Abstract
Overproduction of nitric oxide (NO) can cause neuronal damage, contributing to the pathogenesis of several neurodegenerative diseases and stroke (i.e., focal cerebral ischemia). NO can mediate neurotoxic effects at least in part via protein S-nitrosylation, a reaction that covalently attaches NO to a cysteine thiol (or thiolate anion) to form an S-nitrosothiol. Recently, the tyrosine phosphatase Src homology region 2-containing protein tyrosine phosphatase-2 (SHP-2) and its downstream pathways have emerged as important mediators of cell survival. Here we report that in neurons and brain tissue NO can S-nitrosylate SHP-2 at its active site cysteine, forming S-nitrosylated SHP-2 (SNO–SHP-2). We found that NMDA exposure in vitro and transient focal cerebral ischemia in vivo resulted in increased levels of SNO–SHP-2. S-Nitrosylation of SHP-2 inhibited its phosphatase activity, blocking downstream activation of the neuroprotective physiological ERK1/2 pathway, thus increasing susceptibility to NMDA receptor-mediated excitotoxicity. These findings suggest that formation of SNO–SHP-2 represents a key chemical reaction contributing to excitotoxic damage in stroke and potentially other neurological disorders.
Keywords: nitrosative stress, reactive oxygen species, reactive nitrogen species
Excessive activation of NMDA receptors (NMDARs) activates neuronal nitric oxide (NO) synthase (NOS; nNOS) (1) and results in overproduction of NO in the brain, contributing to neuronal damage in a number of neurodegenerative diseases and cerebral ischemia (2, 3). The NO group can react with specific cysteine thiols to regulate protein activity in a process called S-nitrosylation (4, 5). As a ubiquitous posttranslational modification, S-nitrosylation has been found to modulate a broad spectrum of proteins affecting neuronal development, synaptic plasticity, protein folding, and cell death (3). Recent discoveries have demonstrated that excessive NO has a negative effect on neuronal survival via S-nitrosylation of a number of neurodegenerative disease-associated proteins, including parkin, protein disulfide isomerase, GAPDH, Cdk5, and dynamin-related protein 1 (6-11).
The Src homology-2 domain-containing phosphatase (SHP-2), a member of the ubiquitously expressed protein-tyrosine phosphatase (PTP) family, contains a cysteine residue at its active site (12). SHP-2 is known to localize in the cytosol and nucleus, and plays important biological functions in response to various growth factors, hormones, and cytokines (13). Recent studies have shown that activation of SHP-2 increases survival of various cell types, including neural progenitor cells and neurons, through activation of ERK1/2 (14, 15). SHP-2 is thought to promote ERK signaling by dephosphorylating negative regulators of the Ras-ERK pathway, such as PAG/Cbp, Ras-GAP, or GAP-binding sites on receptor tyrosine kinases or Sprouty proteins (16–20). Additionally, transient activation of the ERK1/2 signaling cascade has been implicated in regulating neuronal survival after stroke (21–24). ERK1/2 can influence the activity of a number of transcription factors, including myc and c-fos by direct phosphorylation, as well as cAMP response element-binding factor and myocyte-enhancer factor 2C via downstream Ser/Thr protein kinases (25, 26). Furthermore, in mouse and yeast models, protective effects of ERK1/2 can increase tolerance to oxidative stress and thereby extend lifespan (27).
Reactive oxygen species (ROS) are known to oxidize cysteine residue(s) of many PTPs, resulting in impairment of their activity (4, 28). Additionally, reactive nitrogen species have been shown to S-nitrosylate a number of phosphatases, including SHP-1 and SHP-2, in nonneuronal cells (29, 30). Here, we hypothesized that S-nitrosylation also occurs at the active-site cysteine residue of SHP-2 in neuronal cells, and that NO could therefore affect neuronal survival under neuropathological conditions. In this report, we present evidence that S-nitrosylation inhibits the activity of SHP-2, resulting in increased vulnerability of neurons to NMDAR-mediated excitotoxic damage and death in vitro and in vivo in an animal model of transient focal cerebral ischemia (i.e., stroke). Our data also show that S-nitrosylation of SHP-2 [forming S-nitrosylated SHP-2 (SNO–SHP-2)] down-regulates the neuroprotective ERK pathway, thereby enhancing NO-mediated excitotoxicity. This study provides a mechanistic link between SNO–SHP-2 formation in response to NMDAR activation and inhibition of the neuroprotective ERK1/2 pathway in cerebral ischemia.
