Cancer is a consequence of the accumulation of mutations and epigenetic alterations in oncogenes and tumor-suppressor genes. Recent advances in high-throughput sequencing methods and methylation array technology has led to genome-wide analyses of gene alterations in a wide variety of cancers, including colorectal cancer (1). These studies have revealed that there are hundreds to thousands of DNA alterations in the average colorectal cancer genome, and that there are roughly 80–100 genes that are commonly altered by nonsynonymous mutations and roughly 15 mutations in candidate “driver” genes per genome. (“Driver genes” are those genes that induce the formation of cancer.) These studies have also revealed considerable heterogeneity between colorectal cancers and that the majority of gene mutations are likely passenger events, which are bystanders in cancer formation. Thus, one of the major challenges facing cancer biologists now is how to identify the mutated genes that are functionally relevant in the tumorigenesis process. The traditional approach to deciphering which genes are functionally relevant assumes mutations in genes will lead to constitutive tumor-promoting or tumor-suppressing biological effects. Thus, mutations in genes for the tyrosine kinases (e.g., EGFR, BRAF, and so forth) have typically been classified as oncogenic because they often induce a constant state of inappropriate proliferation, whereas other genes, such as BRCA2, are most often classified as tumor-suppressor genes because these mutations impair DNA fidelity.
In this context of deciphering the functional consequences of altered genes in the colorectal cancer genome, in PNAS Genevois et al. (2) have identified TrkC (also called NTRK3) as a tumor-suppressor gene in colorectal cancer that is commonly silenced by aberrant DNA methylation (“The dependence receptor TrkC is a putative colon cancer tumor suppressor”). TrkC is a member of the neurotrophin receptor family and is the receptor for the neurotrophin NT-3 (Fig. 1). This family of receptors was identified following the discovery of the neurotrophins, NGF, BDNF, NT-3, and NT-4/5, and consists of TrkA, TrkB, TrkC, and p75NTR (also known as NTRK1, NTRK2, NTRK3, and NGFR, respectively). These receptors are single-pass transmembrane tyrosine kinases that were initially found to mediate neurotrophin-induced cell survival in the nervous system. Although initially identified in the nervous system, the kinases are ubiquitously expressed and are involved in a variety of diseases, including cancer (3, 4).
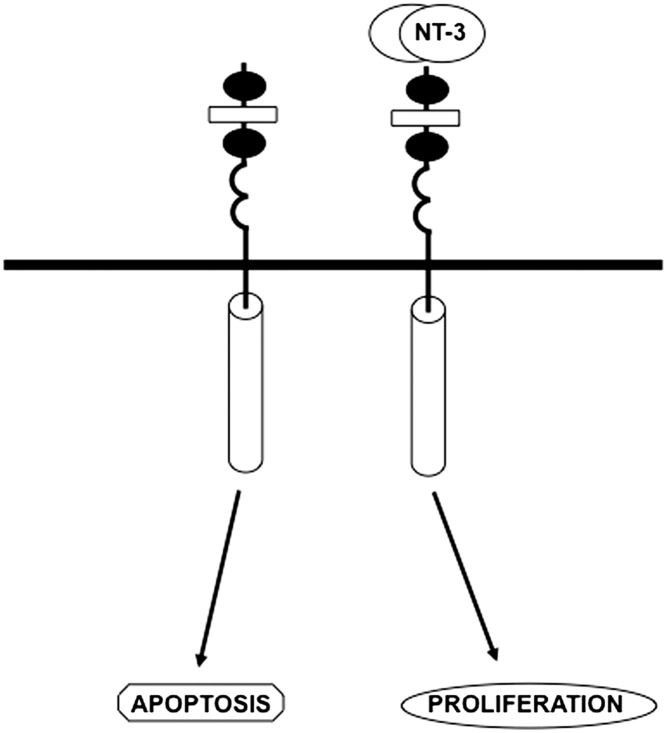
Fig. 1.
Schematic representation of the biological effects induced when TrkC is bound and not bound by NT-3. In the unbound state, TrkC can induce apoptosis through caspase-mediated cleavage. The receptor has two cystein-rich domains (black ovals), a leucine-rich domain (rectangle), two Ig like domains (half-circles), and cytoplasmic tyrosine kinase.
