE11-mediated DNA sensing may be helpful for better understanding the mechanisms of DNA-associated autoimmunity.
In conclusion, we have demonstrated a critical function of MRE11 in intracellular dsDNA responses. Our data provide knowledge about the role of the MRN complex in the recognition of dsDNA in the cytoplasm. Although the consequences of activity of the MRN complex in innate immune responses in vivo remain to be elucidated, these findings suggest a rationale for further studies, which will enable us to understand the precise mechanisms of nucleic acid recognition, and the biological significance of this on host defense against pathogens, as well as responses to damaged host or tumor cells.
Materials and Methods
RNA Interference.
Double-stranded RNA duplexes corresponding to mouse Mre11 (MSS206766 and MSS206767) and Rad50 (MSS208386 and MSS276712) mRNA were purchased from Invitrogen. A nonspecific siRNA (45–2001; Invitrogen) was used as a negative control. MEFs were transfected with 50 nM siRNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. At 60 h after transfection, the cells were used for further experiments.
Quantitative RT-PCR.
Total RNA of cultured cells was isolated using TRIzol reagent (Invitrogen) and reverse transcribed with ReverTra Ace (Toyobo) according to the manufacturer's instructions. PCR analysis was performed with power SYBR Green (Applied Biosystems) and the primers described in SI Materials and Methods.
Statistical Analysis.
Differences were analyzed for statistical significance using Student’s t test. A P value of less than 0.05 was considered significant.
Additional Information.
The details are available in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank M. Nishino and C. Funamoto for technical assistance, the members of the Laboratory of Host Defense for valuable discussion, and E. Kamada and M. Kageyama for secretarial assistance. This study was supported by a KAKENHI Grant-in-Aid for Research Activity (A) (23689030) and supported by the Special Coordination Funds of the Japanese Ministry of Education, Culture, Sports, Science and Technology; as well as the Ministry of Health, Labour, and Welfare in Japan and the Japan Society for the Promotion of Science through the Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST Program).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1222694110/-/DCSupplemental.
References
- 1.Hornung V, Latz E. Intracellular DNA recognition. Nat Rev Immunol. 2010;10(2):123–130. doi: 10.1038/nri2690. [DOI] [PubMed] [Google Scholar]
- 2.Desmet CJ, Ishii KJ. Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nat Rev Immunol. 2012;12(7):479–491. doi: 10.1038/nri3247. [DOI] [PubMed] [Google Scholar]
- 3.Hemmi H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408(6813):740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 4.Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med. 2007;13(5):543–551. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- 5.Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140(5):619–630. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Okabe Y, Kawane K, Akira S, Taniguchi T, Nagata S. Toll-like receptor-independent gene induction program activated by mammalian DNA escaped from apoptotic DNA degradation. J Exp Med. 2005;202(10):1333–1339. doi: 10.1084/jem.20051654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishii KJ, et al. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat Immunol. 2006;7(1):40–48. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]
- 8.Stetson DB, Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24(1):93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Takaoka A, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448(7152):501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 10.Ishii KJ, et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature. 2008;451(7179):725–729. doi: 10.1038/nature06537. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461(7265):788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saitoh T, et al. Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response. Proc Natl Acad Sci USA. 2009;106(49):20842–20846. doi: 10.1073/pnas.0911267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unterholzner L, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11(11):997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Z, et al. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat Immunol. 2011;12(10):959–965. doi: 10.1038/ni.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim T, et al. Activation of interferon regulatory factor 3 in response to DNA-damaging agents. J Biol Chem. 1999;274(43):30686–30689. doi: 10.1074/jbc.274.43.30686. [DOI] [PubMed] [Google Scholar]
- 16.Karpova AY, Trost M, Murray JM, Cantley LC, Howley PM. Interferon regulatory factor-3 is an in vivo target of DNA-PK. Proc Natl Acad Sci USA. 2002;99(5):2818–2823. doi: 10.1073/pnas.052713899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weitzman MD, Lilley CE, Chaurushiya MS. Genomes in conflict: Maintaining genome integrity during virus infection. Annu Rev Microbiol. 2010;64:61–81. doi: 10.1146/annurev.micro.112408.134016. [DOI] [PubMed] [Google Scholar]