Results
NO Induces S-Nitrosylation of SHP-2.
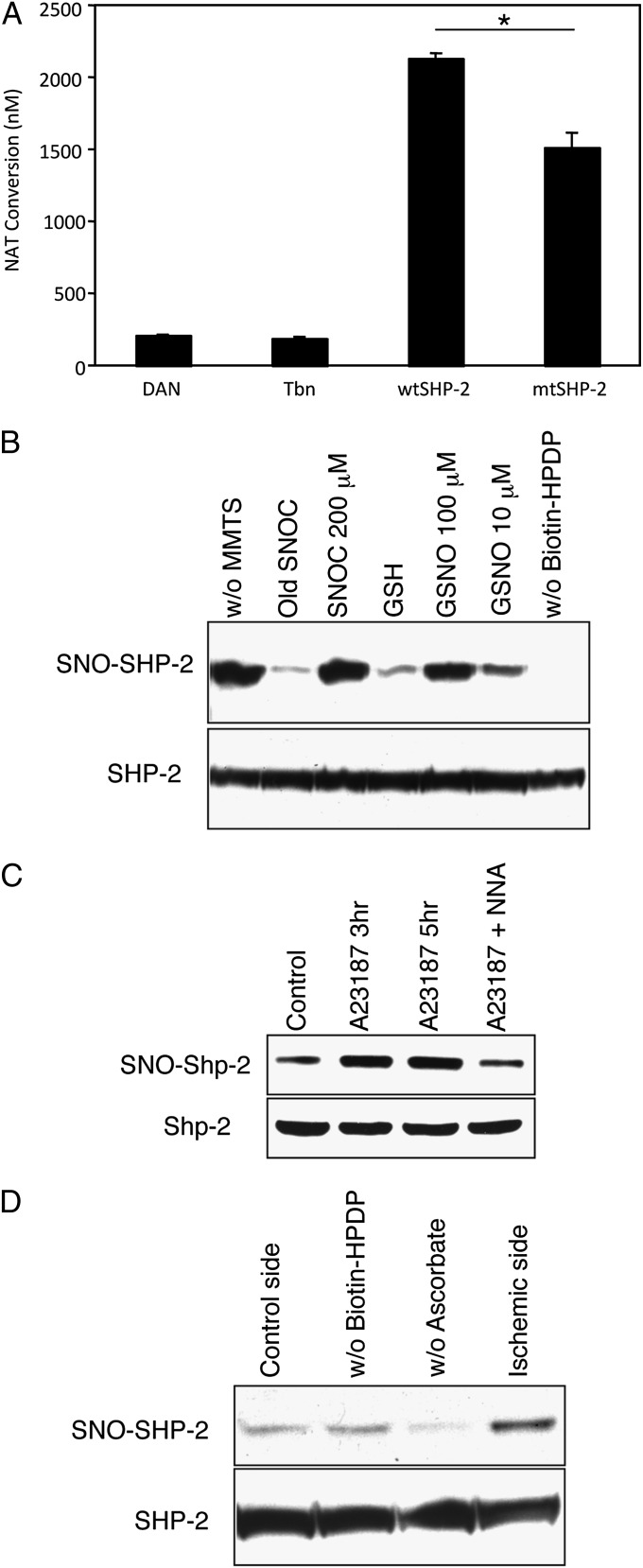
To assess SNO–SHP-2 formation, we initially performed an in vitro chemical assay using 2,3-diaminonaphthalene (DAN) on recombinant SHP-2 proteins. The DAN assay revealed that exposure to the physiological NO donor S-nitrosocysteine (SNOC) induced S-nitrosylation of wild-type SHP-2 (wtSHP-2) (Fig. 1A). Consistent with previous reports (29, 31), mutation of the active-site cysteine to serine drastically curtailed the generation of SNO–SHP-2, suggesting that S-nitrosylation occurred on this cysteine residue (Cys-459; Fig. 1A). Next, by using a biotin-switch assay, we demonstrated formation of SNO–SHP-2 in HEK293 lysates in vitro and in intact cells ex vivo (Fig. 1B). Exposure of cell lysates to a second NO donor, S-nitrosoglutathione, produced similar results (Fig. 1B). To investigate whether endogenously generated NO can also engender formation of SNO–SHP-2, we used HEK293 cells stably expressing nNOS (HEK-nNOS cells). These cells were subjected to the biotin-switch assay after activation of nNOS by the calcium ionophore A23187. Under these conditions, we found that endogenous NO increased S-nitrosylated SHP-2 levels by ∼2.5-fold; moreover, the NOS inhibitor N-nitro-L-arginine (NNA) blocked this reaction (Fig. 1C). As expected, hydrogen peroxide failed to S-nitrosylate SHP-2, as it generates ROS that oxidize the active-site cysteine by non–NO-related chemistry (Fig. S1). To extend these findings to a model of ischemia in vivo, S-nitrosylation of SHP-2 was studied in the cerebrocortex after transient middle cerebral artery occlusion/reperfusion (tMCAO/R) in mice. The biotin-switch assay revealed that focal cerebral ischemia was associated with a ∼2.5-fold increase in SNO–SHP-2 in the mouse brain (Fig. 1D). Taken together, these results clearly suggest that, under pathophysiological conditions, enhanced nitrosative stress results in S-nitrosylation of SHP-2.
Fig. 1.
NO induces S-nitrosylation of SHP-2 at its active site cysteine. (A) Recombinant wtSHP-2 or mutant SHP-2 (mtSHP-2) were exposed to SNOC (200 μM) and then incubated for 30 min at RT. S-Nitrosylated SHP-2 was assessed with the DAN assay by spectrofluorometrically monitoring DAN conversion to fluorescent 2,3-naphthyltriazole (NAT). In mtSHP-2, the active site cysteine was substituted with serine. Thrombin (Tbn) was used to remove the GST-tag from SHP-2 and thus used as a negative control for the assay. Values are mean ± SEM (n ≥ 3; *P < 0.05). (B) (Upper) Cell lysates from HEK293 cells were incubated with SNOC, S-nitrosoglutathione (GSNO), old SNOC, or reduced glutathione (GSH) at RT. Thirty minutes later, SNO–SHP-2 formation was detected by the biotin-switch assay. Old SNOC and GSH were controls in the absence of an NO donor; without methyl methanethiosulfonate (MMTS; without the free thiol blocker) and without N-[6-(biotinamido)hexyl]-3′-(2′-pyridyldithio)-propionamide (biotin-HPDP) are positive and negative reaction controls, respectively. (Lower) Total SHP-2 in cell lysates identified by immunoblot. (C) S-Nitrosylation of SHP-2 by endogenous NO. (Upper) Cell lysates from HEK-nNOS cells that had been exposed to the Ca2+ ionophore A23187 for 3 or 5 h were subjected to the biotin-switch assay. The NOS inhibitor NNA suppressed SNO–SHP-2 formation. (Lower) SHP-2 loading control. (D) Middle cerebral artery occlusion results S-nitrosylation of SHP-2 in mice. SNO–SHP-2 formation was detected by biotin-switch assay in the ischemic side but not in the control side of the mouse brain. Without ascorbate represents negative reaction control for the ischemic side of the brain.
S-Nitrosylation of SHP-2 Suppresses Its Phosphatase Activity and Inhibits Its Ability to Protect Neurons from NMDAR-Mediated Excitotoxicity.