With regards to TrkC and colorectal cancer, one of the first insights into the potential role of neurotrophin receptors in colorectal cancer came when Bardelli et al. found that NTRK3 is a commonly mutated kinase in colorectal cancer (5). Given the classic view of tyrosine kinases as being oncogenic, the authors proposed that the NTRK3 mutations (i.e., G608S, I695V, and L760I) were likely tumor promoting. However, subsequently it has been shown that the neurotrophin receptors, and TrkC in particular, can function as both tumor-suppressor genes and oncogenes (4, 6). This contradiction led Genevois et al. (2) to carry out a series of careful studies to define the role of TrkC in colorectal cancer. This group chose to study TrkC in colorectal cancer because they had earlier shown that TrkC can function as a “dependence receptor” and because other dependence receptors, such as Deleted in Colon Cancer (DCC) and UNC5H, can act as tumor-suppressor genes in colorectal cancer (7, 8). It is the dependence-receptor aspect of the studies by Genevois et al. that make their findings particularly interesting and of importance to field of cancer biology. The concept of dependence receptors is a controversial one that was first introduced by Rabizadeh and Bredesen to explain neuron death induced by the absence of NGF (9, 10). Unlike with classic receptor biology, in which a receptor is in the “off” position when not bound with a ligand, dependence receptors are biologically active in both the ligand-bound (on) and -unbound (off) state (11). Initially, this phenomenon was demonstrated for the p75NTR neurotrophin receptor, but several investigators, including Patrick Mehlen, have provided evidence for other receptors functioning as dependence receptors (e.g., DCC, RET, Patched, UNC5H, IR) (12–14). The concept of dependence receptors is not universally accepted because it is plausible that the effects attributed to the ligand-free receptor are actually a default program intrinsic to the cell. In the case of the neurotrophins, it is well established that these ligands enhance cell survival in neurons, but it is less clear how the absence of ligand induces cell death. Mehlen’s research group has produced a series of well-designed studies that support the mechanism being based on dependence-receptor function (8, 15, 16).
After providing support for the dependence-receptor concept in the nervous system, for the last decade Mehlen’s team has been assessing the role of this class of receptors in cancer. In studies from over a decade ago, they resolved the mystery behind the role of DCC in colorectal cancer. Although DCC was one of the first putative tumor-suppressor genes found in colon cancer, mouse-model studies and other functional studies failed to reveal tumor-suppressor activity (17). Mehlen’s group revealed that the reason for this discrepancy was because DCC is a dependence receptor and that it had opposing effects on intestinal epithelial cells, depending on whether its ligand, netrin-1, was present (18). Genevois et al. and other investigators have further demonstrated a role for other dependence receptors in the pathogenesis of colorectal cancer, including RET and UNC5H (6, 19, 20).
In PNAS, Genevois et al. (2) have now demonstrated that TrkC has tumor-suppressor activity in the colon and, perhaps more importantly, they have provided evidence that TrkC is a conditional tumor suppressor that mediates its effects through its dependence-receptor activities (21). The authors found that the majority of colorectal cancers silence NTRK3 through aberrant DNA methylation and that reconstitution of TrkC in colorectal cancer cell lines suppressed hallmark behaviors of cancer (2). Having established that NTRK3 acts as a tumor-suppressor gene in the colon, they then showed that the addition of the ligand for TrkC, NT-3, rescues the cells from the tumor-suppressor effects of TrkC. The authors also showed that a naturally occurring NTRK3 mutation, E543D, lacks the proapoptotic activity of TrkC. These last sets of experiments demonstrated the conditional nature of TrkC’s tumor-suppressor activity and its role as a dependence receptor in colorectal cancer.
The significance of these findings is twofold. Genevois et al. (2) have found a unique tumor-suppressor gene for colorectal cancer and have provided support for the dependence receptor concept in cancer biology. Their results also extend a context-dependence model of mutations in cancers by reinforcing the idea that tumor-suppressor genes can be conditional tumor-suppressor genes, the effects of which vary depending on not only the state of other mutant genes in the cell, but also on the presence of ligands in the case of dependence receptors.
Although the studies by Genevois et al. (2) provide strong evidence that TrkC is inactivated in colon cancer and that it functions as a tumor-suppressor gene, their findings also raise a number of questions. Given that TrkC is a member of a family of receptors, it would help to know the expression levels of the other neurotrophins and their receptors in colorectal cancer. The expression of p75NTR and sortilin is particularly germane, as these receptors can bind the unprocessed form of neurotrophins, called proneurotrophins, and can induce cell death (22). It is possible that some of the ligand-independent effects of TrkC are actually secondary to p75NTR activity. In addition, the expression of TrkC and NT-3 in the normal colon and in colon adenomas remains to be defined. This information would provide a richer understanding of the consequences of TrkC deregulation in colorectal cancer formation and would address how NT-3 and TrkC interact to create a selective advantage for TrkC inactivation in cancer cells. The studies by Genevois et al. (2) have provided compelling evidence to justify further study of the neurotrophins in colorectal cancer. These additional studies will further define the potential for neurotrophins and their receptors to be the targets of anticancer therapies.
Footnotes
The author declares no conflict of interest.
See companion article on page 3017.