- 18.Ablasser A, et al. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat Immunol. 2009;10(10):1065–1072. doi: 10.1038/ni.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiu YH, Macmillan JB, Chen ZJ. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell. 2009;138(3):576–591. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uziel T, et al. Requirement of the MRN complex for ATM activation by DNA damage. EMBO J. 2003;22(20):5612–5621. doi: 10.1093/emboj/cdg541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stracker TH, Petrini JH. The MRE11 complex: Starting from the ends. Nat Rev Mol Cell Biol. 2011;12(2):90–103. doi: 10.1038/nrm3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dupré A, et al. A forward chemical genetic screen reveals an inhibitor of the Mre11-Rad50-Nbs1 complex. Nat Chem Biol. 2008;4(2):119–125. doi: 10.1038/nchembio.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart GS, et al. The DNA double-strand break repair gene hMRE11 is mutated in individuals with an ataxia-telangiectasia-like disorder. Cell. 1999;99(6):577–587. doi: 10.1016/s0092-8674(00)81547-0. [DOI] [PubMed] [Google Scholar]
- 24.Moreau S, Ferguson JR, Symington LS. The nuclease activity of Mre11 is required for meiosis but not for mating type switching, end joining, or telomere maintenance. Mol Cell Biol. 1999;19(1):556–566. doi: 10.1128/mcb.19.1.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arthur LM, et al. Structural and functional analysis of Mre11-3. Nucleic Acids Res. 2004;32(6):1886–1893. doi: 10.1093/nar/gkh343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buis J, et al. Mre11 nuclease activity has essential roles in DNA repair and genomic stability distinct from ATM activation. Cell. 2008;135(1):85–96. doi: 10.1016/j.cell.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JH, et al. Regulation of Mre11/Rad50 by Nbs1: Effects on nucleotide-dependent DNA binding and association with ataxia-telangiectasia-like disorder mutant complexes. J Biol Chem. 2003;278(46):45171–45181. doi: 10.1074/jbc.M308705200. [DOI] [PubMed] [Google Scholar]
- 28.Fernet M, et al. Identification and functional consequences of a novel MRE11 mutation affecting 10 Saudi Arabian patients with the ataxia telangiectasia-like disorder. Hum Mol Genet. 2005;14(2):307–318. doi: 10.1093/hmg/ddi027. [DOI] [PubMed] [Google Scholar]
- 29.Ito A, et al. Expression of full-length NBS1 protein restores normal radiation responses in cells from Nijmegen breakage syndrome patients. Biochem Biophys Res Commun. 1999;265(3):716–721. doi: 10.1006/bbrc.1999.1737. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi J, et al. NBS1 localizes to gamma-H2AX foci through interaction with the FHA/BRCT domain. Curr Biol. 2002;12(21):1846–1851. doi: 10.1016/s0960-9822(02)01259-9. [DOI] [PubMed] [Google Scholar]
- 31.Li T, Diner BA, Chen J, Cristea IM. Acetylation modulates cellular distribution and DNA sensing ability of interferon-inducible protein IFI16. Proc Natl Acad Sci USA. 2012;109(26):10558–10563. doi: 10.1073/pnas.1203447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts ZJ, et al. The chemotherapeutic agent DMXAA potently and specifically activates the TBK1-IRF-3 signaling axis. J Exp Med. 2007;204(7):1559–1569. doi: 10.1084/jem.20061845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134(4):587–598. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao J, Liu CC, Chen PL, Lee WH. RINT-1, a novel Rad50-interacting protein, participates in radiation-induced G(2)/M checkpoint control. J Biol Chem. 2001;276(9):6105–6111. doi: 10.1074/jbc.M008893200. [DOI] [PubMed] [Google Scholar]
- 35.Arasaki K, Taniguchi M, Tani K, Tagaya M. RINT-1 regulates the localization and entry of ZW10 to the syntaxin 18 complex. Mol Biol Cell. 2006;17(6):2780–2788. doi: 10.1091/mbc.E05-10-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.You Z, Chahwan C, Bailis J, Hunter T, Russell P. ATM activation and its recruitment to damaged DNA require binding to the C terminus of Nbs1. Mol Cell Biol. 2005;25(13):5363–5379. doi: 10.1128/MCB.25.13.5363-5379.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kerur N, et al. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell Host Microbe. 2011;9(5):363–375. doi: 10.1016/j.chom.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rathinam VA, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11(5):395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McWhirter SM, et al. A host type I interferon response is induced by cytosolic sensing of the bacterial second messenger cyclic-di-GMP. J Exp Med. 2009;206(9):1899–1911. doi: 10.1084/jem.20082874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burdette DL, et al. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478(7370):515–518. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Science. 2010;330(6003):517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.