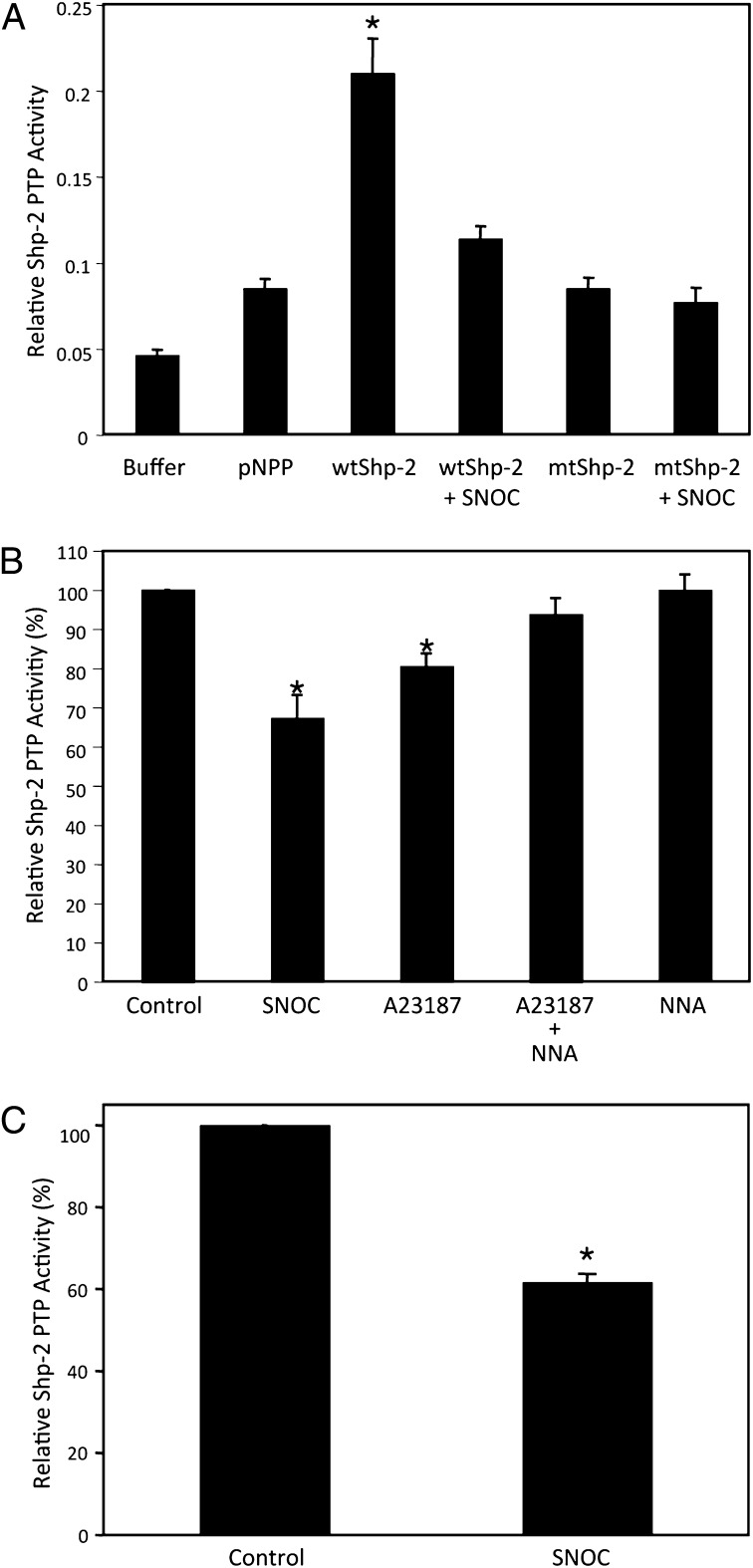
Because S-nitrosylation of SHP-2 occurs at its active site cysteine, we tested whether NO would influence SHP-2 phosphatase activity as expected in vitro and in intact cells. We monitored in vitro phosphatase activity in a standard assay that uses p-nitrophenyl phosphate (pNPP) as a substrate of SHP-2, as previously described (32). wtSHP-2 exhibited high phosphatase activity toward pNPP, whereas mutation in the active-site cysteine totally abrogated the activity (Fig. 2A). S-Nitrosylation of recombinant wtSHP-2 by SNOC also significantly inhibited phosphatase activity (Fig. 2A). Next, to determine if exogenous and endogenous NO could affect SHP-2 phosphatase activity in intact cells, we monitored enzymatic function in HEK-nNOS cells and rat cortical neurons. Exposure of cells to SNOC or A23187 to activate nNOS drastically attenuated SHP-2 phosphatase activity; the effect of A23187 was abrogated by addition of NNA (Fig. 2 B and C). These results are consistent with the notion that S-nitrosylation inhibits the enzymatic function of SHP-2.
Fig. 2.
S-Nitrosylation impairs SHP-2 PTP activity in vitro and in intact cells. (A) Recombinant wtSHP-2 or mtSHP-2 were expressed and purified from bacteria, and subjected to standard in vitro PTP assay. Substrate for the PTP assay, pNPP, and buffer were used as controls. (B and C) HEK293-nNOS cells activated with Ca2+ ionophore A23187 for 3 or 5 h (B) or rat primary cortical neurons in culture exposed to SNOC (C) were assayed for SHP-2 PTP activity as described in Materials and Methods. Values are mean ± SEM (n ≥ 3; *P < 0.05).
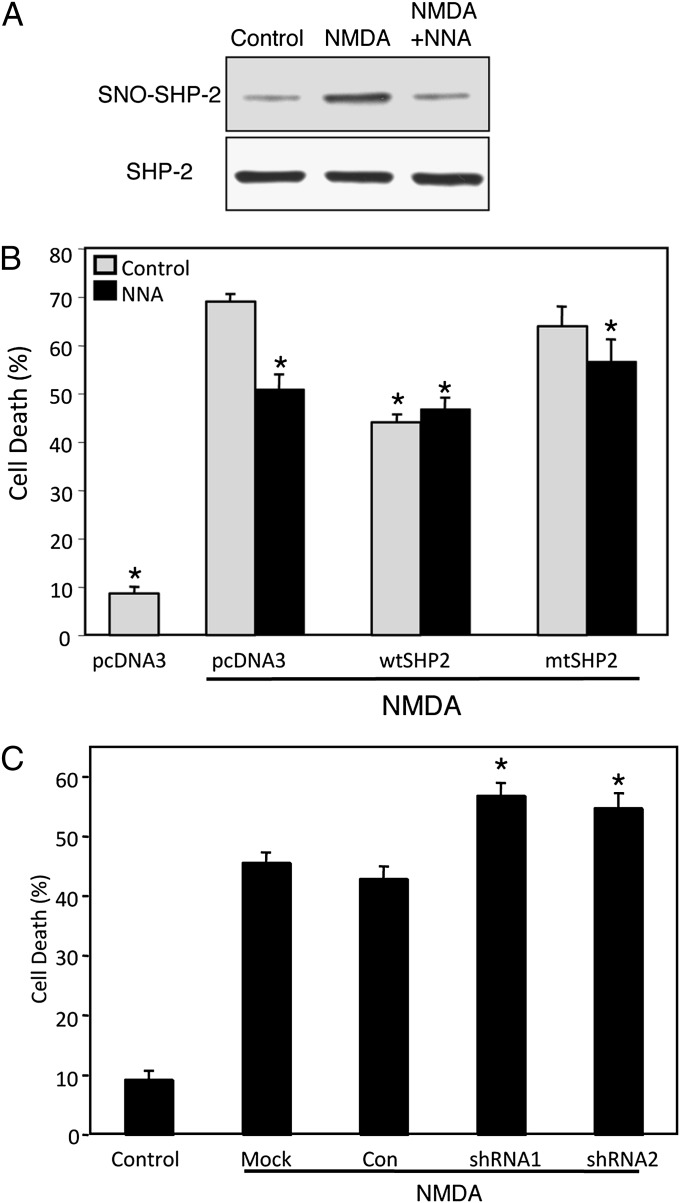
From these results, we reasoned that formation of SNO–SHP-2 might inhibit a prosurvival pathway mediated by SHP-2 activation of the ERK1/2 neuroprotective cascade. To test this concept further, we investigated the effect of S-nitrosylation of Shp-2 during NMDAR-mediated excitotoxicity. Excessive activation of NMDARs is a well-recognized cellular model to stimulate nNOS, which generates NO. Overproduction of NO may lead to neuronal damage and thereby contribute to the pathogenesis of a number of neurodegenerative diseases and focal cerebral ischemia (2). By using the biotin-switch assay, we demonstrated that exposure to NMDA indeed enhanced S-nitrosylation of SHP-2 by ∼2.5-fold in a NOS activity-dependent fashion (Fig. 3A).