References
- 1.Leary RJ, et al. Integrated analysis of homozygous deletions, focal amplifications, and sequence alterations in breast and colorectal cancers. Proc Natl Acad Sci USA. 2008;105(42):16224–16229. doi: 10.1073/pnas.0808041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Genevois A-L, et al. Dependence receptor TrkC is a putative colon cancer tumor suppressor. Proc Natl Acad Sci USA. 2013;110:3017–3022. doi: 10.1073/pnas.1212333110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hondermarck H. Neurotrophins and their receptors in breast cancer. Cytokine Growth Factor Rev. 2012;23(6):357–365. doi: 10.1016/j.cytogfr.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Ivanov SV, Panaccione A, Brown B, et al. TrkC signaling is activated in adenoid cystic carcinoma and requires NT-3 to stimulate invasive behavior. Oncogene. 2012 doi: 10.1038/onc.2012.377. in press. [DOI] [PubMed] [Google Scholar]
- 5.Bardelli A, et al. Mutational analysis of the tyrosine kinome in colorectal cancers. Science. 2003;300(5621):949. doi: 10.1126/science.1082596. [DOI] [PubMed] [Google Scholar]
- 6.Bouzas-Rodriguez J, et al. Neurotrophin-3 production promotes human neuroblastoma cell survival by inhibiting TrkC-induced apoptosis. J Clin Invest. 2010;120(3):850–858. doi: 10.1172/JCI41013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehlen P, Llambi F. Role of netrin-1 and netrin-1 dependence receptors in colorectal cancers. Br J Cancer. 2005;93(1):1–6. doi: 10.1038/sj.bjc.6602656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tauszig-Delamasure S, et al. The TrkC receptor induces apoptosis when the dependence receptor notion meets the neurotrophin paradigm. Proc Natl Acad Sci USA. 2007;104(33):13361–13366. doi: 10.1073/pnas.0701243104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rabizadeh S, et al. Induction of apoptosis by the low-affinity NGF receptor. Science. 1993;261(5119):345–348. doi: 10.1126/science.8332899. [DOI] [PubMed] [Google Scholar]
- 10.Rabizadeh S, Bredesen DE. Ten years on: Mediation of cell death by the common neurotrophin receptor p75(NTR) Cytokine Growth Factor Rev. 2003;14(3-4):225–239. doi: 10.1016/s1359-6101(03)00018-2. [DOI] [PubMed] [Google Scholar]
- 11.Mehlen P, Fearon ER. Role of the dependence receptor DCC in colorectal cancer pathogenesis. J Clin Oncol. 2004;22(16):3420–3428. doi: 10.1200/JCO.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 12.Ellerby LM, et al. Kennedy’s disease: Caspase cleavage of the androgen receptor is a crucial event in cytotoxicity. J Neurochem. 1999;72(1):185–195. doi: 10.1046/j.1471-4159.1999.0720185.x. [DOI] [PubMed] [Google Scholar]
- 13.Bordeaux MC, et al. The RET proto-oncogene induces apoptosis: A novel mechanism for Hirschsprung disease. EMBO J. 2000;19(15):4056–4063. doi: 10.1093/emboj/19.15.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thibert C, et al. Inhibition of neuroepithelial patched-induced apoptosis by sonic hedgehog. Science. 2003;301(5634):843–846. doi: 10.1126/science.1085405. [DOI] [PubMed] [Google Scholar]
- 15.Mehlen P, et al. The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature. 1998;395(6704):801–804. doi: 10.1038/27441. [DOI] [PubMed] [Google Scholar]
- 16.Mehlen P, Bredesen DE. The dependence receptor hypothesis. Apoptosis. 2004;9(1):37–49. doi: 10.1023/B:APPT.0000012120.66221.b2. [DOI] [PubMed] [Google Scholar]
- 17.Arakawa H. Netrin-1 and its receptors in tumorigenesis. Nat Rev Cancer. 2004;4(12):978–987. doi: 10.1038/nrc1504. [DOI] [PubMed] [Google Scholar]
- 18.Mazelin L, et al. Netrin-1 controls colorectal tumorigenesis by regulating apoptosis. Nature. 2004;431(7004):80–84. doi: 10.1038/nature02788. [DOI] [PubMed] [Google Scholar]
- 19.Luo Y, Tsuchiya KD, Il Park D, et al. RET is a potential tumor suppressor gene in colorectal cancer. Oncogene. 2012 doi: 10.1038/onc.2012.225. 10.1038/onc.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin SK, et al. Epigenetic and genetic alterations in Netrin-1 receptors UNC5C and DCC in human colon cancer. Gastroenterology. 2007;133(6):1849–1857. doi: 10.1053/j.gastro.2007.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehlen P. The dependence receptor notion: Another way to see death. Cell Death Differ. 2005;12(8):1003. doi: 10.1038/sj.cdd.4401708. [DOI] [PubMed] [Google Scholar]
- 22.Yang L, et al. Targeted disruption of Smad4 in mouse epidermis results in failure of hair follicle cycling and formation of skin tumors. Cancer Res. 2005;65(19):8671–8678. doi: 10.1158/0008-5472.CAN-05-0800. [DOI] [PubMed] [Google Scholar]