Fig. 3.
NMDA-induced formation of SNO–SHP-2 regulates cell death of rat cortical neurons. (A) Activation of the NMDAR results in S-nitrosylation of SHP-2 in rat cortical cultures. (Upper) Rat cortical cells in primary culture were incubated with NMDA or NMDA plus NOS inhibitor (NNA; 1 mM) and assayed for SNO–SHP-2 formation. SNO–SHP-2 was detected by the biotin-switch assay. (Lower) Total SHP-2 in cell lysates by Western analysis. (B) Expression of SHP-2 inhibits NMDA-induced excitotoxicity in transiently transfected cortical neurons. Rat cortical neurons were transiently transfected with pcDNA3 (empty vector), wtSHP-2, or mtSHP-2, together with GFP. Neurons were visualized by costaining with MAP-2 plus NeuN antibodies. Cell death was assayed with Hoechst staining, measuring the ratio of apoptotic neurons (dead cells) to GFP-positive neurons (transfected cells). Values are mean ± SEM (n ≥ 3; *P < 0.05 vs. vector control exposed to NMDA). (C) Knockdown of SHP-2 expression sensitizes cortical neurons to NMDA-induced excitotoxicity. Rat cortical neurons were transiently transfected with SHP-2 or control shRNA, and neuronal cell death was evoked by exposure to NMDA. Cell death was assayed by counting the ratio of neurons with apoptotic nuclei (dead) to GFP-positive (transfected) neurons. Values are mean ± SEM (n ≥ 3; *P < 0.05).
As S-nitrosylation of SHP-2 inhibits its enzymatic activity, and SHP-2 dominant-negative transgenic mice have been shown to be more susceptible to focal cerebral ischemia (33), a process known to be mediated at least in part by excessive NMDAR activity, we next tested if overexpression of SHP-2 could prevent neuronal cell death elicited by NMDA. Assessment of apoptotic nuclear morphology revealed that exogenous expression of SHP-2 decreased NMDA-evoked cell death by ∼35% compared with control plasmid-transfected neurons (Fig. 3B). Expression of a catalytically impaired cysteine mutant of SHP-2 (mtSHP-2), which mimics SNO–SHP-2 formation and also acts as a dominant negative (34), failed to protect neurons from NMDA toxicity. These data suggest that rescue of cortical neurons by SHP-2 expression requires its catalytic activity and that S-nitrosylation of SHP-2 might inhibit the neuroprotective pathway. Further implicating NO in this process, we observed that the NOS antagonist NNA inhibited NMDA-evoked cell death in the absence of SHP-2 overexpression (Fig. 3B).
To extend our observation that SHP-2 inhibits neuronal cell death, we asked if down-regulation of endogenous SHP-2 could increase the sensitivity of cortical neurons to NMDA. To test this premise, we prepared two mammalian constructs that express shRNAs against mouse/rat SHP-2 (SHP-2 shRNA1 and shRNA2). By immunoblot analyses, we confirmed that expression of these SHP-2 shRNAs in mouse embryonic fibroblasts decreased the level of endogenous SHP-2 compared with control-shRNA transfection (Fig. S2A). Immunocytochemistry also revealed that the shRNAs could abrogate SHP-2 expression in transfected cerebrocortical neurons (Fig. S2B). Moreover, when the SHP-2 shRNAs, but not control shRNA, were expressed in cortical neurons, we found an increased percentage of apoptotic cells after exposure to NMDA (Fig. 3C). Taken together, these results support the notion that SHP-2 protects cortical neurons from NMDA-mediated excitotoxicity, and that NO produced by NMDA stimulation can abrogate SHP-2 activity via S-nitrosylation and thus contribute to neuronal damage.
To determine whether a pathophysiologically relevant amount of SNO–SHP-2 is formed in vivo, we calculated the ratio of SNO–SHP-2 to total SHP-2, as we have described previously (6, 10). We found that the levels of SNO–SHP-2 compared with controls were comparable in HEK-nNOS cells manifesting decreased SHP-2 phosphatase activity, in rat cortical neurons undergoing apoptotic cell death, and in the mouse brain during stroke in vivo. Under these conditions, SNO–SHP-2 increased 2.0 ± 0.3-fold in HEK-nNOS cells (n = 4), 2.0 ± 0.3-fold in rat cortical neurons (n = 3), and 1.7 ± 0.4-fold with focal cerebral ischemia (n = 3; Fig. S3). These results are consistent with the notion that pathophysiologically relevant amounts of SNO–SHP-2 were formed in vivo during stroke.
Activation of MEK/ERK1/2 Signaling Pathway Mediates Neuroprotective Effect of SHP-2.
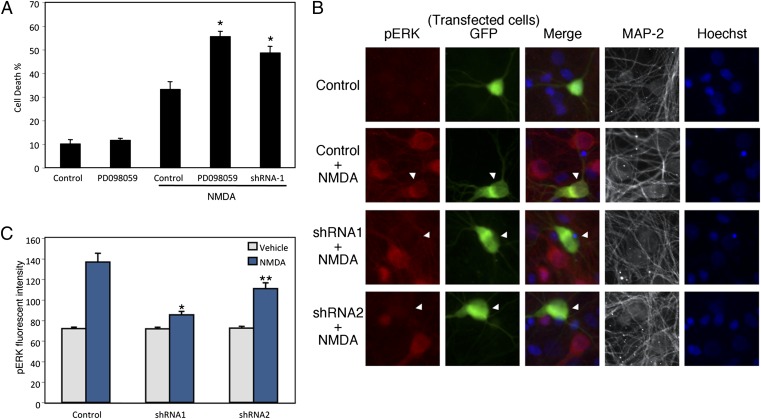
We and others have recently shown that transient activation of the ERK1/2 signaling cascade mediates prosurvival effects of SHP-2 in mouse embryonic fibroblasts and in neural progenitor cells (14, 15). To determine whether SHP-2 activity functions downstream of NMDARs and affects the ERK1/2 pathway in cortical neurons, we initially used the highly selective MEK inhibitor PD098059, as MEK is the kinase that activates ERK through dual phosphorylation of threonine and tyrosine residues (35). We found in cortical neurons that inhibition of ERK activation with PD098059 resulted in increased susceptibility to NMDA (Fig. 4A). Additionally, ERK inhibition eliminated the protective effect of nNOS inhibition, consistent with our hypothesis that SNO–SHP-2 contributes to NO neurotoxicity via suppression of ERK activation (Fig. S4). We then measured the levels of phosphorylated ERK1/2 in neurons with SHP-2 knocked down. By analyzing images obtained with quantitative deconvolution microscopy, we found that exposure to NMDA enhanced ERK1/2 phosphorylation, but expression of SHP-2 shRNA1 or shRNA2 abrogated the up-regulation of phosphorylated ERK1/2 engendered by NMDA. These findings are consistent with the notion that NMDA-induced activation of ERK1/2 is at least partly dependent on SHP-2 activity (Fig. 4 B and C). As a control, immunoblot analyses revealed that exposure to NMDA did not affect the expression level of ERK1/2 in cortical neurons (Fig. S5). Taken together, these results suggest a possible role of SNO–SHP-2 in negatively regulating the neuroprotective ERK1/2 pathway, thus enhancing NMDA/NO-mediated excitotoxicity (Fig. 5).
Fig. 4.
Inhibition of the ERK pathway sensitizes cortical neurons to NMDA-induced excitotoxicity. (A) Rat cortical neurons were pretreated with the MEK inhibitor PD098059 (50 μM) before NMDA exposure. SHP2-shRNA: cells were transfected with SHP-2 shRNA 2 d before NMDA exposure. Cell death was assayed 16 h after NMDA exposure by scoring the ratio of apoptotic nuclei (dead) to GFP-positive (transfected) neurons. Values are mean ± SEM (n ≥ 3; *P < 0.05). (B and C) Reduced activation of ERK1/2 after SHP-2 knockdown in cortical neurons. Rat cortical neurons were transfected with shRNA plasmids and, 2 d later, neurons were exposed to NMDA for 10 min. Images of activated (phosphorylated) ERK (red) in MAP-2–positive cortical neurons (white) expressing GFP and SHP-2 shRNA (green) (B). Quantitative data summarizing the effect of NMDA on phosphorylated ERK1/2 levels (C). Values are mean ± SEM (n ≥ 3; *P < 0.001 and **P < 0.01 vs. control plus NMDA).
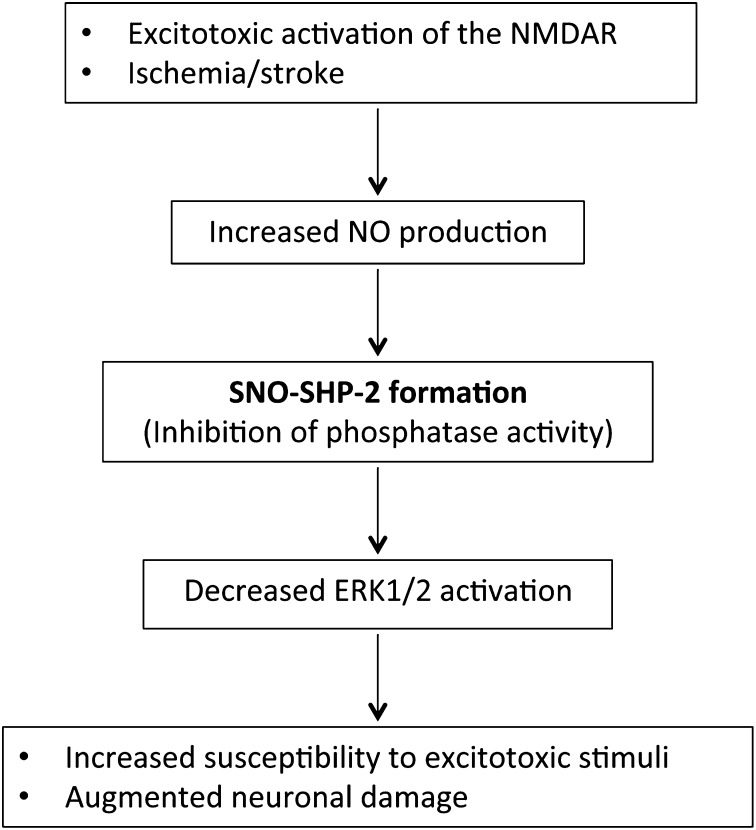
Fig. 5.
Schematic model of increased excitotoxic neuronal damage via SNO–SHP-2 formation. Excitotoxic stimuli or ischemic conditions increase the production of NO and subsequent generation of SNO–SHP-2. S-Nitrosylation inhibits SHP-2 phosphatase activity, leading to decreased activation of ERK1/2, thus contributing to enhanced neuronal damage.
Discussion
In the present study, we demonstrate that SHP-2 can be S-nitrosylated by endogenous NO in neuronal cells. S-Nitrosylation of SHP-2 inhibits the enzyme’s phosphatase activity, thereby diminishing the neuroprotective effect of SHP-2 via reduced activation of the ERK1/2 pathway. These data provide insight into the mechanism whereby nitrosative stress impairs neuroprotective pathways mediated by SHP-2, particularly in the setting of excitotoxic insult and cerebral ischemia. In agreement with recent studies demonstrating an effect of S-nitrosylation on PTPs in nonneuronal cells (29, 31), our results show that S-nitrosylation occurs at the catalytic cysteine of SHP-2. As other PTPs contain a conserved cysteine residue at their active sites, we speculate that nitrosative stress may result in S-nitrosylation of additional neuronal PTPs, such as PTP1B, and that NO may thereby regulate neuronal survival via S-nitrosylation of other of PTPs as well (4, 31, 36). Additionally, ROS can reversibly or irreversibly oxidize the active site cysteine of PTPs to form sulfinic acid (-SO2H) or sulfonic acid (-SO3H) derivatives, significantly inhibiting their activity (28). Because we and others have recently demonstrated that S-nitrosylation can often facilitate further oxidation by ROS to sulfinic or sulfonic acid derivatives (8, 10, 37), it is conceivable that the S-nitrosylated cysteine residue in SHP-2 can also initiate further oxidation in this manner.
Previously, transient activation of the ERK1/2 signaling pathway had been shown to contribute to neuronal survival via regulation of transcription factors, including c-myc and c-fos (23–26). Consistent with these observations, we found that the ERK1/2 pathway contributed to neuroprotection against NMDAR-mediated excitotoxicity. Conversely, we observed that inhibition of SHP-2 activity by S-nitrosylation significantly reduced activation of ERK1/2 and thus increased susceptibility to NMDAR-mediated cell death. It should be noted, however, that other compelling evidence suggests a detrimental role of persistent activation of the ERK1/2 signaling pathway in neurons (38–40). Specifically, in cultured cortical neurons, glutamate can induce transient ERK1/2 activation, which may represent the protective effect of ERK1/2, followed by sustained/pathological ERK1/2 activation that contributes to glutamate toxicity (41–43). Our results are thus consistent with the notion that SHP-2 mediates the initial transient activation of ERK1/2 (e.g., 10 min after NMDA exposure in Fig. 4 B and C), thereby providing neuroprotection from NMDA exposure to cortical neurons, and that inhibition of SHP-2 activity by NO can abrogate this effect. Thus, despite previous work showing that ERK1/2 can be activated by NO via direct interaction with p21ras (44), we found evidence for inhibition of the ERK1/2 pathway by NO-mediated S-nitrosylation of SHP-2. This inhibition of SHP-2 activity by S-nitrosylation attenuates the neuroprotective pathway mediated by transient activation of ERK1/2 signaling.
In summary, the present study suggests that formation of SNO–SHP-2 and subsequent dysregulation of ERK1/2 activity may provide a molecular mechanism linking generation of reactive nitrogen species to neuronal damage, particularly in acute ischemic stroke. Importantly, as inhibition of SHP-2 in vivo is known to augment damage in focal cerebral ischemia (33), and we demonstrate here that S-nitrosylation inhibits SHP-2 activity, the increase in SNO–SHP-2 we observed in vivo during stroke is likely to contribute to this pathology. Thus, we propose that denitrosylation of SNO–SHP-2 may constitute a viable strategy for treating stroke and possibly other disorders associated with excitotoxicity.
Materials and Methods
Plasmids, Cell Culture, Western Blot Analysis, and RNAi-Mediated Knockdown of SHP-2.
Detailed information on plasmids, cell culture, Western blot analysis, and SHP-2 knockdown experiments are provided in SI Materials and Methods.
Fluorometric Measurement of S-Nitrosylated Proteins with DAN Assay.
Generation of SNO–SHP-2 was followed chemically with a modified DAN assay, as we and others have described previously (10, 11, 37, 45). Briefly, recombinant SHP-2 proteins were expressed and purified from Escherichia coli BL21(DE3). After removal of GST by thrombin digestion, recombinant SHP-2 was exposed to 200 µM SNOC or other physiological NO donors, and then incubated for 30 min at room temperature (RT). After passage through a desalting column (Sigma), the protein was incubated with 200 μM HgCl2 and 200 μM DAN for 30 min in the dark. NO release from S-nitrosylated SHP-2 was followed spectrofluorometrically, reflecting conversion of DAN to fluorescent 2,3-naphthyltriazole.
Biotin-Switch Analysis.
Analysis of SNO–SHP-2 with the biotin-switch assay was performed as described previously (10, 46). Briefly, cells were lysed with 1% Triton X-100 in HEN buffer (250 mM Hepes, 1 mM EDTA, 0.1 mM neocuproine). Free thiols were blocked with methyl methanethiosulfonate. Cell extracts were precipitated with acetone and resuspended in HEN buffer with 1% SDS. Nitrosothiols were selectively reduced by ascorbate to reform the thiol group and then biotinylated with 1 mM N-[6-(biotinamido)hexyl]-3′-(2′-pyridyldithio)-propionamide (Pierce). The biotinylated proteins were pulled down with streptavidin–agarose beads and analyzed by immunoblotting. Controls were performed lacking ascorbate to ensure specificity of the observed biotinylated bands.
tMCAO/R Mouse Model of Stroke.
All animal experiments were performed in accordance with institutional guidelines concerning care and treatment. Adult male mice weighing between 20 and 25 g were used for the present study. Mice were anesthetized with 1.5% to 2% (vol/vol) isoflurane in a mixture of 70% nitrous oxide and 30% oxygen. The animals’ body temperatures were maintained at 37 °C with a heating blanket and feedback system (Harvard Apparatus). Transient focal cerebral ischemia was induced by occlusion of the left middle cerebral artery using the intraluminal filament model (47). Reperfusion was achieved after withdrawal of the filament 1 h after occlusion. Relative cerebral blood flow was monitored during occlusion of the middle cerebral artery using a laser Doppler flowmeter (Perimed). Only mice with a relative cerebral blood flow decrease of <80% of baseline upon occlusion and recovery to >80% of baseline upon reperfusion were deemed to have undergone successful tMCAO/R; failures were excluded from further experimentation. For the biotin-switch assay, cortical hemispheres (n = 3) were harvested from the control or ischemic side of the brain immediately after reperfusion.
SHP-2 PTP Activity Assay.
In vitro phosphatase activity of recombinant Shp-2 with pNPP (Sigma) as a substrate was measured in a 30-μL reaction mixture containing 20 mM pNPP, 50 mM sodium citrate (pH 4.8), and 1 μM recombinant SHP-2 (32). After 30 min incubation at 37 °C, the reaction was terminated by addition of 1 M NaOH (270 μL). The amount of released p-nitrophenolate anion was determined by measuring the absorbance at 405 nm. PTP activity of immunoprecipitated SHP-2 was measured as previously described (48). Briefly, SHP-2 was immunoprecipitated from HEK-nNOS cells or rat primary cerebrocortical cell cultures with anti–SHP-2 antibody (Santa Cruz Biotechnology) at 4 °C for 2 h, and immunocomplexes were rinsed with 20 mM Hepes, pH 7.5, 150 mM NaCl, 0.1% Triton X-100, and 10% (vol/vol) glycerol. Immunoprecipitates were then incubated with 2 μg 32P-labeled myelin basic protein (MBP; Sigma) in PTP buffer (50 mM imidazole and 0.1% β-mercaptoethanol). For preparation of 32P-labeled MBP, 32P was incorporated into MBP by incubation with v-Abl (10 U: Invitrogen) and [γ-32P]ATP (300 μCi). PTP activity was determined by measuring the radioactivity of released 32P from MBP following trichloroacetic acid precipitation.
Immunocytochemistry and Apoptotic Cell Death Analysis of Cortical Neurons.
Primary cortical cultures were fixed in 4% (wt/vol) paraformaldehyde in PBS solution for 10 min and then permeabilized with 0.3% Triton X-100 and blocked with 5% (wt/vol) BSA for 1 h. Monoclonal anti–MAP-2 antibody (Sigma), monoclonal anti-NeuN antibody (Chemicon), and chicken polyclonal anti–MAP-2 antibody (Abcam) were used at 1:400 dilution (4 °C; overnight) to visualize neuronal cells. Monoclonal mouse anti–phospho-ERK antibody and anti-ERK antibody were from Cell Signaling. Alexa 555- or Alexa 647-conjugated secondary antibodies (Molecular Probes) were used for fluorescence labeling at a dilution of 1:200 (RT; 90 min). GFP expressing neurons were scored as transfected cells. The number of apoptotic nuclei was evaluated by costaining with Hoechst 33342 and manual scoring performed by using conventional fluorescence microscopy; nuclei that displayed characteristic morphology and chromatin condensation were scored as apoptotic. In some experiments, apoptotic cell death was assayed by TUNEL (Roche). To determine phospho-ERK levels in cortical neurons, deconvolved z-stack images were acquired using a Zeiss AX10 Observer.Z1 microscope equipped with SlideBook 5.0 software (Intelligent Imaging Innovations). The area of GFP-transfected neurons (n = 11–25) was measured by outlining GFP fluorescence in the cell body; the fluorescence intensity of phospho-ERK within the same pixel areas was then quantified.
Statistics.
Multiple comparisons were performed by ANOVA using a Scheffé post hoc test. All experimental points are expressed as mean ± SEM.
Supplementary Material
Acknowledgments
We thank Traci Fang-Newmeyer for preparing cultures. This work was supported in part by a postdoctoral fellowship of the Spanish Ministry of Education and Science Programa Nacional de Movilidad de Recursos Humanos del Plan Nacional de Investigación, Desarrollo e innovación 2008–2011 (to C.R.S.); and National Institutes of Health Grants R01 EY05477, P01 HD29687, P01 ES016738, and P30 NS076411 (to S.A.L.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1215501110/-/DCSupplemental.
References
- 1.Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347(6295):768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- 2.Huang Z, et al. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265(5180):1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura T, Lipton SA. S-Nitrosylation and uncompetitive/fast off-rate (UFO) drug therapy in neurodegenerative disorders of protein misfolding. Cell Death Differ. 2007;14(7):1305–1314. doi: 10.1038/sj.cdd.4402138. [DOI] [PubMed] [Google Scholar]
- 4.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: Purview and parameters. Nat Rev Mol Cell Biol. 2005;6(2):150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 5.Lipton SA, et al. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993;364(6438):626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- 6.Cho DH, et al. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324(5923):102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung KK, et al. S-nitrosylation of parkin regulates ubiquitination and compromises parkin’s protective function. Science. 2004;304(5675):1328–1331. doi: 10.1126/science.1093891. [DOI] [PubMed] [Google Scholar]
- 8.Hara MR, et al. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat Cell Biol. 2005;7(7):665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- 9.Qu J, et al. S-Nitrosylation activates Cdk5 and contributes to synaptic spine loss induced by β-amyloid peptide. Proc Natl Acad Sci USA. 2011;108(34):14330–14335. doi: 10.1073/pnas.1105172108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uehara T, et al. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature. 2006;441(7092):513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- 11.Yao D, et al. Nitrosative stress linked to sporadic Parkinson’s disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc Natl Acad Sci USA. 2004;101(29):10810–10814. doi: 10.1073/pnas.0404161101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang ZY. Protein tyrosine phosphatases: Structure and function, substrate specificity, and inhibitor development. Annu Rev Pharmacol Toxicol. 2002;42:209–234. doi: 10.1146/annurev.pharmtox.42.083001.144616. [DOI] [PubMed] [Google Scholar]
- 13.Qu CK. Role of the SHP-2 tyrosine phosphatase in cytokine-induced signaling and cellular response. Biochim Biophys Acta. 2002;1592(3):297–301. doi: 10.1016/s0167-4889(02)00322-1. [DOI] [PubMed] [Google Scholar]
- 14.Ke Y, et al. Deletion of Shp2 in the brain leads to defective proliferation and differentiation in neural stem cells and early postnatal lethality. Mol Cell Biol. 2007;27(19):6706–6717. doi: 10.1128/MCB.01225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi ZQ, Yu DH, Park M, Marshall M, Feng GS. Molecular mechanism for the Shp-2 tyrosine phosphatase function in promoting growth factor stimulation of Erk activity. Mol Cell Biol. 2000;20(5):1526–1536. doi: 10.1128/mcb.20.5.1526-1536.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang SQ, et al. Shp2 regulates SRC family kinase activity and Ras/Erk activation by controlling Csk recruitment. Mol Cell. 2004;13(3):341–355. doi: 10.1016/s1097-2765(04)00050-4. [DOI] [PubMed] [Google Scholar]
- 17.Chan RJ, Feng GS. PTPN11 is the first identified proto-oncogene that encodes a tyrosine phosphatase. Blood. 2007;109(3):862–867. doi: 10.1182/blood-2006-07-028829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang SS, et al. Coordinated regulation by Shp2 tyrosine phosphatase of signaling events controlling insulin biosynthesis in pancreatic beta-cells. Proc Natl Acad Sci USA. 2009;106(18):7531–7536. doi: 10.1073/pnas.0811715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarvis LA, Toering SJ, Simon MA, Krasnow MA, Smith-Bolton RK. Sprouty proteins are in vivo targets of Corkscrew/SHP-2 tyrosine phosphatases. Development. 2006;133(6):1133–1142. doi: 10.1242/dev.02255. [DOI] [PubMed] [Google Scholar]
- 20.Agazie YM, Hayman MJ. Molecular mechanism for a role of SHP2 in epidermal growth factor receptor signaling. Mol Cell Biol. 2003;23(21):7875–7886. doi: 10.1128/MCB.23.21.7875-7886.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Brain Res Rev. 2007;54(1):34–66. doi: 10.1016/j.brainresrev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Nozaki K, Nishimura M, Hashimoto N. Mitogen-activated protein kinases and cerebral ischemia. Mol Neurobiol. 2001;23(1):1–19. doi: 10.1385/MN:23:1:01. [DOI] [PubMed] [Google Scholar]
- 23.Szatmari E, Kalita KB, Kharebava G, Hetman M. Role of kinase suppressor of Ras-1 in neuronal survival signaling by extracellular signal-regulated kinase 1/2. J Neurosci. 2007;27(42):11389–11400. doi: 10.1523/JNEUROSCI.3473-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270(5240):1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 25.Frödin M, Gammeltoft S. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol Cell Endocrinol. 1999;151(1-2):65–77. doi: 10.1016/s0303-7207(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Liu L, Xia Z. Brain-derived neurotrophic factor stimulates the transcriptional and neuroprotective activity of myocyte-enhancer factor 2C through an ERK1/2-RSK2 signaling cascade. J Neurochem. 2007;102(3):957–966. doi: 10.1111/j.1471-4159.2007.04606.x. [DOI] [PubMed] [Google Scholar]
- 27.Yan L, et al. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell. 2007;130(2):247–258. doi: 10.1016/j.cell.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 28.Tonks NK. Protein tyrosine phosphatases: From genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7(11):833–846. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- 29.Barrett DM, et al. Inhibition of protein-tyrosine phosphatases by mild oxidative stresses is dependent on S-nitrosylation. J Biol Chem. 2005;280(15):14453–14461. doi: 10.1074/jbc.M411523200. [DOI] [PubMed] [Google Scholar]
- 30.Mikkelsen RB, Wardman P. Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene. 2003;22(37):5734–5754. doi: 10.1038/sj.onc.1206663. [DOI] [PubMed] [Google Scholar]
- 31.Hsu MF, Meng TC. Enhancement of insulin responsiveness by nitric oxide-mediated inactivation of protein-tyrosine phosphatases. J Biol Chem. 2010;285(11):7919–7928. doi: 10.1074/jbc.M109.057513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu W, Gong D, Bar-Sagi D, Cole PA. Site-specific incorporation of a phosphotyrosine mimetic reveals a role for tyrosine phosphorylation of SHP-2 in cell signaling. Mol Cell. 2001;8(4):759–769. doi: 10.1016/s1097-2765(01)00369-0. [DOI] [PubMed] [Google Scholar]
- 33.Aoki Y, et al. Increased susceptibility to ischemia-induced brain damage in transgenic mice overexpressing a dominant negative form of SHP2. FASEB J. 2000;14(13):1965–1973. doi: 10.1096/fj.00-0105com. [DOI] [PubMed] [Google Scholar]
- 34.Jakob S, et al. Nuclear protein tyrosine phosphatase Shp-2 is one important negative regulator of nuclear export of telomerase reverse transcriptase. J Biol Chem. 2008;283(48):33155–33161. doi: 10.1074/jbc.M805138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases: Specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19(4):2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forrester MT, Foster MW, Stamler JS. Assessment and application of the biotin switch technique for examining protein S-nitrosylation under conditions of pharmacologically induced oxidative stress. J Biol Chem. 2007;282(19):13977–13983. doi: 10.1074/jbc.M609684200. [DOI] [PubMed] [Google Scholar]
- 37.Gu Z, et al. S-nitrosylation of matrix metalloproteinases: Signaling pathway to neuronal cell death. Science. 2002;297(5584):1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 38.Chu CT, Levinthal DJ, Kulich SM, Chalovich EM, DeFranco DB. Oxidative neuronal injury. The dark side of ERK1/2. Eur J Biochem. 2004;271(11):2060–2066. doi: 10.1111/j.1432-1033.2004.04132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hetman M, Gozdz A. Role of extracellular signal regulated kinases 1 and 2 in neuronal survival. Eur J Biochem. 2004;271(11):2050–2055. doi: 10.1111/j.1432-1033.2004.04133.x. [DOI] [PubMed] [Google Scholar]
- 40.Luo Y, DeFranco DB. Opposing roles for ERK1/2 in neuronal oxidative toxicity: Distinct mechanisms of ERK1/2 action at early versus late phases of oxidative stress. J Biol Chem. 2006;281(24):16436–16442. doi: 10.1074/jbc.M512430200. [DOI] [PubMed] [Google Scholar]
- 41.Rusanescu G, Yang W, Bai A, Neel BG, Feig LA. Tyrosine phosphatase SHP-2 is a mediator of activity-dependent neuronal excitotoxicity. EMBO J. 2005;24(2):305–314. doi: 10.1038/sj.emboj.7600522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stanciu M, et al. Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J Biol Chem. 2000;275(16):12200–12206. doi: 10.1074/jbc.275.16.12200. [DOI] [PubMed] [Google Scholar]
- 43.Basso M, et al. Transglutaminase inhibition protects against oxidative stress-induced neuronal death downstream of pathological ERK activation. J Neurosci. 2012;32(19):6561–6569. doi: 10.1523/JNEUROSCI.3353-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yun HY, Gonzalez-Zulueta M, Dawson VL, Dawson TM. Nitric oxide mediates N-methyl-D-aspartate receptor-induced activation of p21ras. Proc Natl Acad Sci USA. 1998;95(10):5773–5778. doi: 10.1073/pnas.95.10.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wink DA, et al. Detection of S-nitrosothiols by fluorometric and colorimetric methods. Methods Enzymol. 1999;301:201–211. doi: 10.1016/s0076-6879(99)01083-6. [DOI] [PubMed] [Google Scholar]
- 46.Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. Protein S-nitrosylation: A physiological signal for neuronal nitric oxide. Nat Cell Biol. 2001;3(2):193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- 47.Kermer P, et al. BAG1 over-expression in brain protects against stroke. Brain Pathol. 2003;13(4):495–506. doi: 10.1111/j.1750-3639.2003.tb00480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qu CK, et al. A deletion mutation in the SH2-N domain of Shp-2 severely suppresses hematopoietic cell development. Mol Cell Biol. 1997;17(9):5499–5507. doi: 10.1128/mcb.17.9.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.